Abstract
In the past decade, studies of the human tumor suppressor LKB1 have uncovered a novel signaling pathway that links cell metabolism to growth control and cell polarity. LKB1 encodes a serine/threonine kinase that directly phosphorylates and activates AMPK, a central metabolic sensor. AMPK regulates lipid, cholesterol and glucose metabolism in specialized metabolic tissues such as liver, muscle, and adipose, a function that has made it a key therapeutic target in patients with diabetes. The connection of AMPK with several tumor suppressors suggests that therapeutic manipulation of this pathway with established diabetes drugs warrants further investigation in patients with cancer.
Introduction
A fundamental requirement of all cells is that they couple the availability of nutrients to signals emanating from growth factors to drive proliferation only when nutrients are in sufficient abundance to guarantee successful cell division. Although a connection between cellular metabolism and tumorigenesis was first proposed 100 years ago by Otto Warburg, the molecular mechanisms interconnecting the signaling pathways controlling metabolism and cell growth have only begun to be decoded in the past decade, making this an active area of investigation in cancer research. One of the newly uncovered links directly connecting cell metabolism and cancer came from the discovery that that the serine/threonine kinase LKB1 (Liver Kinase B1; also known as Serine/Threonine Kinase 11 - STK11), a known tumor suppressor, was the key upstream activator of the AMP-activated protein kinase (AMPK)1-4. AMPK is a central metabolic switch found in all eukaryotes that governs glucose and lipid metabolism in response to alterations in nutrients and intracellular energy levels.
LKB1 was identified originally as the tumor suppressor gene on human chromosome 19p13 responsible for the inherited cancer disorder Peutz-Jeghers Syndrome (PJS)5. Importantly, LKB1 is also one of the most commonly mutated genes in sporadic human lung cancer, particularly in multiple subtypes of non-small cell lung carcinoma (NSCLC)6, where at least 15-35% of cases have this lesion7. LKB1 was also recently found to be somatically mutated in 20% of cervical carcinomas8, making it the first known recurrant genetic alteration in this cancer which is caused by the human papilloma virus. Together, LKB1 and AMPK control cell growth in response to environmental nutrient changes, which, as we discuss in this Review, potentially identifies new targets and drugs for cancer therapy owing to the fact that the activity of AMPK can be targeted with drugs already in use for diabetes treatment. In addition to controlling cell growth and metabolism, both LKB1 and AMPK play conserved roles in cell polarity, disruption of which is also implicated in carcinogenesis. As LKB1 is one of the few serine/threonine kinases known to be inactivated through mutation during carcinogenesis, a critical early question lay in the identification of its substrates.
LKB1 is a master kinase
The search for substrates of LKB1 that mediate its tumor suppressor function led to the identification of AMPK as a direct substrate1-4. AMPK is a heterotrimer composed of a catalytic (AMPKα subunit and two regulatory (AMKPβ and AMPKγ) subunits (Fig. 1). AMPK is activated when intracellular ATP declines and intracellular AMP increases, such as during nutrient deprivation or hypoxia. Biochemical and genetic analyses in worms, flies and mice have revealed that LKB1 is the major kinase phosphorylating the AMPKα activation loop under conditions of energy stress9.
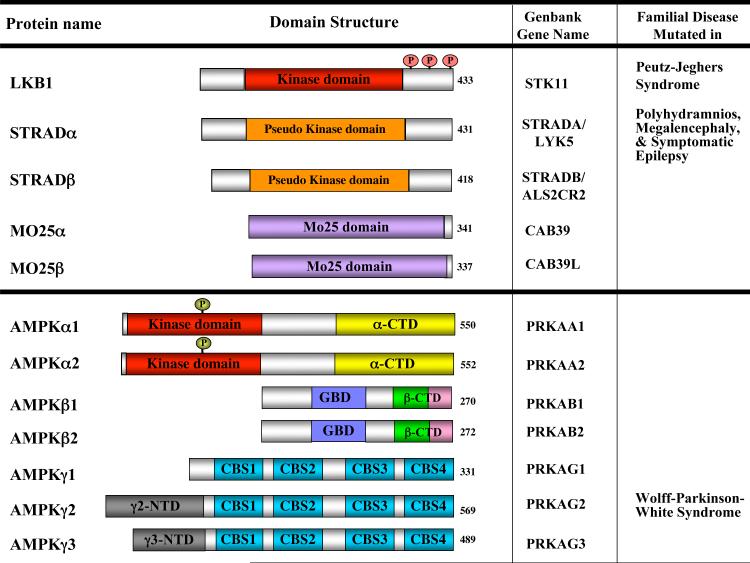
Figure 1. Schematic of the proteins in the LKB1 and AMPK kinase complexes.
Both LKB1 and AMPK exist in heterotrimeric protein complexes. Inactivating mutations in LKB1 underlie the inherited cancer disorder Peutz-Jeghers Syndrome. Most mutations affect the function of the kinase domain, indicating that the tumor suppressor function of LKB1 requires its kinase activity. In addition to deletions or frameshifts, several missense mutations have been found and most cluster to the kinase domain resulting in loss of kinase activity. A handful of mutations lie outside the kinase domain and some of these have been shown to result in decreased kinase activity due to disruption of protein-protein interactions between LKB1 and its regulatory subunits STRAD (STE20-related adapter protein) and Mo25, which appear to be necessary for its kinase activity186. Together, the genetic evidence indicate that the tumor suppressor function of LKB1 requires its kinase activity. While there is a single LKB1 gene in mammals, two STRAD and two Mo25 family members exist and mutations in STRADα underlie the development of an inherited epileptic disorder187. There are two known splice forms of LKB1 differing in the very C-terminal amino acids188, 189, and evidence suggests STRAD proteins undergo extensive alternative splicing as well190. Like LKB1, AMPK is composed of a catalytic subunit (α) and two regulatory subunits. The beta subunits contain a conserved glycogen binding domain which also modulates AMPK activity191. The gamma subunits contain a series of tandem repeats of crystathionine-β-synthase (CBS) domains to which molecules of AMP directly bind as revealed in recent X-ray crystallography studies192. Binding of AMP to AMPKγ is thought to promote phosphorylation of the critical activation loop threonine (Thr172) in AMPKα, which is required for AMPK activity, largely through suppression of phosphatase activity towards Thr172193. Mutation of some of these AMP-binding pockets in the AMPKγ2 gene lead to hypertrophic cardiomyopathy that is associated with Wolff-Parkinson-White syndrome194.
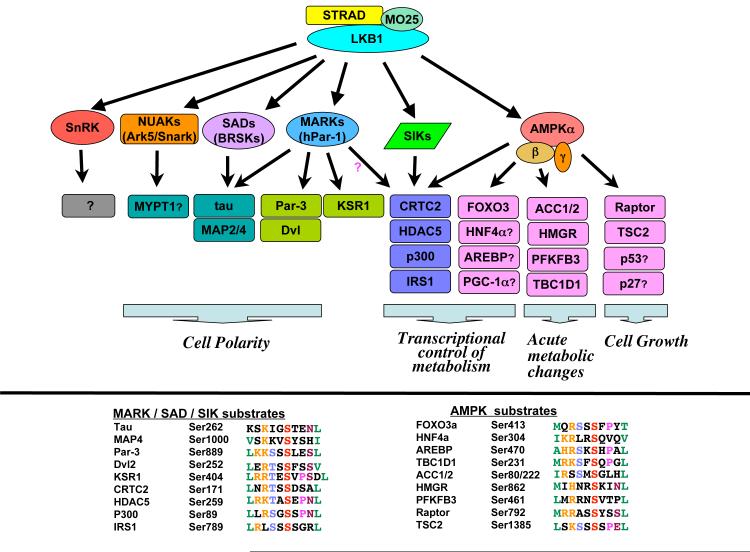
LKB1 also phosphorylates and activates 12 kinases closely related to AMPK10, 11 (Fig. 2). Of the 14 kinases, most current data suggest that only AMPKα1 and AMPKα2 are activated under low ATP conditions, probably because only they interact with AMPKγ12. Interestingly, four of these 14 kinases are mammalian members of the MAP/microtubule affinity regulating kinase (MARK)/Par-1 family, which are mammalian homologs of the C. elegans par-1 kinase that is required for early embryonic partitioning and polarity. Par-4 encodes the C. elegans ortholog of LKB113. The ability of LKB1 (or its orthologs) to act as master upstream kinases that activate AMPK, MARK/par-1, and several additional AMPK-related kinases appears to be widely conserved across eukaryotes.
Figure 2. LKB1-dependent signaling.
