Abstract
Eupnea is normal breathing. If eupnea fails, as in severe hypoxia or ischemia, gasping is recruited. Gasping can serve as a powerful mechanism for autoresuscitation. A failure of autoresuscitation has been proposed as a basis of the sudden infant death syndrome. In an in vitro preparation, endogenous serotonin is reported to be essential for expression of gasping. Using an in situ preparation of the Pet-1 knockout mouse, we evaluated such a critical role for serotonin. In this mouse, the number of serotonergic neurons is reduced by 85–90% compared with animals without this homozygous genetic defect. Despite this reduction in the number of serotonergic neurons, phrenic discharge in eupnea and gasping of Pet-1 knockout mice was not different from that of wild-type mice. Indeed, gasping continued unabated, even after administration of methysergide, a blocker of many types of receptors for serotonin, to Pet-1 knockout mice. We conclude that serotonin is not critical for expression of gasping. The proposal for such a critical role, on the basis of observations in the in vitro slice preparation, may reflect the minimal functional neuronal tissue and neurotransmitters in this preparation, such that the role of any remaining neurotransmitters is magnified. Also, rhythmic activity of the in vitro slice preparation has been characterized as eupnea or gasping solely on the basis of activity of the hypoglossal nerve or massed neuronal activities of the ventrolateral medulla. The accuracy of this method of classification has not been established.
Keywords: phrenic discharge, eupnea, phrenic bursts, norepinephrine, methysergide, WB-4101
in severe hypoxia or asphyxia, normal eupneic breathing may cease. Under these conditions, a second pattern of automatic ventilatory activity, gasping, may be recruited. Gasping can serve as a powerful mechanism for autoresuscitation and restoration of eupnea (2, 9, 15). A failure of gasping and/or its capacity for restarting eupnea has been proposed as the basis of the sudden infant death syndrome (6, 7).
On the basis of studies using an in vitro slice preparation of the medulla of the mouse, in which one pattern of rhythmic hypoglossal activity is considered to be gasping, it was reported that endogenous serotonin was essential for expression of gasping (13). We did not confirm this finding using an in situ perfused preparation of the juvenile rat. In this in situ preparation, the elicitation of gasping was not altered after administration of a variety of blockers of multiple groups of receptors for serotonin (16, 18). We proposed that this difference between results in vitro and in situ reflected the reduced nature of the slice preparation and, it was assumed, reduced levels of neurotransmitters of the in vitro preparations. This proposal was based on the observation that conductances through persistent sodium channels, which are essential for gasping, may be markedly influenced by exogenous levels of multiple neuromodulators, including serotonin (3, 4). In in vitro slice preparations, endogenous neurotransmitters may be substantially reduced, and an alteration in sensitivity to remaining neurotransmitters may be established (3, 4). Hence, any remaining neurotransmitters would assume a greater importance for gasping than in more intact preparations.
In addition to a difference in sensitivity of in vitro and in situ preparations to neurotransmitters, we cannot completely exclude the possibility that the various blockers of receptors for serotonin that were administered to the in situ preparations did not reach medullary neurons that are critical for the neurogenesis of gasping and/or did not reach these neurons in sufficient concentrations. Thus we have used Pet-1 knockout mice to further explore the possible role of endogenous serotonin in the genesis of gasping. In these animals, the genesis of the serotonergic nervous system is impaired, and the number of serotonergic neurons in the central nervous system is reduced by 85–90% compared with animals without this homozygous genetic defect (1, 5). This reduction in the number of serotonergic neurons resulted in reduced levels of serotonin in multiple regions of the brain (5).
At birth, Pet-1 mice have lower respiratory frequencies and more spontaneous apneas than wild-type littermates (1). Moreover, the elimination of such apneas and stabilization of rhythmic breathing are delayed in the Pet-1 mice. Within 10 days after birth, the frequency and pattern of breathing have become similar in Pet-1 and wild-type mice (1). However, no information is available concerning the neurogenesis of gasping in these mice, which lack a substantial fraction of the neurons that synthesize serotonin.
In addition to examining deficiencies in serotonin, we have further examined the potential role of norepinephrine in the regulation of gasping in mice (16). As in previous studies in rats (16, 18), the results of the present studies do not support the proposal that either serotonin or norepinephrine is critical for the expression of gasping.
