Abstract
Multiple sclerosis (MS) is chronic inflammatory demyelinating disease of the central nervous system that is mediated by activated lymphocytes, macrophages/microglia, and complement. In MS, the myelin-forming oligodendrocytes (OLGs) are the targets of the immune attack. Experimental evidence indicates that C5b-9 plays a role in demyelination during the acute phase of experimental allergic encephalomyelitis (EAE). Terminal complement C5b-9 complexes are capable of protecting OLGs from apoptosis. During chronic EAE complement C5 promotes axonal preservation, remyelination and provides protection from gliosis. These findings indicate that the activation of complement and C5b-9 assembly can also have protective roles during demyelination.
Keywords: Oligodendrocyte, C5b-9 complement complex, apoptosis, experimental allergic encephalomyelitis, multiple sclerosis, insulin-like growth factor binding proteins
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS). Although the cause of this disease remains unknown, it is generally accepted that the associated cell death and tissue damage are the result of immune system activation and subsequent inflammation (Frohman et al., 2006). Acute MS lesions are characterized by active demyelination and inflammatory infiltrates that include T-cells, macrophages, B-cells, and activated microglia. In addition to the important role of inflammatory cells in the disease process, antibodies and complement activation have also been shown to contribute to autoimmune demyelination. Deposition of immunoglobulins and terminal complement C5b-9 complexes has been observed in both MS (Barnett et al., 2009; Barnett and Prineas, 2004; Lucchinetti et al., 2000) and Devic’s disease lesions (Lucchinetti et al., 2002).
Strong evidence exists that complement, and specifically the assembly of the C5b-9 complex, is involved in demyelination and that myelin is vulnerable to complement attack (Liu et al., 1983; Seil, 1977). However, oligodendroctyes (OLGs) are able to survive limited, or sublytic, complement attack by shedding their membrane regions that contain C5b-9 complexes (Scolding et al., 1989). Thus, the overall effect of C5b-9 on OLGs is dependent on the number of terminal complement complexes inserted into the cell membrane. C5b-9 complexes have been shown to induce the lysis of OLGs and oligodendrocyte progenitor cells (Wren and Noble, 1989). The assembly of sublytic C5b-9 in vitro has been found to enhance OLG survival (Rus et al., 1996). In addition, the apoptosis of OLGs that has been observed in most acute MS lesions (Barnett and Prineas, 2004; Dowling et al., 1997; Lucchinetti et al., 2000) has in some cases been associated with complement activation and C5b-9 deposition on altered myelin and OLGs (Barnett and Prineas, 2004). In this review, we summarize the evidence indicating a role for the terminal complement pathway in OLG survival and discuss the implications of this evidence for the pathophysiology of MS and its model system, experimental allergic encephalomyelitis (EAE).
2. Complement activation and assembly of the C5b-9 complex
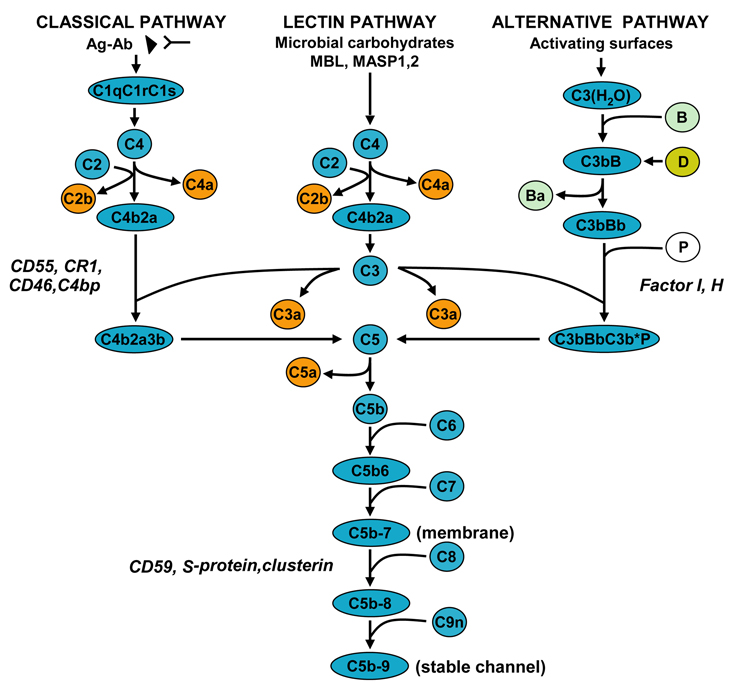
The complement system consists of more than 30 soluble proteins, cell receptors, and control proteins that provide an important defense against infections and immune complex-mediated disease (Frank, 2001; Shin et al., 1996; Walport, 2001) The complement components in the central nervous system (CNS) are synthesized mainly by astrocytes and neurons, and their expression is up-regulated by inflammatory mediators (Rus et al., 2006). The complement system can be activated by the classical, alternative, or lectin pathways. All three pathways converge at the point of C3 cleavage and then generate the membrane attack complex C5b-9, leading to cytolysis (Figure 1). Complement activation leads to the formation of opsonins (C3b and C4b) and anaphlatoxins (C3a and C5a), which are involved in the induction of local and systemic inflammatory reactions.
Figure 1. Complement activation pathways and assembly of the terminal pathway.
The classical pathway is initiated by the binding of the C1 complex to antibody bound by antigen, leading to the formation of the C4b2a enzyme complex, known as the C3 convertase. The lectin pathway is activated by the binding of either MBL or ficolin and MAPS1, 2, and 3, respectively, to an array of mannose groups on the surface of bacterial cells and the generation of C3 convertase of the classical pathway. The alternative pathway is initiated by hydrolyzed C3 and factor B and the subsequent formation of the alternative pathway C3 convertase, C3bBb. Generation of C3 convertase allows the formation of the C5 convertase enzyme, which initiates the formation of the C5b-9 terminal complement complex.
The complement system is regulated at several levels: CD55, CR1, CD46, C4bp, and factors I and H regulate the activity of the C3 convertase and C5 convertase, and other proteins such as CD59 block the final assembly of the pores by preventing the binding of C9. The S protein/vitronectin binds to C5b-7 and leads to the formation of a cytolytically inactive SC5b-9 complex.
The activation of C5 through C9 and the assembly of C5b-9 begin when the C5 convertase cleaves C5 to generate C5a and C5b. The C5b6 complex then binds reversibly to the cell membrane. Subsequently, the interaction of C7, C8, and C9 with C5b6 complexes leads to the assembly of C5b-9 complex, which forms transmembrane pores (Shin et al., 1996). The binding of C7 to C5b6 creates a metastable C5b-7 complex, which associates with and is integrated into the phospholipid membrane bilayer. Addition of C8 to C5b-7 induces the membrane insertion of C8β and C8α and forms unstable pores. Binding of C9to C8α initiates the binding and polymerization of multiple C9 molecules to form stable membrane-inserted pores. This C5b-9 complex, which is effective in inducing cell lysis, is also called the membrane attack complex (Shin et al., 1996). Lytic C5b-9 induces cell death through a multi-hit process (Koski et al., 1983; Shin et al., 1996). In addition to its lytic effect, C5b-9 can form a sublytic complex that plays an important role in the stimulation and activation of target cells (Niculescu and Rus, 2001).
3. Oligodendrocyte cell death in multiple sclerosis
In MS, demyelination is accompanied by extensive destruction and loss of OLGs (Ozawa et al., 1994). Failure to remyelinate during the recovery phase after an acute attack occurs in part because of the death of injured OLGs and the failure of OLG progenitors to mature and remyelinate axons (Wolswijk, 2000; Kuhlmann et al., 2008). The role of apoptosis in OLG cell death remains a subject of vigorous debate (Frohman et al., 2006; Raine, 2008) . In acute MS, apoptosis of OLGs, has been documented in multiple studies (Barnett and Prineas, 2004; Dowling et al., 1997; Lucchinetti et al., 2000) but has only rarely been seen in chronic MS lesions (Bonetti and Raine, 1997; Breij E. C. W., 2008) (Table 1). Barnett and coworkers have proposed that the apoptotic death of OLGs is the initial event in new lesion formation and the primary cause of inflammation in MS (Barnett and Prineas, 2004). By examining relapsing-remitting MS lesions within 24 hours of an acute attack, these authors concluded that OLG death precedes the activation of complement and microglia. This reaction was followed by demyelination and phagocytosis of complement-opsonized myelin sheets by macrophages. Thus, major OLG loss is almost certainly an early event occurring in the initial stages of the acute MS (Cannella et al., 2007). In established MS lesions, OLG apoptosis was absent or rare (Breij E. C. W., 2008; Bonetti and Raine , 1997). One possible explanation for the small number of apoptotic OLGs in chronic lesions is the rapid removal of apoptotic cells in vivo. In addition, a less prominent role for apoptosis in OLG depletion in chronic lesions cannot be excluded (Raine, 2008).