LKB1 in complex with its two regulatory subunits STRAD and Mo25 directly phosphorylates and activates a family of 14 AMPK-related kinases. These kinases in turn directly phosphorylate a number of downstream substrates to mediate effects on cell polarity, metabolism, and growth control. All well-established substrates of AMPK and its related family members are shown, and those for which further in vivo data is needed are shown with a question mark. It is important to note that many of the known substrates are expressed in a tissue-specific manner and may not explain ubiquitous effects of LKB1 and its downstream kinases in all cell types. Bottom: The sequences flanking the best-characterized phosphorylation site in each substrate with those residues selected for from in vitro peptide library and alanaine scanning peptide mutagenesis studies highlighted. Importantly, to date there is no substantive mutational data from human tumors to specifically support any of the downstream kinases, including the two AMPK catalytic genes, as being a particularly critical target of LKB1 in tumor suppression. One confounding issue with the lack of mutations found in these downstream kinases is that there is a great deal of redundancy among them, suggesting that loss of any one of them may be compensated for by other family members, unlike the case for LKB1 for which no other specific kinase has been shown to compensate in vivo.
From tissue-specific knockouts of LKB1 in mice (Table 1), it appears that LKB1 dictates most of the AMPK activation in all tissues examined thus far, with the exception of some hypothalamic neurons14, T-cells15, and endothelial cells16 in which CAMKK2 appears to play a key role, although only in response to changes in the concentration of calcium17-19. Thus LKB1 uniquely mediates the prolonged and adaptive activation of AMPK following energy stress, which allows it to serve as a metabolic checkpoint.
Table 1. Genetically engineered mouse models of Lkb1 function in tumorigenesis.
| Tissue examined | Transgenic mouse model | Phenotype | Significance | Ref |
|---|---|---|---|---|
| Heterozygous throughout | Lkb1+/- | Benign GI hamartomas Multi-focal osteoblastomas, paralysis |
Genetic and histological phenocopy of PJS - evidence for unexpected role in bone? |
115-8 195 |
| Heterozygous throughout combined with p53 loss | Lkb1+/-, p53-/- Lkb1+/- or Lkb1+/-, p53+/- |
GI hamartomas greatly accelerated hepatocellular carcinomas in one strain | p53 loss cooperates Infectious agent or strain difference? |
197 196 |
| 10% function thoughout | Lkb1 hypomorph | No tumor phenotype | 10% LKB1 thoughout but still no tumors so unlikely polyps haploinsufficient-unless this hypomorph has compensation | 137 |
| 10% function thoughout & Pten heterozygous | Lkb1 hypomorph X Pten +/- | Lymphomagenesis greatly accelerated compared to Pten +/- | In presence of reduced Pten, 10% LKB1 not enough to prevent tumorigenesis | 137 |
| GI smooth muscle cells | SM22-Cre-Lkb1lox/+ or lox/los | Benign GI hamartomas | GI Polyps arising from smooth muscle - not epithelium? | 119 |
| Adult GI epithelium | Cyp2a1- Lkb1lox/lox | Altered differentiation of Paneth and goblet cells in adult GI | Altered differentiation? Is deletion in relevant cell population for polyps? | 198 |
| Lung epithelium | Lox-Stop-Lox-KrasG12D, Lkb1lox/lox delivered by inhalation of adeno-Cre | Non-small cell lung cancer: aggressive lung tumors of adeno-, squamous,& large cell origin; metastasis. | LKB1 highly synergizes with K-ras mt Appearance of Squamous tumors / metastasis in adenocarcinomas |
7 |
| Endometrial epithelium | Lkb1+/- Lkb1lox/loxintrauterine inject. of adeno-Cre |
Invasive endometrial adenocarcinoma | Endometrium highly sensitive to LKB1? | 121 |
| Prostate epithelium | P450CYP1A1-Cre-Lkb1lox/lox | Prostate hyperplasia & neoplasia | Sex-hormone regulated growth affected? | 199 |
| Skin Epithelium | Lkb1+/- with DMBA administered to skin K14-Cre-Lkb1lox/lox with or without DMBA administered to skin |
Squamous cell carcinoma of skin (and occasionally lung) | LKB1 highly synergizes with H-ras mutations induced by DMBA? | 124 |
| Pancreatic precursors | Lkb1+/- or Pdx1-Cre-Lkb1lox/lox | Benign pancreatic cystadenomas | Altered junctions, development | 200 |
A LKB1-AMPK-mTORC1 checkpoint
Prior to its identification as a substrate for LKB1, AMPK was known to regulate lipid, cholesterol and glucose metabolism in specialized metabolic tissues such as liver, muscle and adipose20. Work from several laboratories in the past 5 years has revealed that one of the major growth regulatory pathways controlled by LKB1-AMPK is the mammalian target-of-rapamycin (mTOR) pathway. mTOR is a central integrator of nutrient and growth factor inputs that controls cell growth in all eukaryotes and is deregulated in most human cancers21.
mTOR is found in two biochemically and functionally discrete signaling complexes22. mTOR complex 1 (mTORC1) includes raptor, which acts as a scaffold to recruit downstream substrates such as 4EBP1 and ribosomal S6 kinase (p70S6K1) that contribute to mTORC1-dependent regulation of protein translation23. mTORC1 controls the translation of a number of cell growth regulators, including cyclin D1, hypoxia inducible factor 1a (HIF-1α, and c-myc, which in turn promote processes including cell cycle progression, cell growth and angiogenesis, all of which can become deregulated during tumorigenesis21. mTORC1 is nutrient-sensitive and acutely inhibited by rapamycin, though recent studies reveal that rapamycin does not fully suppress mTORC1 activity in many cell types24-26. In contrast, mTORC2 contains the rictor subunit and is neither sensitive to nutrients nor acutely inhibited by rapamycin21.
Cancer genetics and Drosophila genetics led to the discovery of upstream components of mTORC1 including the tuberous sclerosis complex 2 (TSC2) tumor suppressor and its obligate partner TSC1 27. TSC2 inhibits mTORC1 indirectly via regulation of the small GTPase Rheb, such that loss of TSC1 or TSC2 leads to hyperactivation of mTORC128. When levels of ATP, glucose or oxygen are low, AMPK directly phosphorylates TSC2 on conserved serine sites29-32and primes serine residues close by for subsequent phosphorylation by GSK-333. Wnt signaling inhibits phosphorylation of TSC2 by GSK-3, making TSC2 activity a biochemical coincidence detector of the activation state of AMPK and GSK-3 that dictates the amount downstream mTORC1 signaling.
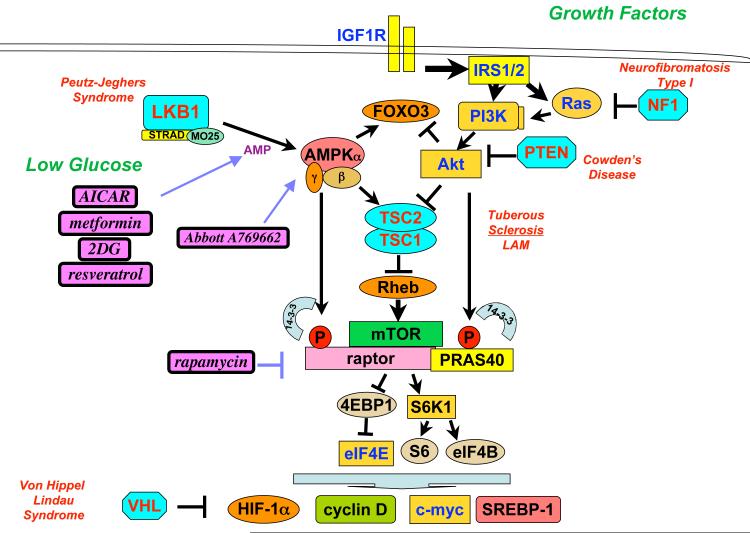
While TSC2 is clearly a central receiver of inputs that regulate mTORC1, cells lacking TSC2 still partially suppress mTORC1 following AMPK activation34, 35. In agreement with these data, raptor has been identified as a direct substrate of AMPK in vivo. Phosphorylation of two conserved serines in raptor by AMPK induced binding to 14-3-3 and resulted in suppression of mTORC1 kinase activity35. Phosphorylation of raptor was shown to be required for downregulation of mTOR and efficient G2/M cell cycle arrest following AMPK activation35. Taken together, the current data indicate that energy stress results in LKB1-dependent activation of AMPK, which directly phosphorylates both TSC2 and raptor to inhibit mTORC1 activity by a dual mechanism, although it remains possible that additional substrates of AMPK contribute to the regulation of mTOR (Fig. 3). Importantly, mTORC1 is currently the only signaling pathway downstream of LKB1 that has been shown to be deregulated in tumors arising in humans and mouse models of both Peutz-Jeghers syndrome31, 36 and NSCLC7, 37.