METHODS
Experimental Groups
All procedures were approved by the Institutional Animal Care and Use Committee of Dartmouth College and Dartmouth Medical School. The Animal Resource Center at Dartmouth Medical School is an American Association for Laboratory Animal Care-approved facility.
Pet-1 heterozygous mice were mated, and the pups were genotyped using standard PCR protocols. Mice were studied 3–4 wk after birth. Two groups were used: Pet-1 homozygous mice, which, as noted above, have a severe deficiency of serotonergic neurons, and a mixed population of wild-type and heterozygous Pet-1 and C57BL/6 mice. Data were overlapping for individual animals of the groups of heterozygous Pet-1 and C57BL/6 mice; therefore, data for all were pooled.
Experimental Preparation
The preparation was similar to that described previously (16, 18), with surgical procedures being performed under deep enflurane anesthesia, as assessed by the absence of a withdrawal response to noxious pinching of a paw. When anesthetized, the mouse was bisected below the diaphragm, and the rostral portion of the body was immersed in ice-cold mock cerebrospinal fluid. The skull was opened, and a precollicular decerebration was performed. The phrenic nerve was sectioned.
The descending aorta was occluded, and the left ventricle was cannulated. Perfusate was delivered through this cannula. The perfusate contained (in mM) 1.25 MgSO4, 1.25 KH2PO4, 3.0 KCl, 24 NaHCO3, 125 NaCl, 2.5 CaCl2, 10 dextrose, and 0.1785 Ficoll 70 in distilled water. Gallamine triethiodide was added to the perfusate to block neuromuscular transmission. The temperature of the perfusate as it entered the aorta was 31°C. The perfusate was equilibrated with a gas mixture of 95% O2-5% CO2 (hyperoxic normocapnia).
Experimental Protocol
Efferent activity of the phrenic nerve was recorded with a suction electrode, filtered (0.6–6.0 kHz), and integrated (50-ms time constant) in all preparations. In some studies, a smoothing function was applied to the integrated signal to remove the artifact from the electrocardiogram. With the perfusate equilibrated with a hyperoxic-normocapnic gas mixture (95% O2-5% CO2), integrated phrenic discharge had the incrementing pattern of eupnea. This phrenic discharge was recorded for ≥20 min in all groups of mice. Recordings then continued without addition of drugs to the perfusate or after addition of a blocker of receptors for serotonin and/or norepinephrine. Methysergide was added as an antagonist of multiple types of serotonin (5-HT) receptors, including 5-HT1, 5-HT2, 5-HT4, 5-HT5, 5-HT6, and 5-HT7. WB-4101 was used to block the α1-adrenergic receptor (16). The concentrations of antagonists are based on previous studies and/or preliminary experiments in which drugs were delivered in increasing concentrations until a clear change in the phrenic discharge of eupnea was observed. Concentrations above and below this level were then tested. All drugs were obtained from Sigma or Tocris.
The pattern of phrenic discharge was altered from eupnea to gasping by production of ischemia, which involved termination of perfusion for 40 s. Perfusion was then recommenced. The duration of ischemia was chosen on the basis of preliminary studies in which we established that, with this duration, gasping was systematically produced and, yet, eupnea could be consistently reestablished. Gasping was apparent as a change in the pattern of integrated phrenic discharge from incrementing to decrementing (10, 11, 17).
Analyses of Data
Integrated phrenic activity was analyzed for the duration of the burst (neural inspiration), period between bursts, peak height, and time to reach peak height, expressed as a percentage of neural inspiration. All these variables were defined in eupnea and gasping. In addition, we defined the time from the onset of ischemia to the first gasp and the period without phrenic discharge that intervened after the recommencement of perfusion until the first phrenic burst reappeared. Statistical evaluations were made using t-tests or one-way ANOVAs as appropriate. When an ANOVA indicated significant differences between groups, specific preplanned comparisons were made using P values adjusted by Bonferroni's method. Thus all statistical comparisons were between the various groups of mice. Data are presented as means ± SE. Some of the data were not normally distributed, and, where noted, the median is reported as well. Moreover, statistical comparisons of nonnormally distributed data were performed only after a logarithmic transformation of the data.
RESULTS
Eupnea and Gasping
Control preparations.
