Abstract
Systemic administration of dopamine D1-like (SCH23390) and, to a lesser degree D2-like (raclopride), receptor antagonists significantly reduce the acquisition and expression of fructose-conditioned flavor preferences (CFP) in rats. Given the role of dopamine in the amygdala (AMY) in the processing and learning of food reward, the present study examined whether dopamine D1-like or D2-like antagonists in this site altered acquisition and/or expression of a fructose-CFP. In Experiment 1, food-restricted rats with bilateral AMY cannulae were trained to drink a fructose (8%) + saccharin (0.2%) solution mixed with one flavor (e.g., grape, CS+/Fs) and a less-preferred 0.2% saccharin solution mixed with another flavor (e.g., cherry, CS-/s) during one-bottle (16 ml) sessions. Two-bottle tests with the two flavors mixed in saccharin solutions (CS+/s, CS-/s) occurred 10 min following total bilateral AMY doses of 0, 12, 24 and 48 nmol of SCH23390 or raclopride. Preference for CS+/s over CS-/s was significantly reduced relative to vehicle baseline by the 48 nmol doses of SCH23390 and raclopride (from 77% to 66% and 68%), but not lower doses. In Experiment 2, rats received bilateral AMY injections (12 nmol) of SCH23390 (D1 group) or raclopride (D2 group) 10 min prior to one-bottle training sessions with CS+/Fs and CS-/s. Yoked control rats received vehicle and were limited to the CS intakes of the D1 and D2 groups; untreated controls were not injected or limited to drug group intakes during training. Subsequent two-bottle tests revealed initial preferences of CS+/s over CS-/s in all groups that remained stable in untreated and yoked controls, but were lost over the 6 tests sessions in the D1 group, but not in the D2 group. These data indicate that dopamine D1-like and D2-like antagonists significantly attenuated the expression of the previously-acquired fructose-CFP, and did not block acquisition of the fructose-CFP. D1-like antagonism during training hastened extinction of the fructose-CFP. The results are similar to those produced by dopamine D1-like and D2-like antagonist injections into the nucleus accumbens shell which suggests that flavor conditioning involves a regionally-distributed brain network.
Keywords: Flavor-flavor learning, sweet taste, saccharin, SCH23390, Raclopride
1. Introduction
Animals use flavor cues (taste, odor, texture) to guide their selection of nutritious foods and avoidance of toxic foods and learning shapes this selection (Capaldi, 1996). One type of learning, called flavor-flavor conditioning, occurs when a preference is acquired for an arbitrary flavor cue (e.g., banana extract) paired with an already-liked flavor (e.g., sweet taste of saccharin) (Holman, 1975). The sweet taste is considered to be an unconditioned stimulus that reinforces the animal's preference for the added flavor, which represents the conditioned stimulus (CS). One neurochemical candidate that is implicated in the reward value of sweet taste is dopamine, primarily because sweet taste activates mesolimbic dopamine circuits that are involved in the mediation of natural as well as drug rewards (e.g., Genn et al., 2004; Hajnal et al., 2003). Dopamine receptor antagonism suppresses the intake of sweet solutions in rats (Geary and Smith, 1985; Muscat and Willner, 1989; Xenakis and Sclafani, 1981), potentially because it reduces the hedonic value (Schneider, 1989; Smith, 1995) or incentive salience (Berridge and Robinson, 1998; Ikemoto and Panksepp, 1999; Salamone et al., 1997) of sweet taste.
Dopamine antagonists also alter the ability of sweet solutions to reinforce conditioned flavor preferences (CFP). Rats reduced their preference for a flavored 10% sucrose solution paired with an injection of the dopamine D2-like antagonist raclopride, relative to a differently-flavored sucrose solution paired with a vehicle injection (Hsiao and Smith, 1995). Sucrose can reinforce flavor preferences based on its sweet taste as well as its post-oral nutritive actions through the processes of flavor-flavor and flavor-nutrient conditioning, respectively (Sclafani, 1995). Our laboratories (Azzara et al., 2000, 2001; Yu et al., 1999, 2000a, 2000b) have used different training procedures to separate flavor-flavor and flavor-nutrient conditioning. Flavor-nutrient learning was investigated using an intragastric (IG) infusion procedure in which rats were trained to drink differently flavored saccharin solutions paired with IG infusions of sucrose and water, respectively. Systemic treatment with a dopamine D1-like antagonist (SCH23390) but not a D2-like antagonist (raclopride) blocked flavor conditioning by IG sucrose infusions (Azzara et al., 2001). Neither drug had much systemic effect on the expression of a previously learned flavor preference.
Flavor-flavor learning was initially investigated using a sham-feeding procedure in which rats fitted with a gastric cannula were trained to drink a flavored 16% sucrose solution and a less preferred flavored 0.2% saccharin solution. Because gastric sham-feeding greatly reduces the post-oral actions of sucrose, a preference for the sucrose-paired flavor (the CS+) over the saccharin-paired flavor (the CS-) was attributed to the sugar's more palatable taste. Rats treated systemically with dopamine D1-like (SCH23390) or D2-like (raclopride) receptor antagonists during sham-feeding training sessions subsequently displayed preferences for the CS+ flavor comparable to control animals (Yu et al., 2000a). However, both antagonists reduced the preference for the CS+ flavor when administered prior to the choice test, indicating that D1 and D2 receptor signaling are involved in the expression of the flavor preference conditioned by sweet taste (Yu et al., 2000a, 2000b). A limitation of the sham-feeding study was that the animals consumed substantially more of the flavored sucrose solution than the flavored saccharin solution during training, and therefore were more familiar with the CS+ flavor. In a subsequent study, we investigated flavor-flavor conditioning by training rats to “real-feed” similar amounts of a fructose solution and a less preferred saccharin solution each containing distinctive CS flavors (Baker et al., 2003). Fructose rather than sucrose or glucose was used because, unlike these other sugars, fructose has minimal post-oral flavor conditioning effects (Sclafani and Ackroff, 1994; Sclafani et al., 1993, 1999). Therefore a preference for a flavor mixed into a fructose solution is assumed to result from flavor-flavor conditioning. Using this training procedure, our laboratory (Baker et al., 2003) observed that systemic treatment with SCH23390 and, to a lesser degree, raclopride blocked acquisition of a fructose-conditioned preference. Both drugs also significantly reduced the expression of the flavor preference.
