Abstract
Caspase-independent cell death, an important death pathway in many cells including neurons, is executed via apoptosis-inducing factor (AIF), an oxidoreductase, localized to the mitochondrial intermembrane space. AIF is processed and released from mitochondria following mitochondrial permeability transition pore (mPTP) formation, and translocates to the nucleus to induce DNA fragmentation and cell death. The release of AIF requires cleavage of its N-terminus anchored in the inner mitochondrial membrane. The protease responsible for this AIF truncation has not been established, although there is considerable evidence suggesting a role for μ-calpain. We previously found that a pool of μ-calpain is localized to the mitochondrial intermembrane space, the submitochondrial compartment in which AIF truncation occurs. The close submitochondrial proximity of mitochondrial μ-calpain and AIF gives support to the hypothesis that mitochondrial μ-calpain may be the protease responsible for processing AIF prior to its release. In the present study, AIF was released from rat liver mitochondria following mPTP induction by atractyloside. This release was inhibited by the cysteine protease inhibitor MDL28170, but not by more specific calpain inhibitors PD150606 and human erythrocyte calpastatin. Atractyloside caused swelling in rat brain mitochondria, but did not induce AIF release. In a mitochondrial fraction from SH-SY5Y neuroblastoma cells, incubation with 5 mM Ca2+ resulted in the activation of μ-calpain but not in AIF truncation. In summary, the localization of μ-calpain to the mitochondrial intermembrane space is suggestive of its possible involvement in AIF processing, but direct experimental evidence supporting such a role has been elusive.
Introduction
Cell death mechanisms can be broadly classified as programmed and non-programmed, with programmed cell death being further subdivided into caspase-dependent and caspase-independent mechanisms (Boujrad, et al., 2007, Kroemer and Martin, 2005). Caspase-independent cell death (CICD) occurs following the cleavage and release of apoptosis-inducing factor (AIF) from mitochondria and the subsequent translocation of AIF to the nucleus, resulting in DNA fragmentation (Boujrad, et al., 2007, Cregan, et al., 2002). CICD is of particular importance in adult neurodegeneration (Stoka, et al., 2006). CICD and AIF translocation can be induced by DNA damage resulting in the activation of poly(ADP-ribose) polymerase-1 (PARP-1), reviewed by Dawson et al in this issue, or by excessive mitochondrial Ca2+ uptake, the focus of the present study.
The N-terminus of mature 62 kDa AIF is anchored in the inner mitochondrial membrane, with remainder of the protein projecting into the intermembrane space. AIF release requires proteolysis near the N-terminus to generate a 57 kDa fragment (Otera, et al., 2005). μ-Calpain is an attractive candidate for the protease responsible for this cleavage (Liou, et al., 2005, Polster, et al., 2005). μ-Calpain, composed of the calpain 1 large subunit and calpain small subunit, cleaves the 62 kDa AIF to a 57 kDa fragment (Polster, et al., 2005). Calpain inhibitors such as calpeptin block the release of AIF from rat and mouse liver mitochondria following opening of the mitochondrial permeability transition pore by Ca2+ or atractyloside (Polster, et al., 2005, Yuste, et al., 2005).
One difficulty with the above hypothesis is that μ-calpain was thought to be a cytosolic enzyme, which would require permeabilization of the outer mitochondrial membrane to gain access to AIF. The recent localization of μ-calpain to the mitochondrial intermembrane space avoids this issue and provides further support for the putative role of μ-calpain in the truncation of AIF (Badugu, et al., 2008, Cao, et al., 2007, Garcia, et al., 2005, Norberg, et al., 2008).
Mitochondria have a finite capacity for Ca2+ uptake, which when exceeded results in the opening of a non-specific mitochondrial permeability transition pore (mPTP) in the inner mitochondrial membrane. mPTP opening allows the passage of molecules less than 1.5 kDa and results in loss of mitochondrial membrane potential, release of Ca2+ from the mitochondrial matrix, mitochondrial swelling, and rupture of the outer mitochondrial membrane (Bernardi and Rasola, 2007). The localization of μ-calpain to the intermembrane space positions this protease to be activated by Ca2+ released following mPTP opening, and in the same subcellular compartment as the C-terminal region of AIF (Fig. 1). In this study we evaluated the hypothesis that AIF processing and release is mediated by mitochondrial μ-calpain.
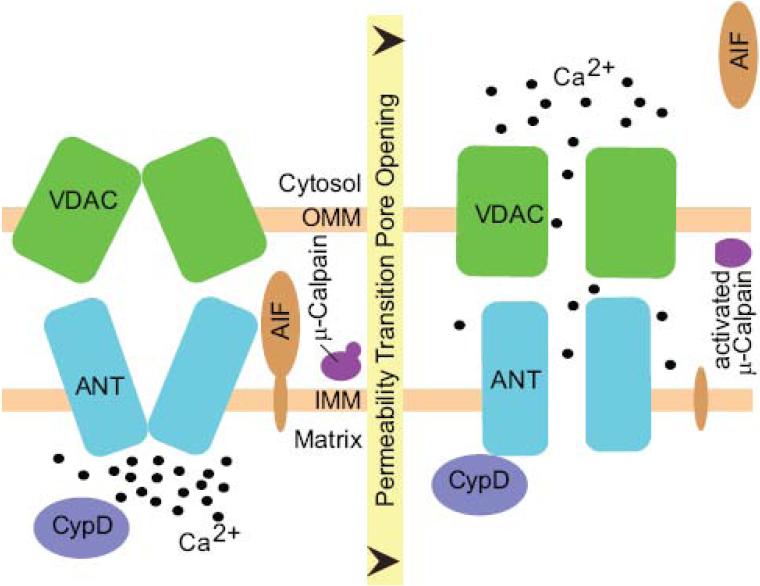
Figure 1. Mitochondrial permeability transition.
