Abstract
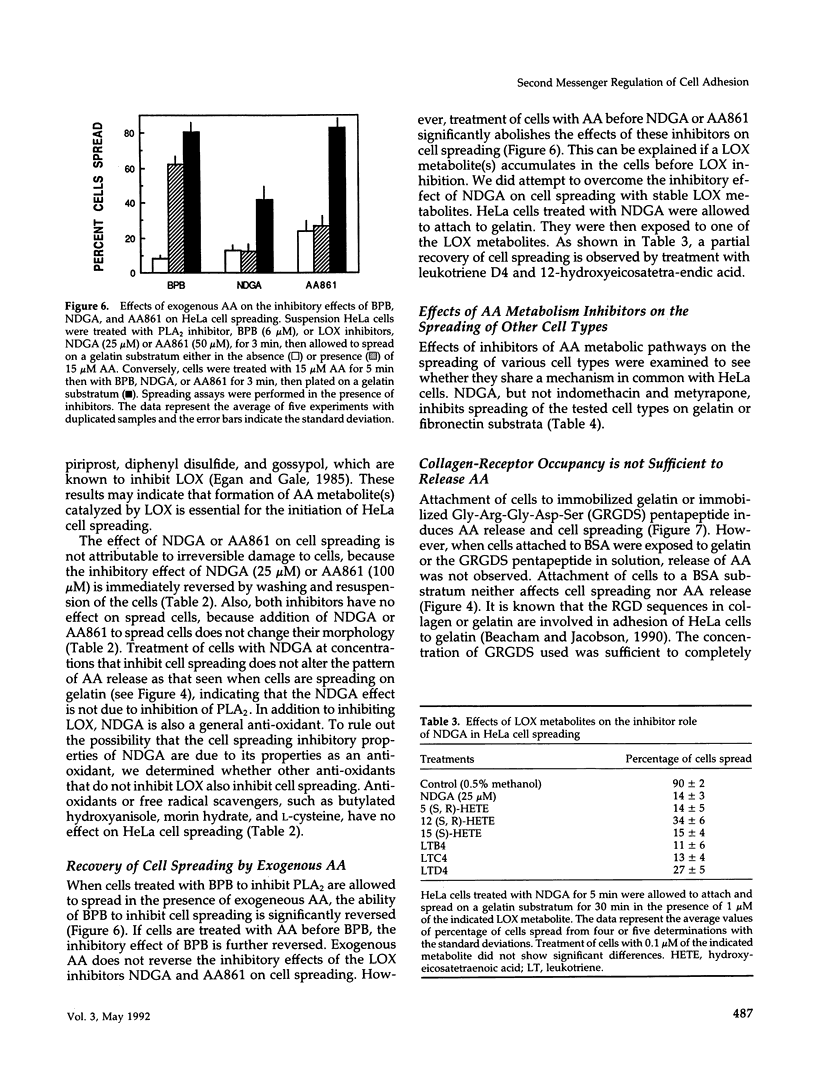
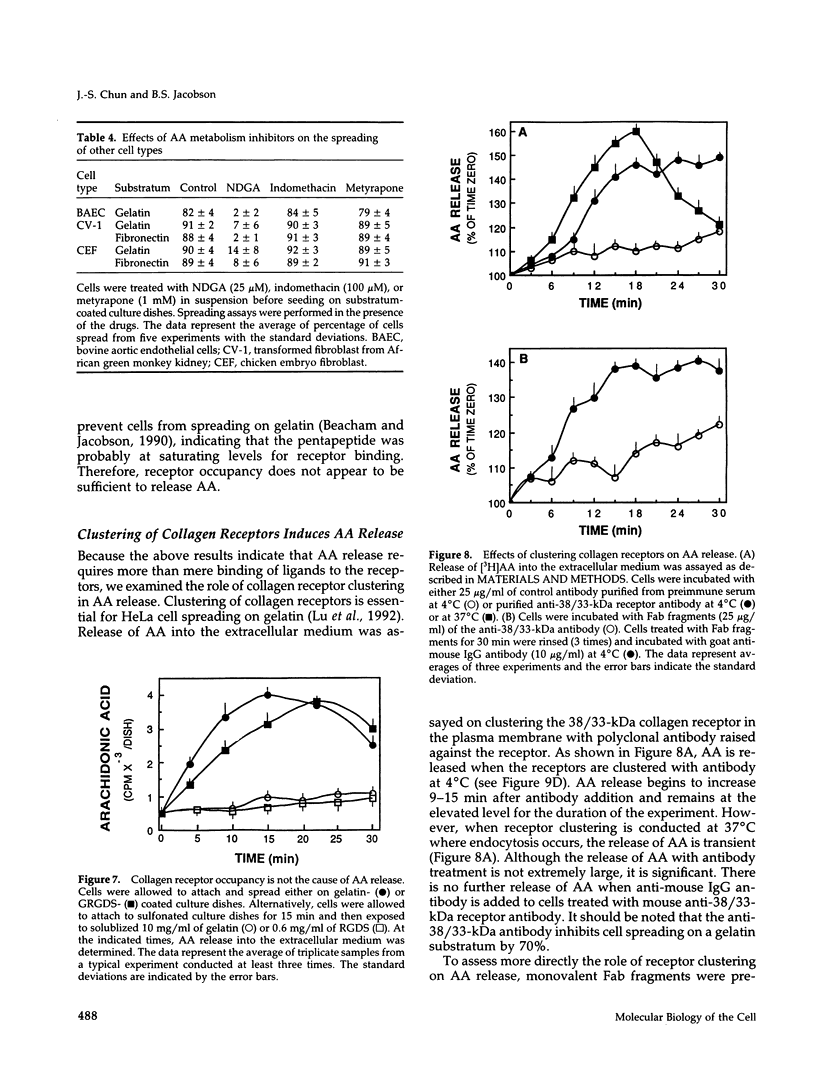
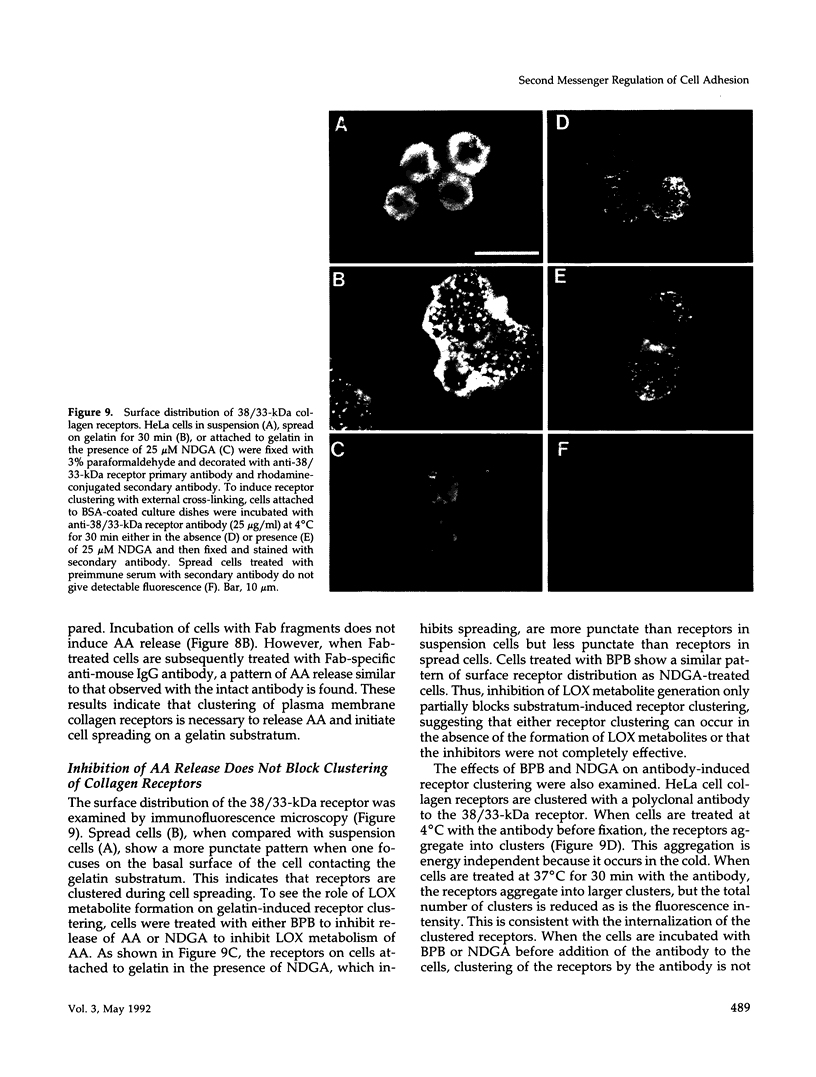
HeLa cells attach to a variety of substrata but spread only on collagen or gelatin. Spreading is dependent on collagen-receptor upregulation, clustering, and binding to the cytoskeleton. This study examines whether second messengers are involved in initiating the spreading process on gelatin. The levels of cytosolic free calcium ([Ca++]i), cAMP, and cytoplasmic pH (pHi) do not change during cell attachment and spreading. However, a basal level of [Ca++]i and an alkaline pH(i) are required for spreading. There is an activation of protein kinase C (PKC) and a release of arachidonic acid (AA) on attachment and before cell spreading. Inhibition of PKC does not block cell spreading, indicating that PKC activation is not essential for spreading. Inhibition of phospholipase A2 blocks cell spreading, whereas addition of exogeneous AA overcomes this inhibitory effect. Among AA metabolic pathways, inhibitors of lipoxygenase (LOX) block cell spreading, suggesting that a LOX product(s) formed from AA initiates spreading. Clustering receptors for collagen with polyclonal antibodies, or with anti-collagen-receptor antigen-binding fragments (Fab) in combination with a secondary antibody, induce AA release. Also, AA is released when cells attach to either immobilized gelatin or immobilized Arg-Gly-Asp (RGD) peptide. Thus, AA is released whenever receptor clustering is observed. Receptor occupancy is not sufficient to release AA; when cells are treated with gelatin or RGD peptide in solution or anti-collagen-receptor Fab fragments without secondary antibody, conditions where receptor clustering is not observed, AA is not released. Thus, a LOX metabolite(s) of AA formed by collagen-receptor clustering is a second messenger(s) that initiates HeLa cell spreading. LOX inhibitors also block the spreading of bovine aortic endothelial cells, chicken embryo fibroblasts, and CV-1 fibroblasts on gelatin or fibronectin, indicating that other cells might use the same second messenger system in initiating cell-substratum adhesion.