In examining the potential central anatomical sites of action for dopaminergic modulation of a fructose-based CFP, our laboratory (Bernal et al., 2008) examined the role of the nucleus accumbens shell (NAcS) because this is a site in which sweet taste and palatable foods stimulates dopamine efflux (Bassareo and DiChiara, 1997, 1999a, 1999b; Bassareo et al., 2002; Cheng and Feenstra, 2006; Genn et al., 2004), and in which dopamine antagonists suppressed lithium chloride-conditioned saccharin aversions (Fenu et al., 2001). NAcS administration of the D1-like antagonist, SCH23390 or the D2-like antagonist, raclopride significantly reduced, but did not eliminate the expression of a fructose-CFP. NAcS administration of these antagonists during training did not prevent the rats from acquiring a fructose-CFP, but resulted in a more rapid extinction of the conditioned preference as compared to control groups not treated with the drugs (Bernal et al., 2008). Because SCH23390 and, to a lesser degree, raclopride eliminated the acquisition and expression of fructose-CFP following systemic administration (Baker et al., 2003), these data suggest that site(s) outside the NAcS contribute to the acquisition of a fructose-CFP. The amygdala (AMY) is a site implicated in flavor aversion learning (Bures et al., 1998), flavor preference learning (Gilbert et al., 2003; Touzani and Sclafani, 2005), and in Pavlovian and instrumental reward learning (Baxter and Murray, 2002; Cardinal et al., 2002). Moreover, feeding and gastric nutrient infusions increased AMY DA turnover or efflux (Hajnal and Lenard, 1997; Heffner et al., 1980), and a Pavlovian CS for food elicited AMY DA efflux (Harmer and Phillips, 1999). Further, AMY microinfusions of amphetamine facilitated learning to respond to a food-related CS (Hitchcott et al., 1997), whereas AMY inactivation modulated feeding-stimulated DA efflux in the NAc (Ahn and Phillips, 2002). Therefore, the present experiment examined whether dopamine transmission within AMY is involved in the modulation of fructose-CFP learning. To this end, the dopamine D1-like (SCH23390) or D2-like (raclopride) antagonists were administered bilaterally into the AMY prior either to training (acquisition) or testing (expression) sessions. Large volumes (0.5 μl) of the antagonists were used to target the whole AMY rather than specific subdivisions because smaller volumes of SCH23390 in either the basolateral or central nucleus of the AMY produced lesser attenuations of flavor preference learning induced by the post-oral action of glucose relative to larger-volume injections (Touzani et al., 2009).
2. Methods
2.1. Subjects, Surgery and Histology
The experimental protocols in the two experiments were approved by the Queens College Institutional Animal Care and Use Committee certifying that all subjects and procedures are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. Male Sprague-Dawley rats (260-300 g, Charles River Laboratories, Wilmington, MA) were housed individually in wire mesh cages and maintained on a 12:12 h light/dark cycle with chow (5001, PMI Nutrition International, Brentwood, MO) and water available ad libitum, except as noted below. Each rat was pretreated with chlorpromazine (3 mg/kg, i.p.) and anesthetized with Ketamine HCl (120 mg/kg, i.m.). Stainless steel guide cannulae (26-gauge, Plastics One, Inc., Roanoke, VA) were aimed stereotaxically (Kopf Instruments) at a range of bilateral placements in the AMY using the following coordinates: incisor bar (-3.3 mm), 2.8 mm anterior to the bregma suture, 4.1-4.5 mm lateral to the sagittal suture, and 8.0-8.4 mm from the top of the skull to sample the full extent of the AMY across placements and conditions. The cannulae were secured to the skull by four anchor screws with dental acrylic. The animals were allowed at least two weeks to recover from stereotaxic surgery before behavioral testing began. At the end of the experiment, all rats were overdosed with an anesthetic (Euthasol) and were injected transcardially with potassium chloride (15 mg/ml, 0.9% saline). Transcardiac perfusions were performed with 0.9% normal saline followed by 10% buffered formalin. Coronal 40-μm sections, stained with Cresyl violet, were examined by light microscopy by an observer unfamiliar with the behavioral data; all animals with confirmed cannula placements were included in the data analysis.
2.2. Test Solutions
The training solutions consisted of 8% fructose (Sigma Chemical Co., St. Louis, MO) and 0.2% sodium saccharin (Sigma Chemical Co.) mixture or a 0.2% sodium saccharin solution, each flavored with 0.05% unsweetened grape or cherry Kool-Aid (General Foods, White Plains, NY). Half of the rats in each group had the cherry flavor added to the fructose+saccharin solution and the grape flavor added to the saccharin only solution; the flavors were reversed for the remaining rats. In the two-bottle preference tests, the cherry and grape flavors were each presented in a 0.2% saccharin solution. The fructose+saccharin-paired flavor is referred to as the CS+, and the saccharin-paired flavor as the CS- because 8% fructose is preferred to 0.2% saccharin (Sclafani and Ackroff, 1994). CS+/Fs refers to the flavored fructose+saccharin solution used in training, and CS+/s refers to the same flavor presented in saccharin only during choice testing. The CS-/s refers to the flavored saccharin solution used in training and testing. All testing took place in the rat's home cage during the mid-light phase of the light:dark cycle. Two weeks before testing began, the rats were placed on a food restriction schedule that maintained their body weights at 85-90% of their ad libitum level. The rats were initially trained to drink an unflavored 0.2% saccharin solution from sipper tubes during daily 1-h sessions. The sipper tube was mounted on the front of the cage held by a taut steel spring, and was positioned 3-6 cm above the cage floor. This training procedure was repeated daily until all rats approached the sipper tubes with short (< 1 min) latency, typically within three days. The limited food rations were given 1 h after each training session.
2.3. Experiment 1: Expression Procedure
Twenty-nine rats were given ten one-bottle training sessions (30 min/day) with 16 ml of the CS+/Fs solution presented on odd-numbered days, and 16 ml of the CS-/s solution presented on even-numbered days. On days 9 and 10, the rats had access to a second sipper tube containing water. This familiarized the rats to the presence of two sipper tubes used during the choice tests; water intake was negligible in these training trials. Training intakes were limited to 16 ml/session to minimize the difference between CS+/Fs and CS-/s intakes. The left-right position of the CS and water sipper tubes was counterbalanced over the two days. Following training, the rats were given eight two-bottle choice test sessions (30 min/day) with unlimited (50 ml) access to the CS+/s and CS-/s solutions. Solution intakes during the training and testing were measured by weighing (0.1 g) the bottles before and after the 30-min sessions.
