Abstract
The effect of elevated muscle temperature on mechanical efficiency was investigated during exercise at different pedal frequencies in young and older women. Eight young (24 ± 3 yr) and eight older (70 ± 4 yr) women performed 6-min periods of cycling at 75% ventilatory threshold at pedal frequencies of 45, 60, 75, and 90 rpm under control and passively elevated local muscle temperature conditions. Mechanical efficiency was calculated from the ratio of energy turnover (pulmonary O2 uptake) and mechanical power output. Overall, elevating muscle temperature increased (P < 0.05) mechanical efficiency in young (32.0 ± 3.1 to 34.0 ± 5.5%) and decreased (P < 0.05) efficiency in older women (30.2 ± 5.6 to 27.9 ± 4.1%). The different effect of elevated muscle temperature in young and older women reflects a shift in the efficiency-velocity relationship of skeletal muscle. These effects may be due to differences in recruitment patterns, as well as sarcopenic and fiber-type changes with age.
Keywords: energy turnover, efficiency/velocity relationship, elderly, pedal frequency
it is well established that muscle function and locomotory performance decline from the fifth decade onward, with even greater declines from the age of 65 yr (33). One of the main factors responsible for this decline is a reduction in the ability to develop power, which is caused by a reduction in both force generation and speed of contraction (e.g., Ref. 36). While the importance of “explosive” power output for functional ability of the older population is well established (for review, see Ref. 27), perhaps as important, although less well understood, is the efficiency of the muscles of older people during “sustained” development of power over longer periods of time, e.g., periods of walking, climbing a longer set of stairs. Improving efficiency is important during locomotion in that, for a given amount of energy, more work can be performed, or vice versa. This would be of benefit to the individual, as a more efficient muscle would result in an improved exercise tolerance and greater fatigue resistance.
Temperature is widely recognized as an important determinant of skeletal muscle function (6, 30). The main effect of heating is to alter the force-velocity and, consequently, the power-velocity relationship, such that maximum power output is increased (10). The rate of ATP consumption is also temperature dependent (22), which is most likely due to a temperature-related increase in myofibrillar ATPase activity (22, 35). As a consequence, it is logical that efficiency is also temperature sensitive, which has been observed in single human muscle fibers (22). Like force and power, muscle efficiency is dependent on contraction velocity and demonstrates a parabolic relationship (39), where peak efficiency occurs at a slightly lower contraction velocity than maximum power (22). Furthermore, the interrelationship between muscle temperature and contraction speed has been demonstrated for mechanical efficiency in young male subjects during sustained submaximal exercise (15).
Surprisingly, the effect of a change in muscle temperature on the efficiency of muscle contraction in humans in vivo has received little attention, especially in the older population. This is despite the fact that the temperature of human skeletal muscle can vary over a wide range, depending on the metabolic heat liberated by the muscle itself during contraction (3, 32) and the environmental conditions. This latter aspect is particularly significant for older people, who may experience lower than normal muscle temperature due to physiological changes with age, such as lower metabolic rate (24) and impaired peripheral circulation (12), and the difficulties that the older population encounter in maintaining a warm living environment.
The purpose of the present study was, therefore, to determine whether an increase in muscle temperature affected efficiency during sustained dynamic exercise in young and older women. It was hypothesized that increasing muscle temperature would increase efficiency in young and older people. It was also hypothesized that these changes would be dependent on the contraction speed at which the exercise was performed, as previously suggested (15). We have tested this hypothesis by estimating net and mechanical efficiency during cycling at 45, 60, 75, and 90 rpm at normal muscle temperature and following passive heating of the working muscle before exercise. We have also examined whether the changes in mechanical efficiency are related to muscle fiber composition.