Table 1.
OLG apoptosis in MS brain
| Lesion type | OLG Apoptosis | Methods | References |
|---|---|---|---|
| Acute and chronic | Present | TUNEL | Dowling et al., 1997 |
| Chronic active or silent | Absent | TUNEL | Bonetti and Raine, 1997 Cannella et al., 2007 |
| Active | Present in Pattern III | TUNEL | Lucchinetti et al., 2000 |
| Chronic | Rare | TUNEL/caspase-3 | Prineas et al., 2001 |
| New active | Present | Morphological Changes | Barnett and Prineas, 2004 |
| Chronic active | Rare or absent | TUNEL | Breij E. C. W., 2008 |
Experimental data point to an important role for caspases in the OLGs apoptosis associated with autoimmune demyelination. Caspase-1-deficient mice develop a less severe form of EAE (Furlan et al., 1999), a finding that is consistent with data obtained from transgenic mice in which the caspase-inhibitory protein p35 is over-expressed (Hisahara et al., 2000). These transgenic mice are less susceptible to EAE and have fewer apoptotic cells. Also, OLGs from these mice are resistant to cytotoxicity induced by FasL, TNFα, or IFNγ in vitro.
Further evidence supporting a role for caspases in EAE has been obtained by using mice deficient in the expression of caspase-11, an apical caspase that activates caspases 3 and 1 (Hisahara et al., 2001). In caspase 11 knockout mice, the incidence and severity of EAE are significantly reduced when compared to wild-type mice, with fewer OLGs being positive for caspase 3. These experimental data clearly suggest an important role for caspases in OLG apoptosis associated with autoimmune demyelination. Recently, another potent regulator of glial apoptosis, alpha-B-crystallin, has been found to be a negative regulator of inflammation in EAE (Ousman et al., 2007). In acute and chronic MS, significantly elevated caspase 1 mRNA is found in the brain (Ming et al., 2002). The presence of caspase-1 mRNA has been shown to correlate with increased protein expression in OLGs. Expression of caspase-1 mRNA is also increased in the peripheral blood mononuclear cells of MS patients when compared to those in healthy controls (Khurana et al., 2004).
4. Mediators of oligodendrocyte cell death
Effector cells
The mechanisms triggering OLG loss in MS are not well understood. A significant number of studies have shown that adaptive and innate immune system constituents can induce OLG cell death. In vitro studies have shown that OLGs are not susceptible to MHC class II-restricted lysis, but myelin-reactive CD4 αβ T-cells can mediate nonrestricted lysis when they express the cell-surface antigen CD56 (Antel et al., 1998). In addition, idiotype-specific CD4 T cells from the CSF and blood can induce the apoptosis of human OLG cell lines in vitro in a manner that requires cell-cell contact and involves the Fas/Fas ligand pathway. Similarly, activated CD4 T cells specific for glutamic acid decarboxylase 65 can also induce apoptosis of OLGs, suggesting that killing does not depend on cognate interaction between T cells and target cells but rather on the activation status of the T cells (Hestvik et al., 2009). CD8 T cells are present in both MS and EAE lesions, and MBP-reactive CD8 αβ T-cells can induce MHC class I-restricted cytotoxicity of OLGs (Jurewicz et al., 1998). Experimental evidence has also demonstrated the potential of CD8 T cells to induce OLG lysis in vivo as a likely consequence of direct antigen recognition (Saxena et al., 2008).
Cells of the innate immune system use non-MHC-restricted lytic mechanisms to mediate target cell injury. NK cells and γδ T-cells are constituents of this system and are present in MS lesions (Traugott and Raine, 1984; Wucherpfennig et al., 1992). OLGs have been found to be susceptible to injury mediated by IL-2-activated NK cells. The inflammatory milieu in MS lesions could provide the conditions required for the activation of NK cells, and such effector cells can bypass the putative protective effects of self-MHC class I molecules that may be expressed on OLGs (Morse et al., 2001).
γδT cells have been implicated in the pathogenesis of MS (Selmaj et al., 1991a); these cells display both innate and adaptive characteristics and activity (Blink and Miller, 2009) and are cytotoxic toward human OLGs (Zeine et al., 1998). OLGs express ligands for the NKG2D receptors that are present on γδ T and NK cells. The cytotoxic effect is partially inhibited by disruption of the NKG2D – NKG2D ligand, suggesting a potential role for this system in cytotoxic responses mediated by activated immune effector cells in the inflamed CNS (Saikali et al., 2007). The death of target cells that is induced by these cytotoxic lymphocytes involves cell lysis and/or activation of one or more of the apoptotic pathways. Interestingly, the same effector molecule can induce both apoptosis and lysis, depending on the biochemical microenvironment (Chavez-Galan et al., 2009). For instance, perforin can induce the lysis of OLGs (Zeine et al., 2001), while at the same time playing a critical role in the initiation of the apoptotic process (Chavez-Galan et al., 2009).
Multiple effector molecules can mediate the death of OLGs through apoptosis and cell lysis (Table 2). In the sections below, we discuss several of the mediators involved in the death of OLGs.
Table 2.
Mediators of OLG cell death.
| Mediator | Mechanisms | In vitro/ experimental model | References |
|---|---|---|---|
| FasL | Cell death | hOLG | D’Souza et al., 1996 |
| Apoptosis | EAE | Heppner et al., 2005 | |
| TNFα | Cell death | rOLG | Robbins et al., 1987 |
| Cell necrosis | mOLG | Selmaj and Raine, 1988 | |
| Apoptosis | hOLG | Jurewicz et al., 2005 | |
| Apoptosis | EAE | Heppner et al., 2005 | |
| Apoptosis | TNF transgenic mice | Akassoglou et al., 1998 | |
| Lymphotoxin | Apoptosis | bOLG culture | Selmaj et al., 1991b |
| TRAIL | Apoptosis | hOLG | Matysiak et al., 2002 |
| IFNγ | Apoptosis | rOLG | Vartanian et al., 1995 |
| Apoptosis | IFN transgenic mice | Lin et al., 2005 | |
| NGF | Apoptosis | rOLG | Casaccia-Bonnefil et al., 1996 |
| Apoptosis | mOLG | Beattie et al., 2002 | |
| CD4+ T cells | Cell lysis | hOLG | Ruijs et al., 1993 |
| Apoptosis | hOLG | Hestvik et al., 2009 | |
| CD8+ T cells | Cell lysis | hOLG | Jurewicz et al., 1998 |
| γδ T Cells | Cell lysis | hOLG | Freedman et al., 1991; Zeine et al., 1998 |
| NK Cells | Cell lysis | hOLG | Morse et al., 2001 |
| Nitric oxide | Cell death | rOLG | Merrill et al., 1993 |
| Apoptosis | hOLG | Jack et al., 2007 | |
| ROS | Apoptosis | hOLG | Jana and Pahan, 2007 |
| Glutamate | Cell death | rOLG | Matute et al., 1997 |
| excitotoxicity | Apoptosis | rOLG | Sanchez-Gomez et al., 2003 |
| Cell loss | EAE | Pitt et al., 2000 | |
| Lytic C5b-9 | Cell lysis | rOLG | Wren and Noble, 1989 |
| Perforin | Cell lysis | mOLG | Scolding et al., 1990 |
| Cel lysis | rOLG | Zeine et al., 2001 |
Cell lysis: lysis was measured by 51Cr-release assay or propidium iodide uptake, Cell death: non-apoptotic cell death, Cell loss: mechanism of cell death was not investigated, hOLG: human oligodendrocyte; rOLG: rat oligodendrocyte, mOLG: mouse oligodendrocyte, bOLG: bovine oligodendrocyte, ROS: reactive oxygen species.