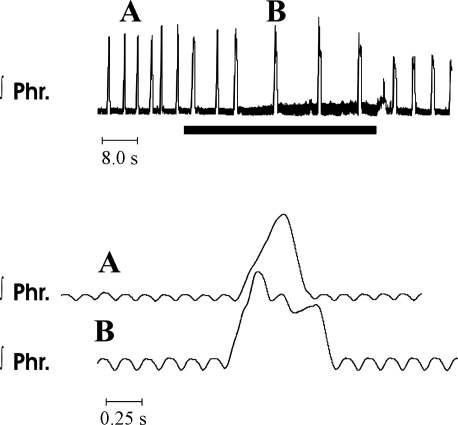
In control preparations (n = 15), phrenic discharge during eupnea was characterized by a sudden onset and rise to a peak value during the last half of the burst (70 ± 1.4% of neural inspiration). With a termination of perfusion, phrenic discharge was altered, such that peak discharge was achieved in the first portion of the burst (34 ± 2.8% of neural inspiration). This decrementing pattern of phrenic discharge is typical of gasping (Fig. 1 ). The delay between the onset of ischemia and the first gasp averaged 20.9 ± 1.8 s. As shown by the records of multiple respiratory cycles in Fig. 2, phrenic discharge was distinctly different in eupnea and gasping in each respiratory cycle.
Fig. 1.
Activity of the phrenic nerve in eupnea (trace A) and gasping (trace B) in the mouse. Top: integrated phrenic (Phr) activity, with perfusate equilibrated with a hyperoxic gas mixture (trace A). Bottom: in expanded trace A, note incrementing pattern of discharge. Small oscillations in baseline activity represent artifacts of electrocardiogram. During the period designated by the solid horizontal bar, perfusion was terminated, and the pattern of phrenic discharge was altered to gasping. Note decrementing pattern of integrated phrenic discharge in B. On a recommencement of perfusion (top traces), phrenic discharge returned within 5 s.
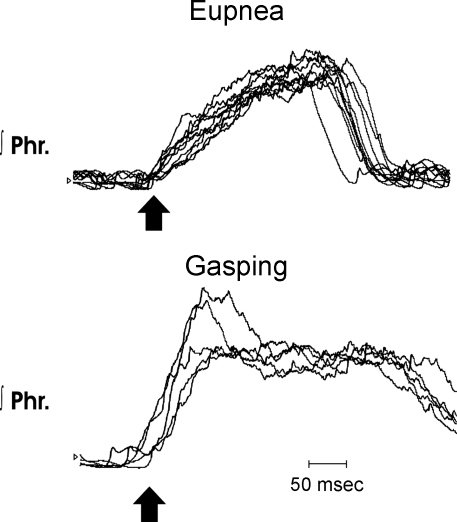
Fig. 2.
Rates of rise of integrated phrenic discharge in eupnea and gasping in a wild-type mouse. Top: 10 cycles of integrated phrenic discharge aligned at the start of each burst (arrow) during eupnea. Note incrementing rise to reach a peak close to the end of the burst. Bottom: 5 cycles aligned (arrow) during gasping. Peak height was reached soon after onset, and the pattern was decrementing in most cycles.
As exemplified by the records of Figs. 1 and 2 and mean data of Fig. 3, the duration of the phrenic burst, period between bursts, and peak height were significantly greater in gasping than in eupnea. After perfusion was recommenced, the time before the first rhythmic burst of phrenic discharge varied from 3 to 80 s in individual mice. Mean time was 22.7 ± 6.0 s, and median was 10.0 s; these data were not normally distributed.
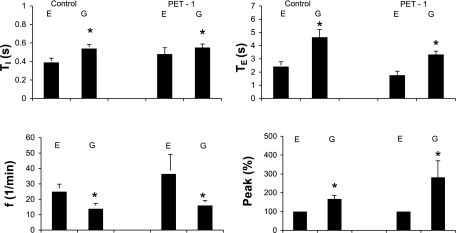
Fig. 3.
Alterations in variables of phrenic activity [duration of the phrenic burst (Ti), period between bursts (Te), respiratory frequency (f), and peak height of integrated phrenic discharge, expressed as a percentage of value during eupnea] with change from eupnea (E) to gasping (G) in control (n = 15) and Pet-1 (n = 8) mice. Values are means ± SE. In both groups of mice, Ti and Te were significantly longer in gasping than in eupnea, whereas f was significantly less. Peak height was significantly greater in gasping. *P < 0.05.
Pet-1 preparations.