Figure 3. AMPK and PI3K signaling converge to antagonistically regulate a number of downstream effectors, including the mTORC1 complex.
A number of inherited hamartoma and cancer predispotion syndromes all share in common hyperactivation of mTORC1 or HIF-1α. Tumor suppressors inactivated in human cancer shown in light blue, oncogenes hyperactivated in human cancer shown in gold. Conditions that lower intracellular ATP levels (low glycolytic rates from low glucose or inhibitors like 2-deoxyglucose [2DG] or oxidative phosphorylation inhibitors like metformin and related biguanides) will lead to activation of AMPK in an LKB1-dependent manner. AICAR is a precursor of ZMP, which acts as an AMP-mimetic and is thought to directly bind the AMP-binding pockets of the AMPKγ subunit. A769662 is the only known small molecule that directly binds AMPK inducing its activity, though it is not currently known where the compound binds on the AMPK heterotrimer.
LKB1-AMPK control of other growth regulators
LKB1 has also been reported to regulate other key cancer-related pathways beyond mTORC1. Most notably, several connections have been made between LKB1, AMPK and the tumor suppressor p53. Before any direct substrates for LKB1 were identified, LKB1 reconstitution into LKB1-deficient tumor cells was reported to stimulate p53 activity and increase levels of Cdkn1a mRNA, which encodes the cyclin dependent kinase inhibitor p2138, 39. In addition, AMPK has been shown to modulate p53-dependent apoptosis40 and directly phosphorylate p53 on serine 1541, which is the established p53 site phosphorylated by the ATM, ATR and DNA-PK DNA-damage response kinases42. Several studies indicate that AMPK is also activated downstream of p5343 and this lead to the discovery of sestrin 1 and sestrin 2 — p53 target genes that inhibit mTOR signaling44. Overexpression of sestrin1 or sestrin 2 leads to increased AMPK activation and suppression of mTORC1 signaling, whereas mice that lack sestrin2 fail to downregulate mTORC1 following exposure to carcinogens. The molecular mechanism by which sestrins activate AMPK in this context remains to be fully elucidated. In addition to the sestrins, PRKAB1, which encodes the AMPKβ1 regulatory subunit, is a p53-responsive gene, suggesting another mechanism through which p53 can inhibit mTOR45.
Importantly, AMPK has been demonstrated to phosphorylate a conserved serine in FOXO3a, the transcriptional factor targeted by PI3K/Akt signaling which plays key roles in cell survival and metabolism46. Of note is that the best mapped AMPK site in FOXO3a matches the consensus for 14-3-3 binding, which is also the case for the best mapped AMPK site in TSC2 (Fig. 2). The parallel regulation of both FOXO and mTOR signaling by AMPK and Akt signaling suggests further study is warranted into the functional overlap between these central pathways controlling both cell growth and metabolism.
AMPK has also been reported to phosphorylate Thr198 of the cyclin dependent kinase inhibitor p2747, 48. However, Thr198 has also been reported to be phosphorylated by Rsk, Akt and Pim kinases, which promote cell growth. Why these pro-growth and anti-growth signals would both target the same phosphorylation site has yet to be established. Several additional AMPK substrates have been suggested to have a role in growth regulation49 50, however future studies with rigorously validated phospho-specific antibodies for each phosphorylation site and careful analysis of early time points following acute energy stress in wild-type or AMPK-deficient cells should help to assign which of these candidate targets are bona fide direct AMPK substrates in vivo.
LKB1 and metabolism of glucose and lipid
Although critical in the suppression of diabetes, the reprogramming of glucose and lipid metabolism by LKB1-dependent kinases is also likely to be important for the growth and tumor-suppressive effects of LKB1. AMPK acutely inhibits fatty acid and cholesterol synthesis through direct phosphorylation of the metabolic enzymes Acetyl-CoA carboxylase (ACC) and HMG-CoA reductase (HMGR)51. Thus activation of AMPK provides an endogenous mechanism to inhibit HMGR activity, akin to the pharmaceutical inhibition of HMGR by the statin family of compounds52. As ACC1 and HMGR are ubiquitously expressed, LKB1-deficient cells of all tissue types would be expected to exhibit enhanced rates of lipid and cholesterol synthesis. In line with recent RNAi studies showing that ACC1 and fatty acid synthase (FASN) are essential for survival in a number of cultured tumor cell lines53-55, chemical inhibitors of FASN and ACC have been shown to suppress the growth of prostate and lung cancer xenografts56, 57. Indeed, a variety of FASN inhibitors are being considered for clinical trails in cancer treatment58 and it remains plausible that suppression of lipogenesis is an important part of the tumor suppressor function of LKB1.
Beyond these lipogenic enzymes, AMPK has been suggested to acutely modulate glycolysis though phosphorylation of multiple isoforms of phosphofructo-2 kinase (PFK2)59, 60. The data are particularly compelling for the inducible-PFK2 (PFKFB3) isoform, whose expression is dramatically upregulated in some types of human cancer61. Indeed, genetic ablation of Pfkfb3 in mouse lung fibroblasts suppressed KRAS-dependent transformation62 and small molecule inhibitors of PFKFB3 block the growth of lung cancer xenografts63.
More broadly, LKB1-dependent kinases may also control cell growth and metabolism through phosphorylation of widely expressed transcriptional coactivators. The p300 histone acetyltransferase (HAT)64, several Class IIa histone deacetyltransferases (HDACs)65-67, and the CRTC (previously TORC)68-71 family of CREB coactivators have all been shown to be substrates of AMPK and related LKB1-dependent kinases (Fig. 2). Current data suggest that in response to distinct stimuli, subsets of LKB1-dependent kinases may target the same phosphorylation sites in these downstream effectors72. AMPK and its related kinases have been reported to phosphorylate Class II HDACs and CRTCs leading to their cytoplasmic sequestration and inactivation through 14-3-3 binding, similar to several other substrates of AMPK and its relatives. Though the best studied transcriptional targets of Class II HDACs and CRTCs are metabolic genes in muscle and liver respectively, these proteins may play wider roles in cell proliferation and tumorigenesis73 74. AMPK has recently been shown to enhance SIRT1 activity by increasing cellular NAD+ levels75, resulting in the regulation of many downstream SIRT1 targets including FOXO3 and PPAR gamma coactivator 1 (PGC1) (also known as PPARGC1A), both of which have also been proposed as direct substrates of AMPK46, 76. As SIRT1 itself is also implicated in tumorigenesis77, this connection between AMPK and SIRT1 may further illuminate how nutrients control cell growth.
AMPK also suppresses mTOR-dependent transcriptional regulators to inhibit cell growth and tumorigenesis. Two mTORC1 regulated transcription factors involved in cell growth are the sterol-regulatory element binding protein 1 (SREBP-1) and hypoxia-inducible factor 1a (HIF-1α. SREBP-1 is a sterol-sensing transcription factor that drives lipogenesis in many mammalian cell types. mTORC1 signaling is required for nuclear accumulation of SREBP-1 and the induction of SREBP-1 target genes78 and this can be inhibited by rapamycin or AMPK agonists78, 79. Consistent with this, mice bearing a liver-specific Lkb1 deletion had increased expression of SREBP-1 target genes, and hepatic lipid accumulation and steatosis71. Moreover, SREBP-1 seems to be critical for cell growth in both Drosophila and mammalian cells78 suggesting that it may be an important target of LKB1, AMPK and mTOR signaling. Additional studies are needed to examine whether SREBP-1 is upregulated in LKB1-deficient tumors and how important SREBP-1 is for tumor formation under these conditions.
HIF is a heterodimer composed of constitutive β (ARNT) subunits and α-subunits whose protein levels are stabilized through hypoxic inactivation of the von Hippel-Lindau (VHL) E3 ligase that targets HIF-α subunits for destruction80. In addition to being increased via hypoxia, HIF-1α protein levels are highly dependent on mTORC1 signaling. mTORC1 hyperactivation from mutations in oncogenes and tumor suppressors are sufficient to promote HIF-1α protein levels and expression of its downstream targets in mouse cancer models and cells in vitro81. Well-established HIF-1 transcriptional targets containing hypoxia-responsive elements (HREs) in their promoters include angiogenic factors such as VEGF and angiopoetin-2, a number of glycolytic enzymes, and multiple members of the GLUT family of glucose transporters82. In this fashion, HIF-1α activation in tumors may be responsible for the Warburg effect — the propensity of tumor cells to rely on glycolysis instead of oxidative phosphorylation83. Indeed, this regulation of glucose metabolism by HIF-1α contributes to tumorigenesis in multiple settings84, 85. Consistent with earlier studies in TSC-deficient fibroblasts86, we have recently shown that levels of HIF-1α and its targets GLUT1 and hexokinase are increased in LKB1- and AMPK-deficient fibroblasts in a rapamycin-reversible manner36. Similarly, the epithelium of gastrointestinal hamartomas from Peutz-Jeghers patients or Lkb1+/- mice (Table 1) also show increased expression of HIF-1α and HIF-1 target genes compared with the surrounding normal tissue, suggesting that Hif-1α may be a relevant target downstream of LKB1-deficiency in Peutz-Jeghers syndrome36. The increase in glucose uptake in tumours from patients with PJS could also be used to guide surgical resection of hamartomas in the GI tract. FDG-PET imaging studies on Lkb1+/- mice showed that their gastrointestinal hamartomas are specifically labeled in a rapamycin-sensitive manner. Given this, it will be interesting to examine whether the presence of LKB1 mutations dictates the level of FDG-PET signal in other tumor models, particularly in NSCLC and cervical cancer.
