Abstract
PURPOSE
To determine the mechanism through which topical mitomycin C prevents and treats corneal haze after photorefractive keratectomy (PRK) and to examine the effects of dosage and duration of exposure.
METHODS
In 224 New Zealand rabbits, −9.0 diopter PRK with mitomycin C or balanced salt solution was performed. Haze level was graded at the slit-lamp. Rabbits were sacrificed at 4 hours, 24 hours, 4 weeks, or 6 months after surgery and immunohistochemistry was performed with TUNEL assay, Ki67 and α-SMA.
RESULTS
TUNEL-positive apoptotic cells marginally increased in all mitomycin C groups whereas Ki67-positive mitotic cells decreased significantly following mitomycin C application. A greater decrease in myofibroblasts was noted with prophylactic mitomycin C treatment than therapeutic mitomycin C treatment. There was, however, an anterior stromal acellular zone (approximately 20% of the total stroma) in eyes treated with mitomycin C, which persisted to the maximum follow-up of 6 months.
CONCLUSIONS
Mitomycin C treatment induces apoptosis of keratocytes and myofibroblasts, but the predominate effect in inhibiting or treating haze appears to be at the level of blocked replication of keratocytes or other progenitor cells of myofibroblasts. Treatment with 0.002% mitomycin C for 12 seconds to 1 minute appears to be just as effective as higher concentrations for longer duration in the rabbit model. However, a persistent decrease in keratocyte density in the anterior stroma could be a warning sign for future complications and treatment should be reserved for patients with significant risk of developing haze after PRK.
Photorefractive keratectomy (PRK) has proven to be a safe and effective procedure to correct low to moderate levels of myopia, hyperopia, or astigmatism. It continues to represent a good alternative to LASIK for many patients, and in some situations, PRK remains the procedure of choice.1 However, PRK continues to have downsides, including increased postoperative pain, a stronger healing response, and most significantly, the possibility of subepithelial corneal opacity or “haze” formation following corrections for high myopia.2,3
The native conformation of the extracellular matrix is altered after PRK. Along with changes in cellular density and phenotype, there is variable production of disorganized extracellular matrix components and generation of myofibroblasts.4 The end result is a decrease in tissue transparency associated with subepithelial corneal haze, which in some patients, is clinically significant. Several different clinical factors likely contribute to haze formation including the depth of ablation, delayed epithelial healing, and the smoothness of the stromal surface after the ablation.5,6 Recent studies have confirmed that postoperative smoothness of the stromal surface is an important factor contributing to subepithelial haze formation.7 In addition, different modulators of the corneal wound healing response and potential medications have been suggested for treatment.8–11
The use of topical mitomycin C during surface ablation procedures has gained considerable attention over the past few years. The mitomycins are potent antibiotics that belong to the family of anti-tumor quinolones.12 In 1956, mitomycin A and B were isolated from the broth of Streptomyces caespitosus, and shortly thereafter mitomycin C was discovered.12 Mitomycin C forms a covalent linkage with deoxyribonucleic acid (DNA) and inhibits DNA synthesis, functioning as a powerful alkylating agent.12,13 At high concentrations, cellular RNA and protein synthesis can also be suppressed.12,13 As a consequence of these cyclostatic effects, mitomycin C is believed to trigger apoptosis and inhibit proliferation of virtually all cells which it enters in sufficient concentrations, including corneal epithelial cells, stromal cells, endothelial cells, conjunctival cells, Tenon’s capsule fibroblasts, and retinal pigment epithelial cells.14–17
The potent effects of mitomycin C on cell replication during the wound healing response gained the attention of vision researchers and its potential benefits in preventing or inhibiting scar formation suggested several possible applications to clinicians. Watanabe et al18 demonstrated that conjunctival cell replication was markedly inhibited by the topical administration of mitomycin C, suggesting potential applications during trabeculectomy surgery. Topical mitomycin C treatment has also been used in the treatment of recurrent pterygium and conjunctival and corneal intraepithelial neoplasia. Subsequently, topical mitomycin C treatment was proposed as an alternative therapy for corneas with previous scar formation secondary to refractive surgical procedures.19 In 1991, Talamo et al20 suggested the use of topical mitomycin C as a modulator of the corneal wound healing response after excimer laser photoablation. These investigators reported that rabbits treated with mitomycin C following laser ablation had markedly reduced formation of subepithelial collagen. Majmudar et al19 first proposed the use of mitomycin C to treat patients with subepithelial fibrosis secondary to previous PRK and radial keratotomy. These authors reported a significant improvement in corneal clarity with a single, 2-minute intraoperative application of 0.02% mitomycin C. Intraoperative use of topical mitomycin C was subsequently used prophylactically to prevent subepithelial haze formation following PRK. Carones et al21 reported a statistically significant decrease in subepithelial haze formation and more accurate refractive outcomes after the prophylactic use of 0.02% mitomycin C during PRK in a group of 60 patients with a spherical equivalent between −6.0 and −10.0 diopters (D) of myopia. Since these early reports, many clinicians have begun using mitomycin C prophylactically—even for low corrections. However, several questions regarding the optimal dosage and exposure time and the long-term safety and efficacy of topical mitomycin C treatment remain unanswered. Even the primary mechanism of action of mitomycin C in the cornea remains uncertain.
The purpose of this study was to examine the effect of topical mitomycin C used at different concentrations and for varying exposure times on corneal cells in situ and the efficacy of prophylactic and therapeutic mitomycin C treatments on subepithelial corneal haze following PRK for high myopia in a rabbit model.
MATERIALS AND METHODS
ANIMAL EXPERIMENTS
Animals
The Animal Control Committee at the Cleveland Clinic Foundation approved all animal studies described in this work. All animals were treated in accordance with the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Anesthesia was obtained by intramuscular injection of ketamine hydrochloride (30 mg/kg) and xylazine hydrochloride (5 mg/kg). In addition, topical proparacaine hydrochloride 1% (Alcon, Ft Worth, Tex) was applied to each eye just prior to surgery. Euthanasia was performed using an intravenous injection of 100 mg/kg pentobarbital while the animal was under general anesthesia.
Two hundred twenty-four 12- to 15-week-old female New Zealand white rabbits weighing 2.5 to 3.0 kg each were included in this study. One eye of each rabbit was selected at random to be in the study. Nine groups were included (Table 1). All study and control eyes had −9.0 D PRK with a 6.0 mm ablation zone performed with an Apex Plus Summit Laser (Alcon). The PRK had a depth of 108 µm (421 pulses). This treatment has been shown to produce marked subepithelial haze in 100% of rabbit eyes.4
Table 1.
Summary of Prophylactic, Therapeutic, and Control Groups That Underwent −9.0 D PRK
| Prophylactic Group |
Therapeutic Group |
||||
|---|---|---|---|---|---|
| PRK Correction Immediately Followed by |
Previous PRK Correction (4 weeks prior) Followed by |
||||
| Groups | No. of Eyes per Time Point (n) |
MMC | BSS | Epithelial Scrape + MMC |
Epithelial Scrape + BBS |
| P-I | 6 | 0.02% for 2 min | — | — | |
| P-II | 6 | 0.02% for 1 min | — | — | |
| P-III | 6 | 0.02% for 12 s | — | — | — |
| P-IV | 6 | 0.002% for 2 min | — | — | |
| P-V | 6 | 0.002% for 12 s | — | — | — |
| T-I | 6 | — | — | MMC 0.02% for 2 min | — |
| T-II | 6 | — | — | — | BSS for 2 min |
| C | 6 | — | BSS 12 s to 2 min | — | — |
Three time points were examined in both prophylactic and therapeutic groups: 4 hours, 24 hours, and 4 weeks after application of mitomycin C or balanced salt solution (BSS). There were six eyes in each group at each time point. To observe long-term effects, another time point at 6 months was added to prophylactic group I (P-I; 0.02% mitomycin for 2 minutes) and the control group (C). Time points following treatment were selected on the basis of peak occurrence of the events under investigation in a previous study.4 Thus, the 4-hour time point was selected to study the approximate peak of apoptosis, the 24-hour time point was selected to study the approximate peak of proliferation of fibroblast cells, and the 4-week time point was selected to study the approximate peak of myofibroblast generation.
To study the prophylactic effects of mitomycin C, PRK was performed followed by application of mitomycin C or BSS. The prophylactic groups (Table 1) were: P-I, mitomycin C 0.02% in BSS for 2 minutes; P-II, mitomycin C 0.02% in BSS for 1 minute; P-III, mitomycin C 0.02% in BSS for 12 seconds; P-IV, mitomycin C 0.002% in BSS for 2 minutes; P-V, mitomycin C 0.002% in BSS for 1 minute; and P-VI, mitomycin C 0.002% in BSS for 12 seconds. The control group, C, had BSS alone for 12 seconds, 1 minute, or 2 minutes. The contralateral eye of each animal was an unoperated control eye.
To generate subepithelial corneal haze to study the therapeutic effect of mitomycin C, PRK for high myopia (−9.0 D) was performed and the rabbits followed for 4 weeks before epithelial scrape and application of 0.05% mitomycin C for 2 minutes (TI) or BSS for 2 minutes (T2).