METHODS
Participants
A total of 16 women volunteered to participate in the investigation. Women were recruited because it has been considered that women should be the initial target of studies and interventions to help maintain or increase the ability to perform everyday tasks (33). Eight older women (O; aged 70 ± 4 yr, height 159.6 ± 3.0 cm, body mass 66.8 ± 4.4 kg) were selected according to the exclusion criteria used to define “healthy” older participants for exercise studies, as previously proposed (19). All older participants were physically active and were required to undergo a general medical examination and ECG test. A control population of 8 young women (Y; age 24 ± 3 yr, height 168.5 ± 2.3 cm, body mass 64.4 ± 7.0 kg) were also recruited. The Y participants were recreationally active and performed a minimum of 30-min moderate-intensity exercise at least three times per week. All participants provided written, informed consent, and the investigation was approved by the University of Strathclyde Ethics Committee.
Experimental Design
Participants completed a series of cycling exercises, in a randomized order. Each exercise was performed under a control muscle temperature condition or after the legs had been passively heated. All control exercises were performed during a single visit to the laboratory with sufficient (∼1 h) recovery between each exercise. Each heated trial required a longer preparation time, due to the time taken to heat the muscle. Therefore, the heated trials were performed over two visits to the laboratory, again with sufficient (∼1 h) recovery between each trial. The 1-h recovery period is also sufficient to allow muscle temperature to return to normal levels (16). The order of control and heated sessions was counterbalanced, and the order of trials (at each pedal frequency) within that framework was randomized. There was a minimum of 5 days separating each visit. Participants arrived at the laboratory fasted and were given a commercially available cereal bar at least 1 h before their first and third trials. Subjects were allowed to consume water whenever they wanted.
Preexperimental Procedures
At least 7 days before the main trials, all participants completed an incremental exercise test on an electromagnetically braked cycle ergometer (Lode, Gronigen, Netherlands) to determine their ventilatory threshold (Tvent), performed at a pedal frequency of 60 rpm. These tests were terminated at a submaximal intensity by identification of Tvent (4) using respiratory exchange ratio (RER) values and the O2 uptake (V̇o2)/CO2 production (V̇co2) relationship (as ascertained by the online gas analysis system). Following a suitable recovery period, participants performed a number of habituation trials that familiarized them with the nature of the exercise (i.e., pedal frequency) involved.
Experimental Protocol
Each exercise protocol involved a 6-min exercise bout on the cycle ergometer at an external power output equivalent to 75% Tvent. Each exercise protocol was performed at four pedal frequencies (45, 60, 75, and 90 rpm) in a randomized order under both control and heated muscle temperature conditions. In the control condition, participants rested in a seated position at normal room temperature (∼22°C) for at least 30 min. In the heated condition, muscle temperature was increased by sitting in a standard bath at a water temperature of 42°C for 30 min. Only the lower limbs up to the small of the back were immersed.
Procedures.
In the control trials after changing into shorts and tee-shirt, the participants rested in a seated position for ∼30 min. They then moved to a couch where the muscle temperature probe was inserted, muscle temperature measured, and the probe then removed. After this, they moved to the cycle ergometer and were instrumented for measurement of pulmonary gas exchange. They then rested for 3 min before beginning the 6-min exercise. Immediately after exercise, participants returned to the chair for at least 1-h recovery before repeating the process.
In the heated trials, immediately after exiting the bath, participants dried themselves and changed into shorts and tee-shirt. They then lay semisupine on a couch with the lower half of the body wrapped in a heated electric blanket to maintain an elevated muscle temperature. At an appropriate time, the blanket was opened to allow insertion of the muscle temperature probe. The blanket was then closed, and temperature was monitored until it reached an appropriate level or had stabilized. This was usually 5 min. After this time, the blanket was removed, the temperature probe was also removed, and the participants moved to the cycle ergometer to perform the exercise test as in the control condition. Immediately after exercise, participants returned to a chair for at least 1 h of recovery before repeating the process.
Measurements
Efficiency.