LKB1-AMPK and cell polarity
Par4, Par1 and Ampk Drosophila mutants have polarity defects during embryogenesis87-90 and oogenesis91. In mammalian cells, inducible activation of LKB1 is sufficient to promote full polarization of tumor cells, including apical and basolateral cell sorting, an actin cap and a full brush border, even in the absence of cell-cell contacts92. In cultured hippocampal neurons, overexpression of LKB1 induces multiple axons and RNAi depletion of LKB1 or its subunit STRAD block axonal differentiation93. Consistent with these findings, tissue-specific deletions in mice of LKB1 or brain-specific kinase 1 (BRSK1) or BRSK2 (orthologues of C.elegans SAD1 kinase and downstream targets of LKB1) result in loss of axonal specification during neuronal polarization in the developing mammalian cerebral cortex94. It is important to note that LKB1 does not appear to be required for polarization of all tissues, as several tissue-specific deletions of Lkb1 in the mouse do not show obvious disruptions of cellular polarity or tissue organization95. The requirement of LKB1 for establishment of polarity as opposed to maintenance of polarity is an additional consideration for the interpretation of these experiments. Cell polarity is known to be established through the action of a number of conserved antagonistic polarity protein complexes, and LKB1 and its downstream MARK/par-1 kinases contribute to this regulation (see Box 1).
Box 1 Polarity protein complexes.
Studies across a wide range of metazoans have revealed that molecular control of cell polarity is commonly established through the opposing function of a handful of polarity protein complexes that mutually exclude the others’ localization172. In addition to LKB1 and the Par-1/MARK kinases, other highly conserved polarity genes include Par-3 and Par-6, which form a quaternary complex with the small GTPase cdc42 and atypical PKC (aPKC) subfamily of kinases (referred to as the “Par” complex). The binding of the small GTPase cdc42 to the Par complex results in activation of aPKC kinase activity, which in turn directly phosphorylates the MARK family of kinases on a conserved C-terminal threonine, leading to their association with 14-3-3 and exclusion from the apical domain of the cell178-180 (see Fig. 4). Reinforcing the mutual exclusion of the polarity complexes, the MARK kinases have been reported to directly phosphorylate and cause relocalization of the Discs Large (DLG) polarity proteins181 and the Par-3 scaffolding protein182. Whether this hypothesized mutual exclusion of the MARKs and Par complex can explain observed effects of LKB1 loss on GSK-3 and cdc42 activity in different settings183, 184 including NSCLC cell lines185 remains to be determined.
LKB1 might also influence cell polarity and migration through a number of substrates of its downstream kinases involved in cytoskeletal remodelling. For example, MARK-dependent phosphorylation of microtubule associated proteins (MAPs) is thought to play a role in cell migration96 and may be relevant to the increased metastatic nature of NSCLC lung tumors specifically lacking LKB17. MARKs phosphorylate serine residues in the microtubule binding domain of MAPs, resulting in increased dynamic instability of cellular microtubules97.
Another set of conserved MARK substrates are the Dishevelled (Dvl) proteins, which are key mediators of the Wnt signaling pathway98. Although MARK phosphorylation of Dvl regulates the membrane localization of Dvl, this is not required for canonical Wnt signaling in Xenopus99, and the MARK phosphorylation sites in Dvl do not seem to be required for the MARKs to affect Wnt signaling99, 100. This suggests that there must be additional unidentified MARK substrates involved in Wnt signaling. Interestingly, canonical and non-canonical Wnts were recently shown to induce cytoskeletal remodeling through Dvl binding to the Par complex, promoting atypical PKC mediated inactivation of the MARKs101-103. Thus Wnt-dependent signals, which promote tumorigenesis in several tissues including colon and breast cancer, may modulate LKB1-dependent signaling through multiple mechanisms, and vice-versa (see Fig. 4).
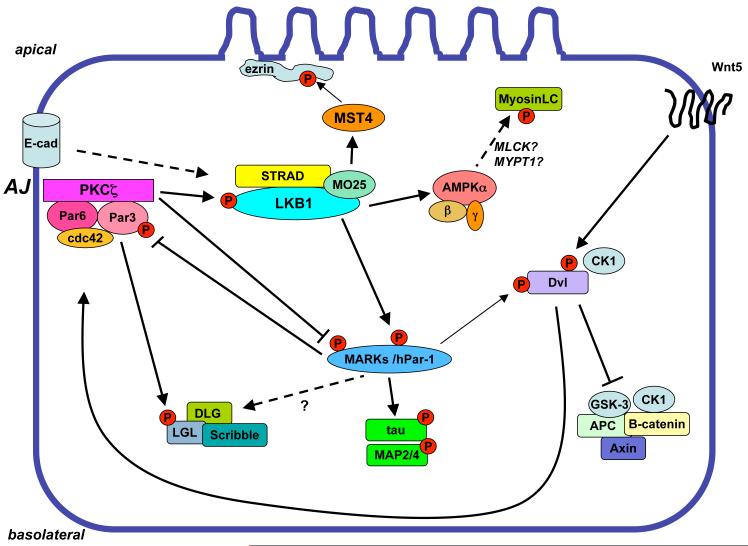
Figure 4. Control of cell polarity by LKB1-dependent signaling.
The Par complex, composed of an atypical PKC family member, the Par-3 scaffold, the cdc42-binding Par-6, and cdc42 phosphorylates a number of downstream polarity proteins, including LKB1, the MARK family, and Lethal giant larvae (LGL). LKB1 also requires a signal from E-cadherin to be recruited and competent to phosphorylate AMPK at the adherens junction. LKB1-dependent AMPK activation is known to modulate the phosphorylation state of myosin light chain (MLC) in Drosophila mutants, which may be through indirect regulation of the kinase (MLCK) and phosphatase (MYPT1) for MLC. LKB1-dependent MARK kinases in turn phosphorylate the Par-3 scaffold, hence leading to the mutual exclusion of the Par complex and the MARK kinases within the cell. MARKs also are well-established to phosphorylate MAPs including tau, MAP2, and MAP4, and have been reported to phosphorylate DLG and Dishevelled (DVL) proteins in some contexts.
AMPK has also recently been reported to modulate cell polarity in Drosophila and mammalian cells. AMPK activation in MDCK cells led to an increase in tight junctions104, 105 and treatment of a colon cancer cell line with the glycolytic inhibitor 2DG led to an AMPK-dependent increase in the number of polarized cells89. In addition, LKB1 and its regulatory subunit STRAD localize to adherens junctions in MDCK cells in an E-cadherin-dependent manner106. Loss of E-cadherin leads to specific loss of AMPK activation at adherens junctions. Studies of AMPK mutants in Drosophila showed mislocation of the Par complex as well as other polarity markers, including loss of myosin light chain (MLC) phosphorylation89. It was suggested in this paper that MLC may be a downstream substrate of AMPK; this seems unlikely as the sites do not conform to the optimal AMPK substrate motif found in all other established in vivo AMPK substrates. However, AMPK and its related family members have been reported to modulate the activity of kinases and phophatases that regulate MLC (MLCK107, MYPT1108), so the full molecular detail of the mechanism requires further study. Given the overlapping substrate specificity of AMPK and its related kinases (see Fig. 2), it seems likely that AMPK may control cell polarity by targeting some of the same substrates as other AMPK family members, such as the MARKs, phosphorylate under other conditions.
Finally, it was recently shown that LKB1 promotes brush border formation on the apical surface of epithelial cells by the activation of the MST4 kinase. MST4 binds the LKB1 partner Mo25, and this interaction is conserved back through to budding yeast109. LKB1-dependent polarization resulted in MST4 translocation and subsequent phosphorylation of the cytoskeletal linker protein ezrin. This function of MST4 was needed for brush border induction but not other aspects of polarization.