PRK With Mitomycin C or Balanced Salt Solution Technique
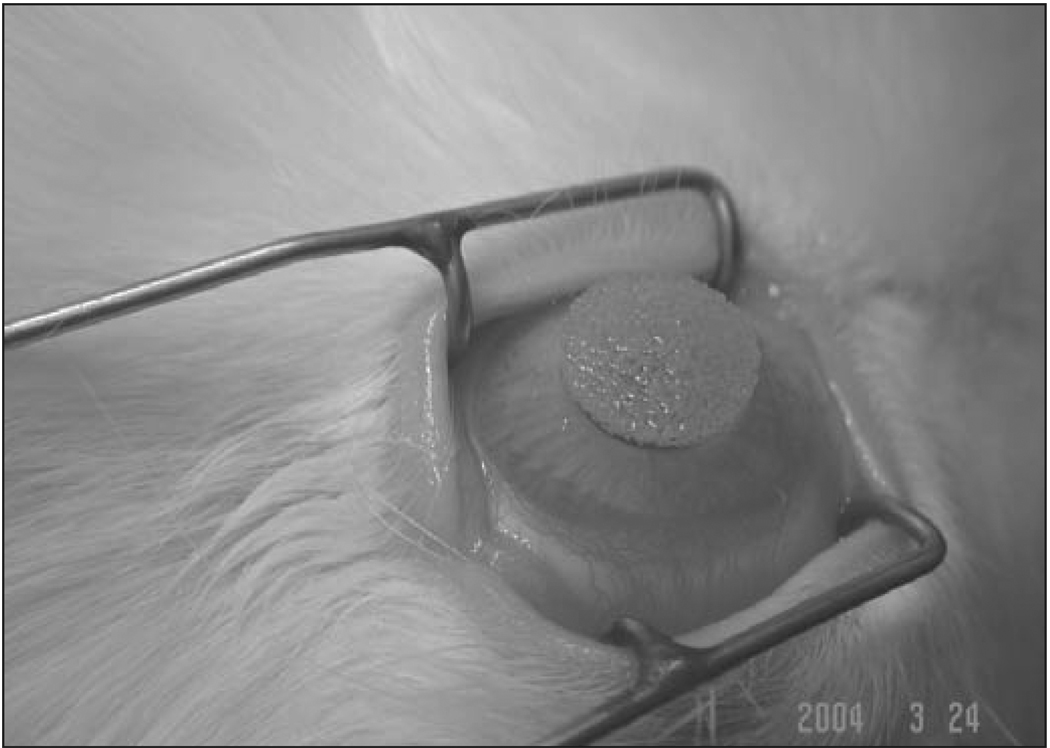
With the animal under general and local anesthesia, a wire lid speculum was placed in the eye and a 7-mm diameter area of epithelium overlying the pupil was removed with a #64 blade (Beaver; Becton-Dickinson, Franklin Lake, NJ). The −9.0 D PRK was immediately followed by the topical application of mitomycin C or BSS to the ablated area using a soaked circular sponge (Fig 1). The mitomycin C or BSS treatment was terminated with copious irrigation with 30 mL of BSS. Two drops of ciprofloxacin hydrochloride 0.3% (Ciloxan, Alcon) were instilled into the eye at the end of the procedure. Any eye that developed infiltration suggestive of infection or an epithelial defect persisting beyond 1 week after surgery was excluded from the study and an additional animal included in its place.
Figure 1.
Topical application of mitomycin C on the rabbit cornea using a soaked circular sponge.
Tissue Fixation and Sectioning
Rabbits were euthanized and the corneoscleral rims of treated and unoperated control eyes removed with 0.12 forceps and sharp Westcott scissors. For histological analyses, the corneas were embedded in liquid OCT compound (Sakura FineTek, Torrance, Calif) within a 24×24×5 mm mold (Fisher, Pittsburgh, Pa). The tissue specimens were centered within the mold so that the block could be bisected and transverse sections cut from the center of the cornea. The mold and tissue were rapidly frozen in 2-methyl butane within a stainless steel crucible suspended in liquid nitrogen. The frozen tissue blocks were stored at −85°C until sectioning was performed.
Central corneal sections (7-µm thick) were cut using a cryostat (HM 505M; Micron GmbH, Walldorf, Germany). Sections were placed on microscope slides (Superfrost Plus, Fisher) and maintained at −85°C until staining was performed.
TUNEL Assays and Immunocytochemistry Assays
To detect fragmentation of DNA associated with apoptosis, tissue sections were fixed in acetone at −20°C for 2 minutes, dried at room temperature for 5 minutes, and then placed in BSS.22 A fluorescence-based TUNEL assay was performed according to the manufacturer’s instructions (ApopTag, Cat No S7165; Intergen, Purchase, NY). Positive control (4 hours after mechanical corneal scrape) and negative control (unwounded) cornea slides were included in each assay. Photographs were obtained with a fluorescence microscope (Nikon E600, Melville, NY).
Immunocytochemistry was performed as previously reported.23 The monoclonal antibody for either Ki67 (Zymed, San Francisco, Calif), a marker of cells in G1-M phase of mitosis, or anti-α-smooth muscle actin (DAKO; Carpentaria, Calif), a marker of myofibroblasts, was placed on the sections and incubated at room temperature for either 2 hours (anti-Ki67) or 1 hour (anti-α-smooth muscle actin). The working concentrations for anti-Ki67 and anti-α-smooth muscle actin were 214.6 mg/L (1% BSA, pH 7.4), and 85 mg/L (1% BSA), respectively. The secondary antibody, fluorescein-conjugated donkey antimouse IgG (Jackson ImmunoResearch, West Grove, Pa), was applied for 1 hour at room temperature. Coverslips were mounted with Vectashield (Vector Laboratories Inc, Burlingame, Calif) containing DAPI to observe all cell nuclei in the tissue. Negative controls with secondary antibody alone or irrelevant isotype-matched antibodies were included in each experiment. The sections were viewed and photographed with a Nikon Eclipse E800 microscope equipped with a digital SPOT camera (Micro Video Instruments, Avon, Mass).
Slit-lamp Microscopy Analysis and Subepithelial Haze Grading
Animals were evaluated at the slit-lamp (Haag-Streit BM900, Haag-Streit, Switzerland) under general anesthesia to grade subepithelial corneal haze. Corneal haze was graded according to a system previously reported by Fantes et al24: grade 0, completely clear cornea; grade 0.5, trace haze, seen with careful oblique illumination with slit-lamp microscopy; grade 1, more prominent haze, not interfering with visibility of fine iris details; grade 2, mild obscuration of iris details; grade 3, moderate obscuration of iris details and lens; and grade 4, completely opaque stroma in the area of the ablation with no visibility of iris details.
Cell Counting for Quantitation of TUNEL Assays and Immunocytochemistry Assays
Six corneas with each surgical procedure were used for cell counting at each time point. In each cornea, all of the stained cells in seven non-overlapping, full-thickness columns extending from the anterior stromal surface to the posterior stromal surface were counted by a single observer on the section under the microscope. The diameter of each column was that of a 400× microscope field. The columns in which counts were performed were selected at random from the central cornea of each specimen. In sections stained for alpha smooth muscle actin and DAPI, the counts of for α-smooth muscle actin + cells only included individual cells that had a DAPI + nucleus.
IN VITRO EXPERIMENT
Keratocyte and Myofibroblast Culture
Primary corneal keratocytes were isolated from rabbit corneas (Pel Freeze, Rogers, Ariz) using a previously reported enzymatic method25 and seeded in four-chamber slides (1×104 cells/chamber) or 100-mm tissue culture plates (30×104 cells/plate) and grown for 7 days in serum-free MEM medium (group I). To generate myofibroblasts (group II), 1 ng/mL TGFβ1 (R&D Systems, Minneapolis, Minn) was added to the plate with fresh medium and replaced at 2-day intervals for 1 to 2 weeks.
Keratocyte and myofibroblast cultures were divided into groups with or without 0.02% mitomycin C for 2 minutes followed by copious irrigation with 200 mL of BSS. The nuclei of keratocytes and myofibroblasts were stained with DAPI (Vector Laboratories Inc). TUNEL assay and immunocytochemistry for α-smooth muscle actin was subsequently performed, as described above.
STATISTICAL ANALYSIS
Data were analyzed using statistical software (Statview 4.5; Abacus Concepts, Berkeley, Calif). Variations were expressed as standard errors of the mean (SEM). Statistical comparisons between the groups were performed using analysis of variance (ANOVA) with the Bonferonni-Dunn adjustment for repeated measures. All statistical tests were conducted at an alpha level of 0.05.
RESULTS
SUBEPITHELIAL CORNEAL HAZE (SLIT-LAMP MICROSCOPY)
All prophylactic and therapeutic groups displayed some variability in subepithelial corneal haze formation (Table 2). Corneal haze usually became noticeable at 2 weeks after surgery and peaked around 4 weeks. Subepithelial corneal haze was confined to the area of laser photoablation, with sharp demarcation between ablated and unablated stroma and independent of the area of the epithelial removal in preparation for PRK (Fig 2). Corneal haze was subepithelial, with occasional extension posteriorly into the stroma to a maximum of approximately 20% depth. The BSS group had significantly (P<.001) more subepithelial corneal haze at 4 weeks after surgery than any of the prophylactic mitomycin C groups, regardless of mitomycin concentration or exposure time (Table 2). However, all prophylactic mitomycin C groups had some corneas with grade 0.5 haze (trace) at 4 weeks and a few corneas in the 0.002% mitomycin C groups with 12-second or 1-minute exposure times developed grade 1 haze at 4 weeks after surgery. However, a statistically significant (P<.05) difference in haze formation was observed only between prophylactic groups P-I (mitomycin C 0.02% for 2 minutes) and P-VI (mitomycin C 0.002% for 12 seconds).