Pulmonary V̇o2 and V̇co2 were measured for 3 min preexercise and throughout the 6-min exercise bout using an online gas analysis system (Oxycon Pro, Jeager). Breath-by-breath V̇o2 data were computed and then averaged over each 30-s period of sampling. Exercise V̇o2 was then averaged over the final 3 min of exercise, which best represents the steady-state values, confirmed by the observation that RER remained < 1.0. Net V̇o2 during exercise was calculated by subtracting resting preexercise V̇o2 from exercise V̇o2. The net rate of energy turnover (kJ/min) was calculated using the caloric content of V̇o2 at the given RER. Net efficiency (%) was calculated as the ratio between external power output delivered to the ergometer (W converted to kJ/min) and the net rate of energy turnover. To take into account both the external and so-called “internal” power output, which is thought to represent the inertial and gravitational forces opposing movement of the limbs (20, 38), total mechanical power output was calculated using the method described previously (28), in which internal power was estimated as 0.153 × frequency3, where frequency is in Hertz. Consequently, mechanical efficiency (%) was defined as the ratio between total mechanical power output (W converted to kJ/min) and the net rate of energy turnover (kJ/min).
Muscle temperature.
Quadriceps muscle temperature was measured immediately before each exercise trial using a flexible muscle temperature probe (MAA flexible probe, Ellab, Denmark) inserted through an indwelling venflon cannula (18G) to ∼2 cm depth of the vastus lateralis.
Myosin heavy chain composition.
Approximately 3 wk after the last exercise trial, a single muscle sample was obtained at rest. Under local anesthesia (2% xylocaine), the sample was obtained from the medial vastus lateralis using the needle microbiopsy technique (Pro-Mag 2.2, Medical Products) and immediately frozen in liquid nitrogen. Myosin heavy chain (MHC) composition of the homogenate muscle sample was determined by SDS-PAGE using a method derived from that previously described (13). Protein bands were visualized by silver staining, using a modified method (29), and quantified by densitometry (Bio-Rad GS800 calibrated densitometer). Whole muscle samples were classified according to the relative expression of the three MHC isoforms: types I, IIA, and IIX.
Statistical Analyses
Repeated-measures ANOVA results were used to evaluate the effects of temperature (control and heated) and contraction frequency (45, 60, 75, 90 rpm), with between-groups measures to determine any effects of the age of the subjects. Where a significant effect was detected, differences were located with post hoc Bonferroni corrected paired t-tests. Relationships between variables were determined with Pearson correlations. Significance was accepted at a P < 0.05, and data are presented as means ± SD.
RESULTS
Muscle Temperature
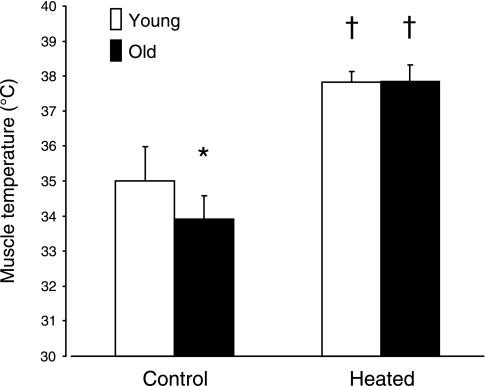
Resting (preexercise) muscle temperature was lower in O compared with Y (P < 0.05). Heating increased muscle temperature in both populations to a similar level (Fig. 1).
Fig. 1.
Muscle temperature measured immediately before moderate-intensity exercise in control (no prior heating) and heated muscle temperature conditions. Muscle temperature data for each participant are the average of each measurement under each temperature condition. Values are means ± SD. *Significant (P < 0.05) difference between young (Y) and older subjects (O). †Significant (P < 0.05) difference from control condition.
External Power Output
The external power output at Tvent was lower (P < 0.05) in O (45 ± 16 W) compared with Y (98 ± 26 W).