Whether the control of cell polarity plays any role in LKB1-dependent tumor suppression also awaits further study. Suggestive of its importance though was a recent study showing LKB1 RNAi in MCF10A mammary acini in 3-D culture led to a loss of polarity and promoted oncogenic mycdependent cell proliferation110, an effect that cannot be seen in standard tissue culture plates111-113. Dissection of the role of LKB1 in cell polarity is hence perhaps best examined in the context of mouse models of LKB1 deficiency.
LKB1 and mouse models of cancer
Consistent with the regulation of cell growth, metabolism and polarity, genetic studies on the loss of function of LKB1 in the mouse have revealed a number of cancerous phenotypes (see Table 1). Like PJS patients, mice heterozygous for Lkb1 develop gastrointestinal polyposis114-118. Strikingly, mice in which Lkb1 is specifically deleted in gastrointestinal smooth muscle cells also develop polyps much like Lkb1+/- mice 119. These mice had alterations in transforming growth factor β (TGFβ signaling, implicating this pathway in hamartoma formation 120 and have raised the possibility that loss of LKB1 in the smooth muscle compartment and not the epithelial cells might be the initiating event. Future studies are needed to further test this model. In addition to GI hamartomas, PJS patients are also predisposed to a number of other malignancies, including breast, ovarian, endometrial and pancreatic tumors, and some of these have been studied in specific Lkb1 mouse models (see table 1). Given the recent discovery of prevalent LKB1 somatic mutations in cervical cancer and their association with poor prognosis8, is it of particular note that deletion of LKB1 in endometrial epithelium of female mice results in highly invasive adenocarcinomas121.
As LKB1 is frequently co-mutated with KRAS in NSCLC122, 123, mice bearing a conditional activated allele of Kras were crossed with mice bearing a conditionally inactivated allele of LKB1. The Kras;Lkb1lox/lox mice showed a dramatic increase in their tumor incidence and metastasis resulting in rapid acceleration of death (25 weeks for Kras alone vs. 10 weeks for Kras;Lkb1lox/lox)7. Furthermore, these mice develop all subtypes of NSCLC, as seen in humans, including squamous lung tumors which have not been previously observed in any genetic mouse model of lung cancer. Mechanistically, whether loss of LKB1 allows a distinct cell population to grow out and form squamous tumors or whether LKB1 loss impacts a lung stem cell compartment and alters their differentiation has yet to be investigated. Loss of LKB1 in skin keratinocytes was also recently reported to promote the development of squamous cell carcinomas, which was greatly accelerated by DMBA treatment124. Given the frequent mutation of Hras by DMBA, this further suggests that Ras-dependent signals and LKB1 loss may display a specific synergy that is selected for in tumour cells.
Therapeutic Implications
AMPK agonists as cancer therapeutics
Because of its long-established roles in various aspects of metabolic physiology, AMPK has received a great deal of pharmaceutical interest as a target for type 2 diabetes and other aspects of the metabolic syndrome125. Metformin (Glucophage), is the most widely used type 2 diabetes drug in the world and is thought to act by decreasing hepatic gluconeogenesis126. Metformin and its more potent analog phenformin inhibit complex I of the mitochondrial respiratory chain, resulting in reduced ATP production and LKB1-dependent activation of AMPK127. Indeed, this pathway is required for the therapeutic ability of metformin to lower blood glucose levels71. More recently, as metformin has been more widely prescribed for different diseases, for example, the treatment of insulin resistance in individuals with polycystic ovary syndrome, polymorphisms in LKB1 have been found in metformin non-responders128. More investigation is needed to determine the effect of these polymorphisms. Similarly, genetic polymorphisms in cell-surface transporter Oct1, which is required for efficient metformin uptake in hepatocytes, have been shown to underlie metformin resistance in some type 2 diabetics129.
The fact that AMPK activation not only reprograms metabolism, but also enforces a metabolic checkpoint on the cell cycle through effects on p53 and mTORC1 signaling, suggests that AMPK activating drugs may be useful as cancer therapeutics. Interestingly, well before the mode of action or key targets of metformin were known, it had been shown to suppress naturally-arising tumors in transgenic mice and in carcinogen-treated rodent cancer models130, 131. More recently, metformin has been shown to inhibit the growth of a wide variety of tumor cells in culture in an AMPK-dependent manner132, 133 and AMPK activation by metformin or aminoimidazole carboxamide ribonucleotide (AICAR) suppresses the growth of tumor xenografts134-136. Similarly, treatment of ES cells with metformin results in growth suppression, an effect that is lost in LKB1-deficient ES cells137. Given the known pharmacokinetics and widespread long-term clinical use of metformin, its potential utility for chemotherapy deserves further attention. Phenformin is a more potent inhibitor of mitochondrial complex I and consequently more potently activates AMPK than metformin 138. Despite the withdrawal of phenformin from clinical use owing to the likely on-target side effect of fatal lactic acidosis 139, it might find modern utility as an anti-cancer agent as the dosing and duration of its use for cancer would be quite distinct from that for diabetes. The anti-tumor efficacy of metformin has been directly compared to that of either phenformin or the AMPK-binding140 small molecule Abbott A769662141 in Pten+/- mice that spontaneously-develop lymphomas. While all three compounds resulted in delayed tumor onset, phenformin and A769662 showed greater efficacy, which correlated with their ability to activate AMPK and suppress mTORC1 in a wider number of tissues in vivo than metformin137. Perhaps an additional key to the success observed in this study is the fact that tumors initiated through loss of Pten have activation of PI3K, making mTORC1 hyperactivation one of the biochemical initiating events for this tumor type and increasing the impact of suppression of mTORC1 from endogenous AMPK activation in these tumors. These data also suggest a possible therapeutic window for the use of AMPK agonists to treat tumors arising in patients with TSC or for tumors exhibiting hyperactivation of mTORC1 by other genetic lesions. The fact that the AMPK targeted Abbott compound also did well further suggests that AMPK is in fact a key target of the biguanides in tumor reduction.
Given the number of type 2 diabetics worldwide taking metformin daily (>100 million), epidemiologists have begun examining the effect of metformin on cancer incidence. Initial studies revealed that diabetic patients taking metformin show a statistical reduction in tumor burden compared to patients taking any alternative142, 143. Similarly, a very recent study of breast cancer in type2 diabetics revealed a significant increase in complete pathological responses in patients taking metformin144, and a large phase III clinical trial of metformin as an adjuvant in breast cancer for diabetics and non-diabetics alike is in development145. Importantly, compounds that activate AMPK will not only impact tumor incidence through cell-autonomous effects on cell growth downstream of AMPK, but perhaps also through non-cell autonomous effects of lowering plasma insulin levels, which itself contributes to cancer risk and incidence146. Many additional epidemiological studies are required to determine whether there is indeed a clear tumor suppressive effect of prolonged use of metformin, and if so, whether tumors of specific tissues or bearing specific oncogenic lesions will show the greatest potential response. Critically, the OCT1 transporter which is critical for effective metformin transport into hepatocytes, shows a limited tissue distribution129 consistent with the pattern of AMPK activation in mice treated with metformin137. In contrast, a direct comparison of metformin to phenformin revealed that phenformin exhibited a more broad profile of tissues in which it potently activated AMPK137 indicating that for many tumor types in the whole organism, a direct action of metformin on tumor cells may be less likely than for phenformin. Interestingly, a recent study demonstrated that metformin was effective in treating a mouse model of endometrial hyperplasia and reducing mTORC1 signaling in that context147, though whether that effect was due to direct activation of AMPK in the endometrium or reduced circulating insulin and insulin signaling in the endometrium was not examined. Going forward, further attention needs to be placed on whether effects of metformin in mice and in human epidemiology studies can be attributed to indirect effects on lowered insulin levels from AMPK activation in liver (as will surely contribute in type 2 diabetics), or due to direct effects of AMPK activation in the tumor cells leading to suppression of their growth. These effects need not be mutually exclusive, and in fact are both likely to contribute to therapeutic effects of AMPK agonists on cancer risk.
Even with effective targeting and activation of AMPK within tumor cells, as with other targeted therapeutics, AMPK activating drugs will likely be most useful against tumors of specific genotypes or in combination with other targeted therapeutics. In fact, tumor cells lacking LKB1 are hypersensitive to apoptosis in culture following treatment with energy stress inducing agents, presumably originating from an inability to restore ATP levels due to AMPK deficiency4, 37, 148, 149. Similarly, fibroblasts lacking TSC2 or p53 are also sensitive to apoptosis induced by energy stress28-30,40 and metformin and AICAR both preferentially killed isogenic colon cancer xenografts lacking p53 as opposed to those with intact p53 function135. Though energy stress can promote apoptosis in cells defective in the AMPK pathway, by contrast in cells competent for the AMPK pathway, its activation is well-established to promote cell survival47, 150, 151. Thus treatment of tumors with intact AMPK function with energy stress agents could lead to prolonged survival of tumor cells, consistent with the ability of AMPK promote survival of cells faced with metabolic stress imposed by activated oncogenes115, 152. These findings indicate that transient inactivation of AMPK may serve as a chemosensitizer in some tumor contexts, not unlike what has been proposed for drugs targeting the DNA damage checkpoint,153 which similarly dictates survival and apoptotic decisions following organismal stress.