Table 2.
Corneal Haze Grading With Slit-lamp Microscopy at 4 Weeks After Treatment
| Groups (n, %) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Degree of Haze |
P-I (MMC 0.02% 2 min) |
P-II (MMC 0.02% 1 min) |
P-III (MMC 0.02% 12 s) |
P-IV (MMC 0.002% 2 min) |
P-V (MMC 0.002% 1 min) |
P-VI (MMC 0.002% 12 s) |
T-I (ES+MMC 0.02% 2 min) |
T-II (ES+BSS) |
C (BSS) |
|
| 0 | 3 (50) | 3 (50) | 2 (33.3) | 2 (33.3) | 2 (33.3) | 1 (16.6) | ||||
| 0.5 | 3 (50) | 3 (500) | 4 (66.6) | 4 (66.6) | 3 (50) | 3 (50) | 1 (16.6) | |||
| 1 | 1 (16.6) | 2 (33.3) | 3 (50) | 1 (16.6) | 1 (16.6) | |||||
| 2 | 2 (33.3) | 2 (33.3) | 2 (33.3) | |||||||
| 3 | 3 (50) | 2 (33.3) | ||||||||
| 4 | 1 (16.6) | |||||||||
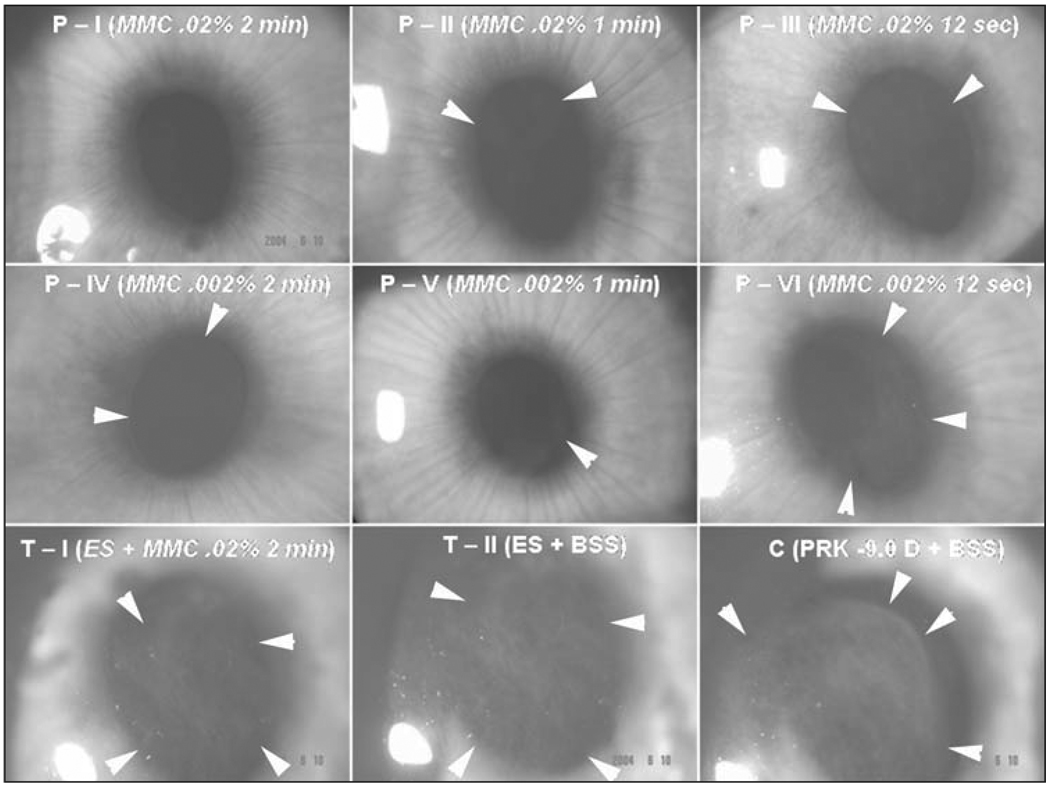
Figure 2.
Representative slit-lamp photographs showing subepithelial haze patterns and locations restricted to the area of corneal ablation. Faint 0.5 to 1.5 subepithelial corneal haze can be seen in the prophylactic mitomycin C groups P-I to P-VI with differing concentrations of mitomycin C and exposure times noted. Grade II subepithelial haze is seen in one specimen (T-1) that was not very effectively treated with therapeutic 0.02% mitomycin C for 2 minutes. Moderate to severe subepithelial corneal haze (grade III) can be noted in the therapeutic control group treated with BSS (T-II) and the prophylactic control group (C). Note that the subepithelial haze tends to terminate exactly at the margin of the excimer laser ablation (arrows in C) (original magnification ×10).
Subepithelial corneal haze tended to diminish with time after 4 weeks in all groups, including prophylactic, therapeutic, and control. Thus, significantly less subepithelial haze was observed in the BSS control group (C), the group with the most intense haze, at 6 months after the PRK procedure compared to 4 weeks after surgery (P<.05). No cornea treated with prophylactic mitomycin C had detectible subepithelial haze at 6 months.
Analysis of the therapeutic groups showed significantly (P<.05) more subepithelial corneal haze in the BSS-treated group (T-II) than the mitomycin C-treated group (T-I) (Table 2) at 4 weeks after treatment. However, the level of subepithelial corneal haze at 4 weeks after treatment in the mitomycin C therapeutic group (T-I) was significantly higher (P<.05) than that at 4 weeks after surgery in any of the mitomycin C prophylactic groups (Table 2).
APOPTOTIC CELLS IN THE STROMA
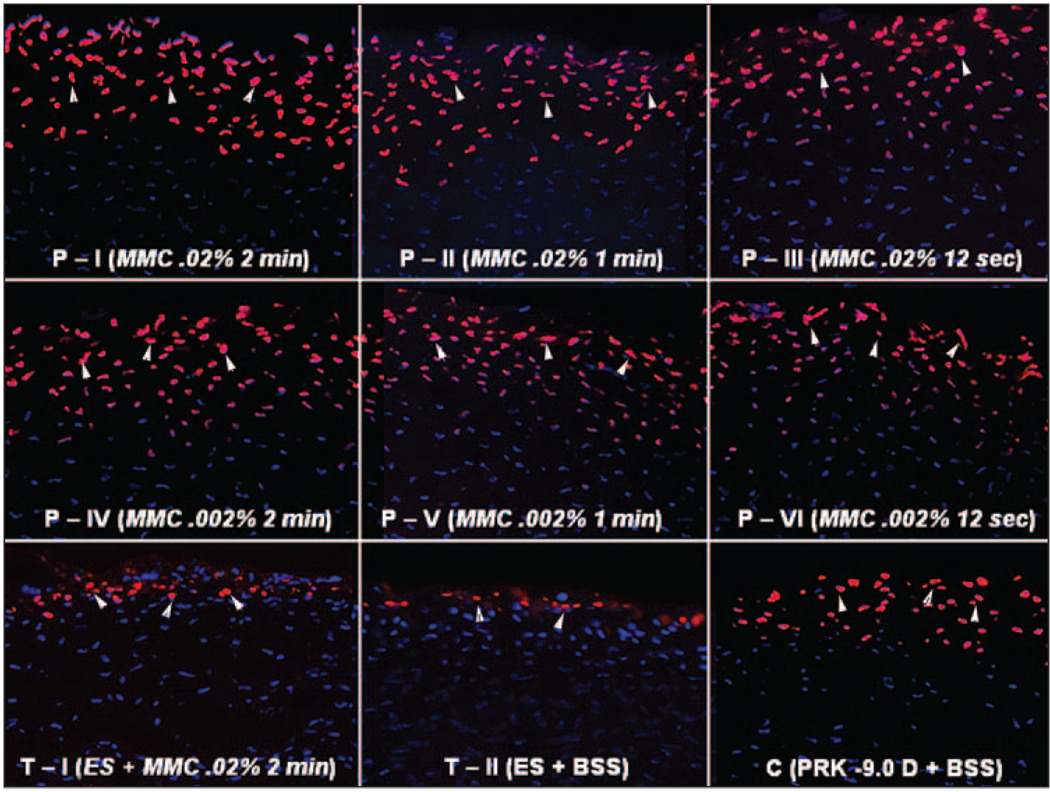
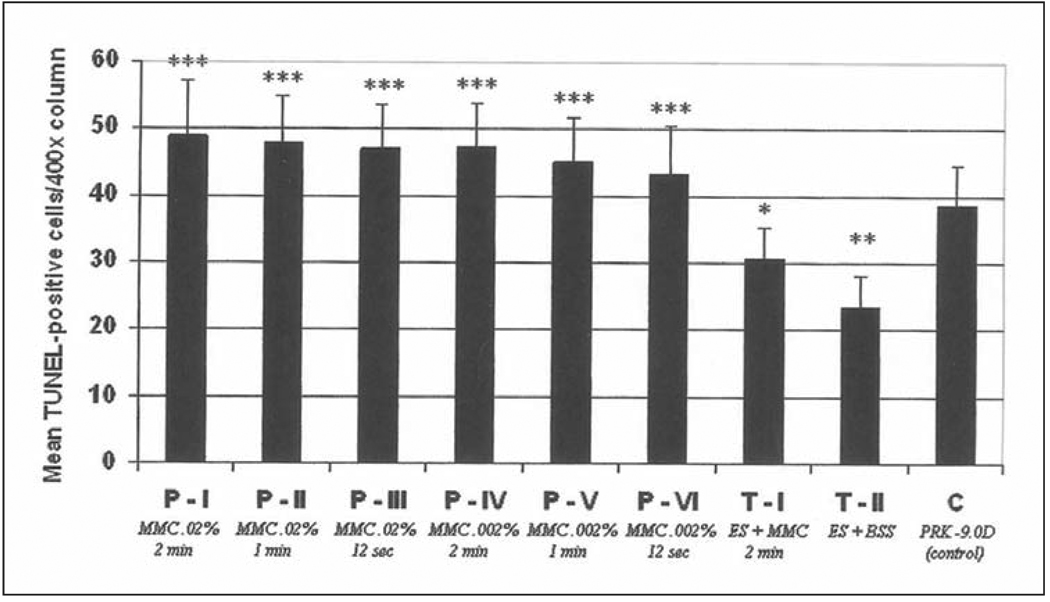
The anterior stroma of all corneas that had −9.0 D PRK with or without mitomycin C had TUNEL-positive cells in the anterior 20% of stroma at 4 hours and 24 hours after the procedure (Fig 3). The posterior stoma and endothelium had only rare TUNEL-positive cells, and no differences in these areas were noted between control and treatment groups. There was a statistically significant increase in the number of cells undergoing apoptosis in the stroma (Fig 4) in the mitomycin C groups with all concentrations and exposure times at 4 hours and 24 hours compared to the control group (P<.05). The numbers of TUNEL-positive cells in the stroma tended to gradually decrease as the mitomycin C concentration and exposure time decreased (see Fig 3 and Fig 4), but these decreases were not statistically significant. No specimens from any group had TUNEL-positive cells at 4 weeks after surgery.