Pulmonary Gas Exchange
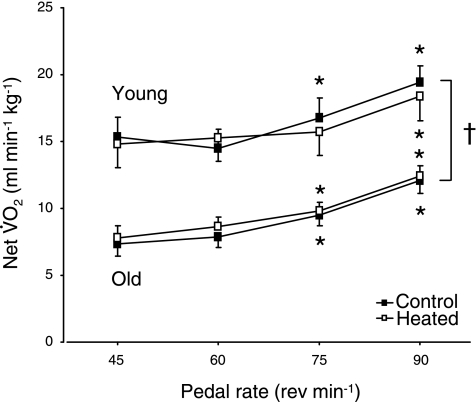
V̇o2 at rest in the control condition was 4.0 ± 0.5 and 3.5 ± 0.7 ml·kg−1·min−1 in Y and O, respectively (P < 0.05). Following heating, V̇o2 at rest was 4.2 ± 0.4 and 3.8 ± 0.6 ml·kg−1·min−1 in Y and O, respectively (P < 0.05). V̇o2 during exercise in both populations achieved a steady state in all conditions. Net V̇o2 during exercise in all conditions was lower (P < 0.05) in O compared with Y (Fig. 2). In both populations and under both temperature conditions, net V̇o2 during exercise was higher (P < 0.05) at 75 and 90 rpm compared with 45 and 60 rpm (Fig. 2).
Fig. 2.
Net O2 uptake (V̇o2) during moderate-intensity exercise at different pedal frequencies in control (no prior heating) and heated muscle temperature conditions. Values are means ± SD. *Significant (P < 0.05) difference from 45 and 60 rpm. †Significant (P < 0.05) difference between Y and O.
Efficiency
Net efficiency.
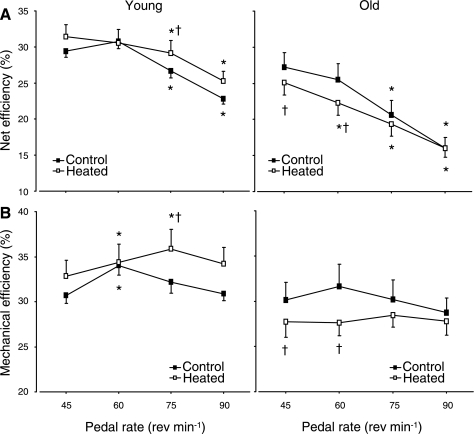
Under control conditions, net efficiency in O was lower (P < 0.05) than in Y. In both populations and under both conditions, net efficiency was lower (P < 0.05) at 75 and 90 rpm compared with 45 and 60 rpm (Fig. 3A). When averaged across the pedal frequencies, net efficiency in Y increased (P < 0.05) from 27.5 ± 4.0% in the control condition to 29.1 ± 5.1% in the heated condition. However, in O, average net efficiency decreased from 22.4 ± 6.9% in the control condition to 20.7 ± 5.5% in the heated condition. When considering the interaction between temperature and pedal frequency in Y, net efficiency was higher (P < 0.05) at 75 rpm in the heated compared with control condition, whereas, in O, net efficiency in the heated condition was lower (P < 0.05) at 45 and 60 rpm compared with the control condition (Fig. 3A).
Fig. 3.
Net efficiency (A) and mechanical efficiency (B) during moderate-intensity exercise at different pedal frequencies in control (no prior heating) and heated muscle temperature conditions in Y and O. Values are means ± SD. *Significant (P < 0.05) difference from 45 rpm. †Significant (P < 0.05) difference from control condition.
Mechanical efficiency.
These results are also reflected when the internal power is taken into consideration to calculate mechanical efficiency. The pattern of mechanical efficiency in relation to pedal frequency was different in that there tended to be a pedal frequency at which mechanical efficiency was optimal. For example, in Y under the control condition, mechanical efficiency was higher at 60 rpm compared with 45, 75, and 90 rpm (Fig. 3B), although this was not significant in O. Under control conditions, mechanical efficiency in O tended (P = 0.06) to be lower than in Y. When averaged across the pedal frequencies, mechanical efficiency in Y increased (P < 0.05) from 32.0 ± 3.1% in the control condition to 34.0 ± 5.5% in the heated condition. However, in O, average mechanical efficiency decreased from 30.2 ± 5.6% in the control condition to 27.9 ± 4.1% in the heated condition. When considering the interaction between temperature and pedal frequency in Y, mechanical efficiency was higher (P < 0.05) at 75 rpm in the heated condition compared with control, whereas, in O, mechanical efficiency in the heated condition was lower (P < 0.05) at 45 and 60 rpm compared with the control condition (Fig. 3B).