For the eight Pet-1 preparations, the pattern of phrenic discharge in eupnea was the same as in control mice (Figs. 4 and 5). Variables of this phrenic discharge were likewise similar to controls (Fig. 3).
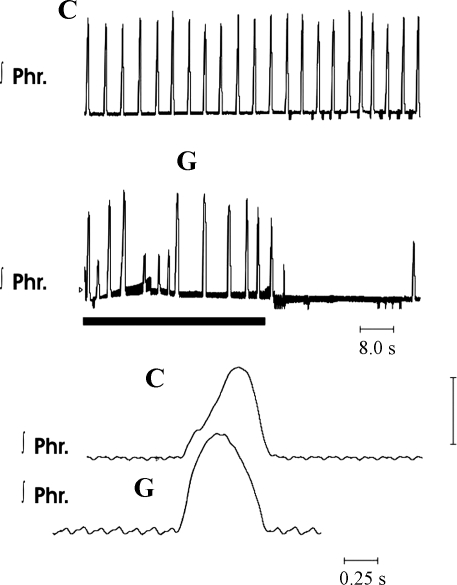
Fig. 4.
Activity of the phrenic nerve in eupnea and gasping in a Pet-1 homozygous mouse. During control recordings (C), phrenic discharge had the incrementing pattern of eupnea. On termination of perfusion (solid horizontal bar), phrenic discharge was changed to the decrementing pattern of gasping (G).
Fig. 5.
Rates of rise of integrated phrenic discharge in eupnea and gasping in a knockout mouse. Top: 10 cycles of integrated phrenic discharge aligned at the start of each burst during eupnea (arrow). Note incrementing rise to reach a peak close to the end of the burst. Bottom: 5 cycles aligned during gasping (arrow). Peak height was reached soon after onset, and pattern was decrementing in most cycles.
On exposure of the preparation to ischemia, the pattern was altered to that of gasping in 19.1 ± 1.6 s. As shown by the overlay of multiple respiratory cycles in Fig. 5, gasping in Pet-1 mice was similar to that in control mice, in that the rate of rise of integrated phrenic discharge was markedly increased. Also, as in control mice, the frequency of phrenic bursts was significantly lower and peak height was higher than in eupnea (Fig. 3). Peak phrenic discharge was attained after 41.1 ± 4.6% of the burst was completed. With the recommencement of perfusion, the time for the return of the first phrenic burst was very variable (range 2–180 s, mean 43 ± 24 s, median 15.0 s). This time for return of rhythmic phrenic activity was not significantly different between Pet-1 and control mice (comparison performed on logarithmically transformed data).
Alterations After Methysergide
Control preparations.
After administration of 1–3 μM methysergide to 14 control preparations, the frequency of phrenic bursts increased significantly, and their peak height significantly declined (Figs. 6 and 7). These changes were not dependent on the concentration of methysergide: when the variables were expressed as a function of concentration, no significant correlation was found (results not shown). The rate of rise of phrenic activity, as defined by the time to reach peak height, was not significantly altered.
Fig. 6.
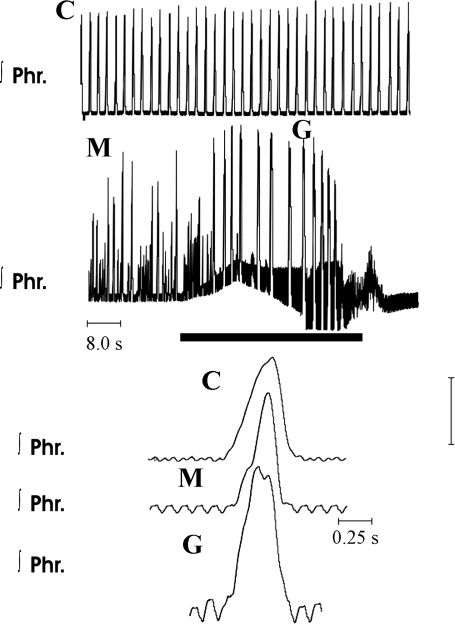
Activity of the phrenic nerve in eupnea, before and after administration of methysergide, and in gasping in a wild-type mouse. C represents traces obtained in eupnea. Note incrementing pattern of incrementing phrenic discharge. M represents recordings 10 min after addition of 2 μM methysergide to the perfusate. Frequency of phrenic bursts had increased and peak height was variable. Solid horizontal bar shows period of ischemia, during which perfusion was terminated. Pattern of phrenic discharge was altered to the decrementing pattern of gasping (G). On a recommencement of perfusion, rhythmic phrenic discharge did not return for 30 s.