Figure 3.
TUNEL assay for apoptotic cells 4 hours after surgery of the central cornea from rabbits that had −9.0 D PRK for myopia followed by mitomycin C or BSS application. Different concentrations and exposure times of mitomycin C are indicated. These are representative results for each group. Cell nuclei are stained blue with DAPI and TUNEL-positive cells are stained red (arrows) (original magnification ×200).
Figure 4.
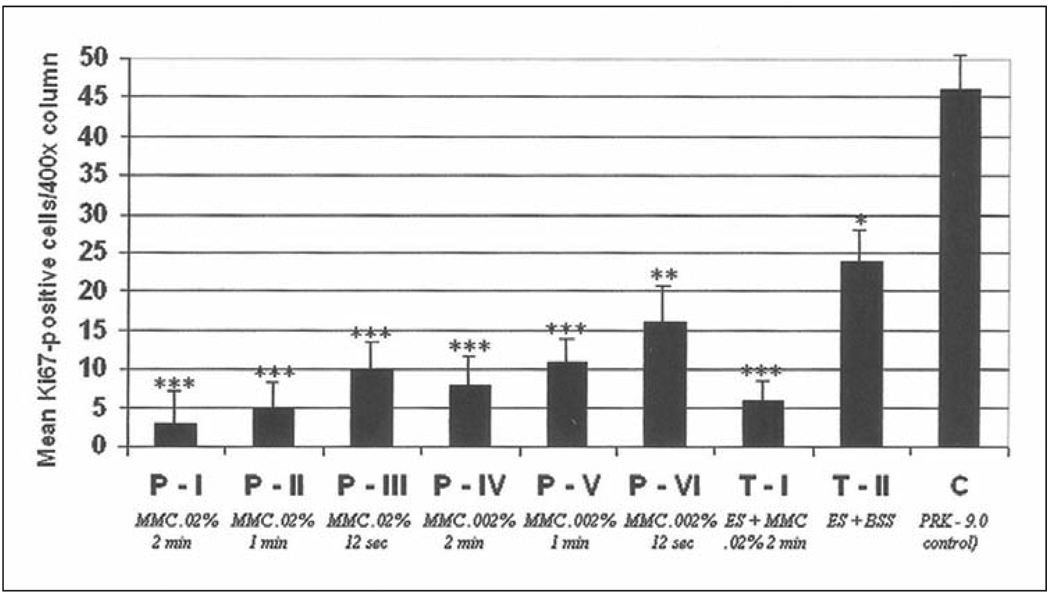
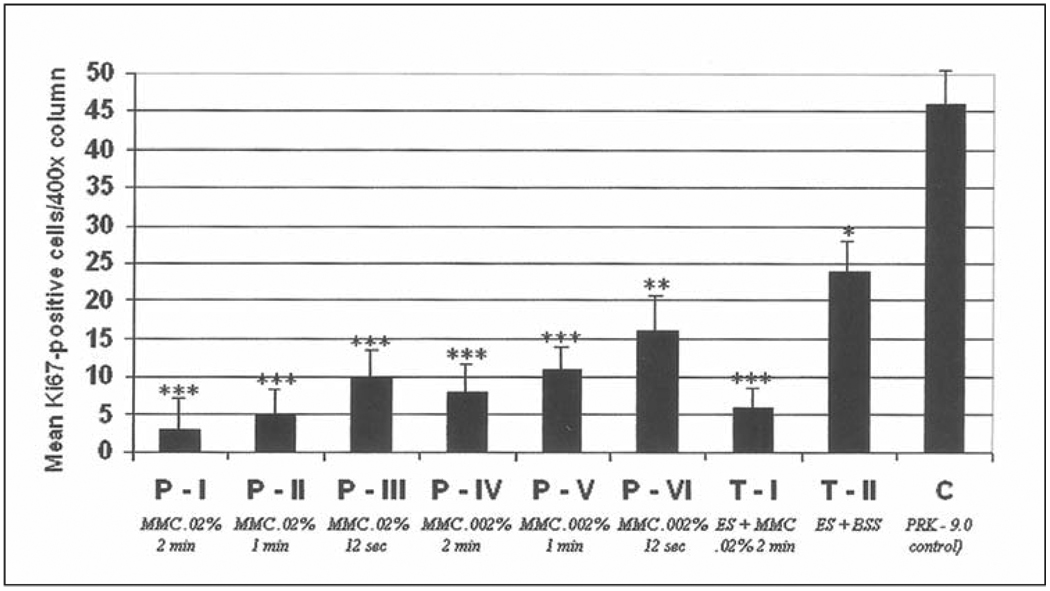
Quantitation of TUNEL-positive apoptotic cells in the anterior stroma (per 400× column) at 4 hours after laser surgery in all prophylactic and therapeutic mitomycin C groups and the control (BSS) group. *, **, *** are used to indicate groups that are significantly different from the control group. In addition, * is significantly different from **, * is significantly different from ***, and ** is significantly different from ***.
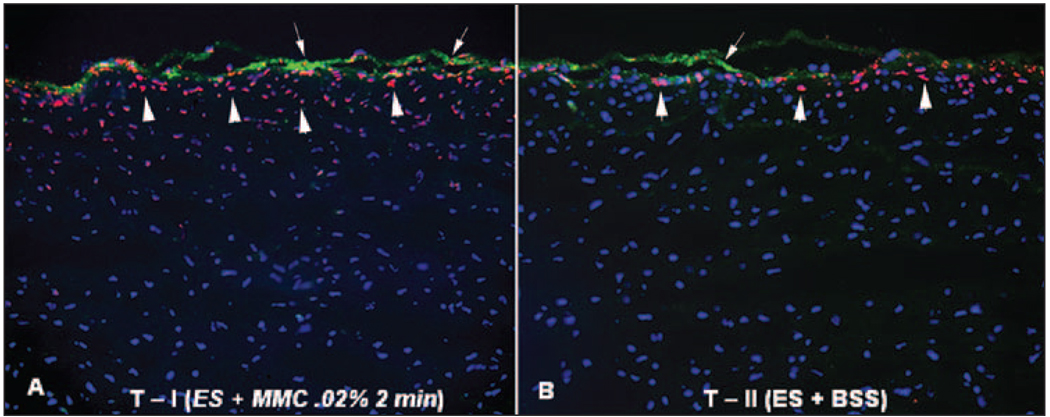
In the therapeutic groups, epithelial scraping with mitomycin C or BSS treatment triggered TUNEL-positive cells in the anterior 10% to 20% of stroma, but significantly more cells underwent apoptosis in the mitomycin C-treated group (T-I) compared to the BSS-treated control group (T-II) (Fig 5). An attempt was also made to use double labeling with the TUNEL assay and immunocytochemistry for α-smooth muscle actin to determine whether mitomycin C produced more apoptosis in myofibroblasts than BSS, but the results were inconclusive (see Fig 5). However, some TUNEL+ and α-smooth muscle actin+ cells were noted.