MHC Composition
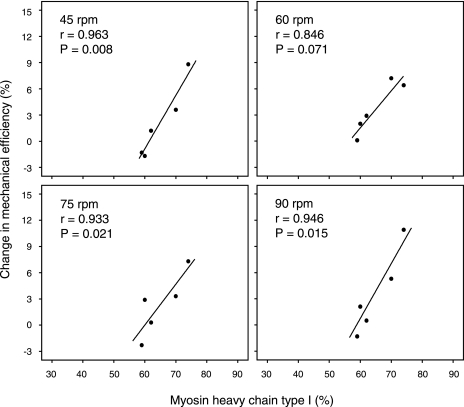
Of the eight participants in each group, five Y and seven O participants consented to provide a muscle biopsy for MHC analysis (Table 1). There were significant correlations between MHC I and mechanical efficiency in the control conditions at 60 rpm (r = 0.80, P < 0.05) and 75 rpm (r = 0.84, P < 0.05) in Y. In O, however, there were no correlations between MHC composition and mechanical efficiency at any pedal rate. There were no correlations between efficiency (net and mechanical) and any other MHC isoform at any pedal frequency. There were significant correlations between MHC I and the change in efficiency with heating at 45, 75, and 90 rpm in Y (Fig. 4). However, there were no such correlations between MHC I and the change in efficiency with heating in O.
Table 1.
MHC composition from the vastus lateralis of young and older participants
| n | MHC I | MHC IIA | MHC IIX | |
|---|---|---|---|---|
| Young | 5 | 65±7 (59–74) | 32±7 (26–41) | 3±0 (0–14) |
| Old | 7 | 57±15 (38–79) | 31±7 (21–38) | 12±16 (0–31) |
Values are means ± SD (range) in percent; n, no. of subjects. MHC, myosin heavy chain.
Fig. 4.
Correlations between proportion of myosin heavy chain I isoform and the change in mechanical efficiency with heating at different pedal frequencies in Y.
DISCUSSION
The present investigation demonstrated that passive local heating of the working muscles before moderate-intensity dynamic exercise significantly altered estimates of net and mechanical efficiency in young and older people. Crucially, the effect of elevated temperature was to increase mechanical efficiency in young people, whereas, in older people, mechanical efficiency actually decreased.
As expected, net efficiency declined as pedal frequency increased in all conditions and ages, which is supported by many previous studies (e.g., Ref. 17). However, the effect of pedal frequency was different, depending on whether the so-called “internal” power was included in the calculation of efficiency. The inclusion of internal power, the validity of which has been previously discussed (20, 23), altered the shape of the relationship with pedal frequency such that there was an increase in mechanical efficiency to a peak value before mechanical efficiency declined again. This more closely reflects the observations made in isolated (22) and in vivo (14) human skeletal muscle of an efficiency-velocity relationship. However, when considering the data from the young participants in the control condition, our values of net efficiency (23–31%) and mechanical efficiency (31–34%) are slightly higher than previously reported. For example, Gaesser and Brooks (17) report net efficiency values of 20 and 25%, while values between 21 and 27% have been reported that take into account the internal power (37). It is not clear why this is the case, but may be related to the sex of subject populations, with the vast majority of previous studies being conducted on men, whereas women were investigated in the present study.