Fig. 7.
Influence of methysergide and WB-4101 on variables of phrenic discharge (see Fig. 3 legend) in gasping in a control group of wild-type and normal mice. Control values are for preparations in which ischemic gasping was produced without addition of methysergide or WB-4101. Values are reproduced from Fig. 3, in which f fell significantly and peak rose significantly in gasping compared with eupnea. Frequencies were slightly higher and peak heights were lower after addition of methysergide or WB-4101 in gasping than in control preparations in gasping. Number of preparations is as follows: n = 15 for control, n = 3 for 1 μM methysergide, 3 μM methysergide, 0.5 μM WB-4101, and 1 μM WB-4101; n = 8 for 2 μM methysergide and 2 μM WB-4101.
Within 20 s after the onset of ischemia, phrenic discharge changed to a decrementing pattern typical of gasping (Fig. 6). Peak phrenic height was reached within the first 40% of neural inspiration. The frequency of phrenic bursts during gasping fell significantly below levels during eupnea before or after administration of methysergide. This fall in frequency was due to significant increases in the durations of neural inspiration and expiration (Fig. 6). Although the frequency of gasping after methysergide administration was above that of gasping in control preparations, the difference was not statistically significant (Fig. 7). Similarly, whereas peak integrated phrenic heights significantly rose compared with values during eupnea before or after administration of methysergide, peak heights in gasping after methysergide administration were not significantly different from those during gasping in control preparations (Fig. 7). None of these changes in variables of phrenic discharge was correlated with the concentration of methysergide.
On resumption of perfusion, rhythmic activity of the phrenic nerve returned. In the preparations that received no drugs, the duration of this apneic period was extremely variable. However, on average, this period was longer than that in control preparations (22.7 ± 6.0 s) after administration of 2 μM (104.7 ± 68 s, n = 8) or 3 μM (47.6 ± 18 s, n = 3) methysergide. One preparation never recovered rhythmic activity after 2 μM methysergide. The apneic period following 1 μM methysergide (28.6 ± 17.0, n = 3) was similar to control.
Pet-1 preparations.
Methysergide at 3 μM was administered to five Pet-1 homozygous mice. Changes in eupnea were similar to those of control preparations, in that the frequency of phrenic bursts increased and the peak height of integrated phrenic discharge fell. The time to reach peak phrenic height was not altered.
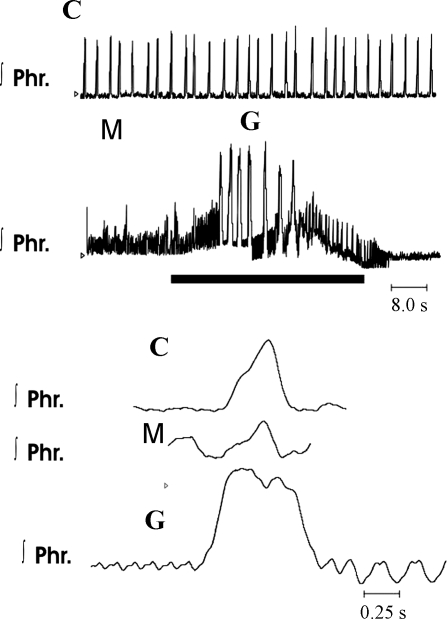
On exposure to ischemia, gasping was induced in each preparation within 13.4 ± 1.6 s (Fig. 8). Gasping was similar to that of control preparations, in that the frequency was lower and phrenic peak height was higher than during eupnea. Differences between control and methysergide-treated preparations were not significant (Fig. 9). On average, the time to recovery of rhythmic phrenic discharge after reestablishment of perfusion was greatly prolonged in methysergide-treated mice (77.6 ± 30 s, range 4–170 s).
Fig. 8.
Alterations in integrated phrenic discharge after administration of methysergide to a Pet-1 homozygous mouse. C represents control recordings; M represents recordings obtained 5 min after addition of 3 μM methysergide to perfusate. Note increase in frequency and decrease in peak height of integrated phrenic bursts. G represents gasps induced during period of ischemia (solid horizontal bar). Rhythmic phrenic bursts did not return until 116 s after recommencement of perfusions. Bottom traces are on a faster time scale than top traces.