Therefore, defining which oncogenic genotypes (such as loss of p53 or LKB1) sensitize tumors to AMPK activating drug treatments in more refined genetically-engineered mouse tumor models within individual tumor types (lung, mammary, etc) is an important goal for future studies.
Rapamycin as a therapeutic for hamartomas and other LKB1-deficient tumors
Mutations in PTEN, NF1, TSC2, or LKB1 tumor suppressor genes are responsible for a number of inherited cancer syndromes, collectively referred to as phakomatoses. They all have overlapping clinical features including the development of hamartomas and aberrant pigmentation defects. Given that each of these tumor suppressors function upstream of mTORC1 (Fig. 3), the underlying hypothesis is that inactivation of these tumor suppressors in individual cells leads to cell-autonomous hyperactivation of mTORC1, ultimately resulting in tumors that are reliant on mTORC1 signaling. Over the past 5 years, rapamycin analogs have been examined in spontaneously arising tumors in Pten+/-154, Nf1+/-155, Tsc2 +/-156, Lkb1+/-36, 157, 158 and activated Akt84 transgenic mice and tumours in these mice have proven to be responsive to this approach.
These encouraging preclinical results have helped spur ongoing phase II and phase III clinical trials for rapamycin analogs159, 160 161, 162. These data suggest that hamartoma syndromes involving hyperactivation of mTORC1 may be particularly responsive to rapamycin analogs as a single agent, although the effects might be cytostatic rather than cytotoxic161. Perhaps new, targeted inhibitors directed at the kinase domain of mTOR will produce greater therapeutic response with targeted cytotoxicity, or perhaps kinase inhibitors that inactivate both mTOR and PI3K would be even more effective, as PI3K provides a survival signal in most epithelial cell types.
The number of patients with inherited hamartoma syndromes is dwarfed by the number of people with sporadic lung tumors containing LKB1 mutations. However, the predicted effectiveness of mTORC1 inhibitors against these tumors is unclear given that most of these tumors have mutated KRAS in addition to loss of LKB1, which might activate survival pathways other than mTORC1. Whether mTORC1 inhibitors might be useful in the treatment of LKB1 mutant tumors of different tissue origins remains to be determined.
Outstanding questions
The existence of a nutrient-regulated tumor suppressor pathway that couples cell growth to glucose and lipid metabolism raises a number of intriguing predictions and unanswered questions. For example, do environmental factors such as diet and exercise that contribute to physiological AMPK activation modulate tumorigenic risk through mTORC1 suppression? It is clear from a large number of epidemiology studies that cancer risk is correlated with metabolic syndrome, obesity or type 2 diabetes163. This association may be due to increased cell proliferation via hyperactivation of mTORC1 downstream of altered LKB1-AMPK signaling. The identity of the cell types most sensitive to growth suppression effects of AMPK and LKB1 may reveal those lineages in which cell growth is most tightly coupled to dietary conditions. Conversely, exercise and caloric restriction, each of which activates AMPK in some lineages, can lower overall cancer risk and improve cancer prognosis164. The mammalian cell types in which exercise and caloric restriction suppress cell growth and cancer risk remain to be delineated. Though much remains to be done to examine whether AMPK mediates some of the beneficial effects of exercise and caloric restriction on cancer risk, a recent study revealed that AMPK was activated, and mTORC1 signaling was suppressed, in some rodent tissues in a dose-dependent manner by increasing amounts of dietary restriction165. Conversely, high fat diet was observed to increase mTOR and decrease AMPK activity in some mouse tissues166. Finally, lower expression levels of metabolic hormones including the adipokine adiponectin — which is a key activator of AMPK in some tissues — have been shown to correlate with increased risk for breast endometrial, prostate and colon cancer167, 168. Strikingly, the incidence of colonic polyps in a colorectal cancer mouse model lacking adiponectin or the adiponectin receptor 1 (AdipoR1), was significantly increased and this correlated with loss of AMPK signaling and increased mTORC in the colonic epithelium169. These effects were only observed in animals on a high fat diet, further enforcing the concept that the metabolic status of the cells and the organism will dictate the conditions where LKB1 is most effective in tumor suppression.
Whether the endogenous metabolic checkpoint imposed by AMPK must be subjugated to allow tumorigenic progression is also unclear. Melanoma cell lines expressing oncogenic BRAF do not activate AMPK following energy stress due to hyperphosphorylation of LKB1 at Erk- and Rsk-phosphorylation sites170. Moreover, Ampkα2 mRNA levels in breast and ovarian cancers are profoundly suppressed by oncogenic PI3K signals 171, suggesting another route through which AMPK signaling can be inhibited. Thus, there is evidence that oncogenic pathways can downregulate LKB1 and AMPK through a variety of mechanisms. When selection against the LKB1-AMPK pathway occurs is also unclear, but it is conceivable that limitations on glucose and oxygen diffusion in pre-angiogenic tumors will result in growth inhibition, possibly due to activation of an AMPK-mediated metabolic growth checkpoint. Whether endogenous AMPK signaling is truly part of the pre-angiogenic checkpoint is a crucial question. Furthermore, whether pre-angiogenic tumors lacking LKB1 or AMPK continue to proliferate faster than AMPK-containing counterparts but then succumb to apoptosis or necrosis due to the inevitable energy shortage remains to be seen. The role and requirement for AMPK in these processes and overall tumor suppression is perhaps best addressed genetically through deletion of AMPK subunits in the context of different well-studied mouse models of tumorigenesis.
Despite the evidence supporting a role for AMPK as metabolic checkpoint in the cell, key mechanistic questions remain regarding which of the kinases downstream from LKB1, and which of their substrates, are required for tumor suppressor activity of LKB1 in different tissue settings. The regulation of mTORC1 and p53 by AMPK make it a likely contributor to LKB1-dependent tumor suppression. However, control of cell polarity is also known to play a role in tumorigenesis172 and in fact suppression of the MARK kinases by the Helicobacter pylori CagA protein is thought to be essential for its pathogenic disruption of gastric epithelial polarity and tumor promotion173. Currently there is minimal mutational data from human tumors to specifically support any single LKB1-dependent kinase as the critical target for LKB1 in tumorigenesis. There is a great deal of redundancy among them, suggesting that in many tissues loss of any one kinase may be compensated for by other family members.
The potency of LKB1 as a tumor suppressor probably derives from its control of multiple growth suppressive pathways. For example, combined loss of LKB1 with KRAS in the mouse lung epithelium causes 3 discrete phenotypes: accelerated tumor progression and tumor growth; the appearance of a novel tumor type, squamous carcinomas; and a dramatic increase in the numbers of metastases. While AMPK and mTORC1 signaling may play a role in the growth component of this acceleration, it also seems probable that loss of cell polarity and increased cytoskeletal signaling upon loss of MARK activity impacts the unique metastatic nature of the LKB1-deficient tumors. The appearance of novel tumor types may also reflect de-differentiation through transcriptional reprogramming downstream of AMPK and several of its related family members. AMPK has also been shown to modulate other tumor suppressive mechanisms, including the promotion of autophagy174 and cellular senescence175 under energy-poor conditions. The absolute requirement for AMPK or LKB1 in the induction of senescence or autophagy in different physiological and pathological contexts in an intact organism remains to be fully investigated.
Another important question is whether LKB1 or AMPK deregulation often contributes to the Warburg effect. Studies from cell culture and targeted mouse knockouts have revealed that mutations in oncogenes and tumor suppressors that drive tumorigenesis stimulate HIF-1α176. Indeed, HIF-1α and its target genes are upregulated in LKB1-, AMPK-, and TSC-deficient fibroblasts even under normoxic conditions, indicating that loss of any one of these genes is sufficient to confer activation of the full HIF-1α transcriptional program and hence alter cell metabolism36, 177. Indeed immunohistochemistry on gastrointestinal tumors from Peutz-Jeghers patients and LKB1+/- mice reveals that both contain elevated HIF-1α and its target GLUT1, and these tumors in LKB1+/- mice are positive by FDG-PET despite their benign nature36. These observations further prompt an examination of physiological or pathological contexts in which LKB1 or AMPK normally act to suppress HIF-1α and whether their inactivation is commonly involved in the glycolytic switch of most tumors. Given the regulation of the LKB1-AMPK pathway by hormones, exercise and diet, future studies should address whether LKB1 or AMPK mediate changes in tumor metabolism and FDG-PET imaging following behavioral or hormonal intervention. Whether LKB1 mutant NSCLC and cervical cancers show altered FDG-PET, and whether that can be used to direct therapeutic interventions in different patient populations, will be important aims for future studies. Regardless, the development of new serum and tissue biomarkers reflective of LKB1 and AMPK activation state will lead to better optimization of future clinical trials aimed at efficacy of targeted therapeutics.