The effect of heating the muscle in the young was to increase mechanical efficiency at the high contraction speeds, whereas heating in the older participants decreased mechanical efficiency at the low contraction speeds. To explain this, it is perhaps useful to consider the recruitment of individual muscle fibers that have diverse contractile and metabolic properties, and the effect that temperature has on these properties. For example, it has been demonstrated (22) that single human muscle fibers have different efficiency-velocity relationships, and, while peak efficiencies were similar in all fiber types, peak efficiency was reached at a higher speed of shortening in fast type IIA fibers compared with slow type I fibers. Moreover, elevated temperature increased efficiency in both fiber types and increased the speed of shortening at which efficiency was optimal (22). It has not been possible to determine the pattern of fiber-type recruitment in the present study. However, it is probable that, in the young subjects, an intensity of 75% Tvent required power that would represent <40% of the maximum power available (18). At the low contraction speeds (45 and 60 rpm), the majority of power would be generated by type I muscle fibers (1). Although the temperature-related shift in efficiency of type I fibers would move the peak mechanical efficiency to a higher velocity, the ascending arm of the relationship would be relatively unchanged, and a change in mechanical efficiency would not necessarily be evident at these lower speeds, as we observed (Fig. 3). At the higher contraction speeds (75 and 90 rpm), there may be a greater contribution from type II fibers, although type I fibers would still remain fully recruited (5). At these higher speeds, the temperature-related increase in single-fiber efficiency would become more evident and result in the increase in mechanical efficiency observed at high pedal frequencies (albeit only significantly at 75 rpm; Fig. 3). In the older population, the pattern of recruitment must be considered alongside the age-related changes in muscle fiber size (including the selective atrophy of type II fibers; Ref. 26), composition (2), and function (8). It is not known how efficiency at the single-fiber level is influenced by age. Although working at the same relative exercise intensity (75% Tvent), it is possible that this represents a greater proportion of an older person's maximal available power (34). Consequently, while power will still be delivered by the type I fiber population, there may be a significant delivery from the available type II fibers, for which the peak of the efficiency-velocity relationship is further to the right to begin with. Therefore, while temperature may still result in a rightward shift in the efficiency-velocity relationship of all fiber types, because of the dominant contribution of the available type II fibers, there would actually be a decrease in mechanical efficiency from the control condition at the lower speeds, as the ascending arm of the relationship will be relatively lower. At the higher speeds, the ascending arm would be relatively unchanged, and a change in mechanical efficiency would not be evident.
So far, this discussion has attempted to explain the changes in mechanical efficiency with heating in relation to the possible pattern of recruitment of different fiber types. We were able to obtain muscle biopsies from a number of young and older participants, and it was observed that there were correlations between type I MHC and the change in mechanical efficiency with heating in the young, but not the older, participants. This would support the speculation that, in the young, much of the change in mechanical efficiency would be due to the dominant contribution to power output of the type I fibers. Indeed, it has been well established that the fiber-type composition is an important determinant of mechanical efficiency (7, 21), especially in the young, as we have also demonstrated. These correlations were not observed in the older population, which may suggest that factors other than fiber-type composition may be important. However, MHC analysis of fiber composition in older people, as opposed to a more traditional histochemical approach, is compounded by the selective atrophy of type II fibers with aging (26). Moreover, Andersen et al. (2) observed that there is an increase in the number of fibers in older people that coexpress more than one MHC isoform, in particular type I and type IIA. Therefore, this analysis must be interpreted with caution, and it cannot be ruled out that the changes in fiber-type composition with aging have an important influence on efficiency, as well as the changes that occur in mechanical efficiency with heating.
Implications for Functional Ability
The reduction in mechanical efficiency in older people as a result of heating is in contrast to the development of maximal instantaneous power output, which has been shown to be enhanced in response to a similar passive heating protocol as the present study (9). This was attributed to temperature-related changes in both force and velocity components of power output. Indeed, one may argue that it is the power-generating capabilities of muscle that are more important for older people (27), as it is these “explosive” tasks, e.g., rising from a chair, climbing stairs, responding to a trip or fall, that are the limiting factors an older person has to overcome to improve quality of life. However, energy consumption in relation to contractile output (i.e., efficiency) must be important for more sustained activities that older people still have to carry out. Clearly, it would be an advantage to minimize energy consumption in older people's daily living in the face of a limited dietary intake (31). However, the reduction in mechanical efficiency in older people must also be considered in light of the lower resting muscle temperature that was observed (Fig. 1), which has also been observed previously (11). The fact that mechanical efficiency declines in older people when temperature is increased may indicate that, if muscle temperature in normal conditions was similar to that in young people (i.e., ∼35°C), mechanical efficiency would be even lower than reported. Thus, in this respect, it may be beneficial for the muscles of older people to be slightly cooler than those of the young to maximize mechanical efficiency.