Fas ligand
Fas, a member of the TNF receptor (TNFR) superfamily, is expressed on the cell surface and is responsible for transducing cell death signals (Sharma et al., 2000). The Fas-FasL system has been shown to be involved in the OLG cell death seen in MS (Dowling et al., 1996) (Figure 2). Fas expression on OLGs is increased in MS lesions, and human OLGs are susceptible to Fas-induced non-apoptotic cell death (D'Souza et al., 1996). In addition, TUNEL-positive cells are co-localized with FasL in MS lesions, and the microglia and lymphocytes in the lesions show intense staining for FasL (D'Souza et al., 1996). Another study has found that apoptosis induced by the Fas-FasL system plays an important role in eliminating infiltrating cells, but not that of myelinating OLGs in chronic MS lesions (Bonetti and Raine , 1997). In vitro, caspase activation and cell death can be induced by FasL in human OLG hybrid cells (Li et al., 2002; Pouly et al., 2000). OLGs derived from mice over-expressing the caspase inhibitor p35 (Hisahara et al., 2000) or transgenic mice deficient in caspase 11 (Hisahara et al., 2001) are resistant to Fas-mediated apoptosis. Enhanced expression of FasL on lymphocytes is correlated with the development of EAE, whereas the expression of Fas on brain cells may play a role in the progression of EAE (Choi and Benveniste, 2004). In addition, Fas inactivation alone, as well as a complete absence of TNF-R1, has been shown to partially protect mice from EAE induced by immunization with myelin OLG glycoprotein. These double-deficient mice, however, show almost no clinical signs of EAE after immunization and exhibit a lack of demyelination and a reduction in lymphocyte infiltration, suggesting that the death receptors Fas and TNF-R1 are major initiators of OLGs apoptosis in EAE (Heppner et al., 2005).
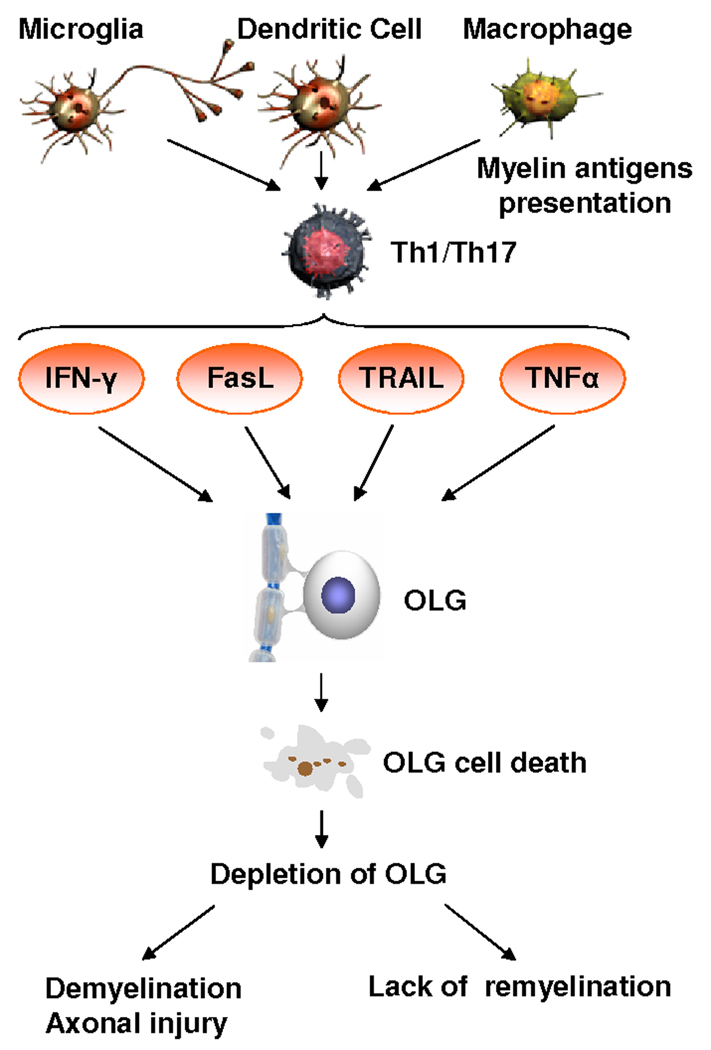
Figure 2. Impact of cytokines on OLGs.
Activated mycroglia, T-lymphocytes, and macrophages present in the CNS during MS produce various cytokines and mediators (TNFα, IFNγ, FasL and TRAIL) that induce OLG cell death. Cytokines belonging to the TNF receptor superfamily and IFNγ produced by inflammatory cells induce caspase activation and also deregulate the balance between the pro-apoptotic and anti-apoptotic members of the Bcl-2 family of proteins. This deregulation leads to mitochondrial dysfunction and induction of OLG cell death. Cell death then contributes to the decrease in OLGs number seen in MS lesions and ultimately leads to increased demyelination and axonal injury.
Another factor that may be involved in the apoptosis of OLGs is p53 (Wosik, 2003). In situ analysis of active MS lesions has revealed an increased expression of p53 in OLG in lesions featuring OLG apoptosis and cell loss. p53 overexpression induces the up-regulation of the death receptors Fas and TNF-related apoptosis-inducing ligand_(TRAIL), with subsequent caspase-mediated apoptosis of the OLGs. Although lower levels of p53 did not induce apoptosis, the increase in death receptor expression was sufficient to render the OLGs susceptible to apoptosis in the presence of exogenous Fas ligand and TRAIL (Wosik, 2003).
Tumor necrosis factor α (TNFα)
TNFα is an important modulator of the immune response. The high levels of TNFα that are found in the cerebrospinal fluid (CSF) of patients with chronic progressive MS are directly correlated with increasing severity and progression of the disease (Sharief and Hentges, 1991). TNFα is able to induce both the apoptotic and necrotic death of OLGs, and inhibition of caspase-1 and caspase-3 protects OLGs against TNFα-induced apoptosis (Hisahara et al., 1997; Louis et al., 1993; Robbins et al., 1987; Selmaj and Raine, 1988). Recently, electrophoresis of OLGs exposed to TNF has revealed large-scale DNA fragmentation characteristic of apoptosis-inducing factor (AIF)-mediated cell death (Jurewicz et al., 2005). AIF depletion using an antisense strategy can prevent TNFα-induced death of OLGs, suggesting that AIF plays an important role in during immune-mediated demyelination (Jurewicz et al., 2005). Intracellular transduction signaling involved in the TNF-induced apoptotic cell death of OLGs is associated with the activation of c-Jun NH2-terminal kinase (JNK) 3 (Jurewicz et al., 2003).
TNFα also reduces the expression of IGF-I in the brain and alters the abundance of a number of IGF system proteins that are capable of modulating the activity of IGF-I, suggesting that reduced IGF-I availability is responsible, at least in part, for the pro-apoptotic effects of TNFα on OLGs. Addition of IGF-I to culture medium inhibits TNFα-induced apoptosis in OLGs both in vivo and in vitro (Bailey et al., 2007).
Microglia may also play a role in mediating the death of OLGs through the release of TNFα (Merrill et al., 1993). Since neutralization of TNFα causes an increase in MS disease activity, and TNF-neutralizing agents provoke inflammatory demyelination (Mohan et al., 2001), TNF-α may act as a pro-inflammatory and demyelinating factor and also protect the host from demyelination.
TRAIL
TRAIL and its receptors are constitutively expressed in a variety of normal tissues and tumor cells. More recently, studies have shown TRAIL and its receptors in human brain tissue. DR4 and DR5 (TRAIL-R1 and TRAIL-R2) are two of five cloned receptors of TRAIL, and two other receptors of TRAIL, DcR1 and DcR2 (TRAIL-R3 and TRAIL-R4), are thought to be protective and to act as decoy receptors. DR6, one of the newer members of the DR family, is widely expressed in human tissues. RT-PCR data indicate that DR6 is abundant in the normal human CNS (Harrison et al., 2000).
Examination of human OLGs in culture has demonstrated the presence and functionality of DR following ligation with TRAIL (Cannella et al., 2007). OLGs obtained from human fetal spinal cord expressed DR3, DR4, DR5, DR6, as well as the decoy receptors DcR1 and DcR2. The findings were somewhat different from those in adult CNS tissue in situ, in which DR4 and DR6 were not observed on OLGs (Cannella et al., 2007). Studies using adult human OLGs have shown that TRAIL-R1 is the receptor responsible for the apoptosis of OLGs (Matysiak et al., 2002). The intracellular transduction signaling involved in the TRAIL-induced apoptotic cell death of OLGs is associated with the activation of JNK (Jurewicz et al., 2006). Other mitogen-activated protein kinases (p38 kinase and ERK) are not activated during TRAIL-induced OLG cell death. OLGs can also be protected from p53-induced cell death by blocking signaling through Fas and/or TRAIL receptors (Wosik, 2003). These data indicate that, in vitro, TRAIL is an important factor in the apoptotic cell death of OLGs.