Figure 5.
TUNEL assay and α-smooth muscle actin double-staining of the central rabbit corneas at 4 hours after epithelial scrape followed by mitomycin C or BSS application in eyes that had −9.0 D PRK 4 weeks earlier with development of significant subepithelial corneal haze (therapeutic treatment with mitomycin C or BSS). All cell nuclei are stained blue with DAPI. TUNEL-positive cells are stained red (arrows) and α-smooth muscle actin-positive cells are stained green (arrowheads). Overall, significantly more TUNEL-positive cells were noted in A) group T-I than in B) group T-II (original magnification ×200).
CELL REPLICATION IN THE STROMA
All groups displayed very few Ki67-positive cells in the stroma at 4 hours after surgery. At 24 hours after surgery (Fig 6 and Fig 7), however, large numbers of Ki67-positive stromal cells undergoing mitosis were present in the anterior 20% to 30% of the stroma of rabbit eyes that had PRK followed by BSS application, with significantly fewer Ki67-positive stromal cells undergoing mitosis in eyes that had PRK followed by mitomycin C (P<.001). The differences between the two concentrations and three exposure times for mitomycin C were not statistically significant, except the 0.002% mitomycin C for 12 seconds group had significantly more Ki67-positive proliferating cells than the other prophylactic mitomycin C treated groups, but still significantly less proliferating stromal cells than the BSS-treated control group (see Fig 7).
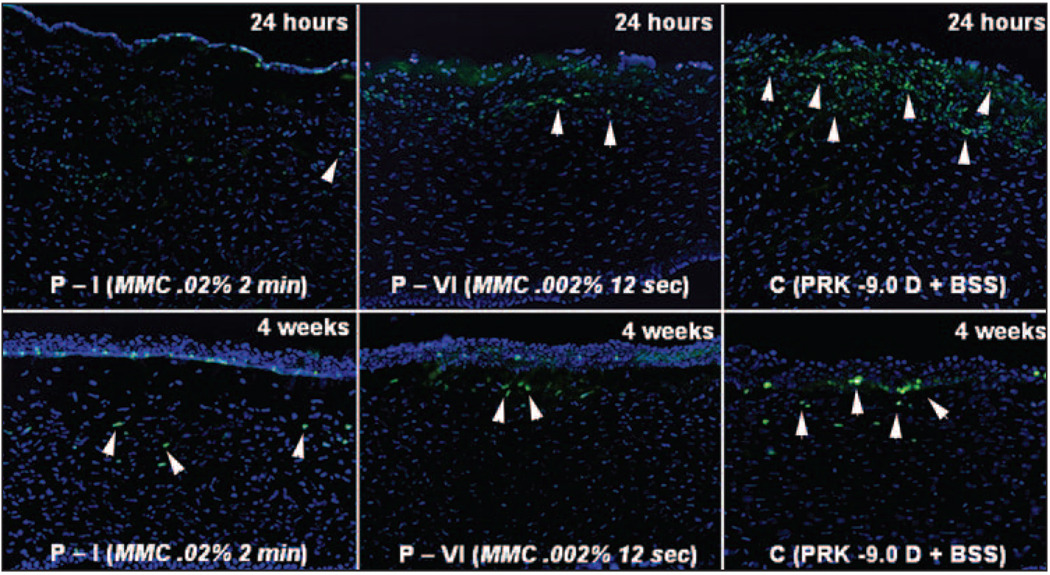
Figure 6.
Immunohistochemical Ki67 staining to detect cell mitosis in the central cornea in eyes that had −9.0 D PRK for myopia followed by mitomycin C or BSS application at 24 hours or 4 weeks after treatment. There is marked cell proliferation (arrowheads) in the anterior stroma in the BSS group (C) at 24 hours, with some still detectable 4 weeks after treatment. Minimal proliferation cells in the anterior stroma are noted in corneas treated with 0.02% mitomycin C for 2 minutes (P-I) at 24 hours after treatment. In corneas treated with 0.002% mitomycin C for 12 seconds (P-VI), there are more anterior stromal cells undergoing mitosis at 24 hours after treatment than the higher concentration mitomycin C group, but still far fewer than in the control group (original magnification ×200).
Figure 7.
Quantitation of Ki67-positive cells in the anterior stroma (per 400× column) at 24 hours after laser surgery in prophylactic and therapeutic mitomycin C-treated groups and the BSS-treated control group. *, **, *** are used to indicate groups that are significantly different from the control group. In addition, * is significantly different from **, * is significantly different from ***, and ** is significantly different from ***.
At 4 weeks, there was a decrease in Ki67-positive cells in the anterior stroma of control rabbit eyes treated with BSS (see Fig 6). The numbers of these Ki67-positive cells was still significantly lower in the mitomycin C-treated groups than the control BSS-treated group, but the difference was small (see Fig 6).
In the therapeutic groups, more Ki67-positive cell were observed in the anterior stroma of rabbit corneas with subepithelial haze that had epithelial scrape followed by treatment with BSS compared to those treated with mitomycin C at 24 hours (P<.001) or 4 weeks (P<.05) after treatment.
Few Ki67-positive cells were observed in any of the groups at 6 months after surgery and no statistically significant differences between the groups were noted (not shown).
MYOFIBROBLASTS IN THE STROMA
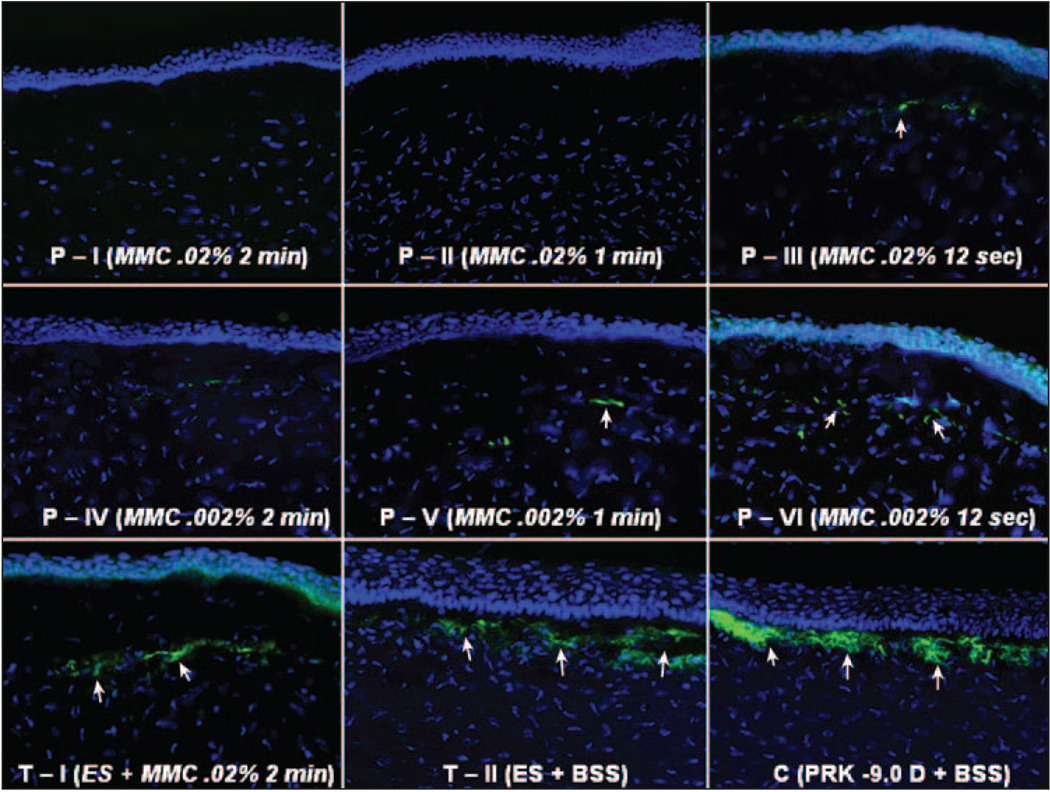
Alpha-smooth muscle actin-positive myofibroblasts were not identified in any groups at the early time points (4 hours and 24 hours). Significant numbers of α-smooth muscle actin-positive cells were present in the anterior 5% of the stroma in the BSS-treated control group at 4 weeks after surgery (Fig 8 and Fig 9). For prophylactic mitomycin C treatment, there was a statistically significant difference between all mitomycin C-treated groups and the BSS-treated control group (P<.001). No significant difference was noted between the different mitomycin C concentrations and times, except the 0.002% mitomycin C for 12 seconds group (P-VI) had significantly more (P<.05) myofibroblasts at 1 month than prophylactic 0.02% mitomycin C for 2 minutes group (P-I). The number of α-smooth muscle actin-positive cells in group P-VI was, however, still significantly less than the BSS-treated control group C (see Fig 9).
Figure 8.