The components and regulation of the mitochondrial permeability transition pore are not fully understood, the following is a working model. In response to elevated Ca2+ in the mitochondrial matrix (and promoted by oxidative stress and reduced adenine nucleotides), Cyclophilin D (CypD) induces a conformational change in adenine nucleotide translocase (ANT), resulting in the opening of a non-specific channel, the mPTP. The voltage-dependent anion channel (VDAC) in the outer mitochondrial membrane may also contribute to pore opening. This results in a rapid efflux of Ca2+ and other small solutes (<1500 Da) from the matrix into the mitochondrial intermembrane space and cytosol, matrix swelling, mitochondrial depolarization, and release of death-effector proteins including apoptosis-inducing factor (AIF). μ-Calpain is also located in the mitochondrial intermembrane space where it is hypothesized to be activated following mPTP opening and to cleave AIF.
Materials and Methods
Reagents Used
Chemicals and reagents were obtained from Sigma Chemical, St. Louis MO, unless otherwise noted. PD150606 and human erythrocyte calpastatin were from Calbiochem. Potassium chloride and Tris-base were obtained from Fisher Scientific. Bromophenol blue and Tween-20 were from Bio-Rad. Complete EDTA free protease inhibitor cocktail tablets were purchased from Roche. Cell culture reagents including minimal essential medium (MEM), L-glutamine, Penstrep (penicillin-streptomycin), and 0.05% Trypsin-EDTA were purchased from Invitrogen (Gibco). Fetal bovine serum was purchased from Atlanta Biologicals. Bicinchoninic acid (BCA) kit was purchased from Pierce. Percoll was purchased through Amersham Biosciences. Calpain 1 domain III monoclonal antibody was from Calbiochem. A polyclonal antibody against the N-terminal of calpain 1 was produced by Triple Point Biologics and purchased through Abcam. AIF monoclonal antibody was from Santa Cruz (E-1 clone, SC-13116). Mitochondrial HSP-70 antibody was from ABR. Secondary antibodies for Western blots were IRDye 700 CW and 800CW conjugated affinity purified anti-rabbit or anti-mouse IgG (H & L) from Rockland, Gilbertsville, PA. All animals were obtained from Harlan (Indianapolis, IN).
Mitochondrial isolation
All experimental protocols involving animals were approved by the University of Kentucky Animal Use and Care Committee. Adult male Sprague-Dawley rats, weighing approximately 250 g, were used in all studies. The rats were anesthetized using carbon dioxide. Mitochondria were isolated from rat cerebral cortex as described previously (Brown, et al., 2006) based on method A of Sims et al. (Sims, 1990) with slight modifications. Briefly, following CO2 asphyxiation animals were decapitated, their brains were quickly removed and the cortices were dissected. Cortical tissue was placed in ice cold mitochondrial isolation buffer (MIB, 75mM Sucrose, 215mM Mannitol, 1mM EGTA, 0.1% BSA, 20 mM HEPES, pH adjusted to 7.2 using KOH). Cortical tissues were placed into a cold glass dounce homogenizer and homogenized with six strokes. A solution consisting of equal amounts of 30% Percoll (v/v) and sample, for a final Percoll concentration of 15%, was placed on a discontinuous gradient of 24% and 40% Percoll. The density gradients were centrifuged for 10min at 30,400g, 4°C in fixed angle SE-12 rotors. The fraction at 24-40% interface which contained the non-synaptic brain mitochondria was removed, placed into separate tubes, and diluted with MIB. The samples were then centrifuged at 16,700g for 15 min. The supernatant was removed and the pellet was resuspended in MIB without EGTA and BSA. It was washed with MIB and centrifuged again at 11,000g for 10 min. The resultant pellet was resuspended in 1ml of MIB without EGTA and BSA, and centrifuged at 10,000g for 10 min. The final mitochondrial pellets were stored on ice.
Rat liver mitochondria were isolated using combination of differential and Percoll discontinuous density gradient methods. Following CO2 asphyxiation, the right lateral lobe of liver was dissected out, placed in MIB. Tissue was homogenized by 6 strokes at 500rpm, centrifuged at 800g for 10 min. The supernatant was centrifuged at 10,000g for 10 min and the pellets resuspended in MIB. Pellets were homogenized in the glass dounce homogenizer, and centrifuged at 3000g for 5 min. The supernatants were then centrifuged at 10,000g for 10 min and the pellet was resuspended in MIB, centrifuged at 9,000g for 10 min and resuspended the pellet in MIB. The mitochondria were then enriched using a discontinuous Percoll density gradient as described for brain mitochondrial isolation.
SH-SY5Y cells were obtained from American Type Culture Collection (Manassas, VA) and propagated in MEM with non-essential amino acids, 10% fetal bovine serum, 1% penicillin-streptomycin until 80% confluent. Mitochondria were isolated as described previously (Garcia, et al., 2005).
Mitochondrial Swelling Assay
Mitochondrial swelling was estimated based on changes in light scattering at 540nm, measured in a Biotek Synergy HT microplate reader (Biotek instruments, Winooski, VT). Isolated mitochondria from rat cerebral cortex or rat liver (1mg/ml) were resuspended in the respiration buffer (125mM KCl, 2mM MgCl2, 20mM HEPES, 2.5mM KH2PO4, pH 7.2) with 5 mM pyruvate, 2.5 mM malate and 150μM ADP. Swelling was estimated after 15 minutes of incubation with atractyloside or alamethicin, or without treatment. Mitochondrial swelling is represented by decreased absorbance.