Study Limitations
Of critical importance to the main findings is the temperature of the working muscle during exercise. Unfortunately, we were unable to measure muscle temperature, either during or shortly after each exercise bout. However, previous studies have demonstrated that muscle temperature is still elevated following exercise in prior-heated compared with normal muscle temperature conditions. For example, it was demonstrated that muscle temperature was still ∼1°C higher in the preheated condition following 10-min intense knee-extensor exercise (16). Moreover, using a similar moderate intensity (i.e., less than lactate threshold) and duration (6 min) as the present study, it was observed (25) that there was still a 3°C difference in muscle temperature at the end of exercise (36.3 vs. 39.2°C) in which muscle temperatures before exercise in the control and heated conditions were of a similar level (∼35 vs. 38°C). On this basis, we are confident that, in the young participants, the required condition of elevated muscle temperature during exercise was accomplished. This is probably the case in the older participants, although it is acknowledged that the increase in muscle temperature during exercise in older people is not known. It may be possible that muscle temperature may be greater due to an impaired circulation that may not dissipate the heat produced. On the other hand, muscle temperature might be lower due to the lower absolute work rate.
In conclusion, we have demonstrated different effects of passively elevated muscle temperature on mechanical efficiency in young and older women. The different responses between young and old most likely reflect possible differences in recruitment patterns, as well as sarcopenic and fiber-type changes with age.
GRANTS
The study was supported by a grant from the Strategic Promotion of Ageing Research Capacity (Biotechnology and Biological Sciences Research Council/Engineering and Physical Sciences Research Council).
Acknowledgments
We gratefully acknowledge the time and commitment of all participants who took part in the study. We thank Stephen Patterson, Mark Robinson, and Moira Watson for excellent technical assistance, and Dr. Andrea Macaluso for medical supervision.
REFERENCES
- 1.Altenberg TM, Degens H, van Mechelen W, Sargeant AJ, De Haan A. Recruitment of single muscle fibers during submaximal cycling exercise. J Appl Physiol 103: 1752–1756, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve 22: 449–454, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Asmussen E, Bøje O. Body temperature and the capacity for work. Acta Physiol Scand 10: 1–22, 1945. [Google Scholar]
- 4.Beaver KL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60: 2020–2027, 1986. [DOI] [PubMed] [Google Scholar]
- 5.Beelen A, Sargeant AJ, Lind A, De Haan A, Kernell D, van Mechelen W. Effect of contraction velocity on the pattern of glycogen depletion in human muscle fibre types. In: Neuromuscular Fatigue, edited by Sargeant AJ, Kernell D. Amsterdam: Royal Netherlands Academy of Arts and Sciences, 1993, p. 93–96.
- 6.Bennett AF Thermal dependence of muscle function. Am J Physiol Regul Integr Comp Physiol 247: R217–R229, 1984. [DOI] [PubMed] [Google Scholar]
- 7.Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibres. Med Sci Sports Ex 24: 782–788, 1992. [PubMed] [Google Scholar]
- 8.D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies CTM, Young K. Effect of heating on the contractile properties of triceps surae and maximal power output during jumping in elderly men. Gerontology 31: 1–5, 1985. [DOI] [PubMed] [Google Scholar]
- 10.De Ruiter CJ, De Haan A. Temperature effect on the force/velocity relationship of the fresh and fatigued human adductor pollicis muscle. Pflugers Arch 440: 163–170, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Dewhurst S, Graven-Neilsen T, De Vito G, Farina D. Muscle temperature has a different effect on force fluctuations in young and older women. Clin Neurophysiol 118: 762–769, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dinenno FA, Seals DR, De Souza CA, Tanaka H. Age-related decreases in basal limb blood flow in humans; time course, determinants and habitual exercise effects. J Physiol 531: 573–579, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fauteck SP, Kandarian SC. Sensitive detection of myosin heavy chain composition in skeletal muscle under different loading conditions. Am J Physiol Cell Physiol 268: C419–C424, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson RA, Ball D, Krustrup P, Aagaard P, Kjær M, Sargeant AJ, Hellsten Y, Bangsbo