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Nagata K., Yamada K. M. Cell surface receptors for extracellular matrix components. Biochim Biophys Acta. 1990 Feb 28;1031(1):91–110. doi: 10.1016/0304-4157(90)90004-v. [DOI] [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Ashendel C. L. The phorbol ester receptor: a phospholipid-regulated protein kinase. Biochim Biophys Acta. 1985 Sep 9;822(2):219–242. doi: 10.1016/0304-4157(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Beacham D. A., Jacobson B. S. Mg2+ mediates the cell-substratum interaction of Arg-Gly-Asp-dependent HeLa cell collagen receptors. Exp Cell Res. 1990 Jul;189(1):69–80. doi: 10.1016/0014-4827(90)90258-c. [DOI] [PubMed] [Google Scholar]
- Breuer D., Wagener C. Activation of the phosphatidylinositol cycle in spreading cells. Exp Cell Res. 1989 Jun;182(2):659–663. doi: 10.1016/0014-4827(89)90268-1. [DOI] [PubMed] [Google Scholar]
- Burke D., Brown M. J., Jacobson B. S. HeLa cell adhesion to microcarriers in high shear conditions: evidence for membrane receptors for collagen but not laminin or fibronectin. Tissue Cell. 1983;15(2):181–191. doi: 10.1016/0040-8166(83)90015-0. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Chacos N., Werringloer J., Prough R. A., Estabrook R. W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Musser J. H., McGregor H. Phospholipase A2: function and pharmacological regulation. Biochem Pharmacol. 1987 Aug 1;36(15):2429–2436. doi: 10.1016/0006-2952(87)90512-0. [DOI] [PubMed] [Google Scholar]
- Cheung E., Brown P. J., Juliano R. L. Altered type I protein kinase in adhesion defective CHO cell variants. J Cell Physiol. 1987 Jan;130(1):118–124. doi: 10.1002/jcp.1041300117. [DOI] [PubMed] [Google Scholar]
- Cody R. L., Wicha M. S. Clustering of cell surface laminin enhances its association with the cytoskeleton. Exp Cell Res. 1986 Jul;165(1):107–116. doi: 10.1016/0014-4827(86)90536-7. [DOI] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Fairman K., Jacobson B. S. Unique morphology of HeLa cell attachment, spreading and detachment from microcarrier beads covalently coated with a specific and non-specific substratum. Tissue Cell. 1983;15(2):167–180. doi: 10.1016/0040-8166(83)90014-9. [DOI] [PubMed] [Google Scholar]
- Forscher P. Calcium and polyphosphoinositide control of cytoskeletal dynamics. Trends Neurosci. 1989 Nov;12(11):468–474. doi: 10.1016/0166-2236(89)90098-2. [DOI] [PubMed] [Google Scholar]
- Grossi I. M., Fitzgerald L. A., Umbarger L. A., Nelson K. K., Diglio C. A., Taylor J. D., Honn K. V. Bidirectional control of membrane expression and/or activation of the tumor cell IRGpIIb/IIIa receptor and tumor cell adhesion by lipoxygenase products of arachidonic acid and linoleic acid. Cancer Res. 1989 Feb 15;49(4):1029–1037. [PubMed] [Google Scholar]