Incubation of Mitochondria with Atractyloside, Alamethicin, and Calcium
BCA protein assay was done on mitochondria from all sources. Mitochondria (1 mg/ml, protein content determined using Pierce BCA method) were resuspended in respiration buffer with pyruvate, malate and ADP. Samples were incubated in the presence or absence of inhibitors including MDL 28170 (20μM), PD150606 (50μM), human erythrocyte calpastatin (20 I.U.) at 4°C for 15 min. Mitochondria were then incubated with either 5mM atractyloside (Atr) for 30 min at 30°C or with 15μM alamethicin (Alm) for 15 min at 37°C. This was followed by centrifugation at 13,400g for 30 min at 4°C. The supernatants and pellets were collected, 3X sample buffer (0.5M Tris- pH 6.8, SDS, Glycerol, β-mercaptoethanol, 1% Bromophenol blue) was added. Samples were boiled at 100 °C for 10 min and were stored at −80 °C prior to western blot analysis.
SH-SY5Y isolated mitochondria were resuspended in calpain activity buffer ( 20mM Tris-HCl, 1mM EDTA, 100mM KCl, 0.1% β-mercaptoethanol, pH 7.5) at 2mg/ml. Calcium chloride (0, 0.5 or 5 mM) was added and the suspension was incubated for two hours at room temperature with shaking. Solubilized mitochondria were treated with 0.2% Triton X-100 prior to incubation with CaCl2. Following incubation, 3X sample buffer was added. Samples were boiled at 100 °C for 10 min and were stored at −80 °C.
Western Blotting
Equal amount of protein, along with molecular weight markers, were separated by SDS-PAGE on 10% Tris-glycine polyacrylamide gels. The proteins were transferred to a 0.45μM nitrocellulose membranes (Bio-Rad) by electrophoresis, and membranes were incubated in 5% nonfat dry milk powder in TBS (50mM Tris, NaCl 0.14M, pH7.5). Membranes were incubated with primary antibodies overnight in 5% non fat milk and TTBS (TBS, 0.05% tween-20, pH 7.5). After three washes for 20 min each in TTBS, membranes were incubated with the species-appropriate secondary antibody for 1 hr at room temperature in the dark. The membranes were washed 3X in TTBS and scanned and quantified using a Li-Cor Biosciences Odyssey infrared imaging system (Lincoln, Nebraska, USA).
Statistics
Statistical calculations were performed using Statview software. Analysis consisted of Student's unpaired t-test or ANOVA followed by Fisher's LSD post hoc test for multiple comparisons. Data is expressed as mean ± S.E.M. from at least three independent experiments. P of <0.05 was considered significant.
Results
Atractyloside induced AIF release from isolated liver mitochondria
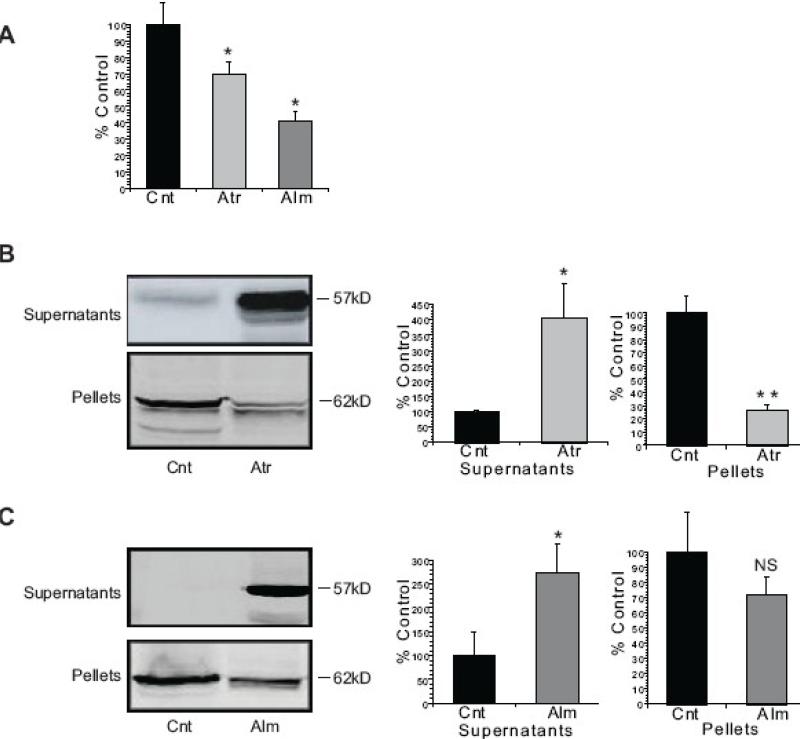
Isolated rat liver mitochondria were monitored for swelling at 540nM following treatment with atractyloside (Atr) and alamethicin (Alm). Atr is a ligand for the adenine nucleotide translocase, and promotes formation of the permeability transition pore (Vieira, et al., 2000). Alm, a peptide antibiotic, forms artificial channels in biological membranes (Brustovetsky, et al., 2002, Ritov, et al., 1992). Both drugs induced significant mitochondrial swelling and AIF release, although AIF release was more pronounced with Atr (Fig. 2). AIF released into the supernatant fraction was of a slightly lower mol.wt. than AIF in the mitochondrial pellets, consistent with the requirement for AIF truncation prior to mitochondrial release and nuclear translocation (Otera, et al., 2005).
Figure 2. Atractyloside and Alamethicin induce AIF release from rat liver mitochondria.
Isolated rat liver mitochondria were incubated with 5mM Atr for 30min at 30°C or 15μM Alm for 30min at 37°C in the KCl-based mitochondrial respiration buffer with pyruvate, malate and ADP. (A) The decrease in absorbance (540 nm) of the mitochondrial suspension indicates that both Atr and Alm induced swelling of the rat liver mitochondria. (B) Incubation with Atr resulted in cleavage of 62 kDa AIF from the mitochondrial pellet into a 57 kDa band released into the supernatant fraction. (C) Incubation of the rat liver mitochondria with Alm also resulted in the release of AIF from the mitochondrial pellet into the supernatant fraction. The data are from at least three independent experiments, represented as mean ± S.E.M, * indicates P < 0.05, ** indicates P < 0.01, NS indicates that differences were not significant at P=0.05, relative to Cnt- Control.