Interferon γ(IFNγ)
IFNγ is produced by lymphocytes in MS lesions, and systemic administration of IFNγ causes an exacerbation of MS (Panitch et al., 1987). IFNγ can also have cytotoxic effects on OLGs in culture through its modulation of cellular responses to injury (Hisahara et al., 2000; Hisahara et al., 2001; Pouly et al., 2000) (Figure 2). Treatment with IFN-γ increases Fas expression on OLGs in vitro and thus enhances their susceptibility to FasL-induced apoptosis (D'Souza et al., 1996). In addition, TNFα increases the IFNγ-induced death of OLG progenitor cells, and this effect can be partially suppressed by caspase inhibitors (Andrews et al., 1998). OLGs from caspase-11 knockout mice are less sensitive to IFNγ-induced cell death, further supporting the requirement for caspase activation in OLGs cell death (Hisahara et al., 2001). Moreover, the induction of IFN-γ expression after demyelinating insults significantly inhibits the remyelination process in EAE (Lin et al., 2006). OLG injury after overexpression of IFN-γ has been related to the triggering of a proapoptotic response (Lin et al., 2005). These data indicate that IFN-γ plays a role in apoptosis in OLGs both in vivo and in vitro.
Excitotoxicity
Alterations in the homeostasis of glutamate could contribute to the death of OLGs through both caspase-dependent and -independent mechanisms (Sanchez-Gomez et al., 2003). While rodent OLGs are vulnerable to injury mediated by glutamate (Matute et al., 1997), normal human adult OLGs express low levels of ionotropic glutamate receptors and are more resistant to excitotoxicity-mediated injury (Wosik et al., 2004). In MS lesions, the levels of glutaminase, a glutamate-synthesizing enzyme, are increased in microglia, whereas the expression of glutamate transporters is decreased in OLGs (Werner et al., 2001). In EAE, blocking of AMPA/kainite receptors ameliorates the course of the disease and promotes OLG survival (Pitt et al., 2000; Smith et al., 2000). In addition, glutamate can sensitize OLGs to complement attack and may further contribute to OLGs loss (Alberdi et al., 2006). Thus, imbalanced glutamate homeostasis contributes to the OLGs cell death in MS.
5. C5b-9 complex-mediated protection of OLGs from apoptotic cell death
Apoptosis initiated in OLGs by serum withdrawal is associated with a rapid decline in phosphatidylinositol-3 kinase (PI3 kinase)/Akt activity, together with the release of cytochrome c, activation of caspase 9, and cleavage of caspase-3 (Soane et al., 2001; Soane et al., 1999). To release cytochrome c, Bad binds to Bcl-xL and causes mitochondrial damage by displacing Bcl-xL and allowing the oligomerization of pro-apoptotic Bax and Bak (Willis and Adams, 2005) (Figure 3). On the other hand, the dissociation of Bad from Bcl-XL and binding of Bad to cytoplasmic 14-3-3 proteins increases cell survival and requires phosphorylation of Bad at Ser112, Ser136, and Ser156 (Willis and Adams, 2005).
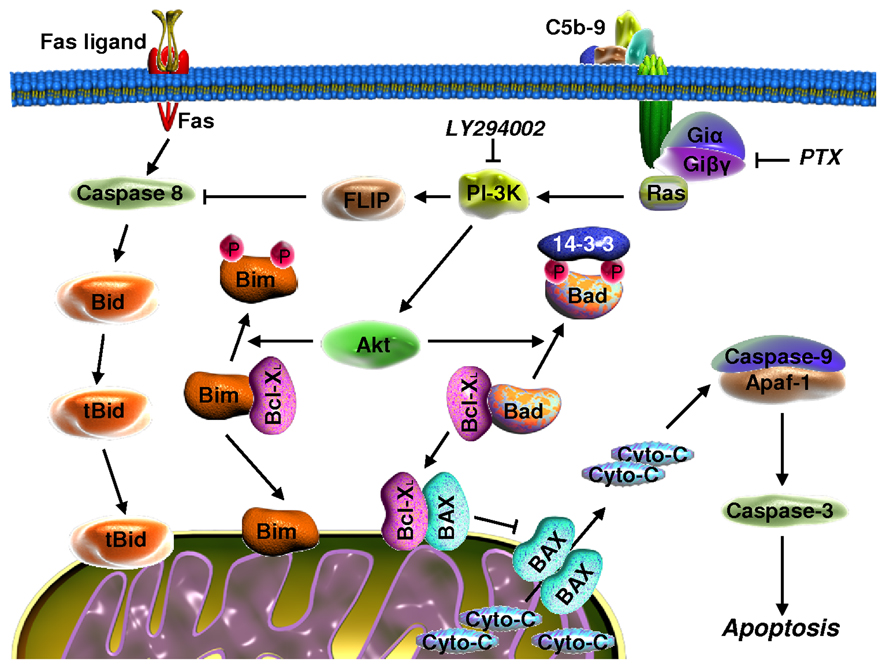
Figure 3. Signaling pathways that are activated by C5b-9 and involved in OLG survival.
Insertion of the sublytic C5b-9 into the cell membrane leads to G-protein activation, followed by the activation of PI3K and Akt. Activation of PI3K induces up-regulation of FLIP and inhibition of caspase-8 processing, thereby inhibiting Fas-induced apoptosis in OLGs. Inhibition of caspase-8 processing is also responsible for the inhibition of Bid cleavage. Akt is able to induce the phosphorylation of Bad and Bim and protects the mitochondria from pore formation by Bax/Bak and cytochrome c release. All these signaling pathways result in the inhibition of apoptosis and OLGs survival.
We have found that these apoptosis-associated activities are inhibited by the activation of complement and assembly of sublytic C5b-9. Studies of upstream signaling have shown that C5b-9 induces strong PI3K/Akt activation and phosphorylation of Bad. C5b-9 increases the phosphorylation of Bad at Ser112 and Ser136 and results in the dissociation of Bad/Bcl-xL complexes (Soane et al., 2001). Both processes can be reversed by inhibiting phosphatidylinositol-3 kinase (PI3K). Therefore, sublytic complement attack appears to increase the survival of OLGs, in part by activating signaling pathways that are important for Bad phosphorylation and subsequent alteration of the association between Bcl-xL and Bad (Figure 3).
To identify the initiators of apoptosis in OLGs, we evaluated the ability of TNFα and FasL to induce apoptosis and examined the effect of C5b-9 on these apoptotic pathways. We found that both TNFα and FasL are able to induce the apoptosis of OLGs and that C5b-9 inhibits FasL- and TNF-α-induced cell death (Cudrici et al., 2005; Soane et al., 1999). This C5b-9 effect is mediated through the inhibition of caspase-8 activation (Cudrici et al., 2006). Since the cleavage of Bid by caspase-8 has been shown to directly trigger the release of cytochrome c from mitochondria (Gonzalvez et al., 2008), we monitored Bid cleavage as well as the levels of c-FLIP, an endogenous inhibitor of caspase-8 (Lavrik et al., 2005). Exposure to C5b-9 inhibited Bid cleavage and caused a significant increase in c-FLIPL expression (Cudrici et al., 2006). These results suggest that C5b-9 prevents caspase-8 processing through a c-FLIPL-dependent mechanism (Figure 3).
Bim is another BH3-only protein and is the most effective of such proteins in terms of cell killing because it can engage all of the Bcl-2 proteins (Ewings et al., 2007). Bim expression is increased after withdrawal of survival factors from OLGs and is significantly reduced by exposure to C5b-9. The C5b-9 effect can be reversed by inhibition of PI3K (Cudrici et al., 2005). These data suggest that Bim is required for the OLG cell death caused by serum withdrawal and that C5b-9 prevents this association by promoting the rapid dissociation of preformed Bim/Bcl-xL complexes (Figure 3). Thus, our data indicate that C5b-9, acting through PI3K signaling, is able to rescue OLGs from apoptosis by up-regulating c-FLIPl and preventing mitochondrial insertion of the pro-apoptotic proteins Bad, Bid, and Bim.
6. Complement C5-mediated protection of OLGs from apoptosis during EAE
We have analyzed the influence of C5 on inflammatory demyelination during the course of chronic EAE (up to 120 days) in C5-deficient (C5-d) mice (Weerth et al., 2003). In the absence of C5, more severe inflammatory demyelination was seen during acute EAE, and this condition eventually progressed to gliosis associated with axonal loss. In contrast, C5-sufficient (C5-s) mice showed a marked degree of myelin repair and axon preservation during the chronic phase of EAE. We also made use of these C5-d mice to analyze the role of C5 in the apoptosis of OLGs during EAE (Niculescu et al., 2004). In acute EAE, C5-d and C5-s mice had similar numbers of total apoptotic cells. During recovery, however, C5-s mice had significantly fewer apoptotic cells than did C5-d mice. In addition, while both groups of mice displayed TUNEL+ OLGs, the number of these cells was significantly lower in C5-s than in C5-d mice during both the acute and recovery phases (Niculescu et al., 2004). These findings are consistent with a role for C5 in protecting OLGs from apoptosis in EAE, potentially by forming C5b-9 complexes and thereby promoting remyelination during recovery. It is important to mention that disruption of the C5a receptor failed to protect against EAE (Reiman et al., 2002), thus suggesting a role for terminal complement complexes in mediating the in vivo survival of OLGs.