Fig. 9.
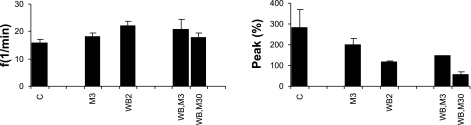
Variables of gasping in Pet-1 mice that received methysergide and/or WB-4101. C, untreated control (n = 8); M3, 3 μM methysergide (n = 2); WB2, 2 μM WB-4101 (n = 2); WB,M3, 2 μM WB-4101 + 3 μM methysergide (n = 2); WB,M30, 2 μM WB-4101 + 30 μM methysergide (n = 3). Compared with control, peak height fell in preparations receiving drugs and fell greatly in those receiving the highest concentrations.
Alterations After WB-4101
Control preparations.
The frequency of phrenic bursts increased after administration of 0.5 μM (n = 3), 1.0 μM (n = 3), and 2.0 μM (n = 4) WB-4101 (Fig. 10). This increase reflected a decline in the interval between bursts. Peak height was unaltered, except for a significant decline at the highest concentration. Rates of rise were unaltered at any concentration.
Fig. 10.
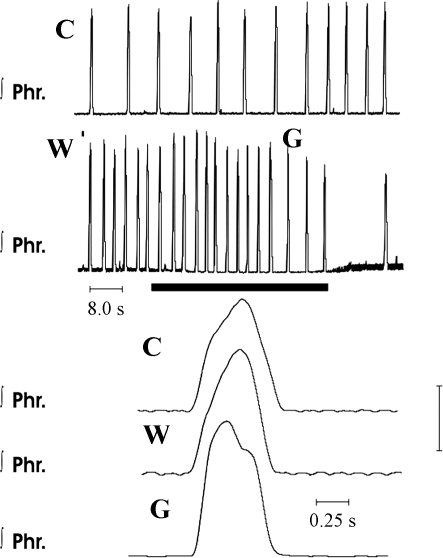
Eupnea and gasping in a wild-type mouse after blockade of α1-adrenergic receptors with WB-4101. C, integrated records of phrenic discharge; W, recordings obtained 10 min after addition of 1.0 μM WB-4101 to perfusate. Incrementing pattern of phrenic discharge continued. During the period designated by the solid horizontal bar, perfusion was terminated and gasping (G) was induced. Note decrementing discharge pattern of gasping.
In ischemia, gasping was elicited after mean delays of 13.3, 10.3, and 14.8 s at the various concentrations. The frequency of gasps was lower than the frequency of eupnea after drug administration, and the peak height was greater (Fig. 10). Again, as observed after administration of methysergide, frequencies in gasping were higher than in control preparations after WB-4101, and peak heights of integrated phrenic discharge were lower than in control preparations, but none of these changes was significant (Fig. 7). The apneic period before the return of rhythmic phrenic discharge (8.2 ± 1.3 s, range 5–16 s) was similar to that of control preparations.
Pet-1 preparations.
Two Pet-1 mice were treated with 2 μM WB-4101. Changes in eupnea were similar to those in control preparations, in that the frequency of phrenic bursts increased and the peak height of integrated phrenic discharge fell. The time to reach peak phrenic height was not altered.
On exposure to ischemia, gasping was induced within 20.5 ± 1.5 s. Again, gasping was similar to that in control preparations, in that the frequency was lower and the peak height was higher than during eupnea. Peak heights were lower in WB-4101-treated than in untreated Pet-1 mice (Fig. 9). Times to recovery of rhythmic phrenic activity on restoration of perfusion were 9 and 22 s.
Alterations After Methysergide and WB-4101 Administration
Two Pet-1 mice received 3 μM methysergide + 2 μM WB-4101, and a second group of three Pet-1 mice received 30 μM methysergide + 2 μM WB-4101. Changes in eupnea and gasping of the first group were similar to those in mice that received no drugs. Specifically, in gasping, frequencies were lower and peak integrated phrenic heights were higher than in eupnea. In two mice of the second group, frequencies and peak heights were greatly reduced during recording in hyperoxia. However, on exposure to ischemia, gasping was elicited in <25 s in each preparation (Fig. 11). Peak heights during gasping were much below those of control preparations, but these changes were not statistically significant (Fig. 9). The time to recovery of rhythmic phrenic discharge was prolonged in two preparations (180 and 220 s) but was only 4 s in the other.