Atractyloside did not induce AIF release from isolated brain mitochondria
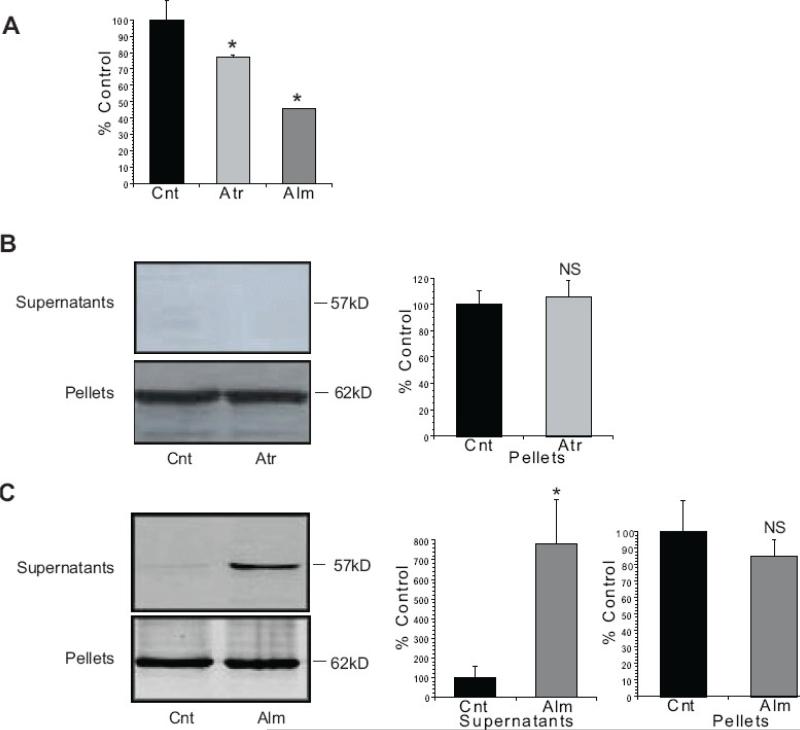
As with liver mitochondria, both Atr and Alm induced swelling in isolated non-synaptic rat brain mitochondria (Fig. 3A). However, in contrast to rat liver mitochondria, Atr failed to induce AIF release from the rat brain mitochondria (Fig. 3B). The lack of response to Atr was also observed in synaptic rat brain mitochondria (results not shown). Incubation of the rat brain mitochondria with Alm did result in release of AIF into the supernatant fraction (Fig. 3C), similar to results obtained with rat liver mitochondria.
Figure 3. AIF is not released from rat brain mitochondria following incubation with Atractyloside.
Mitochondria enriched from the cerebral cortex of rat brain were incubated with 5mM Atr for 30min at 30°C or 15 μM Alm for 30min at 37°C in the KCl-based mitochondrial respiration buffer with pyruvate, malate and ADP. (A) The decrease in absorbance (540 nm) of the mitochondrial suspension indicates that both Atr and Alm induced swelling of the rat brain mitochondria. (B) Incubation with Atr did not result in the cleavage or release of AIF from the mitochondrial pellet into the supernatant fraction. (C) Incubation of the rat brain mitochondria with Alm resulted in the release of AIF from the mitochondrial pellet into the supernatant fraction. The data are from at least three independent experiments, represented as mean ± S.E.M, * indicates P < 0.05, NS indicates that differences were not significant at P=0.05, relative to Cnt- Control.
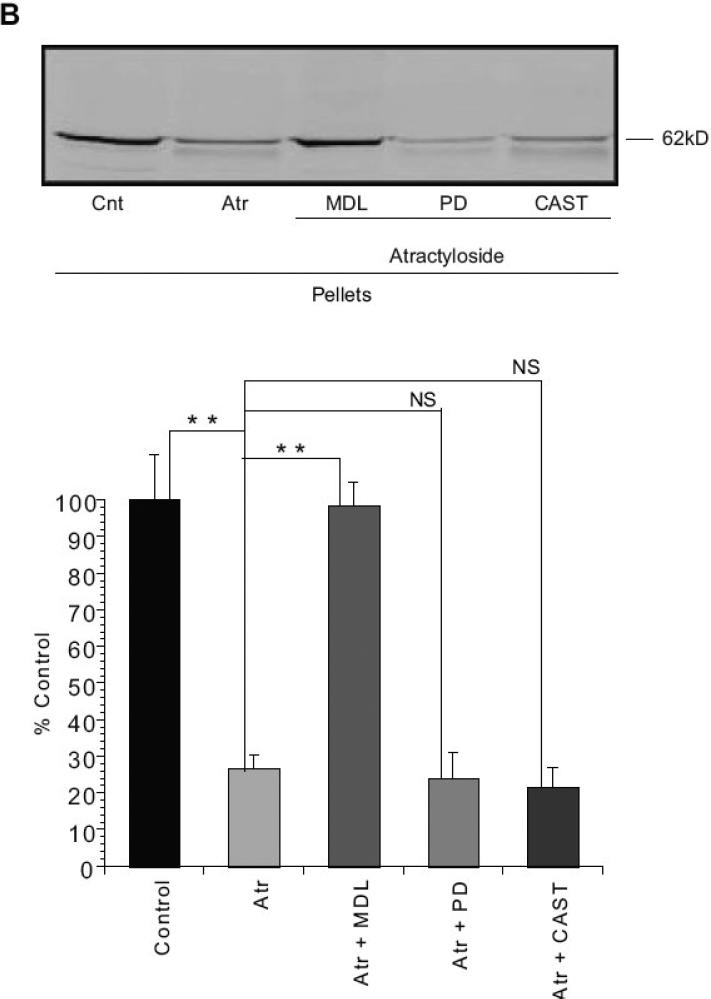
Atractyloside-induced AIF release is inhibited by cysteine protease inhibitor MDL28170
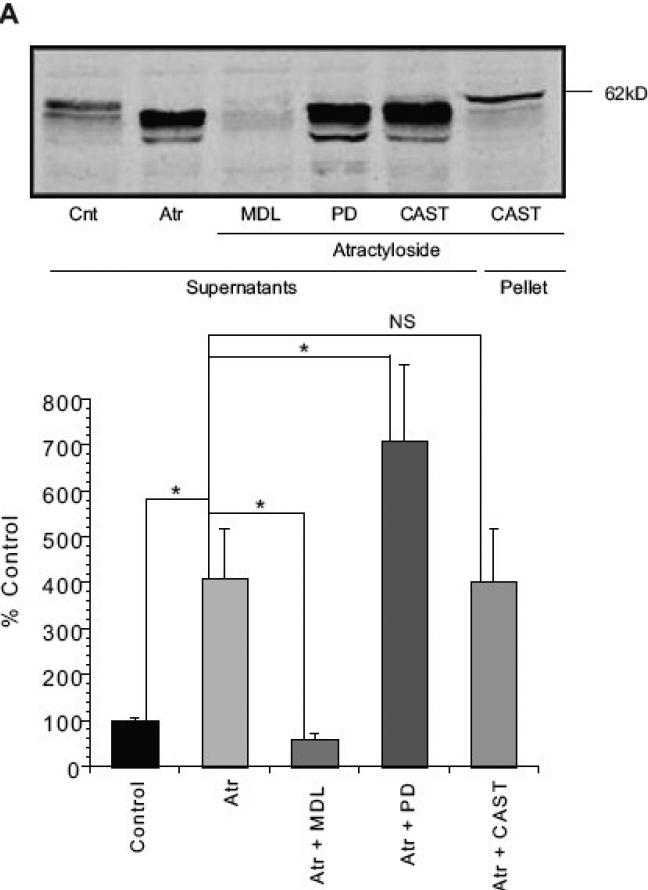
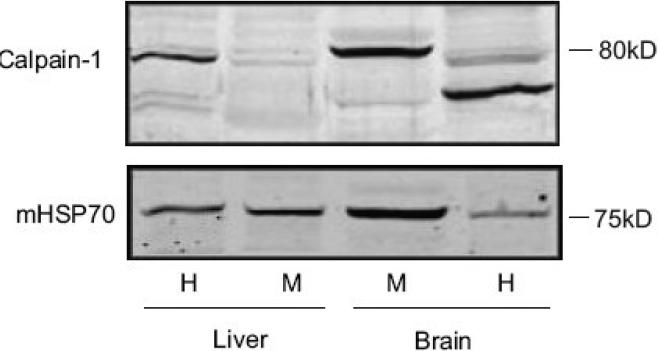
Previous studies have suggested the involvement of a calpain or another cysteine protease in AIF processing and release (Polster, et al., 2005, Yuste, et al., 2005). Preincubation of isolated liver mitochondria with 20 μM MDL-28170 (carbobenzoxy-valylphenylalanial; calpain inhibitor III), inhibited Atr-induced AIF release from the rat liver mitochondria (Fig. 4). However, this drug inhibits both calpains and other cysteine proteases including cathepsin B (Wang and Yuen, 1999). We therefore examined more specific calpain inhibitors including PD150606 (Wang, et al., 1996) and calpastatin (Wendt, et al., 2004). Both failed to prevent AIF release from rat liver mitochondria (Fig 4). Moreover, μ-calpain immunoreactivity was difficult to detect in the mitochondria-enriched fraction from rat liver (Fig. 5), in contrast to mitochondrial fractions from rat cerebral cortex (Fig. 5).
Figure 4. Atractyloside-induced AIF release in rat liver mitochondria was inhibited by cysteine protease inhibitor MDL 28170 but not by specific calpain inhibitors PD150606 or human erythrocyte calpastatin.
Isolated rat liver mitochondria were incubated with 20 μM MDL-28170 (MDL), 50 μM PD150606 (PD), or 20. I.U. human erythrocyte calpastatin (CAST) at 4°C for 15 min on ice. The mitochondria were then incubated with 5mM Atr for 30 min at 30°C in the KCl-based mitochondrial respiration buffer with pyruvate, malate and ADP. Supernatants (A) and Pellets (B) were separated, subjected to SDS-PAGE and western blotting for AIF. MDL28170, but not PD150606 or CAST inhibited the release of AIF from the mitochondrial fraction. Data are from at least three independent experiments, represented as mean ± S.E.M. * indicates P < 0.05, ** indicates P < 0.01, NS indicates that differences were not significant at P=0.05, relative to Cnt- Control.
Fig. 5. Calpain-1 immunoreactivity is difficult to detect in rat liver mitochondria.
Mitochondria were isolated from rat liver and rat cerebral cortex as described in Materials and Methods. The starting homogenate (H) and the final mitochondrial enriched fraction (M) from the two tissues were subjected to SDS-PAGE and western blotting, and probed for calpain-1 (the large subunit of μ-calpain) using an antibody against N terminal, or for mitochondrial HSP70 (mHSP70). The results indicate an enrichment of 80 kDa calpain 1 immunoreactivity in rat brain mitochondria, but very faint calpain-1 immunoreactivity in rat liver mitochondria. Protein loaded 80μg protein/lane.