J. Muscle oxygen uptake and energy turnover during dynamic exercise at different contraction frequencies in humans. J Physiol 536: 261–271, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson RA, Ball D, Sargeant AJ. Effect of muscle temperature on rate of oxygen uptake during exercise in humans at different contraction frequencies. J Exp Biol 205: 981–987, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson RA, Krustrup P, Kjær M, Mohr M, Ball D, Bangsbo J. Effect of muscle temperature on skeletal muscle energy turnover during dynamic knee extensor exercise in humans. J Appl Physiol 101: 47–52, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Gaesser GA, Brooks GA. Muscular efficiency during steady-rate exercise: effects of speed and work rate. J Appl Physiol 38: 1132–1139, 1975. [DOI] [PubMed] [Google Scholar]
- 18.Greig CA, Sargeant AJ, Vollestad NK. Muscle force and fibre recruitment during dynamic exercise in man (Abstract). J Physiol 371: 176P, 1986. [Google Scholar]
- 19.Greig CA, Young A, Skelton DA, Pippet E, Butler FM, Mahmud SM. Exercise studies with elderly volunteers. Age Ageing 23: 185–189, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Hansen EA, Jorgensen LV, Sjogaard G. A physiological counterpoint to mechanistic estimates of “internal power” during cycling at different pedal rates. Eur J Appl Physiol 91: 435–442, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Hansen EA, Sjogaard G. Relationship between efficiency and pedal rate in cycling: significance of internal power and muscle fiber type composition. Scand J Med Sci Sports 17: 408–414, 2007. [DOI] [PubMed] [Google Scholar]
- 22.He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibres with different myosin isoform composition. Biophys J 79: 945–961, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kautz SA, Neptune RR. Biomechanical determinants of pedaling energetics: internal and external work are not independent. Exerc Sport Sci Rev 30: 159–169, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Keys A, Taylor HL, Grande F. Basal metabolism and age of adult man. Metabolism 22: 579–587, 1973. [DOI] [PubMed] [Google Scholar]
- 25.Koga S, Shiojiri T, Kondo N, Barstow TJ. Effect of increased muscle temperature on oxygen uptake kinetics during exercise. J Appl Physiol 83: 1333–1338, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Lexell L, Taylor CC, Sjostrom M. What is the cause of ageing atrophy? Total number, size and proportion of different fibre types studied in whole vastus lateralis muscle from 15- to 83-year old men. J Neurol Sci 84: 275–294, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol 91: 450–472, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Minetti AE, Pinkerton J, Zamparo P. From bipedalism to bicyclism: evolution in energetics and biomechanics of historic bicylces. Proc R Soc Lond 268: 1351–1360, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oakley BR, Kirsch DR, Morris NR. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 105: 361–363, 1980. [DOI] [PubMed] [Google Scholar]
- 30.Rall JA, Woledge RC. Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol Regul Integr Comp Physiol 259: R197–R203, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev 86: 651–667, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Saltin B, Gagge AP, Stolwijk JAJ. Muscle temperature during submaximal exercise in man. J Appl Physiol 25: 679–688, 1968. [DOI] [PubMed] [Google Scholar]
- 33.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 23: 371–377, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Stathokostas L, Jacob-Johnson S, Petrella RJ, Paterson DH. Longitudinal changes in aerobic power in older men and women. J Appl Physiol 97: 781–789, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Steinen GJM, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: fibre types and temperature dependence. J Physiol 493: 299–307, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thom JM, Morse CI, Birch KM, Narici MV. Triceps surae muscle power, volume, and quality in older versus younger healthy men. J Gerontol A Biol Sci 60: 1111–1117, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Tokui M, Hirakoba K. Effect of internal power on muscular efficiency during cycling exercise. Eur J Appl Physiol 101: 565–570, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Winter DA A new definition of mechanical work done in human movement. J Appl Physiol 46: 79–83, 1979. [DOI] [PubMed] [Google Scholar]
- 39.Woledge RC, Curtin NA, Homsher E. Energetic Aspects of Muscle Contraction. New York: Academic, 1985. [PubMed]