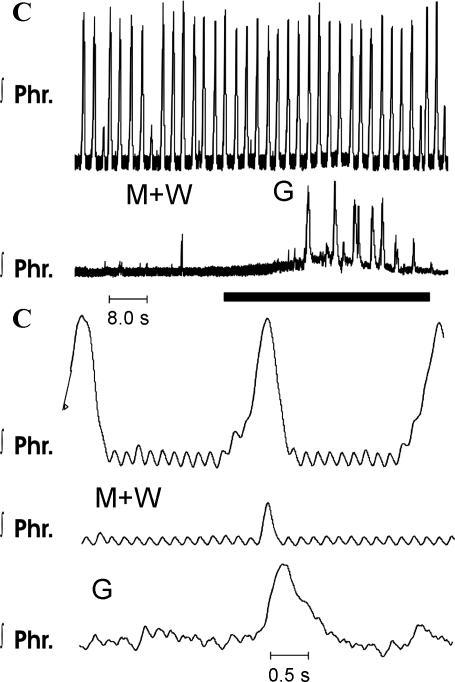
Fig. 11.
Elicitation of gasping after administration of 30 μM methysergide (M) and 2.0 μM WB-4101 (W) to a Pet-1 mouse. C, records of integrated phrenic discharge (Phr) before drugs were given; M+W, recordings obtained 10 min after administration of methysergide and WB-4101. Phrenic discharge was reduced to occasional bursts. In the period designated by the solid horizontal bar, ischemia was induced and gasping (G) was elicited.
DISCUSSION
The major conclusion of this study is that the genesis of gasping is uninfluenced by levels of serotonin in the brain stem of mice. Thus variables of gasping in Pet-1 knockout mice and in control mice were indistinguishable, even though the number of serotonergic neurons is reduced by 80–90% in the Pet-1 knockout mice. With the addition of methysergide, a blocker of many types of receptors for serotonin, gasping was still unaltered in Pet-1 knockout mice and mice without this homozygous genetic defect. The recovery of rhythmic phrenic discharge after a period of ischemia and gasping was, on average, prolonged in Pet-1 knockout mice and in all mice that received a high concentration of methysergide. This finding supports our conclusion from previous studies that serotonin may play a role in restarting respiratory activity after a period of ischemia and gasping, rather than in generating gasping per se (16).
These results and conclusions as to the absence of a role for serotonin in the genesis of gasping in the in situ preparation of the mouse are, with minor exceptions, identical to those of two previous studies using the in situ preparation of the juvenile rat (16, 18). These results are fundamentally different from those derived from studies using an in vitro preparation of the mouse. Serotonin is reported to be essential for the expression of an in vitro rhythm considered to be gasping (19). This in vitro rhythm is characterized on the basis of recordings of activity of the hypoglossal activity or of massed neuronal activities from the ventrolateral medulla of a slice preparation (8, 19). We previously proposed that the difference between findings concerning a role for serotonin may reflect the diminished complexity and connectivity of neural tissue in brain stem slices (16). Similarly, the types and levels of neurotransmitters of the medullary in vitro slice are assumed to be reduced compared with those of the entire pontomedullary brain stem of the in situ preparation. Inasmuch as multiple neurotransmitters may influence mechanisms underlying gasping (3, 4, 16, 18), any of these neurotransmitters remaining in vitro, such as serotonin, will assume a disproportionate importance for rhythm generation in this highly reduced preparation. Finally, findings from the in vitro preparation are predicated on the assumption that activity of the hypoglossal nerve and/or massed medullary neuronal activities provide an unambiguous index of the respiratory rhythm, be it eupnea or gasping (8, 12). However, a detailed comparison of different cranial and spinal neuronal activities in eupnea and gasping in the mouse has not been performed, and the characteristics of eupnea and gasping, defined by activity of the phrenic nerve, may not be accurately transferable to activities of the hypoglossal nerve or massed neuronal activities in medullary slices.