Calpain activation is not sufficient for AIF release
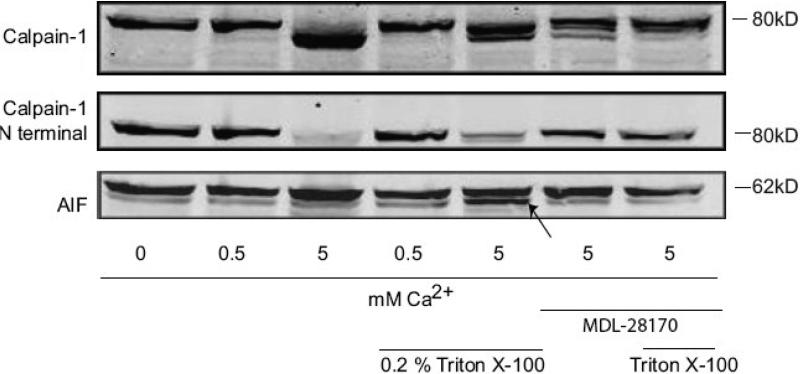
In mitochondria isolated from rat cerebral cortex, incubation with various levels of Ca2+ up to 5 mM did not result in μ-calpain activation or AIF release (results not shown). One possible explanation is that the μ-calpain may have been activated during the euthanasia, brain removal, and mitochondrial isolation procedure (Garcia, et al., 2005), making subsequent activation difficult. To further investigate the possible role of mitochondrial μ-calpain in AIF release, we utilized mitochondria from SHSY-5Y neuroblastoma cells, which we previously demonstrated contain μ-calpain, and in which the calpain 1 immunoreactivity is evident as a single 80 kDa band (Badugu, et al., 2008, Garcia, et al., 2005). Incubation of SH-SY5Y mitochondria with 5mM, but not 0.5 mM, Ca2+ resulted in a lower mol wt. calpain-1 immunoreactive band (Fig. 6), indicative of autoproteolysis and calpain activation (Cong, et al., 1989, Li, et al., 2004). Calpain autolysis was also observed using an antibody specific for the N-terminus of calpain 1, which shows a loss in immunoreactivity following μ-calpain activation (Fig. 6). Under these conditions of calpain activation, we did not detect increased AIF proteolysis in the mitochondrial fraction (Fig. 6). Following solubilization of the mitochondria, AIF was processed to a smaller mol weight fragment following incubation with 5 mM Ca2+ (Fig 6, lower band indicated by arrow). MDL28170 (20 μM) inhibited the Ca2+-induced calpain activation in both intact and solubilized mitochondria, and attenuated the intensity of the lower mol. wt. AIF band following Ca2+ incubation in the Triton-solubilized mitochondria (Fig. 6).
Figure 6. Mitochondrial μ-calpain activation does not result in AIF cleavage in SH-SY5Y mitochondria.
Mitochondria enriched from SH-SY5Y cells were treated with 0.5 or 5 mM CaCl2, with or without Triton-X 100 solubilization, and in the presence or absence of 20 μM MDL28170, in calpain activity buffer for 2hrs at room temperature. Proteins were resolved by SDS-PAGE and western blotting was performed using antibodies against calpain-1 (anti-domain III and anti-N-terminus). In non-solubilized mitochondria, incubation with 5 mM, but not 0.5 mM, CaCl2 resulted in calpain activation as indicated by autolysis of the N-terminal, resulting in a lower mol. wt. calpain-1 band and loss of N-terminal calpain-1 immunoreactivity. These conditions did not, however, result in AIF cleavage. In mitochondria solubilized with 0.2% Triton X-100, 5 mM CaCl2, resulted in both calpain activation and AIF cleavage. The lower mol. wt. AIF band is identified by an arrow in the lower panel. MDL28170 (20 μM) inhibited both the calpain-1 autolysis and AIF truncation induced by 5 mM Ca2+. These blots are representative of three replicate experiments.
Discussion
The results of this study do not support a role for mitochondrial μ-calpain in AIF release. In mitochondria enriched from rat liver, Atr-induced AIF truncation and release was inhibited by the calpain inhibitor MDL-28170 but not by the more specific calpain inhibitors PD150606 and calpastatin. Although MDL-28170 was designed as a calpain inhibitor and is widely used for this purpose, it also inhibits other cysteine proteases including cathepsin B (Mehdi, et al., 1988, Sasaki, et al., 1990, Wang and Yuen, 1999). In addition, it was difficult to detect μ-calpain immunoreactivity in mitochondria enriched from rat liver. This contrasts with the enrichment of μ-calpain in the mitochondrial fractions vs. homogenate obtained from rat cerebral cortex (Garcia, et al., 2005). Ozaki and colleages were able to detect and enrich a μ-calpain like protein from swine liver (Ozaki, et al., 2007). However, they used only differential centrifugation (4,500 × g for 10 min) to enrich mitochondria, in contrast to the combination of differential and discontinuous Percoll density gradient centrifugation utilized in the present study. It is therefore possible that at least some of the μ-calpain detected in their mitochondrial fraction represents cytosolic μ-calpain. Together, these results argue against a role for μ-calpain in the truncation and release of AIF from rat liver mitochondria following mPTP opening with Atr.
For mitochondria enriched from rat brain, we were unable to induce AIF release following incubation with either Ca2+ or Atr, although AIF release was observed following permeabilization with Alm. We were also unable to demonstrate μ-calpain activation under similar conditions. Several proteolytic fragments of calpain 1 are observed in mitochondria isolated from rat brain(Garcia, et al., 2005), indicative of calpain activation prior to or during the isolation procedure which could interfere with the ability to activate calpain in the isolated mitochondria. We therefore examined mitochondria obtained from human SH-SY5Y neuroblastoma cells in which proteolytic calpain 1 fragments are not observed (Garcia, et al., 2005). In the SH-SY5Y mitochondria, incubation with 5 mM, but not 0.5 mM, Ca2+ resulted in μ-calpain activation but not in AIF truncation. In Triton-X 100 solubilized mitochondria, 5 mM Ca2+ resulted in both μ-calpain activation and AIF cleavage. The 5 mM Ca2+ results in Triton-solubilized mitochondria are consistent with the ability of μ-calpain to cleave AIF, but suggest that AIF and μ-calpain are spatially separated within intact mitochondria and that AIF is not a mitochondrial μ-calpain substrate.