Ten min prior to the two-bottle test sessions, the rats were given bilateral AMY injections (0.5 μl/side) through a stainless steel internal cannula (33-gauge, Plastics One) that extended 1.0 mm beyond the tip of the guide cannula. This was accomplished using a Hamilton microsyringe that was connected by polyethylene tubing to the internal cannula. For the first two sessions of two-bottle tests, all rats were given a vehicle (0.9% saline) injection. Based on their CS+/s and CS-/s intakes in these tests, the rats were divided into two matched groups. The D1 group of 15 rats was treated with the D1-like antagonist, SCH23390 (Sigma Chemical Co.) at total doses of 12 (6 nmol/side), 24 (12 nmol/side) and 48 (24 nmol/side) nmol administered into the AMY. Half of the rats were tested with an ascending dose order, and the remaining rats were tested with a descending dose order. The D2 group of 14 rats was similarly tested, but with AMY microinfusions of the D2-like antagonist, raclopride (Sigma Chemical Co.) at total doses of 12, 24, and 48 nmol. The rats were tested twice at each drug dose with the left-right position of the CS+ and CS- solutions counterbalanced across sessions. A one-day rest period separated each pair of drug doses for both groups.
2.4. Experiment 2: Acquisition Procedure
Three groups of rats were matched for their intakes of an unflavored 0.2% saccharin solution prior to training. The rats were given eight one-bottle training sessions (60 min/day) with the CS+/Fs solution presented on odd-numbered sessions, and the CS-/s solution presented on even-numbered sessions. A 1-day break was placed between each of the four pairs of training trials to reduce the impact of repeated bilateral AMY microinjections. Rats in the D1 and D2 groups were given bilateral injections of SCH23390 (12 nmol, 6 nmol/side, n=7) or raclopride (12 nmol, 6 nmol/side, n=7), respectively, into the AMY 10 min prior to each one-bottle training session. These doses were identical to those employed in acquisition testing following microinjections into the NAcS (Bernal et al., 2008). A third group (Yoked Control, n=20) received AMY vehicle injections throughout one-bottle training, and their exposure to the CS+/Fs and CS-/s solutions was limited to the mean 60-min intakes of the D1 and D2 groups. A fourth group of unoperated rats (Control, n=15) was trained as above except without injections and with their CS+/Fs and CS-/s intakes limited to 16 ml/session; the purpose of this group was to evaluate the effectiveness of the training procedure. This group was used as a comparator Control group in a previous study (Bernal et al., 2008). Following training, all groups were given six daily two-bottle choice sessions (60 min/day) with unlimited (50 ml) access to the CS+/s and CS-/s solutions; no drugs were administered prior to these sessions. The positions of the CS+/s and CS-/s solutions were counterbalanced across sessions.
2.5. Data analysis
In the expression study, training intakes were averaged over the five CS+/Fs and five CS-/s sessions and evaluated with a t-test. Intakes during the preference tests were averaged over the two sessions at each dose and evaluated with two-way repeated-measures analyses of variance (ANOVA, CS condition vs. Dose) for the D1 and D2 groups, respectively. Separate ANOVAs evaluated total intakes and percent CS+/s intakes as a function of dose for the two groups.
In the acquisition study, training intakes were averaged over the four CS+/Fs and 4 CS-/s sessions and were analyzed with a two-way randomized-blocks ANOVA (CS conditions x Groups). Intakes during the preference tests were averaged over sessions 1-2, 3-4, and 5-6 (referred to as Tests 1, 2, and 3) to control for side position effects. A three-way randomized-blocks ANOVA compared the CS intakes of D1, D2 and control groups (Group x CS x Test). Separate two-way ANOVAs evaluated total CS intakes and percent CS+/s intakes of the four groups. When main or interaction effects were found, Bonferroni corrected comparisons (p<0.05) detected significant effects.
3. Results
3.1. Histological Verification
Figure 1 is a schematic representation (Paxinos and Watson, 1997) and contains a detailed description of the bilateral cannula placements (n=126) of all 63 animals in experiments 1 and 2. Cannulae were distributed in the rostral (n= 25 animals), middle (n= 25 animals) and caudal (n=13) levels of the AMY. Multiple animals had highly similar cannula placements, and there was considerable overlap of placements for the animals included in the five different groups.
Figure 1.
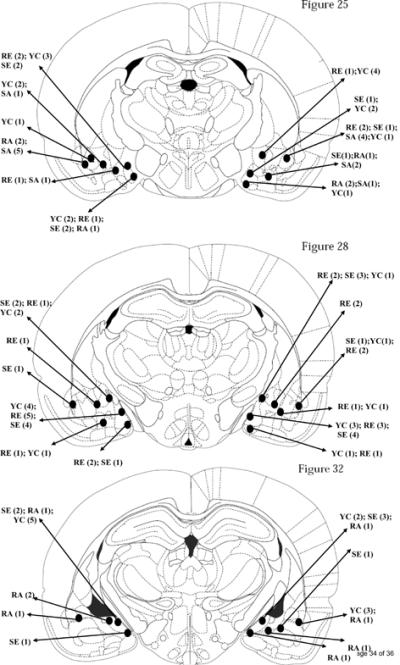
Bilateral representation of cannula sites (n = 126) aimed at the amygdala (AMY) of 63 animals in Experiment 1 (SCH23390 [SE, n=15]; raclopride [RE, n=14]) and Experiment 2 (SCH23390 [SA, n=7], raclopride [RA, n=7]; yoked [YA, n=20] using Figures 25 (Bregma -1.80 mm), 28 (Bregma -2.56 mm) and 32 (Bregma -3.60 mm) of the stereotaxic atlas of Paxinos and Watson (1997). Cannulae were localized in or near the medial AMY nuclei bilaterally (n= 20 animals), centro-medial AMY nuclei bilaterally (n= 5 animals), baso-lateral AMY nuclei bilaterally (n= 4 animals), unilateral medial and centro-medial AMY nuclei (n= 14 animals), unilateral medial and baso-lateral AMY nuclei (n= 18 animals), and unilateral centromedial and baso-lateral AMY nuclei (n= 2 animals). Cannulae were distributed in the rostral (n= 25 animals), middle (n= 25 animals) and caudal (n=13) levels of the AMY. Multiple animals had highly similar cannula placements, and there was considerable overlap of placements for the animals included in the five different groups.