- Hart I. R., Goode N. T., Wilson R. E. Molecular aspects of the metastatic cascade. Biochim Biophys Acta. 1989 Jul 28;989(1):65–84. doi: 10.1016/0304-419x(89)90035-8. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Prusty D., Frangioni J. V., Cragoe E. J., Jr, Lechene C., Schwartz M. A. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J Cell Biol. 1990 May;110(5):1803–1811. doi: 10.1083/jcb.110.5.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Minakuchi R., Takai Y., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase (protein kinase C) from rat brain. Methods Enzymol. 1983;99:288–298. doi: 10.1016/0076-6879(83)99064-x. [DOI] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal B. A., Maxfield F. R. Cytosolic free calcium increases before and oscillates during frustrated phagocytosis in macrophages. J Cell Biol. 1987 Dec;105(6 Pt 1):2685–2693. doi: 10.1083/jcb.105.6.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruskal B. A., Shak S., Maxfield F. R. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 May;83(9):2919–2923. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Holzhütter H. G., Schewe T., Hiebsch C., Rapoport S. M. The mechanism of inactivation of lipoxygenases by acetylenic fatty acids. Eur J Biochem. 1984 Mar 15;139(3):577–583. doi: 10.1111/j.1432-1033.1984.tb08044.x. [DOI] [PubMed] [Google Scholar]
- Labat-Robert J., Bihari-Varga M., Robert L. Extracellular matrix. FEBS Lett. 1990 Aug 1;268(2):386–393. doi: 10.1016/0014-5793(90)81291-u. [DOI] [PubMed] [Google Scholar]
- Linderman J. J., Harris L. J., Slakey L. L., Gross D. J. Charge-coupled device imaging of rapid calcium transients in cultured arterial smooth muscle cells. Cell Calcium. 1990 Feb-Mar;11(2-3):131–144. doi: 10.1016/0143-4160(90)90066-4. [DOI] [PubMed] [Google Scholar]
- Liu B., Timar J., Howlett J., Diglio C. A., Honn K. V. Lipoxygenase metabolites of arachidonic and linoleic acids modulate the adhesion of tumor cells to endothelium via regulation of protein kinase C. Cell Regul. 1991 Dec;2(12):1045–1055. doi: 10.1091/mbc.2.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M. L., Beacham D. A., Jacobson B. S. The identification and characterization of collagen receptors involved in HeLa cell-substratum adhesion. J Biol Chem. 1989 Aug 15;264(23):13546–13558. [PubMed] [Google Scholar]
- Margolis L. B., Rozovskaja I. A., Cragoe E. Intracellular pH and cell adhesion to solid substrate. FEBS Lett. 1988 Jul 18;234(2):449–450. doi: 10.1016/0014-5793(88)80135-2. [DOI] [PubMed] [Google Scholar]