Together, these findings argue against mitochondrial μ-calpain being the protease responsible for AIF cleavage under conditions which cause mPTP opening. In both rat and liver mitochondria, permeabilization of the outer mitochondrial membrane with Alm enabled AIF release. This suggests that a pool of truncated AIF is available for release following outer mitochondrial membrane permeabilization by Bid or related proteins. The requirement of outer membrane permeabilization for AIF release is consistent with several other studies (Landshamer, et al., 2008, Shalbuyeva, et al., 2006, Shulga and Pastorino, 2006). However, release of AIF also requires cleavage of the C-terminal 57 kDa portion from the N-terminal anchor in the inner mitochondrial membrane (Otera, et al., 2005). The protease responsible for AIF cleavage prior to release remains to be determined.
The ability of MDL28170, which inhibits both calpains and cysteine cathepsin proteases, to inhibit AIF release suggests the possible involvement of a cysteine cathepsin, such as cathepsin B (Chaitanya and Babu, 2008, Yuste, et al., 2005). Although cathepsin B is a lysosomal protease, an alternatively spliced variant lacking exons 2 and 3 is targeted to mitochondria (Muntener, et al., 2004). However, this splice variant is catalytically inactive due to improper folding (Baici, et al., 2006). In addition, cathepsin B knockout does not alter AIF release induced by a DNA alklylating agent (Moubarak, et al., 2007).
Although our findings argue against the involvement of mitochondrial μ-calpain, the results do not rule out a role for cytosolic μ-calpain in AIF processing. Cytosolic μ-calpain could gain access to AIF following outer mitochondrial membrane permeabilization. In previous studies involving isolated mitochondria, exogenous μ-calpain was added to achieve mitochondrial AIF proteolysis (Cao, et al., 2007, Liou, et al., 2005, Polster, et al., 2005). Evidence that calpastatin overexpression and calpain 1 knockdown attenuates AIF translocation following excitotoxic insult supports a role for μ-calpain, but does not distinguish between the cytosolic and mitochondrial localizations of the protease (Cao, et al., 2007). In a recent study supporting a role for mitochondrial μ-calpain in AIF proteolysis, the experimental model used was digitonin permeabilized U1810 cells incubated with Ca2+ (Norberg, et al., 2008), which would not distinguish between the involvement of cytosolic and mitochondrial μ-calpain. Their results did demonstrate that blockade of mitochondrial Ca2+ uptake with ruthenium red prevented the AIF release, although this would also inhibit mPTP opening and subsequent outer mitochondrial membrane permeabilization.
In summary, the ability of μ-calpain to degrade mature AIF to a 57 kDa fragment (Liou, et al., 2005, Polster, et al., 2005), inhibition of AIF truncation and release with specific calpain inhibitors or calpain knockdown (Cao, et al., 2007, Moubarak, et al., 2007, Norberg, et al., 2008), and the localization of μ-calpain to the submitochondrial compartment in which AIF cleavage must occur would appear to provide compelling support for the involvement of mitochondrial μ-calpain in AIF processing. However, direct experimental support of mitochondrial μ-calpain's role in AIF truncation has been elusive.
Acknowledgements
This research was supported by NIH grants PO1NS058484, PO1AG010836, and P30NS051220, as well as funding from the Kentucky Spinal Cord and Head Injury Research Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badugu R, Garcia M, Bondada V, Joshi A, Geddes JW. N terminus of calpain 1 is a mitochondrial targeting sequence. J Biol Chem. 2008;283:3409–3417. doi: 10.1074/jbc.M706851200. [DOI] [PubMed] [Google Scholar]
- Baici A, Muntener K, Willimann A, Zwicky R. Regulation of human cathepsin B by alternative mRNA splicing: homeostasis, fatal errors and cell death. Biol Chem. 2006;387:1017–1021. doi: 10.1515/BC.2006.125. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- Boujrad H, Gubkina O, Robert N, Krantic S, Susin SA. AIF-mediated programmed necrosis: a highly regulated way to die. Cell Cycle. 2007;6:2612–2619. doi: 10.4161/cc.6.21.4842. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitanya GV, Babu PP. Multiple apoptogenic proteins are involved in the nuclear translocation of Apoptosis Inducing Factor during transient focal cerebral ischemia in rat. Brain Res. 2008;1246:178–190. doi: 10.1016/j.brainres.2008.09.075. [DOI] [PubMed] [Google Scholar]
- Cong J, Goll DE, Peterson AM, Kapprell HP. The role of autolysis in activity of the Ca2+-dependent proteinases (mu-calpain and m-calpain). J Biol Chem. 1989;264:10096–10103. [PubMed] [Google Scholar]