The alterations in transcriptomic networks that occur in EAE include significant changes in the expression of genes involved in myelination, signal transduction, and the inflammatory response (Comabella and Martin, 2007; Iacobas et al., 2007). To further explore the effects of C5 on post-inflammatory repair of CNS tissue, we recently used oligonucleotide arrays to investigate the transcriptional profile induced by C5 in chronic EAE (Cudrici et al., 2008). The transcriptional profile in the spinal cord was determined by microarray analysis using the Affymetrix Mouse Expression Set 430 2.0 array chip (Affymetrix, Santa Clara, CA). The presence of C5 in mice had a profound effect on the transcriptional profile. When we restricted the profile to those genes with a ≥2-fold change in expression from that in control mice without EAE, we found that 2,511 genes were differentially regulated in C5-s and C5-d mice. During EAE, 916 and 871 genes were differentially regulated in the acute and recovery phases, respectively. During chronic EAE, we found that 390 genes were differentially regulated in C5-s mice and C5-d mice (Cudrici et al., 2008). The differentially regulated genes were further characterized according to their roles in biological processes using the Gene Ontology database (Ashburner et al., 2000). In the case of chronic EAE, more than 25% of these differentially expressed genes were involved in cell growth and maintenance, signal transduction, and cell adhesion.
To determine whether the biological processes that were identified were represented by genes that were significantly overexpressed, we used EASE (Hosack et al., 2003), which performs a statistical analysis of gene categories in the gene list to find those categories that are most overexpressed (and can therefore be described as “themes” of the gene list). The majority of the genes identified in this manner were related to cell growth and signal transduction. Among them, a group of genes belonging to the family of insulin-like growth factor binding proteins (IGFBP) was significantly regulated by C5. These proteins included IGFBP-3, −4, and −6 and connective tissue growth factor (CTGF) (Cudrici et al., 2008).
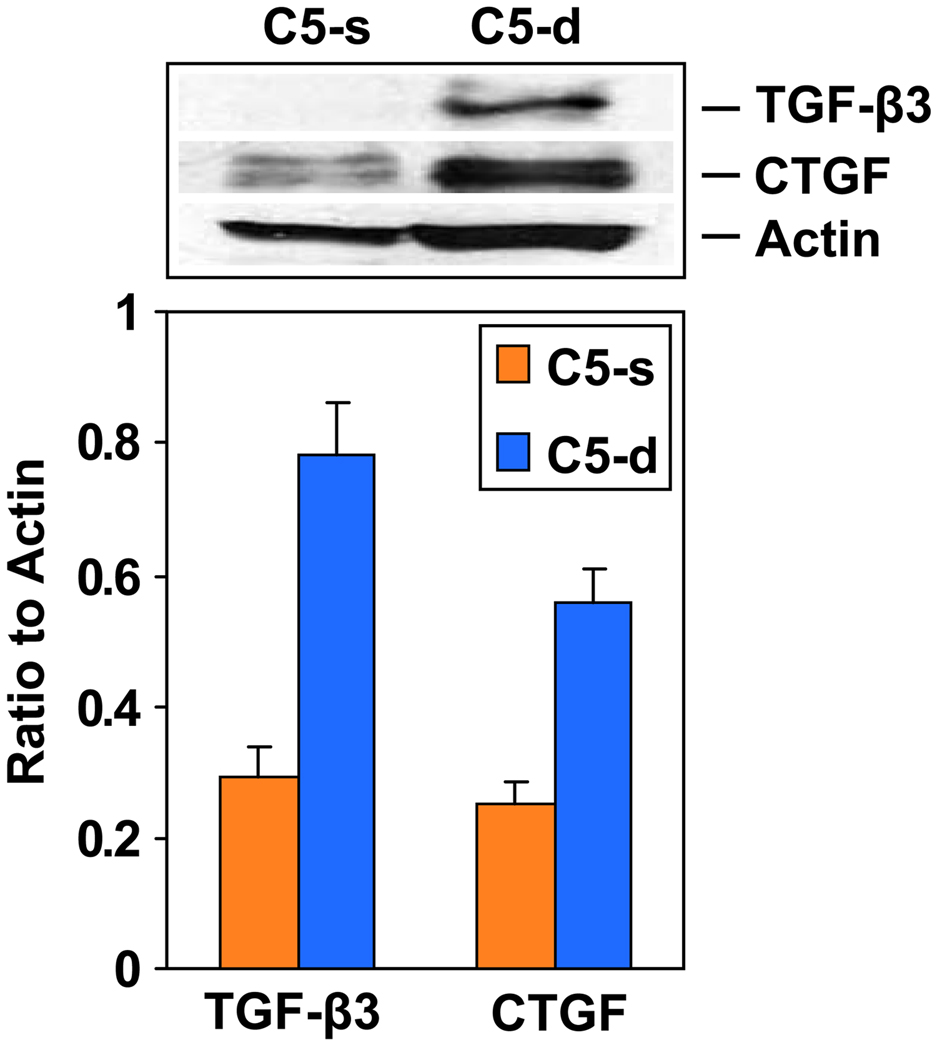
Using real-time PCR and immunoblotting, we confirmed the up-regulation of IGFBP-3 and −4 and the down-regulation of IGFBP-6 in C5-d mice during chronic EAE. Both CTGF and transforming growth factor (TGF)-β3 were also up-regulated in C5-d mice during chronic EAE (Figure 4). Our data clearly show that the changes in the mRNA levels were followed by similar changes in protein levels and further implicate these proteins in the pathogenesis of gliosis seen in C5-d mice. IGFBP-3, IGFBP-6 and CTGF were localized to the infiltrative inflammatory cells during the acute and recovery phases of EAE. In addition, all three proteins were found to stain spinal neurons during the acute phase and recovery and to a lesser extent in chronic EAE. Higher levels of CTGF were found in reactive astrocytes in C5-d than in C5-s mice in chronic EAE (Cudrici et al., 2008).
Figure 4. Expression of TGF-β33 and CTGF in chronic EAE.
Expression of TGF-β3 and CTGF was examined by western blotting. Spinal cords from C5-s and C5-d mice with chronic EAE were lysed in RIPA buffer and fractionated on 10% PAGE gels, followed by transfer to nitrocellulose. Increased levels of TGF-β3 and CTGF were found in C5-d mice.
The dysregulation of genes belonging to the IGFBP and TGF-β families suggests that these proteins might be responsible for the gliosis and lack of remyelination seen in C5-d mice with chronic EAE. IGFBP-3, IGFBP-6, and CTGF were also found to be expressed in MS brain tissue (Gveric et al., 1999; Holley et al., 2003). CTGF expression was confined to reactive astrocytes, axons, and blood vessels. IGFBP-3 was confined to foamy macrophages and microglia. In addition, we found IGFBP-3 and IGFBP-6 expression on axons, and IGFBP-3 and −6 were found to be present in OLGs, with some increased expression at the edges of demyelinated plaques (Wilczak et al., 2008). These data suggest a complex role for the IGFBPs in MS and point to the need for further studies of the potential role of complement system activation in the regulation of their expression. Since we found that TGF-β3 was up-regulated in C5-d mice, it is possible that this molecule plays a role, through regulation of the Notch pathway, in the limited remyelination seen in these mice. In astrocytes, TGF-β was shown to re-induce the expression of Jagged1, which is known to inhibit OLG maturation through the activation of Notch 1 receptors (John et al., 2002).
7. C5b-9 and OLG apoptosis in MS
Increased levels of SC5b-9 have been detected in the spinal fluid of MS patients during relapses (Mollnes et al., 1987; Sanders et al., 1988; Sellebjerg et al., 1998), and these levels have been shown to correlate with neurological disability (Sellebjerg et al., 1998). These data suggest that full activation of the complement cascade during attacks of MS may be restricted to patients with more advanced disease and indicate that long-term activation of complement is detrimental rather than beneficial. In MS, the deposition of C1q, C3d, and C5b-9 is detected on and within macrophages and in the blood vessel walls of active lesions (Barnett and Prineas, 2004; Compston et al., 1989; Storch et al., 1998) but rarely in pure cortical lesions (Brink et al., 2005). One study has used complement activation and OLG apoptosis to define four patterns of MS lesions (Lucchinetti et al., 2000). In the most common pattern, pattern II, immunoglobulins and C5b-9 deposition are found at the sites of active myelin destruction. Moreover, the C5b-9 deposits are found only in pattern II demyelinating lesions (Lassmann, 2007; Lucchinetti et al., 2000). Only pattern III is reported as generally showing a significant number of apoptotic OLGs. In contrast, a recent study has found that the immunopathological appearance of active demyelinating lesions in established MS is uniform (Breij E. C. W., 2008). In this study, complement components and imunoglobulins were consistently associated with macrophages in areas of active demyelination, and MS lesions did not segregate according to OLG behavior and showed a uniform pattern II phenotype. In addition, preferential loss of myelin proteins, extensive hypoxia-like damage, and OLG apoptosis were absent or rare (Breij E. C. W., 2008). IgG and complement immunostaining of disrupted myelin was seen not only in MS but also in other neurological diseases, an indication that the pathologic features that were observed were nonspecific and cannot be interpreted as evidence of a distinct pathogenesis or be used to define particular variants of the disease (Barnett et al., 2009).