3.2. Experiment 1. Expression study
During one-bottle training, the mean intake of the CS+/Fs solution significantly exceeded that of the CS-/s solution (12.3 vs. 8.4 g/30 min, t(28)= 6.84, p<0.0001). In the two-bottle preference tests conducted with the D1 group, overall, CS+/s intakes significantly exceeded CS-/s intakes (F(1,55)= 180.54, p<0.0001) with intakes significantly varying as a function of drug dose (F(3,55)= 4.92, p<0.004) and the interaction between CS conditions and drug doses (F(3,55)= 4.92, p<0.004). CS+/s intake was significantly higher than CS-/s intake following the vehicle and all SCH23390 doses (Figure 2A). The 48 nmol total dose of SCH23390, but not the 12 and 24 mol total doses, significantly reduced CS+/s intake relative to vehicle (Figure 2A). Significant differences in the percent CS+ intakes were observed across doses (F(3,42)= 10.46, p<0.0001), and the preference (66%) following the 48 nmol SCH23390 dose was significantly lower than the preference (77%) following vehicle (Figure 2A). Preferences at the 12 (71%) and 24 (74%) nmol doses of SCH23390 were intermediate, and failed to differ significantly from the vehicle preference. Significant differences in total intake were observed across SCH23390 doses (F(3,42)= 14.99, p<0.0001), and total CS intakes were less following the 12 (14.8 g), 24 (14.8 g) and 48 (12.3 g) nmol total doses relative to vehicle (18.7 g).
Figure 2.
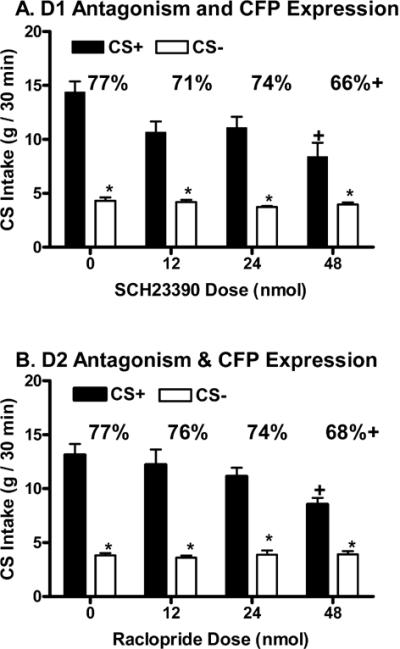
Experiment 1 (expression procedure). Intakes (mean +SEM, g/30 min) of CS+/s and CS-/s solutions in two-bottle tests in animals receiving bilateral AMY microinjections of the D1-like dopamine antagonist, SCH23390 (upper panel) or the D2-like dopamine antagonist, raclopride (lower panel) at total doses of 0, 12, 24 or 48 nmol 10 min prior to testing. Significant differences are denoted between CS+/s and CS-/s intake within an injection condition (*) and between CS+/s intake following a drug dose relative to the vehicle treatment (+). The percentages of CS+/s intake over total intake are denoted above each pair of values with significant differences relative to vehicle treatment (+) noted.
In the two-bottle preference tests conducted with the D2 rats, overall, CS+/s intakes significantly exceeded CS-/s intakes (F(1,52)= 233.07, p<0.0001) with intakes significantly varying as a function of drug dose (F(3,52)= 2.99, p<0.039) and the interaction between CS conditions and drug doses (F(3,52)= 4.48, p<0.007). CS+/s intake was significantly higher than CS-/s intake following vehicle and all raclopride doses (Figure 2B). The 48 nmol total dose of raclopride, but not the 12 and 24 mol doses, significantly reduced CS+/s intake relative to vehicle (Figure 2B). Significant differences in the percent CS+ intakes were observed across doses (F(3,39)= 5.75, p<0.002), and the preference (68%) following the 48 nmol raclopride dose was significantly lower than the preference (77%) following vehicle (Figure 2B). Preferences following the 12 (76%) and 48 (74%) nmol raclopride doses failed to differ from the vehicle preference. Significant differences in total intake were observed across raclopride doses (F(3,39)= 8.09, p<0.0003) with total CS intakes following the 48 (12.3 g), but not the 12 (15.8 g) or 24 (15.0 g), nmol raclopride dose significantly reduced relative to that following vehicle (16.9 g).
3.3. Experiment 2. Acquisition study
In the one-bottle training sessions, overall, CS+/Fs intake significantly exceeded CS-/s intake (12.9 vs. 9.7 g/1 h, F(1,19)= 242.08, p<0.0001), the four groups significantly differed from each other (F(3,57)= 15.53, p<0.0001), and there was a marginally significant interaction between groups and conditions (F(3,57)= 2.653, p=0.057). Overall mean CS intake for the Yoked Control group (9.2 g) was significantly less than the D1 (12.4 g), D2 (11.7 g) and the Control (12.0 g) groups. The yoked controls consumed less than the D1 and D2 groups apparently because they were provided with a lesser amount (12 ml per session) during training trials than the D1, D2 and untreated Control groups (16 ml per session). In particular, CS+/Fs intakes were significant greater than CS-/s intakes for the Control rats (13.4 vs. 10.6 g), Yoked Control rats (10.9 vs. 7.4 g) and D2 rats (13.9 vs. 9.5 g), but not for the D1 rats (13.7 vs. 11.2 g). Moreover, CS+/Fs intakes were significantly higher in the D1, D2 and Control groups relative to the Yoked Control group, and CS-/s intakes were significantly higher in the D1 and Control groups relative to the Yoked Control group.
In the two-bottle preference tests, there were significant differences in CS+/s and CS-/s intakes (F(1,19)= 359.90, p<0.0001), among the four training groups (F(3,57)= 2.92, p<0.042) and among the three tests (F(2,38)= 17.63, p<0.0001). In addition, there were significant interactions between groups and tests (F(6,114)= 17.97, p<0.0004), between CS solutions and tests (F(2,38)= 16.54, p<0.0001), and among groups, CS solutions and tests (F(6,114)= 9.35, p<0.0001). Within-group comparisons revealed that significantly greater consumption of the CS+/s solution relative to the CS-/s solution occurred across all three tests in the Yoked Control (Figure 3C) and Control (Figure 3D) groups as well as the D2 group (Figure 3B). In contrast, although the D1 group consumed significantly more of the CS+/s relative to the CS-/s solution in Test 1, CS intakes failed to differ in Tests 2 and 3, indicating extinction of the CS+/s preference (Figure 3A). In particular, the D1 group displayed a selective and significant reduction in CS+/s intake from the first to the third test (Figure 3A). The CS+/s intakes of the D1 and D2 groups in Test 3 were also significantly lower than those of the Control and Yoked Control groups (Figures 3A, 3B), whereas CS-/s intakes failed to change over testing in any group.
Figure 3.