- Cregan SP, Fortin A, MacLaurin JG, Callaghan SM, Cecconi F, Yu SW, Dawson TM, Dawson VL, Park DS, Kroemer G, Slack RS. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J Cell Biol. 2002;158:507–517. doi: 10.1083/jcb.200202130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Bondada V, Geddes JW. Mitochondrial localization of mucalpain. Biochem Biophys Res Commun. 2005;338:1241–1247. doi: 10.1016/j.bbrc.2005.10.081. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–730. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- Landshamer S, Hoehn M, Barth N, Duvezin-Caubet S, Schwake G, Tobaben S, Kazhdan I, Becattini B, Zahler S, Vollmar A, Pellecchia M, Reichert A, Plesnila N, Wagner E, Culmsee C. Bid-induced release of AIF from mitochondria causes immediate neuronal cell death. Cell Death Differ. 2008;15:1553–1563. doi: 10.1038/cdd.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Thompson VF, Goll DE. Effects of autolysis on properties of mu- and m-calpain. Biochim Biophys Acta. 2004;1691:91–103. doi: 10.1016/j.bbamcr.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Liou AK, Zhou Z, Pei W, Lim TM, Yin XM, Chen J. BimEL up-regulation potentiates AIF translocation and cell death in response to MPTP. Faseb J. 2005;19:1350–1352. doi: 10.1096/fj.04-3258fje. [DOI] [PubMed] [Google Scholar]
- Mehdi S, Angelastro MR, Wiseman JS, Bey P. Inhibition of the proteolysis of rat erythrocyte membrane proteins by a synthetic inhibitor of calpain. Biochem Biophys Res Commun. 1988;157:1117–1123. doi: 10.1016/s0006-291x(88)80989-6. [DOI] [PubMed] [Google Scholar]
- Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Menissier-de Murcia J, Susin SA. Sequential Activation of PARP-1, Calpains, and Bax is Essential in AIF-Mediated Programmed Necrosis. Mol Cell Biol. 2007 doi: 10.1128/MCB.02141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntener K, Zwicky R, Csucs G, Rohrer J, Baici A. Exon skipping of cathepsin B: mitochondrial targeting of a lysosomal peptidase provokes cell death. J Biol Chem. 2004;279:41012–41017. doi: 10.1074/jbc.M405333200. [DOI] [PubMed] [Google Scholar]
- Norberg E, Gogvadze V, Ott M, Horn M, Uhlen P, Orrenius S, Zhivotovsky B. An increase in intracellular Ca(2+) is required for the activation of mitochondrial calpain to release AIF during cell death. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.123. [DOI] [PubMed] [Google Scholar]
- Otera H, Ohsakaya S, Nagaura Z, Ishihara N, Mihara K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005;24:1375–1386. doi: 10.1038/sj.emboj.7600614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki T, Tomita H, Tamai M, Ishiguro S. Characteristics of mitochondrial calpains. J Biochem. 2007;142:365–376. doi: 10.1093/jb/mvm143. [DOI] [PubMed] [Google Scholar]
- Polster BM, Basanez G, Etxebarria A, Hardwick JM, Nicholls DG. Calpain I induces cleavage and release of apoptosis-inducing factor from isolated mitochondria. J Biol Chem. 2005;280:6447–6454. doi: 10.1074/jbc.M413269200. [DOI] [PubMed] [Google Scholar]
- Ritov VB, Tverdislova IL, Avakyan T, Menshikova EV, Leikin Yu N, Bratkovskaya LB, Shimon RG. Alamethicin-induced pore formation in biological membranes. Gen Physiol Biophys. 1992;11:49–58. [PubMed] [Google Scholar]
- Sasaki T, Kishi M, Saito M, Tanaka T, Higuchi N, Kominami E, Katunuma N, Murachi T. Inhibitory effect of di- and tripeptidyl aldehydes on calpains and cathepsins. J Enzyme Inhib. 1990;3:195–201. doi: 10.3109/14756369009035837. [DOI] [PubMed] [Google Scholar]
- Shalbuyeva N, Brustovetsky T, Bolshakov A, Brustovetsky N. Calcium-dependent spontaneously reversible remodeling of brain mitochondria. J Biol Chem. 2006;281:37547–37558. doi: 10.1074/jbc.M607263200. [DOI] [PubMed] [Google Scholar]
- Shulga N, Pastorino JG. Acyl coenzyme A-binding protein augments bid-induced mitochondrial damage and cell death by activating mu-calpain. J Biol Chem. 2006;281:30824–30833. doi: 10.1074/jbc.M602503200. [DOI] [PubMed] [Google Scholar]
- Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Stoka V, Turk V, Bredesen DE. Differential regulation of the intrinsic pathway of apoptosis in brain and liver during ageing. FEBS Lett. 2006;580:3739–3745. doi: 10.1016/j.febslet.2006.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira HL, Haouzi D, El Hamel C, Jacotot E, Belzacq AS, Brenner C, Kroemer G. Permeabilization of the mitochondrial inner membrane during apoptosis: impact of the adenine nucleotide translocator. Cell Death Differ. 2000;7:1146–1154. doi: 10.1038/sj.cdd.4400778. [DOI] [PubMed] [Google Scholar]
- Wang KK, Nath R, Posner A, Raser KJ, Buroker-Kilgore M, Hajimohammadreza I, Probert AW, Jr., Marcoux FW, Ye Q, Takano E, Hatanaka M, Maki M, Caner H, Collins JL, Fergus A, Lee KS, Lunney EA, Hays SJ, Yuen P. An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc Natl Acad Sci U S A. 1996;93:6687–6692. doi: 10.1073/pnas.93.13.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, Yuen P.-w. Calpain substrates, assay methods, regulations, and its inhibitory agents. In: Wang KK, Yuen PW, editors. Calpain: Pharmacologiy and Toxicology of Calpain-Dependent Protease. Taylor & Francis; Philadelphia, PA: 1999. pp. 77–101. [Google Scholar]
- Wendt A, Thompson VF, Goll DE. Interaction of calpastatin with calpain: a review. Biol Chem. 2004;385:465–472. doi: 10.1515/BC.2004.054. [DOI] [PubMed] [Google Scholar]
- Yuste VJ, Moubarak RS, Delettre C, Bras M, Sancho P, Robert N, d'Alayer J, Susin SA. Cysteine protease inhibition prevents mitochondrial apoptosis-inducing factor (AIF) release. Cell Death Differ. 2005;12:1445–1448. doi: 10.1038/sj.cdd.4401687. [DOI] [PubMed] [Google Scholar]