While these and many other questions will take years to fully address, the discovery of this highly conserved pathway has already led to fundamental insights into the mechanisms through which all eukaryotic organisms couple their growth to nutrient conditions and metabolism. A deeper understanding of the key components of this pathway will not only lead to future therapeutic targets for cancer and diabetes, but will reveal the minimal number of steps required to suppress cell growth and reprogram metabolism.
Acknowledgements
We regret being unable to cite the work of many of our colleagues owing to space limitations. The authors wish to thank Katja Lamia for critical reading and editing of the manuscript. The authors’ research is funded by grants from the NIH (R01 DK080425 and P01 CA120964), American Cancer Society, and V. Foundation for Cancer Research to R.J.S. D.B.S. was supported by training grant T32 CA009370 to the Salk Institute Center for Cancer Research. R.J.S. is an Early Career Scientist of the Howard Hughes Medical Institute.
Glossary terms
- Peutz-Jeghers Syndrome (PJS)
PJS is characterized by the development of gastrointestinal hamartomas and an increased predisposition to a number of other malignancies including those arising in colon, breast, ovarian, pancreatic and lung tissue.
- Tuberous sclerosis complex (TCS)
A familial tumour syndrome induced through mutation of the mTORC1 regualators TSC1 and TCS2.
- Steatosis
Excess intracellular lipid accumulation such as occurs pathologically in the liver in diabetic or obese patients
Biography
Biography
Reuben J. Shaw is the Hearst Endowment Assistant Professor in the Molecular and Cell Biology Laboratory at the Salk Institute for Biological Studies. His laboratory, including postdoctoral fellow David B. Shackelford, study the role of LKB1 and AMPK in cancer and diabetes.
re198
de199
fin200
Footnotes
AT A GLANCE
- The LKB1 serine/threonine kinase is inactivated in Peutz-Jeghers syndrome and a large percentage of sporadic non small cell lung carcinomas and cervical carcinomas
- LKB1 acts a master upstream kinase, directly phosphorylating and activating AMPK and a family of 12 related kinases which play critical roles in cell growth, metabolism, and polarity
- The LKB1/AMPK pathway serves as a metabolic checkpoint in the cell, arresting cell growth under conditions of low intracellular ATP such as under conditions of low nutrients
- One the central mitogenic pathways suppressed by LKB1 and AMPK signaling is the mTORC1 target of rapamycin pathway, which is inhibited via AMPK phosphorylation of TSC2 and raptor
- Organismal metabolism and overnutrition can suppress LKB1-AMPK signaling which may contribute to increased cancer risk in obese or diabetic patients. Conversely, activation of LKB1/AMPK signaling may contribute the suppression of cancer risk associated with exercise and caloric restriction. Will AMPK activating drugs including existing diabetes therapeutics find clinical utility as anti-cancer agents?
References
- 1.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci U S A. 2003;100:8839–43. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawley SA, et al. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods A, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–8. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Shaw RJ, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–35. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemminki A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–7. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Cespedes M, et al. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–62. [PubMed] [Google Scholar]
- 7.Ji H, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 8.Wingo SN, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;4:e5137. doi: 10.1371/journal.pone.0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32(Suppl 4):S55–9. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 10.Lizcano JM, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaleel M, et al. Identification of the sucrose non-fermenting related kinase SNRK, as a novel LKB1 substrate. FEBS Lett. 2005;579:1417–23. doi: 10.1016/j.febslet.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 12.Al-Hakim AK, et al. 14-3-3 cooperates with LKB1 to regulate the activity and localization of QSK and SIK. J Cell Sci. 2005;118:5661–73. doi: 10.1242/jcs.02670. [DOI] [PubMed] [Google Scholar]
- 13.Watts JL, Morton DG, Bestman J, Kemphues KJ. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development. 2000;127:1467–75. doi: 10.1242/dev.127.7.1467. [DOI] [PubMed] [Google Scholar]
- 14.Anderson KA, et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–88. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Tamas P, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–70. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta. Mol Cell Biol. 2006;26:5933–45. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley SA, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Woods A, et al. C(Ca2+)/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Hurley RL, et al. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–6. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 20.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–20. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 21.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–80. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–9. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thoreen CC, et al. An ATP-competitive mTOR inhibitor reveals rapamycin-insensitive functions of mTORC1. J Biol Chem. 2009 doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman ME, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 30.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–8. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw RJ, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, et al. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–31. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoki K, et al. TSC2 Integrates Wnt and Energy Signals via a Coordinated Phosphorylation by AMPK and GSK3 to Regulate Cell Growth. Cell. 2006;126:955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 34.Hahn-Windgassen A, et al. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–9. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 35.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shackelford DB, et al. mTOR- and HIF-1α mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0900465106. www.pnas.org□cgi□doi□10.1073□pnas.0900465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carretero J, et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26:1616–25. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
- 38.Karuman P, et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001;7:1307–19. doi: 10.1016/s1097-2765(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 39.Tiainen M, Vaahtomeri K, Ylikorkala A, Makela TP. Growth arrest by the LKB1 tumor suppressor: induction of p21(WAF1/CIP1) Hum Mol Genet. 2002;11:1497–504. doi: 10.1093/hmg/11.13.1497. [DOI] [PubMed] [Google Scholar]
- 40.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–7. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 41.Jones RG, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 42.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–54. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 43.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–75. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 44.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–60. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Z, et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–53. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 46.Greer EL, et al. The Energy Sensor AMP-activated Protein Kinase Directly Regulates the Mammalian FOXO3 Transcription Factor. J Biol Chem. 2007;282:30107–19. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 47.Liang J, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–24. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 48.Short JD, et al. AMP-activated protein kinase signaling results in cytoplasmic sequestration of p27. Cancer Res. 2008;68:6496–506. doi: 10.1158/0008-5472.CAN-07-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baba M, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552–7. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, et al. AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1: involvement in the nuclear import of RNA-binding protein HuR. J Biol Chem. 2004;279:48376–88. doi: 10.1074/jbc.M409014200. [DOI] [PubMed] [Google Scholar]
- 51.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–22. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 52.Sato R, Goldstein JL, Brown MS. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc Natl Acad Sci U S A. 1993;90:9261–5. doi: 10.1073/pnas.90.20.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhan Y, et al. Control of cell growth and survival by enzymes of the fatty acid synthesis pathway in HCT-116 colon cancer cells. Clin Cancer Res. 2008;14:5735–42. doi: 10.1158/1078-0432.CCR-07-5074. [DOI] [PubMed] [Google Scholar]
- 54.Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66:5287–94. doi: 10.1158/0008-5472.CAN-05-1489. [DOI] [PubMed] [Google Scholar]
- 55.Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–25. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- 56.Beckers A, et al. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67:8180–7. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- 57.Orita H, et al. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res. 2007;13:7139–45. doi: 10.1158/1078-0432.CCR-07-1186. [DOI] [PubMed] [Google Scholar]
- 58.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 59.Marsin AS, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–55. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 60.Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 61.Bando H, et al. Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clin Cancer Res. 2005;11:5784–92. doi: 10.1158/1078-0432.CCR-05-0149. [DOI] [PubMed] [Google Scholar]
- 62.Telang S, et al. Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene. 2006;25:7225–34. doi: 10.1038/sj.onc.1209709. [DOI] [PubMed] [Google Scholar]
- 63.Clem B, et al. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther. 2008;7:110–20. doi: 10.1158/1535-7163.MCT-07-0482. [DOI] [PubMed] [Google Scholar]
- 64.Yang W, et al. Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem. 2001;276:38341–4. doi: 10.1074/jbc.C100316200. [DOI] [PubMed] [Google Scholar]
- 65.Berdeaux R, et al. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med. 2007;13:597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- 66.Dequiedt F, et al. New role for hPar-1 kinases EMK and C-TAK1 in regulating localization and activity of class IIa histone deacetylases. Mol Cell Biol. 2006;26:7086–102. doi: 10.1128/MCB.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGee SL, et al. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57:860–7. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- 68.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–11. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 69.Screaton RA, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Jansson D, et al. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc Natl Acad Sci U S A. 2008;105:10161–6. doi: 10.1073/pnas.0800796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu A, Screaton RA. Using kinomics to delineate signaling pathways: control of CRTC2/TORC2 by the AMPK family. Cell Cycle. 2008;7:3823–8. doi: 10.4161/cc.7.24.7241. [DOI] [PubMed] [Google Scholar]