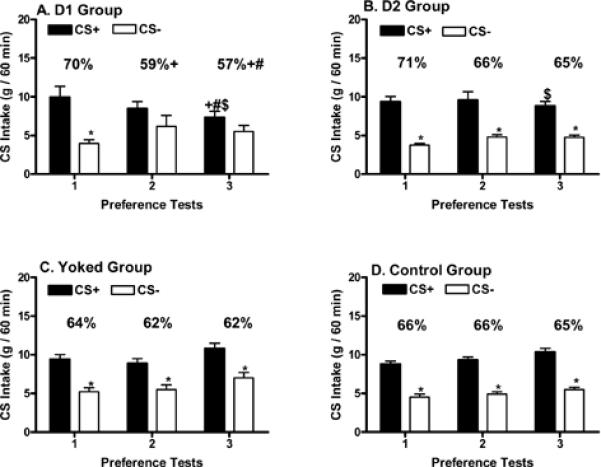
Experiment 2 (acquisition procedure). Intakes (mean +SEM, g/1 h) of CS+/s and CS-/s solutions during two-bottle Tests 1-3. During the training, the D1 group received bilateral AMY microinjections of SCH23390 (12 nmol total dose, Panel A) and the D2 group received bilateral AMY microinjections of raclopride (12 nmol total dose, Panel B); the yoked control group was limited to the CS intakes of the drug groups and received bilateral AMY vehicle microinjections (Panel C); the control group received no injections during training (Panel D). Numbers atop bars represent the mean percent intakes of CS+/s. Significant differences are denoted between CS+/s and CS-/s intake within each test (*). Significant differences in CS intake or percent CS+ intakes between Test 1 and subsequent tests are denoted (+) as are differences in the drug groups relative to the yoked control group ($) or control group (#).
Analysis of the percent CS+/s data failed to reveal any significant overall group difference (F(3,57)= 1.96, ns), but there were significant effects across tests (F(2,38)= 33.36, p<0.0001) and for the interaction between groups and tests (F(6,114)= 8.73, p<0.0001). Whereas percent CS+/s intakes remained stable across the three tests in the Control and Yoked Control groups (Figures 3C, 3D) as well as the D2 group (Figure 3B), the percent CS+ intakes of the D1 group were significantly lower in Tests 2 (59%) and 3 (57%) relative to Test 1 (70%) (Figure 3A). In addition, CS+/s preference of the D1 group in Test 3 (Figure 3A) was significantly below that of the Control group, again suggesting extinction of the CS+/s preference. Finally, significant differences in total CS solution intakes were observed among the four training groups (F(3,57)= 2.92, p<0.042), among the three tests (F(2,38)= 17.63, p<0.0001), and for the interaction between groups and tests (F(6,114)= 17.97, p<0.0001). The Yoked Control group significantly increased total CS intake in Test 3 (17.8 g) relative to the first (14.6 g) and second (14.4 g) Tests, and the Control group significantly increased total CS intake in Test 3 (15.8 g) relative to Test 1 (13.3 g). Total CS intakes in Test 3 were significantly lower in D1 (12.9 g) and D2 (13.6 g) groups compared to the Yoked Control group.
3.4 Differences in the magnitude of fructose-CFP in the acquisition and expression procedures
As in prior work (Bernal et al., 2008), the training procedure used in the acquisition procedure (Experiment 2) was modified from that used in the expression procedure (Experiment 1) so as to reduce the impact of repeated microinjections during training. Whereas the rats in Experiment 1 received 10 training sessions, the rats in Experiment 2 were given 8 training sessions with a rest day between each pair of CS+/Fs and CS-/s sessions although their training sessions were increased in length (from 30 to 60 min). To evaluate the effect of the different training regimens, the two-bottle data obtained in the vehicle tests (combined for both drug groups) in Experiment 1 were compared with the Test 1 data obtained with the untreated control group in Experiment 2. Significant differences in intake were observed between experiments, (F(1,28)= 30.03, p<0.0001), between CS+/s and CS-/s intakes (F(1,28)= 368.96, p<0.0001), and for the interaction between CS intakes and experiments (F(1,28)= 59.24, p<0.0001). With both training procedures CS+/s test intakes exceeded CS-/s intake (acquisition: 8.8 vs. 4.5 g; expression: 13.8 vs. 4.1 g). However, the CS+/s intake with the expression procedure exceeded that with the acquisition procedure; CS-/s intakes did not differ as a function of training procedure. Correspondingly, the percent CS+/s preference was greater with the expression procedure than with the acquisition procedure (77% vs. 66%, t(28)= 5.26, p < 0.0001).
4. Discussion
In this study, we analyzed the role of dopamine transmission within the AMY in flavor preferences conditioned by the sweet taste of fructose. The results showed that dopamine D1-like and D2-like receptor antagonism at high (48 nmol), but not low (12-24 nmol), drug doses significantly attenuated the expression of a previously-learned fructose-CFP. D1-like antagonist treatment (12 nmol dose) during training had no effect on the initial establishment of fructose-CFP, but rather resulted in a rapid extinction of the learned fructose-CFP. These effects cannot be attributed to the spread of the drugs to structures outside the AMY. Large excitotoxic lesions of AMY with 0.7 μl of ibotenic acid never invaded structures outside of the AMY (Touzani and Sclafani, 2005). These results showed that dopamine transmission within the AMY plays an important role in the maintenance of learned fructose-CFP.
Fructose-CFP Expression Effects
The present study demonstrated that bilateral administration into the AMY of either the dopamine D1-like receptor antagonist SCH23390 or the D2-like receptor antagonist raclopride attenuated the expression of a fructose-CFP. These results are quite similar to the results obtained with the same drugs microinjected into the NAcS (Bernal et al., 2008). Thus, both the AMY and NAcS are implicated as central sites of action for the suppressive effects of systemic administration of dopamine D1-like and D2-like antagonists on the expression of flavor preferences conditioned by the sweet taste of sugars (Baker et al., 2003; Yu et al., 2000a, 2000b). However, the central and systemic injections of the D1-like antagonist differed in the degree to which they attenuated the fructose-CFP. SCH23390 (48 nmol) significantly reduced the expression of the fructose-CFP preference from 77% to 66% following AMY administration, and from 76% to 62% following NAcS administration. This is in contrast to the elimination of the fructose-CFP preference from 77% to 39-55% across a 50-800 nmol/kg systemic dose range of SCH23390 (Baker et al., 2003). On the other hand, the central and systemic effects of the D2-like antagonist on the expression of the fructose-CFP were similar. Raclopride significantly reduced the expression of the fructose-CFP preference from 77% to 68% following AMY (48 nmol) administration, from 76% to 63% following NAcS (24 nmol) administration, and from 80% to 66% following systemic (200 nmol/kg) administration.