In another study in which acute MS lesions were examined (Barnett and Prineas, 2004), extensive OLG apoptosis, complement activation, and remyelination were found in the same lesions. Within hours, OLGs throughout the affected tissue appeared apoptotic, myelin sheaths stained positively for the activated complement components C3d and C5b-9, and immunoreactivity with 2´, 3´-cyclic nucleotide 3´-phosphodiesterase and myelin-associated glycoprotein was diminished (Barnett and Prineas, 2004). In addition, the cleavage products C3d, C4d, and C5b-9 were detected along myelin sheaths at the edge of the lesions, indicating complement activation by the myelin (Barnett and Prineas, 2004; Breij E. C. W., 2008; Prineas et al., 2001). The consistent presence of complement, antibodies, and Fcγ receptors in macrophages suggested that antibody- and complement-mediated phagocytosis of myelin is the dominant mechanism of demyelination and myelin clearance in MS (Barnett and Prineas, 2004; Breij E. C. W., 2008). Recently, microglial nodules containing short, linear deposits of C3d on partly demyelinated axons located in normal-appearing periplaque white matter have been described in MS brains and were considered to be unique to this disease (Barnett et al., 2009). In summary, it is possible that apoptotic OLGs are less frequently seen in MS lesions because of the protective effects of the activation of complement and the subsequent assembly of C5b-9 complexes.
Taken together, our data suggest that assembly of the terminal complement pathway may play a protective role by promoting OLG survival and axon preservation and providing protection from gliosis. However, further work is needed to clarify the role of C5b-9 in the protection of OLGs from apoptosis in the MS brain. These neuroprotective effects should be taken into account when drugs that inhibit the activation of the complement system are designed for therapy in MS.
ACKNOWLEDGMENTS
We thank Dr. Deborah McClellan for editing this manuscript. This work was supported in part by US Public Health Grant RO1 NS42011 (to H.R.), by a Veterans Administration Merit Award (to H.R.), and by the Multiple Sclerosis Society Pilot Project PP1422 (to H.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassmann H, Kollias G, Probert L. Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice: models for multiple sclerosis with primary oligodendrogliopathy. Am J Pathol. 1998;153:801–813. doi: 10.1016/S0002-9440(10)65622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi E, Sanchez-Gomez MV, Torre I, Domercq M, Perez-Samartin A, Perez-Cerda F, Matute C. Activation of Kainate Receptors Sensitizes Oligodendrocytes to Complement Attack. J. Neurosci. 2006;26:3220–3228. doi: 10.1523/JNEUROSCI.3780-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews T, Zhang P, Bhat NR. TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–583. doi: 10.1002/(SICI)1097-4547(19981201)54:5<574::AID-JNR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Antel JP, McCrea E, Ladiwala U, Qin YF, Becher B. Non-MHC-restricted cell-mediated lysis of human oligodendrocytes in vitro: relation with CD56 expression. J Immunol. 1998;160:1606–1611. [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Parratt JD, Cho ES, Prineas JW. Immunoglobulins and complement in postmortem multiple sclerosis tissue. Ann Neurol. 2009;65:32–46. doi: 10.1002/ana.21524. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blink SE, Miller SD. The contribution of gammadelta T cells to the pathogenesis of EAE and MS. Curr Mol Med. 2009;9:15–22. doi: 10.2174/156652409787314516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti B, Pohl J, Gao YL, Raine CS. Cell death during autoimmune demyelination: effector but not target cells are eliminated by apoptosis. J Immunol. 1997;159:5733–5741. [PubMed] [Google Scholar]
- Bonetti B, Raine CS. Multiple sclerosis: oligodendrocytes display cell death-related molecules in situ but do not undergo apoptosis. Ann Neurol. 1997;42:74–84. doi: 10.1002/ana.410420113. [DOI] [PubMed] [Google Scholar]
- Breij ECW, BBP, Veerhuis R, Van den Berg C, Vloet R, Yan R, Dijkstra CD, Van der Valk P, Bö L. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann. Neurol. 2008;63:16–25. doi: 10.1002/ana.21311. [DOI] [PubMed] [Google Scholar]
- Brink BP, Veerhuis R, Breij EC, van der Valk P, Dijkstra CD, Bo L. The pathology of multiple sclerosis is location-dependent: no significant complement activation is detected in purely cortical lesions. J Neuropathol Exp Neurol. 2005;64:147–155. doi: 10.1093/jnen/64.2.147. [DOI] [PubMed] [Google Scholar]
- Cannella B, Gaupp S, Omari KM, Raine CS. Multiple sclerosis: Death receptor expression and oligodendrocyte apoptosis in established lesions. J. Neuroimmunol. 2007;188:128–137. doi: 10.1016/j.jneuroim.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Chavez-Galan L, Arenas-DelAngel MC, Zenteno E, Chavez R, Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol. 2009;6:15–25. doi: 10.1038/cmi.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Benveniste EN. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Brain Res Rev. 2004;44:65–81. doi: 10.1016/j.brainresrev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Comabella M, Martin R. Genomics in multiple sclerosis--current state and future directions. J Neuroimmunol. 2007;187:1–8. doi: 10.1016/j.jneuroim.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Compston DA, Morgan BP, Campbell AK, Wilkins P, Cole G, Thomas ND, Jasani B. Immunocytochemical localization of the terminal complement complex in multiple sclerosis. Neuropathol Appl Neurobiol. 1989;15:307–316. doi: 10.1111/j.1365-2990.1989.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Cudrici C, Ito T, Zafranskaia E, Weerth S, Rus V, Chen H, Niculescu F, Soloviova K, Tegla C, Gherman A, Raine CS, Shin ML, Rus H. Complement C5 regulates the expression of insulin-like growth factor binding proteins in chronic experimental allergic encephalomyelitis. J Neuroimmunol. 2008;203:94–103. doi: 10.1016/j.jneuroim.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudrici C, Niculescu F, Jensen T, Zafranskaia E, Fosbrink M, Rus V, Shin ML, Rus H. C5b-9 Terminal Complex Protects Oligodendrocytes from Apoptotic Cell Death by Inhibiting Caspase-8 Processing and Up-Regulating FLIP. J Immunol. 2006;176:3173–3180. doi: 10.4049/jimmunol.176.5.3173. [DOI] [PubMed] [Google Scholar]
- Cudrici C, Summers D, Jansen T, Fosbrink M, Rus H. C5b-9 protects oligodendrocytes apoptosis by regulating BH-3-only proapoptotic proteins. FASEB J. 2005;19:A324. [Google Scholar]
- D’Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP. Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med. 1996;184:2361–2370. doi: 10.1084/jem.184.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling P, Shang G, Raval S, Menonna J, Cook S, Husar W. Involvement of the CD59 (APO-1/Fas) receptor ligand system in multiple sclerosis brain. J Exp Med. 1996;184:1315–1318. doi: 10.1084/jem.184.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling P, Husar W, Menonna J, Donnenfeld H, Cook S, Sidhu M. Cell death and birth in multiple sclerosis brain. J Neurol Sci. 1997;149:1–11. doi: 10.1016/s0022-510x(97)05213-1. [DOI] [PubMed] [Google Scholar]
- Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, Degenhardt K, White E, Cook SJ. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. Embo J. 2007;26:2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MM, Atkinson JP. Complement system. In: Frank MM AK, Atkinson JP, Cantor H, editors. Samter’s Immunologic Diseases. Boston: Lippincott Williams & Wilkins; 2001. pp. 281–298. [Google Scholar]
- Freedman MS, Ruijs TC, Selin LK, Antel JP. Peripheral blood gamma-delta T cells lyse fresh human brain-derived oligodendrocytes. Ann Neurol. 1991;30:794–800. doi: 10.1002/ana.410300608. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple Sclerosis -- The Plaque and Its Pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Furlan R, Martino G, Galbiati F, Poliani PL, Smiroldo S, Bergami A, Desina G, Comi G, Flavell R, Su MS, Adorini L. Caspase-1 regulates the inflammatory process leading to autoimmune demyelination. J Immunol. 1999;163:2403–2409. [PubMed] [Google Scholar]
- Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJA, Petit PX, Vaz FM, Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J.Biol. Chem. 2008;Vol 183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gveric D, Cuzner ML, Newcombe J. Insulin-like growth factors and binding proteins in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1999;25:215–225. doi: 10.1046/j.1365-2990.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- Harrison DC, Roberts* J, Campbell CA, Crook B, Davis R, Deen K, Meakin J, Michalovich D, Price J, Stammers M, Maycox PR. TR3 death receptor expression in the normal and ischaemic brain. Neuroscience. 2000;96:147–160. doi: 10.1016/s0306-4522(99)00502-3. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- Hestvik AL, Skorstad G, Vartdal F, Holmoy T. Idiotope-specific CD4(+) T cells induce apoptosis of human oligodendrocytes. J Autoimmun. 2009;32:125–132. doi: 10.1016/j.jaut.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Hisahara S, Araki T, Sugiyama F, Yagami K, Suzuki M, Abe K, Yamamura K, Miyazaki J, Momoi T, Saruta T, Bernard CC, Okano H, Miura M. Targeted expression of baculovirus p35 caspase inhibitor in oligodendrocytes protects mice against autoimmune-mediated demyelination. Embo J. 2000;19:341–348. doi: 10.1093/emboj/19.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisahara S, Shoji S, Okano H, Miura M. ICE/CED-3 family executes oligodendrocyte apoptosis by tumor necrosis factor. J Neurochem. 1997;69:10–20. doi: 10.1046/j.1471-4159.1997.69010010.x. [DOI] [PubMed] [Google Scholar]
- Hisahara S, Yuan J, Momoi T, Okano H, Miura M. Caspase-11 mediates oligodendrocyte cell death and pathogenesis of autoimmune-mediated demyelination. J Exp Med. 2001;193:111–122. doi: 10.1084/jem.193.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley JE, Gveric D, Newcombe J, Cuzner ML, Gutowski NJ. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathol Appl Neurobiol. 2003;29:434–444. doi: 10.1046/j.1365-2990.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- Hosack DA, Dennis G, Jr, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobas DA, Iacobas S, Werner P, Scemes E, Spray DC. Alteration of transcriptomic networks in adoptive-transfer experimental autoimmune encephalomyelitis. Front Integr Neurosci. 2007;1:10. doi: 10.3389/neuro.07.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C, Antel J, Bruck W, Kuhlmann T. Contrasting potential of nitric oxide and peroxynitrite to mediate oligodendrocyte injury in multiple sclerosis. Glia. 2007;55:926–934. doi: 10.1002/glia.20514. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K. Oxidative stress kills human primary oligodendrocytes via neutral sphingomyelinase: implications for multiple sclerosis. J Neuroimmune Pharmacol. 2007;2:184–193. doi: 10.1007/s11481-007-9066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, Brosnan CF. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, Biddison WE, Antel JP. MHC class I-restricted lysis of human oligodendrocytes by myelin basic protein peptide-specific CD8 T lymphocytes. J Immunol. 1998;160:3056–3059. [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Andrzejak S, Selmaj K. TRAIL-induced death of human adult oligodendrocytes is mediated by JNK pathway. Glia. 2006;53:158–166. doi: 10.1002/glia.20249. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Tybor K, Kilianek L, Raine CS, Selmaj K. Tumour necrosis factor-induced death of adult human oligodendrocytes is mediated by apoptosis inducing factor. Brain. 2005;128:2675–2688. doi: 10.1093/brain/awh627. [DOI] [PubMed] [Google Scholar]
- Jurewicz A, Matysiak M, Tybor K, Selmaj K. TNF-induced death of adult human oligodendrocytes is mediated by c-jun NH2-terminal kinase-3. Brain. 2003;126:1358–1370. doi: 10.1093/brain/awg146. [DOI] [PubMed] [Google Scholar]
- Khurana R, Zhuang Z, Bhardwaj S, Murakami M, De Muinck E, Yla-Herttuala S, Ferrara N, Martin JF, Zachary I, Simons M. Angiogenesis-Dependent and Independent Phases of Intimal Hyperplasia. Circulation. 2004;110:2436–2443. doi: 10.1161/01.CIR.0000145138.25577.F1. [DOI] [PubMed] [Google Scholar]
- Koski CL, Ramm LE, Hammer CH, Mayer MM, Shin ML. Cytolysis of nucleated cells by complement: cell death displays multi-hit characteristics. Proc Natl Acad Sci U S A. 1983;80:3816–3820. doi: 10.1073/pnas.80.12.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Brück W, Lucchinetti C. The Immunopathology of Multiple Sclerosis: An Overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;115:2665–2672. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Maeda Y, Ming X, Cook S, Chapin J, Husar W, Dowling P. Apoptotic death following Fas activation in human oligodendrocyte hybrid cultures. J Neurosci Res. 2002;69:189–196. doi: 10.1002/jnr.10285. [DOI] [PubMed] [Google Scholar]
- Lin W, Harding HP, Ron D, Popko B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-{gamma} J. Cell. Biol. 2005;169:603–612. doi: 10.1083/jcb.200502086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. Interferon- {gamma} inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- Liu WT, Vanguri P, Shin ML. Studies on demyelination in vitro: the requirement of membrane attack components of the complement system. J Immunol. 1983;131:778–782. [PubMed] [Google Scholar]
- Louis JC, Magal E, Takayama S, Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993;259:689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, Trebst C, Weinshenker B, Wingerchuk D, Parisi JE, Lassmann H. A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain. 2002;125:1450–1461. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Sanchez-Gomez MV, Martinez-Millan L, Miledi R. Glutamate receptor-mediated toxicity in optic nerve oligodendrocytes. Proc Natl Acad Sci U S A. 1997;94:8830–8835. doi: 10.1073/pnas.94.16.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matysiak M, Jurewicz A, Jaskolski D, Selmaj K. TRAIL induces death of human oligodendrocytes isolated from adult brain. 2002;Vol 125:2469–2480. doi: 10.1093/brain/awf254. [DOI] [PubMed] [Google Scholar]
- Merrill JE, Ignarro LJ, Sherman MP, Melinek J, Lane TE. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993;151:2132–2141. [PubMed] [Google Scholar]
- Ming X, Li W, Maeda Y, Blumberg B, Raval S, Cook SD, Dowling PC. Caspase-1 expression in multiple sclerosis plaques and cultured glial cells. J Neurol Sci. 2002;197:9–18. doi: 10.1016/s0022-510x(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Mohan N, Edwards ET, Cupps TR, Oliverio PJ, Sandberg G, Crayton H, Richert JR, Siegel JN. Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheum. 2001;44:2862–2869. doi: 10.1002/1529-0131(200112)44:12<2862::aid-art474>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Mollnes TE, Vandvik B, Lea T, Vartdal F. Intrathecal complement activation in neurological diseases evaluated by analysis of the terminal complement complex. J Neurol Sci. 1987;78:17–28. doi: 10.1016/0022-510x(87)90074-8. [DOI] [PubMed] [Google Scholar]
- Morse RH, Seguin R, McCrea EL, Antel JP. NK cell-mediated lysis of autologous human oligodendrocytes. J Neuroimmunol. 2001;116:107–115. doi: 10.1016/s0165-5728(01)00289-2. [DOI] [PubMed] [Google Scholar]
- Niculescu F, Rus H. Mechanisms of signal transduction activated by sublytic assembly of terminal complement complexes on nucleated cells. Immunol Res. 2001;24:191–199. doi: 10.1385/ir:24:2:191. [DOI] [PubMed] [Google Scholar]
- Niculescu T, Weerth S, Niculescu F, Cudrici C, Rus V, Raine CS, Shin ML, Rus H. Effects of Complement C5 on Apoptosis in Experimental Autoimmune Encephalomyelitis. J Immunol. 2004;172:5702–5706. doi: 10.4049/jimmunol.172.9.5702. [DOI] [PubMed] [Google Scholar]