Immunohistochemical staining for α-smooth muscle actin in the central cornea of representative sections of the different groups at 4 weeks after surgery. Cells nuclei are stained blue with DAPI and α-smooth muscle actin-positive myofibroblasts are stained green (arrows). Note that in mitomycin C-treated corneas myofibroblasts lie beneath an acellular to pauci-cellular zone of anterior stroma whereas in BSS-treated corneas (either therapeutic control group T-II or PRK control) the myofibroblasts are localized immediately beneath the epithelium. Also note that more DAPI stained nuclei are present in the anterior stroma of all of the prophylactic group corneas treated with 0.002% mitomycin C (P-IV, P-V, and P-VI) compared to the corneas treated with 0.02% mitomycin C (P-I, P-II, and P-III) (original magnification ×400).
Figure 9.
Quantitation of α-smooth muscle actin-positive cells in the anterior stroma (per 400× column) at 4 weeks after laser surgery in prophylactic and therapeutic mitomycin C groups and the BSS-treated controls. *, **, *** are used to indicate groups that are significantly different from the control group. In addition, * is significantly different from **, * is significantly different from ***, and ** is significantly different from ***.
In the therapeutic groups, α-smooth muscle actin-positive cells were observed at 4 hours after the epithelial scrape with mitomycin C or BSS treatment, and no statistically significant difference was noted between the groups at this early time point. However, at 4 weeks after treatment, there were significantly more α-smooth muscle actin-positive cells in the BSS group than the mitomycin C group (P<.05). Some myofibroblasts persisted in the mitomycin C-treated therapeutic group. When the therapeutic group (mitomycin C 0.02% for 2 minutes) was compared with the corresponding prophylactic group (mitomycin C 0.02% for 2 minutes), there were significantly more myofibroblast cells in the therapeutic group (P<.05).
At 6 months after therapeutic treatment with BSS, a highly significant decrease in the number of α-smooth muscle actin-positive cells was noted compared to 4 weeks after treatment. No α-smooth muscle actin-positive cells were present in the mitomycin C therapeutic treatment group at 6 months after surgery.
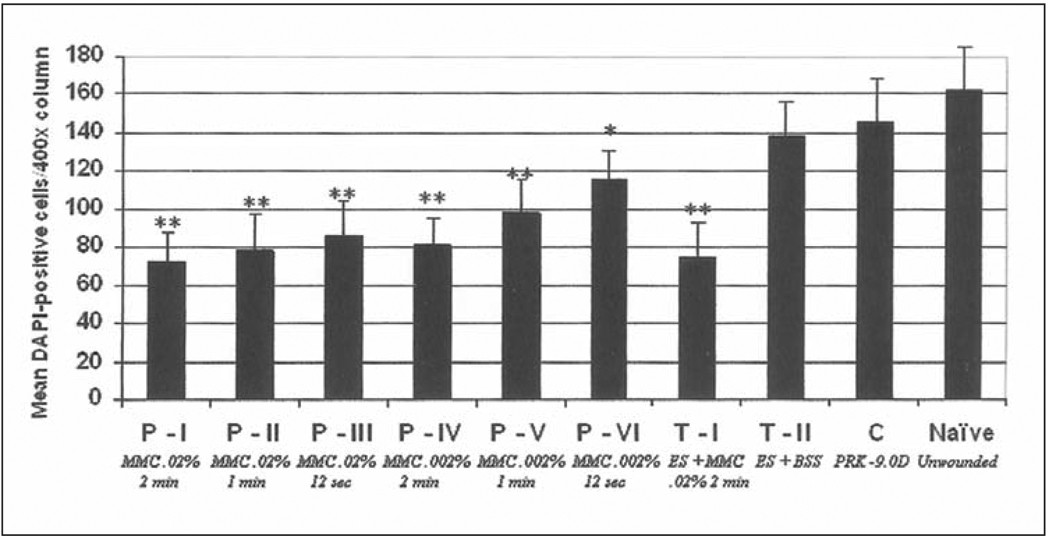
KERATOCYTE CELL DENSITY
DAPI-positive staining of cell nuclei was used to mark stromal cells and determine keratocyte density in the anterior stroma. In all groups treated with PRK, there was a statistically significant decrease in the keratocyte density in approximately the 20% most anterior stroma compared to the unwounded corneas at 4 weeks after surgery (P<.05). There was, however, significantly lower (P<.01) anterior stromal keratocyte density in approximately 20% of the anterior stroma in corneas treated prophylactically with mitomycin C compared to BSS at 4 weeks after surgery (Fig 10). Among the prophylactic mitomycin C-treated groups, the keratocyte density in the anterior stroma at 4 weeks tended to increase with lower concentrations of mitomycin C and shorter exposure times, although only the 0.002% mitomycin C for 12 seconds had significantly greater keratocyte density in the anterior stroma than the other prophylactic groups.
Figure 10.
Quantitation of DAPI-positive cells in the anterior stroma (per 400× column) at 4 weeks after laser surgery in prophylactic and therapeutic mitomycin C-treated groups and BSS-treated control groups. *, **, *** are used to indicate groups that are significantly different from the control group. In addition, * is significantly different from **, * is significantly different from ***, and ** is significantly different from ***.
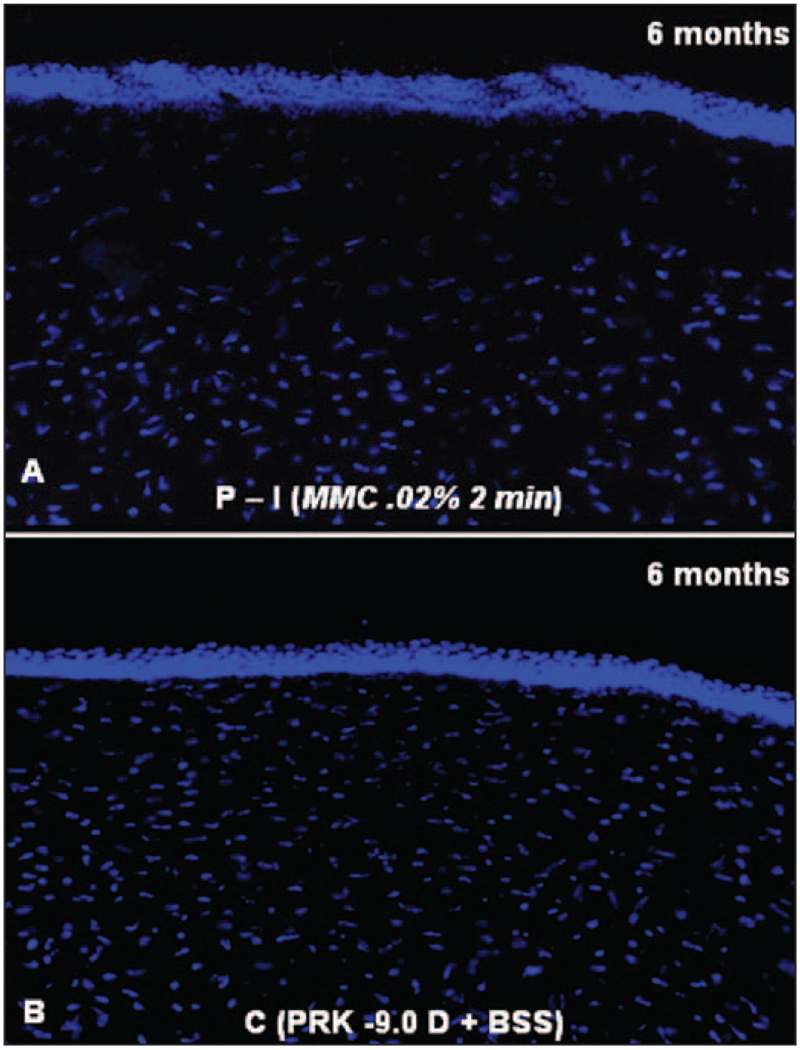
At 6 months after surgery, a highly significant (P<.001) decrease in keratocyte density in the anterior stroma extending to approximately 20% depth persisted in the mitomycin C treated groups. For example, Figure 11 shows typical results for a cornea treated with 0.02% mitomycin C for 2 minutes (P-I) and compared to BSS for 2 minutes (C).
Figure 11.
DAPI staining of cell nuclei in the central corneas from rabbits at 6 months after −9.0 D PRK and prophylactic treatment with A) 0.02% mitomycin C or B) BSS for 2 minutes. Note the markedly diminished keratocyte density in the anterior stroma of the cornea after mitomycin C treatment (original magnification ×400).
IN VITRO EXPERIMENTS: APOPTOSIS IN KERATOCYTES AND MYOFIBROBLASTS
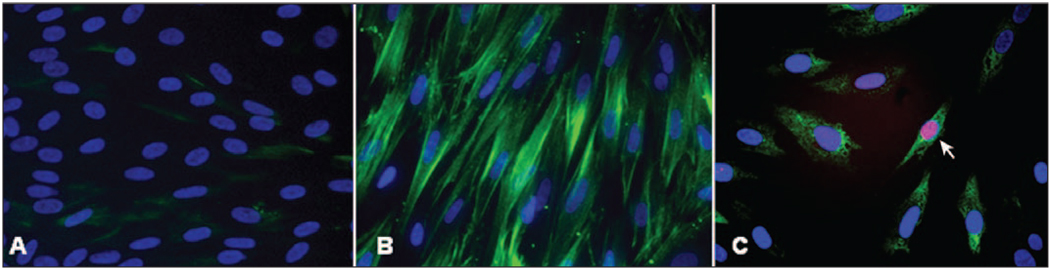
Mitomycin C 0.02% for 2 minutes significantly increases (P<.05) the number of keratocytes undergoing apoptosis in culture compared to BSS-treated controls (not shown). Treatment with mitomycin C 0.02% for 2 minutes also induces apoptosis of TGFβ1-induced myofibroblasts in culture (Fig 12).