Fructose-CFP Acquisition Effects
In addition to attenuating the expression of a previously learned CS+ preference, injection of SCH23390 into the AMY during training influenced the acquisition of the fructose-conditioned CS+ preference. Although the D1 group displayed a significant preference for the CS+ flavor in Test 1 (70%), the preferences declined to 59% by Test 2, and to 57% by Test 3, and were no longer significant. In contrast, the D2 group treated with raclopride during training displayed a significant CS+ preference that persisted over the three pairs of tests. The CS+ preference of the D2 group declined somewhat by Test 3 (to 65%), but it did not differ from that of the Yoked and Control groups that displayed stable preferences of 62% and 66% over the three tests. In contrast to the present results, systemic treatment (200 nmol/kg) with SCH23390 or raclopride completely prevented the acquisition of a fructose-conditioned flavor preference in rats (Baker et al., 2003). Systemic SCH23390, but not raclopride also prevented the acquisition of a flavor preference conditioned by IG sucrose infusions (Azzara et al., 2001).
The results obtained with dopamine antagonism of the AMY showed both similarities and differences with fructose-CFP acquisition results obtained with drug injections into the NAcS (Bernal et al., 2008). First and foremost, injection of SCH23390 into either the AMY or NAcS during training produced similar effects with an initial CS+ preference in Test 1 (70%) giving way to no preference in Test 3 (57%). Both central D1-like antagonist treatments differ from systemic D1-like antagonist treatment, which as noted above, prevented the animals from showing any CS+ preference (Baker et al., 2003). It is possible that the single tested dose (12 nmol) of the D1-like antagonist administered into the AMY was not sufficient to induce effects upon acquisition of the fructose-CFP in the same manner that a systemic dose of 200 nmol/kg SCH23390 during training eliminated either sucrose-conditioned CFP in sham-feeding rats (Yu et al., 2000b) or fructose-conditioned CFP in real-feeding rats (Baker et al., 2003). However, the 12 nmol dose of SCH23390 in the AMY in the expression study reduced total CS intakes to the same degree as that produced by the systemic 200 nmol/kg SCH23390 dose in the systemic injection studies (Baker et al., 2003; Yu et al., 2000b).
Although SCH23390 administered during training did not block learning of the fructose-CFP, D1-like antagonism in the AMY, like that in the NAcS during training hastened the extinction of the fructose-CFP. The reason for this effect remains to be established. It is not readily explained by the drug treatment merely producing a weaker association between the CS+ flavor and fructose taste. If this were the case, the D1 AMY group would have been expected to show a weaker CS+ preference in Test 1 rather than showing a slightly greater preference than that displayed by the control groups (70% vs. 64-67%). However, AMY administration of the 12 nmol dose of SCH23390 during training may have been producing a more “tenuous” association between the CS+ flavor and fructose taste. One potential mechanism of action involves the co-distribution of dopamine D-1 and NMDA/AMPA receptors within the AMY (Pickel et al., 2006). Thus, dopamine D1-like receptor antagonists in the cortico-lateral AMY block the enhancement of long-term potentiation elicited by low-frequency stimulation (e.g., Huang and Kandel, 2007). Further, dopamine receptor-mediated enhancement of hippocampal long-term potentiation requires the NR2B subunit of the NMDA receptor (Stramiello and Wagner, 2008). Thus, using this form of neuronal plasticity underlying learning and memory (see review: Bliss and Collingridge, 1993), a low dose of SCH23390 in the AMY can affect the stability of a NMDA-mediated neuroplasticity within the AMY underlying the acquisition of fructose-CFP learning. Such a mechanism has been proposed (see review: Sutton and Benninger, 1999) in that co-activation of D1-like receptors and NMDA/AMPA receptors contributes to consolidation of learned behavior, and that long-term memory (maintenance of the behavior) may depend upon the molecular cascade mediated by D1-like receptors that are interrupted by SCH23390. Indeed, a role for NMDA receptors in the acquisition of fructose-CFP has been established in that systemic administration of MK-801 and D-cycloserine respectively block and enhance acquisition, but not expression of a fructose-CFP (Golden and Houpt, 2007). Further studies are needed to address this important issue.
In contrast to the identical CS+ preferences observed with the D1 AMY and D1 NAcS groups, treatment with the D2-like antagonist during training produced a somewhat weaker decline in CS+ preference in the AMY rats of the present study (from 71% to 65%) than in the NAcS rats of our prior study (from 73% to 60%: Bernal et al., 2008). Thus, fructose-CFP appears more dependent on D2 receptor activity in the NAcS than in the AMY. As in the case of the 12 nmol dose of SCH23390, the 12 nmol dose of raclopride administered into the AMY may not have been equivalent to the systemic 200 nmol/kg dose of raclopride that blocked flavor conditioning by fructose (Baker et al., 2003).
The dopamine drug effects on flavor conditioning by oral fructose also differ from those produced by IG glucose conditioning. Thus, SCH23390 injections into the AMY or NAcS during training did not block fructose conditioning, but they did block the acquisition of a flavor preference produced by glucose infusions (Touzani et al., 2008, 2009). On the other hand, the expression of an IG glucose-conditioned flavor preference was less affected by SCH23390 injections into the AMY or NAcS (Touzani et al., 2008, 2009) than was the expression of the fructose-CFP (present study, Bernal et al., 2008). It should be noted that the effect of AMY or NAcS injections of raclopride on IG glucose conditioning was not determined because systemic injections of the D2-like receptor antagonist did not block conditioning with IG sucrose (Azzara et al., 2001). Taken together, the central and systemic injection data indicate that there is differential involvement of D1 and D2 receptors in flavor-flavor and flavor-nutrient preference conditioning produced by oral fructose and IG glucose, respectively.
The AMY is a highly heterogeneous aggregate of nuclei. There is extensive evidence that the discretely sub-divided baso-lateral and central nuclei of the AMY (see review: Pitkanen, 2000) play highly distinct and specific roles in different forms of appetitive and aversive learning (see reviews: Davis, 2000; LeDoux, 2000; Gallagher, 2000; Balleine and Killcross, 2006). This would suggest that dopamine transmission within these discrete subdivisions of the AMY plays differential role in fructose-conditioned flavor preferences. Given the relatively large microinfusion volume (0.5 μl) used in the present study, the dopamine antagonists presumably acted both in the baso-lateral and central nuclei of the AMY. Thus, it is not possible to determine whether the effect observed were due to the drugs' action within the baso-lateral or the central nuclei (or both). Recently Touzani et al. (2009) reported that flavor-nutrient conditioning by intragastric glucose infusions was eliminated by SCH23390 injections (12 nmol in 0.5 ul) that involved both the baso-lateral and central AMY nuclei. In contrast, flavor-nutrient conditioning was only attenuated by smaller volume (0.25 μl) infusions of the same drug dose into the baso-lateral or central AMY nuclei. These findings suggested that the baso-lateral and central nuclei are both involved in flavor conditioning, and that additive effects are produced by dopamine antagonism in both nuclei.