In addition to serotonin, which might influence gasping, we again assessed a possible role for norepinephrine (16). As in previous studies, blockade of α1-norepinephrine receptors with WB-4101 caused little alteration of gasping (16). Gasping was also still recorded when blockade of these α1-norepinephrine receptors was combined with blockers of receptors for serotonin. In the Pet-1 knockout mice, in which the number of neurons that synthesize serotonin is greatly reduced, we administered quantities of methysergide and WB-4101 that were sufficient to depress greatly the eupneic rhythm. Yet, even under such extreme treatment, gasping could still be elicited. The return of rhythmic phrenic activity was delayed after this treatment, which may simply reflect the suppression of eupnea. In preparations receiving lesser concentrations of antagonists of receptors for serotonin or norepinephrine, the recovery of rhythmic phrenic activity after gasping was prolonged after methysergide had been given. As opposed to our previous results in rats (16), simultaneous administration of WB-4101 failed to prolong this recovery of rhythmic activity. We have no firm explanation for this difference in results, which could represent a difference in permeability of the blood-brain barrier to drugs in mice compared with rats. Also, a reduced importance of norepinephrine in the establishment of eupnea in mice, as opposed to rats, is possible.
Differences Between Phrenic Discharge in Eupnea and Gasping in the Mouse and Rat
Patterns of phrenic discharge in eupnea and gasping differed markedly in the in situ preparation of the mouse compared with the rat. Not only was the rate of rise of phrenic discharge much greater in gasping than in eupnea, but the frequency of phrenic bursts was significantly less in gasping. In the in situ preparation of the rat, rates of rise of phrenic discharge were again greater in gasping than in eupnea, but the frequency of phrenic bursts was the same for these two patterns (16). Although we have no explanation for this difference between in the situ preparation of the mouse and the rat, the relative frequencies of phrenic bursts in eupnea and gasping are very dependent on the temperature of the preparation (14, 17). Thus, in the rat preparation, frequencies of phrenic bursts were the same in eupnea and gasping only during hypothermia. With elevations of temperature of the in situ rat preparation, the frequency became significantly less in gasping than in eupnea.
Summary
A critical linkage of serotonin and gasping cannot be found in in situ preparations of the mouse or rat (16, 18). No failure of gasping was found in Pet-1 knockout mice, which lack most of the neurons that synthesize serotonin in the brain. Gasping continued even after treatment of the Pet-1 mice with methysergide, which is a relatively promiscuous antagonist of receptors for serotonin.
GRANTS
These studies were supported by National Heart, Lung, and Blood Institute Grant HL-26091. Mice were obtained from a colony supported by funds from National Institute of Child Health and Human Development Grant P01-HD-36379.
REFERENCES
- 1.Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol 159: 85–101, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fewell JE Protective responses of the newborn to hypoxia. Respir Physiol Neurobiol 149: 243–255, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey PJ, Li X, Li Y, Bennett DJ. Endogenous monoamine receptor activation is essential for enabling persistent sodium currents and repetitive firing in rat spinal motoneurons. J Neurophysiol 96: 1171–1186, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37: 233–247, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Hunt CE The cardiorespiratory control hypothesis for sudden infant death syndrome. Clin Perinatol 19: 757–771, 1992. [PubMed] [Google Scholar]
- 7.Leiter JC, Böhm I. Mechanisms of pathogenesis in the sudden infant death syndrome. Respir Physiol Neurobiol 159: 127–138, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci 3: 600–607, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Lumsden T Effects of bulbar anaemia on respiratory movements. J Physiol 59: lvii–lx, 1924. [Google Scholar]
- 10.Paton JFR, Abdala APL, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci 9: 311–316, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Paton JFR, St-John WM. Medullary pacemakers are essential for gasping, but not eupnea in mammals. J Appl Physiol 103: 718–720, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Peña F, Meza-Andrade R, Páez-Zayas V, González-Marín MC. Gasping generation in developing Swiss-Webster mice in vitro and in vivo. Neurochem Res 33: 1492–1500, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St.Jacques R, St-John WM. Sensitivities of eupnea and gasping to alterations in temperature of in vivo and perfused rat preparations. Respir Physiol 123: 215–224, 2000. [DOI] [PubMed] [Google Scholar]
- 15.St John WM Neurogenesis, control, and functional significance of gasping. J Appl Physiol 68: 1305–1315, 1990. [DOI] [PubMed] [Google Scholar]
- 16.St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia-induced depression requires activation of receptors for norepinephrine and serotonin. J Appl Physiol 104: 665–673, 2008. [DOI] [PubMed] [Google Scholar]
- 17.St-John WM, Paton JFR. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol 123: 201–213, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Toppin VAL, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol 103: 220–227, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci 26: 2623–2634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]