- Ousman SS, Tomooka BH, van Noort JM, Wawrousek EF, O/’Conner K, Hafler DA, Sobel RA, Robinson WH, Steinman L. Protective and therapeutic role for [alpha]B-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, Lassmann H. Patterns of oligodendroglia pathology in multiple sclerosis. Brain. 1994;117(Pt 6):1311–1322. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Pouly S, Becher B, Blain M, Antel JP. Interferon-gamma modulates human oligodendrocyte susceptibility to Fas-mediated apoptosis. J Neuropathol Exp Neurol. 2000;59:280–286. doi: 10.1093/jnen/59.4.280. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL, Hoffman B, Morgan BP. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- Raine C. Multiple sclerosis: Classification revisited reveals homogeneity and recapitulation. Ann Neurol. 2008;63:1–3. doi: 10.1002/ana.21314. [DOI] [PubMed] [Google Scholar]
- Reiman R, Gerard C, Campbell IL, Barnum SR. Disruption of the C5a receptor gene fails to protect against experimental allergic encephalomyelitis. Eur J Immunol. 2002;32:1157–1163. doi: 10.1002/1521-4141(200204)32:4<1157::AID-IMMU1157>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Robbins DS, Shirazi Y, Drysdale BE, Lieberman A, Shin HS, Shin ML. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987;139:2593–2597. [PubMed] [Google Scholar]
- Ruijs TC, Louste K, Brown EA, Antel JP. Lysis of human glial cells by major histocompatibility complex-unrestricted CD4+ cytotoxic lymphocytes. J Neuroimmunol. 1993;42:105–111. doi: 10.1016/0165-5728(93)90217-m. [DOI] [PubMed] [Google Scholar]
- Rus H, Cudrici C, David S, Niculescu F. The complement system in central nervous system diseases. Autoimmunity. 2006;39:395–402. doi: 10.1080/08916930600739605. [DOI] [PubMed] [Google Scholar]
- Rus HG, Niculescu F, Shin ML. Sublytic complement attack induces cell cycle in oligodendrocytes. J Immunol. 1996;156:4892–4900. [PubMed] [Google Scholar]
- Saikali P, Antel JP, Newcombe J, Chen Z, Freedman M, Blain M, Cayrol R, Prat A, Hall JA, Arbour N. NKG2D–mediated cytotoxicity toward oligodendrocytes suggests a mechanism for tissue injury in multiple sclerosis. J Neurosci. 2007;27:1220–1228. doi: 10.1523/JNEUROSCI.4402-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gomez MV, Alberdi E, Ibarretxe G, Torre I, Matute C. Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainate receptors. J Neurosci. 2003;23:9519–9528. doi: 10.1523/JNEUROSCI.23-29-09519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Alexander EL, Koski CL, Shin ML, Sano Y, Frank MM, Joiner KA. Terminal complement complexes (SC5b-9) in cerebrospinal fluid in autoimmune nervous system diseases. Ann N Y Acad Sci. 1988;540:387–388. doi: 10.1111/j.1749-6632.1988.tb27109.x. [DOI] [PubMed] [Google Scholar]
- Saxena A, Bauer J, Scheikl T, Zappulla J, Audebert M, Desbois S, Waisman A, Lassmann H, Liblau RS, Mars LT. Cutting Edge: Multiple Sclerosis-Like Lesions Induced by Effector CD8 T Cells Recognizing a Sequestered Antigen on Oligodendrocytes. J. Immunol. 2008;181:1617–1621. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- Scolding NJ, Jones J, Compston DA, Morgan BP. Oligodendrocyte susceptibility to injury by T-cell perforin. Immunology. 1990;70:6–10. [PMC free article] [PubMed] [Google Scholar]
- Scolding NJ, Morgan BP, Houston WA, Linington C, Campbell AK, Compston DA. Vesicular removal by oligodendrocytes of membrane attack complexes formed by activated complement. Nature. 1989;339:620–622. doi: 10.1038/339620a0. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Tissue culture studies of demyelinating disease: a critical review. Ann Neurol. 1977;2:345–355. doi: 10.1002/ana.410020417. [DOI] [PubMed] [Google Scholar]
- Sellebjerg F, Jaliashvili I, Christiansen M, Garred P. Intrathecal activation of the complement system and disability in multiple sclerosis. J Neurol Sci. 1998;157:168–174. doi: 10.1016/s0022-510x(98)00086-0. [DOI] [PubMed] [Google Scholar]
- Selmaj K, Brosnan CF, Raine CS. Colocalization of lymphocytes bearing gamma delta T-cell receptor and heat shock protein hsp65+ oligodendrocytes in multiple sclerosis. Proc Natl Acad Sci U S A. 1991a;88:6452–6456. doi: 10.1073/pnas.88.15.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K, Raine CS, Farooq M, Norton WT, Brosnan CF. Cytokine cytotoxicity against oligodendrocytes Apoptosis induced by lymphotoxin. J Immunol. 1991b;147:1522–1529. [PubMed] [Google Scholar]
- Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Sharma K, Wang RX, Zhang LY, Yin DL, Luo XY, Solomon JC, Jiang RF, Markos K, Davidson W, Scott DW, Shi YF. Death the Fas way: regulation and pathophysiology of CD95 and its ligand. Pharmacol Ther. 2000;88:333–347. doi: 10.1016/s0163-7258(00)00096-6. [DOI] [PubMed] [Google Scholar]
- Shin ML, Rus HG, Niculescu FI. Membranes Attack by Complement: Assembly and Biology of the Terminal Complement Complexes. In: Lee A, editor. Biomembranes. Vol 4. Greenwich, CT: JAI Press; 1996. pp. 123–149. [Google Scholar]
- Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat Med. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- Soane L, Cho HJ, Niculescu F, Rus H, Shin ML. C5b-9 terminal complement complex protects oligodendrocytes from death by regulating Bad through phosphatidylinositol 3-kinase/Akt pathway. J Immunol. 2001;167:2305–2311. doi: 10.4049/jimmunol.167.4.2305. [DOI] [PubMed] [Google Scholar]
- Soane L, Rus H, Niculescu F, Shin ML. Inhibition of oligodendrocyte apoptosis by sublytic C5b-9 is associated with enhanced synthesis of bcl-2 and mediated by inhibition of caspase-3 activation. J Immunol. 1999;163:6132–6138. [PubMed] [Google Scholar]
- Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43:465–471. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- Traugott U, Raine CS. Further lymphocyte characterization in the central nervous system in multiple sclerosis. Ann N Y Acad Sci. 1984;436:163–180. doi: 10.1111/j.1749-6632.1984.tb14788.x. [DOI] [PubMed] [Google Scholar]
- Vartanian T, Li Y, Zhao M, Stefansson K. Interferon-gamma-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med. 1995;1:732–743. [PMC free article] [PubMed] [Google Scholar]
- Walport MJ. Complement First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Weerth SH, Rus H, Shin ML, Raine CS. Complement C5 in experimental autoimmune encephalomyelitis (EAE) facilitates remyelination and prevents gliosis. Am J Pathol. 2003;163:1069–1080. doi: 10.1016/S0002-9440(10)63466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- Wilczak N, Chesik D, Hoekstra D, De Keyser J. IGF binding protein alterations on periplaque oligodendrocytes in multiple sclerosis: Implications for remyelination. Neurochemistry International. 2008;52:1431–1435. doi: 10.1016/j.neuint.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Current Opinion in Cell Biology. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolswijk G. Oligodendrocyte survival, loss and birth in lesions of chronic-stage multiple sclerosis. Brain. 2000;123(Pt 1):105–115. doi: 10.1093/brain/123.1.105. [DOI] [PubMed] [Google Scholar]
- Wosik K, Antel J, Kuhlmann T, Brück W, Massie B, Nalbantoglu J. Oligodendrocyte injury in multiple sclerosis: a role for p53. J. Neurochem. 2003;85:635–644. doi: 10.1046/j.1471-4159.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- Wosik K, Ruffini F, Almazan G, Olivier A, Nalbantoglu J, Antel JP. Resistance of human adult oligodendrocytes to AMPA/kainate receptor-mediated glutamate injury. Brain. 2004;127:2636–2648. doi: 10.1093/brain/awh302. [DOI] [PubMed] [Google Scholar]
- Wren DR, Noble M. Oligodendrocytes and oligodendrocyte/type-2 astrocyte progenitor cells of adult rats are specifically susceptible to the lytic effects of complement in absence of antibody. Proc Natl Acad Sci U S A. 1989;86:9025–9029. doi: 10.1073/pnas.86.22.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci U S A. 1992;89:4588–4592. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeine R, Cammer W, Barbarese E, Liu CC, Raine CS. Structural dynamics of oligodendrocyte lysis by perforin in culture: relevance to multiple sclerosis. J Neurosci Res. 2001;64:380–391. doi: 10.1002/jnr.1089. [DOI] [PubMed] [Google Scholar]
- Zeine R, Pon R, Ladiwala U, Antel JP, Filion LG, Freedman MS. Mechanism of gammadelta T cell-induced human oligodendrocyte cytotoxicity: relevance to multiple sclerosis. J Neuroimmunol. 1998;87:49–61. doi: 10.1016/s0165-5728(98)00047-2. [DOI] [PubMed] [Google Scholar]