Potential Central Mechanisms of Action
As detailed above, D1-like and D2-like receptor antagonism in the AMY and NAcS resulted in similar reductions in, but not elimination of the expression of a fructose-CFP, and D1 receptor antagonism in the AMY and NAcS during training hastened the extinction of the fructose-CFP. These data suggest that dopamine-responsive neurons within the two sites are part of a regional network of brain sites that mediate flavor-flavor conditioning in a manner similar to proposed regional networks of interacting brain sites for other aspects of feeding behavior (e.g., Baldo and Kelley, 2007; Bodnar and Levine, 2008; Will et al., 2003). Several sources of evidence support the existence of an AMY-NAcS dopamine reward network. First, the source of dopamine into the AMY is derived from the mesolimbic dopamine pathway originating in the ventral tegmental area which also provides dopaminergic innervation to the NAcS (e.g., Asan, 1997, 1998; Eliava et al., 2003; Lammel et al., 2008). Second, there is very strong evidence for AMY, particularly baso-lateral and lateral nuclei, projections to the NAc, particularly the shell region (Brog et al., 1993; Christie et al., 1987; Fudge et al., 2002) in an organized and highly compartmentalized fashion (Groenewegen et al., 1999; Phillipson and Griffiths, 1985; Wright and Groenewegen, 1995; Wright et al., 1996). Third, the NAcS sends projections to the AMY (Brog et al., 1993; Mello et al., 1992). Fourth, the NAcS is a site in which sweet taste stimulated dopamine efflux (e.g., Cheng and Feenstra, 2006; Genn et al., 2004), and in which dopamine antagonists suppressed lithium chloride-conditioned saccharin aversions (Fenu et al., 2001). Indeed and importantly, the NAcS is a site in which the motivational valence and novelty of hedonic food stimuli (e.g., Fonzies) is a critical component of dopamine release as compared to the core of the nucleus accumbens in which generic motivational values of food-related stimuli elicit dopamine release (Bassareo and DiChiara, 1997, 1999a, 1999b; Bassareo et al., 2002). Fifth, the AMY is another site in which Pavlovian and instrumental reward learning are supported (Baxter and Murray, 2002; Cardinal et al., 2002), in which feeding and gastric nutrient infusions increased DA turnover or efflux (Hajnal and Lenard, 1997; Heffner et al., 1980), and in which a Pavlovian CS for food elicited DA efflux (Harmer and Phillips, 1999). Further, AMY inactivation modulated feeding-stimulated DA efflux in the NAc (Ahn and Phillips, 2002). Finally, recent findings indicate that AMY and dopamine projections to the NAc interact to promote sugar seeking behavior, and that the AMY responses preceded and indeed excited the responses observed in the NAc (Ambroggi et al., 2008). The integrity of the AMY, and particularly the baso-lateral nucleus is necessary for NAc responsivity. These findings are consistent with a network model of D1 and/or D2 receptor modulation of flavor preference learning, with differential involvement in the acquisition and expression of flavor-flavor and flavor-nutrient associations. Further systematic microinjection studies in the AMY and NAc, as well as other sites (e.g., medial prefrontal cortex, lateral hypothalamus) sites are necessary to identify the underlying system(s) mediating these forms of food-related learning.
ACKNOWLEDGEMENTS
This research was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK071761.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Phillips AG. Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. J Neurosci. 2002;22:10958–10965. doi: 10.1523/JNEUROSCI.22-24-10958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdale neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tiss Res. 1997;288:449–469. doi: 10.1007/s004410050832. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdale. Adv Anat Embryol Cell Biol. 1998;142:1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. Naltrexone fails to block the acquisition or expression of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2000;67:545–557. doi: 10.1016/s0091-3057(00)00395-6. [DOI] [PubMed] [Google Scholar]
- Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D-1 but not D-2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol. Biochem. Behav. 2001;68:709–720. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Baker RM, Shah MJ, Sclafani A, Bodnar RJ. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2003;75:55–65. doi: 10.1016/s0091-3057(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharm. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross S. Parallel incentive processing: an integrated view of amygdala function. Trends Neurosci. 2006;29:272–279. doi: 10.1016/j.tins.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17:851–861. doi: 10.1523/JNEUROSCI.17-02-00851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neurosci. 1999a;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999b;11:4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. J Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behav Brain Res. 2008;190:59–66. doi: 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Levine AS. Role of opioid peptides in regulating energy balance. In: Harvey J, Withers DJ, editors. Neurobilogy and Obesity. Cambridge University Press; United Kingdom: 2008. pp. 232–265. [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- Bures J, Bermúdez-Rattoni F, Yamamoto T. Conditioned Taste Aversion: Memory of a Special Kind. Oxford University Press; Oxford: 1998. [Google Scholar]
- Capaldi ED, editor. Why We Eat What We Eat. American Psychological Association; Washington, DC: 1996. [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cheng J, Feenstra MG. Individual differences in dopamine efflux in nucleus accumbens shell and core during instrumental conditioning. Learn Mem. 2006;13:168–177. doi: 10.1101/lm.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM. Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neurosci. 1987;22:425–439. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. Second Edition Oxford University Press; Oxford, UK: 2000. pp. 213–287. [Google Scholar]
- Eliava M, Yilmazer-Hanke D, Asan E. Interrelationships between monoaminergic afferents and corticotrophin-releasing factor-immunoreactive neurons in the rat central amygdaloid nucleus: ultrastructural evidence for dopaminergic control of amygdaloid stress systems. Histochem Cell Biol. 2003;120:183–197. doi: 10.1007/s00418-003-0557-9. [DOI] [PubMed] [Google Scholar]
- Fenu S, Bassareo V, Di Chiara G. A role for dopamine D1 receptors of the nucleus accumbens shell in conditioned taste aversion learning. J Neurosci. 2001;21:6897–6904. doi: 10.1523/JNEUROSCI.21-17-06897.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neurosci. 2002;110:257–275. doi: 10.1016/s0306-4522(01)00546-2. [DOI] [PubMed] [Google Scholar]
- Gallagher M. The amygdale and associative learning. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. Second Edition Oxford University Press; Oxford, UK: 2000. pp. 311–329. [Google Scholar]