Figure 12.
Mitomycin C-induced apoptosis of myofibroblasts in vitro. A) Keratocytes cultured in the absence of TGFβ1. Nuclei stain with DAPI, with no detectible α-smooth muscle actin-positive cells. B) Myofibroblasts generated in the presence of TGFβ1 stain green for α-smooth muscle actin, with nuclei staining with DAPI. C) After treatment of myofibroblasts with 0.02% mitomycin C for 2 minutes, cells undergo triple staining—immunohistochemistry for α-smooth muscle actin, TUNEL assay, and DAPI staining. Cells are shrunken and the nucleus of one cell (arrow) is stained with the TUNEL assay.
DISCUSSION
The causes of clinically significant subepithelial corneal haze following surface ablation with the excimer laser remain enigmatic. This complication rarely occurs in normal eyes that have PRK corrections for <6.0 D of myopia, as the incidence increases with increasing correction for myopia. In eyes that have not had previous surgery, the incidence does not exceed >2% to 4% of patients in most studies, even with corrections up to −10.0 to −12.0 D.26,27 The disorder almost always occurs bilaterally in the eyes of a single afflicted patient, suggesting that there may be underlying cellular or metabolic disorders or environmental factors in some patients that predispose to the condition. Recent studies in our laboratory in a rabbit animal model of subepithelial haze4 have demonstrated that surface irregularity and associated defects in the regenerated basement membrane are associated with development of haze after PRK, presumably due to increased penetration of epithelium-derived transforming growth factor β, which is important in the generation of myofibroblasts.7 Studies by Stramer et al28 have also supported an important role for the integrity of the basement membrane in development of subepithelial corneal haze.
The possibility of severe subepithelial corneal haze formation has tended to limit surface ablation procedures as options for correcting high refractive errors. Alternative surface ablation techniques have been proposed, including procedures with chemical (laser subepithelial keratomileusis) or mechanical (Epi-LASIK) epithelial flap creation.29,30 However, a small amount of convincing evidence shows that these procedures will be associated with less subepithelial haze formation when used in eyes with similar levels of refractive error.31–34
Over the past few years, intraoperative use of topical mitomycin C after PRK has been suggested as an adjunct drug to prevent subepithelial corneal haze formation19,21 based on experimental studies suggesting efficacy for mitomycin C in minimizing subepithelial corneal haze formation.20,35 Subsequent clinical studies reinforced the potential benefits of mitomycin C in preventing subepithelial corneal haze after PRK.21 Recently, Gambato et al36 reported the results of a contralateral eye study of PRK with mitomycin C or placebo in 36 patients with high myopia with a mean followup of 18 months (range: 12 to 36 months). The results of this study suggested that mitomycin C was effective in reducing subepithelial corneal haze formation after PRK in eyes with high myopia. The authors did not report any toxic side effects from the treatments.
With these studies showing the reproducible effectiveness of mitomycin C in reducing subepithelial corneal haze formation, topical use of the drug has rapidly grown in refractive clinical practice, with many surgeons using prophylactic treatment in all eyes that have PRK, or any other surface ablation technique, regardless of the level of correction. In addition, several different protocols, involving different concentrations and exposure times, have been suggested based on clinical observations without clear scientific support. Importantly, the mechanism of action of mitomycin C in inhibiting subepithelial haze has not been delineated and its effects on corneal tissues have not been examined. Of critical importance, nothing is known about the long-term effects of mitomycin C on corneal structure and function. The purpose of this study was to study the effects of prophylactic and therapeutic mitomycin C on corneal cells and tissues and to better define optimal dosages and exposure times of the drug for use in preventing subepithelial haze formation.
The results of this study demonstrate that prophylactic or therapeutic treatment with mitomycin C is effective in preventing or treating subepithelial haze, respectively, in the rabbit model that closely follows the human clinical situation except that subepithelial haze formation tends to occur at higher incidence and with more intensity in the rabbit. Prophylactic treatment was routinely more effective than therapeutic treatment. It has been our observation that therapeutic mitomycin C treatment is most effective when stromal opacity is simultaneously removed with phototherapeutic keratectomy or some other surgical modality (Netto and Wilson, unpublished data, 2004). With regards to the concentration and exposure time for prophylactic mitomycin C, the lower concentration of 0.002% was as effective as the 0.02% concentration and the shorter exposure times, down to 12 seconds, were as effective as the longer exposure time of 2 minutes in reducing subepithelial haze and myofibroblast density in the anterior stroma at 1 month after surgery in the prophylactic groups. The 0.002% mitomycin C for 12 seconds group (P-VI) had significantly more (P<.05) myofibroblasts at 1 month after treatment than the 0.02% mitomycin C for the 2 minutes group (Table 2); however, it is doubtful this difference would be clinically relevant. With larger numbers of animals at each time point in each prophylactic group, statistically significant differences in subepithelial haze might have been detected between the different concentrations of mitomycin C or exposure times, but these differences would most likely not have been clinically relevant.
Mitomycin C appears to trigger augmented keratocyte apoptosis, as well as myofibroblast apoptosis in the case of therapeutic treatment, in comparison to epithelial scrape alone. This was confirmed by the in vitro experiments included in this study, corroborating the work of Kim et al.37 However, this study demonstrates that the dominate mechanism through which mitomycin C inhibits subepithelial haze formation is likely through inhibition of proliferation of keratocytes, and possibly other progenitor cells, that give rise to repopulating keratocytes and haze-associated myofibroblasts that are generated in some eyes that have high PRK. Reduction or complete inhibition of myofibroblast regeneration results in reduced abnormal stromal matrix deposition associated with altered transparency, as well as the diminished transparency attributable to the myofibroblasts themselves.38,39 Variations in subepithelial corneal haze intensity were found within each group treated with prophylactic mitomycin C, emphasizing individual variability of the corneal wound healing response among different animals within the same species. This same variability exists among humans, leading to the sporadic incidence of subepithelial corneal haze following PRK.40
An alarming finding of our study was decreased cellularity of the anterior stroma at 1 month after treatment, also noted by Kim et al,37 that persisted in the current study out to maximum 6-month follow-up of treatment with mitomycin C after PRK compared to controls. In vivo studies performed with confocal microscopy have reported a decrease in the keratocyte density following excimer laser ablation without mitomycin C.41 Consistent with this, all mitomycin C groups in the present study had a highly significant decrease in keratocytes in the anterior stroma, corresponding to the area treated with mitomycin C, compared to the control group treated with BSS. There were significantly more keratocytes in the anterior stroma at 1 month and 6 months after treatment in the 0.002% mitomycin C for 12 seconds group than the other mitomycin C-treated prophylactic groups. No corneal abnormalities were detected with the slit-lamp in these corneas, consistent with clinical observations in patients treated with mitomycin C after PRK over the past decade. However, even longer follow-up of patients will be needed to ensure that acellularity does not lead to long-term opacity, melting, or other abnormalities of the corneal stroma, or overlying epithelium modulated by keratocyte-derived cytokines.
A few clinical recommendations can be made based on the results of this laboratory study and information available in the literature. First, based on the uncertainties associated with long-term diminished keratocyte density in the anterior stroma following prophylactic mitomycin C treatment and the repeated observations by many researchers that clinically significant subepithelial haze almost never occurs in eyes that have PRK for myopia < −6.0 D or hyperopia < +4.0 D,3,26,27 it is our suggestion that prophylactic mitomycin C rarely be used in normal eyes that have not had prior surgery within this range. Secondly, if humans have similar responses to rabbits, which appears likely based on prior studies, then when prophylactic mitomycin C is used, it appears that 0.002% concentration for 12 seconds provides nearly the same efficacy in inhibiting subepithelial haze formation as 0.02% for 12 seconds, 1 minute, or 2 minutes, while potentially having a lesser effect on long-term keratocyte density in the anterior stroma. Mitomycin C has been used in the cornea for 10 years without serious complications being noted. However, reducing the dosage and exposure time to the lowest levels that remain effective in preventing subepithelial haze is probably better for corneal health.
Acknowledgments
Supported in part by US Public Health Service grants EY10056 and EY15638 from the National Eye Institute, National Institutes of Health, Bethesda, Md.
Footnotes
The authors have no financial or proprietary interest in the materials presented herein.