- McClay D. R., Ettensohn C. A. Cell adhesion in morphogenesis. Annu Rev Cell Biol. 1987;3:319–345. doi: 10.1146/annurev.cb.03.110187.001535. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Greengard P. Lipoxygenase metabolites of arachidonic acid in neuronal transmembrane signalling. Trends Pharmacol Sci. 1990 Sep;11(9):367–373. doi: 10.1016/0165-6147(90)90182-8. [DOI] [PubMed] [Google Scholar]
- Pritchard K. A., Jr, Tota R. R., Stemerman M. B., Wong P. Y. 14, 15-Epoxyeicosatrienoic acid promotes endothelial cell dependent adhesion of human monocytic tumor U937 cells. Biochem Biophys Res Commun. 1990 Feb 28;167(1):137–142. doi: 10.1016/0006-291x(90)91741-a. [DOI] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Pozzan T. Using quin2 in cell suspensions. Cell Calcium. 1985 Apr;6(1-2):133–144. doi: 10.1016/0143-4160(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Both G., Lechene C. Effect of cell spreading on cytoplasmic pH in normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4525–4529. doi: 10.1073/pnas.86.12.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A., Cragoe E. J., Jr, Lechene C. P. pH regulation in spread cells and round cells. J Biol Chem. 1990 Jan 25;265(3):1327–1332. [PubMed] [Google Scholar]
- Schwartz M. A., Lechene C., Ingber D. E. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin alpha 5 beta 1, independent of cell shape. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7849–7853. doi: 10.1073/pnas.88.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. F., Chap H., Douste-Blazy L. Selective inhibition of human platelet phospholipase A2 by buffering cytoplasmic calcium with the fluorescent indicator quin 2. Evidence for different calcium sensitivities of phospholipases A2 and C. Biochim Biophys Acta. 1986 Feb 12;875(2):157–164. doi: 10.1016/0005-2760(86)90164-5. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Watson P. A. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 1991 Apr;5(7):2013–2019. doi: 10.1096/fasebj.5.7.1707019. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Yokoyama C., Ochi K., Yamamoto S., Maki Y., Ashida Y., Terao S., Shiraishi M. 2,3,5-Trimethyl-6-(12-hydroxy-5,10-dodecadiynyl)-1,4-benzoquinone (AA861), a selective inhibitor of the 5-lipoxygenase reaction and the biosynthesis of slow-reacting substance of anaphylaxis. Biochim Biophys Acta. 1982 Nov 12;713(2):470–473. [PubMed] [Google Scholar]