- Geary N, Smith GP. Pimozide decreases the positive reinforcing effect of sham fed sucrose in the rat. Pharmacol Biochem Behav. 1985;22:787–790. doi: 10.1016/0091-3057(85)90528-3. [DOI] [PubMed] [Google Scholar]
- Genn RF, Ahn S, Phillips AG. Attenuated dopamine efflux in the rat nucleus accumbens during successive negative contrast. Behav Neurosci. 2004;118:869–873. doi: 10.1037/0735-7044.118.4.869. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Campbell A, Kesner RP. The role of the amygdala in conditioned flavor preference. Neurobiol Learn Mem. 2003;79:118–121. doi: 10.1016/s1074-7427(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Golden GJ, Houpt TA. NMDA receptor in conditioned flavor-taste preference learning: blockade by MK-801 and enhancement by D-cycloserine. Pharmacol Biochem Behav. 2007;86:587–596. doi: 10.1016/j.pbb.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann NY Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Lenard L. Feeding-related dopamine in the amygdala of freely moving rats. Neuroreport. 1997;8:2817–2820. doi: 10.1097/00001756-199708180-00033. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol. 2003;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience. 1999;90:119–130. doi: 10.1016/s0306-4522(98)00464-3. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Hartman JA, Seiden LS. Feeding increases dopamine metabolism in the rat brain. Science. 1980;208:1168–1170. doi: 10.1126/science.7375926. [DOI] [PubMed] [Google Scholar]
- Hitchcott PK, Harmer CJ, Phillips GD. Enhanced acquisition of discriminative approach following intra-amygdala d-amphetamine. Psychopharmacology. 1997;132:237–246. doi: 10.1007/s002130050341. [DOI] [PubMed] [Google Scholar]
- Holman EW. Immediate and delayed reinforcers for flavor preferences in the rat. Learning and Motivation. 1975;6:91–100. [Google Scholar]
- Hsiao S, Smith GP. Raclopride reduces sucrose preference in rats. Pharmacol. Biochem. Behav. 1995;50:121–125. doi: 10.1016/0091-3057(95)00315-n. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Low-frequency stimulation induces a pathway-specific late phase of LTP in the amygdale that is mediated by PKA and dependent on protein synthesis. Learn Mem. 2007;14:497–503. doi: 10.1101/lm.593407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons with a dual mesocorticaolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala and emotion: a view through fear. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. Second Edition Oxford University Press; Oxford, UK: 2000. pp. 289–310. [Google Scholar]
- Mello LE, Tan AM, Finch DM. Convergence of projections from the rat hippocampal formation, medial geniculate and basal forebrain onto single amygdaloid neurons: an in vivo extra- and intracellular electrophysiological study. Brain Res. 1992;587:24–40. doi: 10.1016/0006-8993(92)91425-e. [DOI] [PubMed] [Google Scholar]
- Muscat R, Willner P. Effects of selective dopamine receptor antagonists on sucrose consumption and preference. Psychopharm. 1989;99:98–102. doi: 10.1007/BF00634461. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Compact Third Ed. Academic Press; London: 1997. [Google Scholar]
- Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neurosci. 1985;16:275–296. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Colago EE, Mania I, Molosh AI, Rainnie DG. Dopamine D1 receptors co-distribute with N-methyl-D-aspartic acid type-1 subunits and modulate synaptically-evoked N-methyl-D-aspartic currents in rat baso-lateral amygdala. Neurosci. 2006;142:671–690. doi: 10.1016/j.neuroscience.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala: A Functional Analysis. Second Edition Oxford University Press; Oxford, UK: 2000. pp. 31–115. [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: Empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Schneider LH. Orosensory self-stimulation by sucrose involves brain dopaminergic mechanisms. Ann NY Acad Sci. 1989;575:307–319. doi: 10.1111/j.1749-6632.1989.tb53252.x. [DOI] [PubMed] [Google Scholar]
- Sclafani A. How food preferences are learned: laboratory animal models. Proc. Nutrition Soc. 1995;54:419–427. doi: 10.1079/pns19950011. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: taste versus post-oral conditioning. Physiol. Behav. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am. J. Physiol. 1993;265:R320–R325. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Smith GP. Dopamine and food reward. In: Fluharty S, Morrison AM, editors. Progress in Psychobiology and Physiological psychology. Vol. 16. Academic Press; New York: 1995. pp. 83–144. [Google Scholar]
- Stramiello M, Wagner JJ. D1/5 receptor-mediated enhancement of LTP requires PKA, Src family kinases, and NR2B-containing NMDARs. Neuropharmacol. 2008;55:871–877. doi: 10.1016/j.neuropharm.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Benninger RJ. Psychopharmacology of conditioned reward: evidence for a rewarding signal at D1-like dopamine receptors. Psychopharmacol. 1999;144:95–110. doi: 10.1007/s002130050982. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Activation of dopamine D1 receptors in nucleus accumbens is critical for the acquisition, but not the expression, of glucose-conditioned flavor preference in rats. Eur J Neurosci. 2008;27:1525–1533. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Dopamine D1 receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preference in rats. Eur. J. Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06829.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Sclafani A. Critical role of amygdala in flavor but not taste preference learning in rats. Eur. J. Neurosci. 2005;22:1767–1774. doi: 10.1111/j.1460-9568.2005.04360.x. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regultate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Beijer AV, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic and basal amygdaloid afferents. J Comp Neurol. 1995;361:383–403. doi: 10.1002/cne.903610304. [DOI] [PubMed] [Google Scholar]
- Xenakis S, Sclafani A. The effects of pimozide on the consumption of a palatable saccharinglucose solution in the rat. Pharmacol Biochem Behav. 1981;15:435–442. doi: 10.1016/0091-3057(81)90274-4. [DOI] [PubMed] [Google Scholar]
- Yu W-Z, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of naltrexone. Pharmacol Biochem Behav. 1999;64:573–584. doi: 10.1016/s0091-3057(99)00124-0. [DOI] [PubMed] [Google Scholar]
- Yu W-Z, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of dopamine receptor antagonists. Pharmacol Biochem Behav. 2000a;65:635–647. doi: 10.1016/s0091-3057(99)00239-7. [DOI] [PubMed] [Google Scholar]
- Yu W-Z, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Role of D1 and D2 dopamine receptors in the acquisition and expression of flavor preference conditioning in sham-feeding rats. Pharmacol Biochem Behav. 2000b;67:537–544. doi: 10.1016/s0091-3057(00)00396-8. [DOI] [PubMed] [Google Scholar]