REFERENCES
- 1.Rajan MS, Jaycock P, O’Brart D, Nystrom HH, Marshall J. A long-term study of photorefractive keratectomy; 12-year followup. Ophthalmology. 2004;111:1813–1824. doi: 10.1016/j.ophtha.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 2.El-Maghraby A, Salah T, Waring GO, 3rd, Klyce SD, Ibrahim O. Randomized bilateral comparison of excimer laser in situ keratomileusis and photorefractive keratectomy for 2.50 to 8.00 diopters of myopia. Ophthalmology. 1999;106:447–457. doi: 10.1016/S0161-6420(99)90102-1. [DOI] [PubMed] [Google Scholar]
- 3.Hersh PS, Stulting RD, Steinert RF, Waring GO, III, Thompson KP, O’Connell M, Doney K, Schein OD. Results of phase III excimer laser photorefractive keratectomy for myopia. The Summit PRK Study Group. Ophthalmology. 1997;104:1535–1553. doi: 10.1016/s0161-6420(97)30073-6. [DOI] [PubMed] [Google Scholar]
- 4.Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 5.Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998;17:627–639. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Vinciguerra P, Azzolini M, Airaghi P, Radice P, De Molfetta V. Effect of decreasing surface and interface irregularities after photorefractive keratectomy and laser in situ keratomileusis on optical and functional outcomes. J Refract Surg. 1998;14:S199–S203. doi: 10.3928/1081-597X-19980401-12. [DOI] [PubMed] [Google Scholar]
- 7.Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaji Y, Amano S, Oshika T, Obata H, Ohashi T, Sakai H, Shirasawa E, Tsuru T, Yamashita H. Effect of anti-inflammatory agents on corneal wound-healing process after surface excimer laser keratectomy. J Cataract Refract Surg. 2000;26:426–431. doi: 10.1016/s0886-3350(99)00358-2. [DOI] [PubMed] [Google Scholar]
- 9.Thom SB, Myers JS, Rapuano CJ, Eagle RC, Jr, Siepser SB, Gomes JA. Effect of topical anti-transforming growth factor-beta on corneal stromal haze after photorefractive keratectomy in rabbits. J Cataract Refract Surg. 1997;23:1324–1330. doi: 10.1016/s0886-3350(97)80110-1. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa H, Nakayasu K, Gotoh T, Watanabe Y, Takano T, Ishikawa T, Kanai A. Effect of topical tranilast and corticosteroids on subepithelial haze after photorefractive keratectomy in rabbits. J Refract Surg. 1997;13:S457–S458. doi: 10.3928/1081-597X-19970801-20. [DOI] [PubMed] [Google Scholar]
- 11.Lohmann CP, Marshall J. Plasmin- and plasminogen-activator inhibitors after excimer laser photorefractive keratectomy: new concept in prevention of postoperative myopic regression and haze. Refract Corneal Surg. 1993;9:300–302. [PubMed] [Google Scholar]
- 12.Galm U, Hager MH, Van Lanen SG, Ju J, Thorson JS, Shen B. Antitumor antibiotics: bleomycin, enediynes, and mitomycin. Chem Rev. 2005;105:739–758. doi: 10.1021/cr030117g. [DOI] [PubMed] [Google Scholar]
- 13.Fujita T, Tamura T, Yamada H, Yamamoto A, Muranishi S. Pharmacokinetics of mitomycin C (MMC) after intraperitoneal administration of MMC-gelatin gel and its anti-tumor effects against sarcoma-180 bearing mice. J Drug Target. 1997;4:289–296. doi: 10.3109/10611869708995844. [DOI] [PubMed] [Google Scholar]
- 14.Jampel HD. Effect of brief exposure to mitomycin C on viability and proliferation of cultured human Tenon’s capsule fibroblasts. Ophthalmology. 1992;99:1471–1476. doi: 10.1016/s0161-6420(92)31781-6. [DOI] [PubMed] [Google Scholar]
- 15.Nuyts RM, Pels E, Greve EL. The effects of 5-fluorouracil and mitomycin C on the corneal endothelium. Curr Eye Res. 1992;11:565–570. doi: 10.3109/02713689209001812. [DOI] [PubMed] [Google Scholar]
- 16.Wu KY, Hong SJ, Huang HT, Lin CP, Chen CW. Toxic effects of mitomycin-C on cultured corneal keratocytes and endothelial cells. J Ocul Pharmacol Ther. 1999;15:401–411. doi: 10.1089/jop.1999.15.401. [DOI] [PubMed] [Google Scholar]
- 17.Kang SG, Chung H, Yoo YD, Lee JG, Choi YI, Yu YS. Mechanism of growth inhibitory effect of Mitomycin-C on cultured human retinal pigment epithelial cells: apoptosis and cell cycle arrest. Curr Eye Res. 2001;22:174–181. doi: 10.1076/ceyr.22.3.174.5513. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe J, Sawaguchi S, Fukuchi T, Abe H, Zhou L. Effects of mitomycin C on the expression of proliferating cell nuclear antigen after filtering surgery in rabbits. Graefes Arch Clin Exp Ophthalmol. 1997;235:234–240. doi: 10.1007/BF00941765. [DOI] [PubMed] [Google Scholar]
- 19.Majmudar PA, Forstot SL, Dennis RF, et al. Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery. Ophthalmology. 2000;107:89–94. doi: 10.1016/s0161-6420(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 20.Talamo JH, Gollamudi S, Green WR, De La Cruz Z, Filatov V, Stark WJ. Modulation of corneal wound healing after excimer laser keratomileusis using topical mitomycin C and steroids. Arch Ophthalmol. 1991;109:1141–1146. doi: 10.1001/archopht.1991.01080080101040. [DOI] [PubMed] [Google Scholar]
- 21.Carones F, Vigo L, Scandola E, Vacchini L. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. J Cataract Refract Surg. 2002;28:2088–2095. doi: 10.1016/s0886-3350(02)01701-7. [DOI] [PubMed] [Google Scholar]
- 22.Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–283. [PubMed] [Google Scholar]
- 23.Zieske JD, Wasson M. Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J Cell Sci. 1993;106:145–152. doi: 10.1242/jcs.106.1.145. [DOI] [PubMed] [Google Scholar]
- 24.Fantes FE, Hanna KD, Waring GO, 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108:665–675. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
- 25.Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- 26.Siganos DS, Katsanevaki VJ, Pallikaris IG. Correlation of subepithelial haze and refractive regression 1 month after photorefractive keratectomy for myopia. J Refract Surg. 1999;15:338–342. doi: 10.3928/1081-597X-19990501-10. [DOI] [PubMed] [Google Scholar]
- 27.Shah SS, Kapadia MS, Meisler DM, Wilson SE. Photorefractive keratectomy using the summit SVS Apex laser with or without astigmatic keratotomy. Cornea. 1998;17:508–516. doi: 10.1097/00003226-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- 29.Camellin M. Laser epithelial keratomileusis for myopia. J Refract Surg. 2003;19:666–670. doi: 10.3928/1081-597X-20031101-09. [DOI] [PubMed] [Google Scholar]
- 30.Pallikaris IG, Katsanevaki VJ, Kalyvianaki MI, Naoumidi II. Advances in subepithelial excimer refractive surgery techniques: epi-LASIK. Curr Opin Ophthalmol. 2003;14:207–212. doi: 10.1097/00055735-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kervick G. Subepithelial scarring after laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2005;31:647–648. doi: 10.1016/j.jcrs.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Netto MV, Ambrosio R, Jr, Chalita MR, Krueger RR, Wilson SE. Corneal wound healing response following different modalities of refractive surgical procedures. Arq Bras Oftalmol. 2005;68:140–149. doi: 10.1590/s0004-27492005000100027. [DOI] [PubMed] [Google Scholar]
- 33.Taneri S, Zieske JD, Azar DT. Evolution, techniques, clinical outcomes, and pathophysiology of LASEK: review of the literature. Surv Ophthalmol. 2004;49:576–602. doi: 10.1016/j.survophthal.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Anderson NJ, Beran RF, Schneider TL. Epi-LASEK for the correction of myopia and myopic astigmatism. J Cataract Refract Surg. 2002;28:1343–1347. doi: 10.1016/s0886-3350(02)01461-x. [DOI] [PubMed] [Google Scholar]
- 35.Schipper I, Suppelt C, Gebbers JO. Mitomycin C reduces scar formation after excimer laser (193 nm) photorefractive keratectomy in rabbits. Eye. 1997;11:649–655. doi: 10.1038/eye.1997.171. [DOI] [PubMed] [Google Scholar]
- 36.Gambato C, Ghirlando A, Moretto E, Busato F, Midena E. Mitomycin C modulation of corneal wound healing after photorefractive keratectomy in highly myopic eyes. Ophthalmology. 2005;112:208–218. doi: 10.1016/j.ophtha.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Kim TI, Tchah H, Lee SA, Sung K, Cho BJ, Kook MS. Apoptosis in keratocytes caused by mitomycin C. Invest Ophthalmol Vis Sci. 2003;44:1912–1917. doi: 10.1167/iovs.02-0977. [DOI] [PubMed] [Google Scholar]
- 38.Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995;36:809–819. [PubMed] [Google Scholar]
- 39.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 40.Maldonado MJ. Intraoperative MMC after excimer laser surgery for myopia. Ophthalmology. 2002;109:826–828. doi: 10.1016/s0161-6420(02)01007-2. [DOI] [PubMed] [Google Scholar]
- 41.Erie JC, Patel SV, McLaren JW, Hodge DO, Bourne WM. Keratocyte density in the human cornea after photorefractive keratectomy. Arch Ophthalmol. 2003;121:770–776. doi: 10.1001/archopht.121.6.770. [DOI] [PubMed] [Google Scholar]