- 73.Wu L, et al. Transforming activity of MECT1-MAML2 fusion oncoprotein is mediated by constitutive CREB activation. Embo J. 2005;24:2391–402. doi: 10.1038/sj.emboj.7600719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Canettieri G, et al. The coactivator CRTC1 promotes cell proliferation and transformation via AP-1. Proc Natl Acad Sci U S A. 2009;106:1445–50. doi: 10.1073/pnas.0808749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Canto C, et al. AMPK regulates energy expenditure by modulating NAD(+) metabolism and SIRT1 activity. Nature. 2009 doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brooks CL, Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer. 2009;9:123–8. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Porstmann T, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–36. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 81.Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 82.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–13. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 83.Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39:231–4. doi: 10.1007/s10863-007-9081-2. [DOI] [PubMed] [Google Scholar]
- 84.Majumder PK, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 85.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–34. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 86.Brugarolas J, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–84. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 88.Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–92. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Lee JH, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–20. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 90.Tomancak P, et al. A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nat Cell Biol. 2000;2:458–60. doi: 10.1038/35017101. [DOI] [PubMed] [Google Scholar]
- 91.Shulman JM, Benton R, St Johnston D. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell. 2000;101:377–88. doi: 10.1016/s0092-8674(00)80848-x. [DOI] [PubMed] [Google Scholar]
- 92.Baas AF, et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–66. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 93.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–77. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 94.Barnes AP, et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell. 2007;129:549–63. doi: 10.1016/j.cell.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 95.Hezel AF, Bardeesy N. LKB1; linking cell structure and tumor suppression. Oncogene. 2008;27:6908–19. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 96.Kojima Y, et al. Suppression of tubulin polymerization by the LKB1-microtubule-associated protein/microtubule affinity-regulating kinase signaling. J Biol Chem. 2007;282:23532–40. doi: 10.1074/jbc.M700590200. [DOI] [PubMed] [Google Scholar]
- 97.Biernat J, et al. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol Biol Cell. 2002;13:4013–28. doi: 10.1091/mbc.02-03-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun TQ, et al. PAR-1 is a Dishevelled-associated kinase and a positive regulator of Wnt signalling. Nat Cell Biol. 2001;3:628–36. doi: 10.1038/35083016. [DOI] [PubMed] [Google Scholar]
- 99.Ossipova O, Dhawan S, Sokol S, Green JB. Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev Cell. 2005;8:829–41. doi: 10.1016/j.devcel.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 100.Elbert M, Cohen D, Musch A. PAR1b promotes cell-cell adhesion and inhibits dishevelled-mediated transformation of Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3345–55. doi: 10.1091/mbc.E06-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178:355–61. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang X, et al. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat Cell Biol. 2007;9:743–54. doi: 10.1038/ncb1603. [DOI] [PubMed] [Google Scholar]
- 103.Narimatsu M, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci U S A. 2006;103:17272–7. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2007;104:819–22. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sebbagh M, Santoni MJ, Hall B, Borg JP, Schwartz MA. Regulation of LKB1/STRAD localization and function by E-cadherin. Curr Biol. 2009;19:37–42. doi: 10.1016/j.cub.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Horman S, et al. AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem. 2008;283:18505–12. doi: 10.1074/jbc.M802053200. [DOI] [PubMed] [Google Scholar]
- 108.Yamamoto H, et al. Identification of a novel substrate for TNFalpha-induced kinase NUAK2. Biochem Biophys Res Commun. 2008;365:541–7. doi: 10.1016/j.bbrc.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 109.ten Klooster JP, et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell. 2009;16:551–62. doi: 10.1016/j.devcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 110.Partanen JI, Nieminen AI, Makela TP, Klefstrom J. Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc Natl Acad Sci U S A. 2007;104:14694–9. doi: 10.1073/pnas.0704677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aranda V, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–45. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- 112.Dow LE, et al. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–82. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- 113.Nolan ME, et al. The polarity protein Par6 induces cell proliferation and is overexpressed in breast cancer. Cancer Res. 2008;68:8201–9. doi: 10.1158/0008-5472.CAN-07-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ylikorkala A, et al. Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science. 2001;293:1323–6. doi: 10.1126/science.1062074. [DOI] [PubMed] [Google Scholar]
- 115.Bardeesy N, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–7. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 116.Miyoshi H, et al. Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res. 2002;62:2261–6. [PubMed] [Google Scholar]
- 117.Jishage K, et al. Role of Lkb1, the causative gene of Peutz-Jegher’s syndrome, in embryogenesis and polyposis. Proc Natl Acad Sci U S A. 2002;99:8903–8. doi: 10.1073/pnas.122254599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rossi DJ, et al. Induction of cyclooxygenase-2 in a mouse model of Peutz-Jeghers polyposis. Proc Natl Acad Sci U S A. 2002;99:12327–32. doi: 10.1073/pnas.192301399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Katajisto P, et al. LKB1 signaling in mesenchymal cells required for suppression of gastrointestinal polyposis. Nat Genet. 2008;40:455–9. doi: 10.1038/ng.98. [DOI] [PubMed] [Google Scholar]
- 120.Vaahtomeri K, et al. Lkb1 is required for TGFbeta-mediated myofibroblast differentiation. J Cell Sci. 2008;121:3531–40. doi: 10.1242/jcs.032706. [DOI] [PubMed] [Google Scholar]
- 121.Contreras CM, et al. Loss of Lkb1 provokes highly invasive endometrial adenocarcinomas. Cancer Res. 2008;68:759–66. doi: 10.1158/0008-5472.CAN-07-5014. [DOI] [PubMed] [Google Scholar]
- 122.Carretero J, Medina PP, Pio R, Montuenga LM, Sanchez-Cespedes M. Novel and natural knockout lung cancer cell lines for the LKB1/STK11 tumor suppressor gene. Oncogene. 2004 doi: 10.1038/sj.onc.1207502. [DOI] [PubMed] [Google Scholar]
- 123.Makowski L, Hayes DN. Role of LKB1 in lung cancer development. Br J Cancer. 2008;99:683–8. doi: 10.1038/sj.bjc.6604515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gurumurthy S, Hezel AF, Berger JH, Bosenberg MW, Bardeesy N. LKB1 deficiency sensitizes mice to carcinogen-induced tumorigenesis. Cancer Res. 2008;68:55–63. doi: 10.1158/0008-5472.CAN-07-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hardie DG. AMP-Activated Protein Kinase as a Drug Target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 126.Hundal RS, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–9. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hardie DG. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology. 2006;131:973. doi: 10.1053/j.gastro.2006.07.032. author reply 974-5. [DOI] [PubMed] [Google Scholar]
- 128.Legro RS, et al. Ovulatory response to treatment of polycystic ovary syndrome is associated with a polymorphism in the STK11 gene. J Clin Endocrinol Metab. 2008;93:792–800. doi: 10.1210/jc.2007-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shu Y, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117:1422–31. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schneider MB, et al. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology. 2001;120:1263–70. doi: 10.1053/gast.2001.23258. [DOI] [PubMed] [Google Scholar]
- 131.Anisimov VN, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–93. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 132.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 133.Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Phila Pa) 2008;1:369–75. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 134.Swinnen JV, et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–8. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- 135.Buzzai M, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 136.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15:833–9. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 137.Huang X, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–21. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 138.Dykens JA, et al. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol Appl Pharmacol. 2008;233:203–10. doi: 10.1016/j.taap.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 139.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–14. [PMC free article] [PubMed] [Google Scholar]
- 140.Scott JW, et al. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol. 2008;15:1220–30. doi: 10.1016/j.chembiol.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 141.Cool B, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–16. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 142.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. Bmj. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 144.Jiralerspong S, et al. Metformin and Pathologic Complete Responses to Neoadjuvant Chemotherapy in Diabetic Patients With Breast Cancer. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in Breast Cancer: Time for Action. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- 146.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 147.Erdemoglu E, Guney M, Giray SG, Take G, Mungan T. Effects of metformin on mammalian target of rapamycin in a mouse model of endometrial hyperplasia. Eur J Obstet Gynecol Reprod Biol. 2009 doi: 10.1016/j.ejogrb.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 148.Memmott RM, et al. Phosphatidylinositol ether lipid analogues induce AMP-activated protein kinase-dependent death in LKB1-mutant non small cell lung cancer cells. Cancer Res. 2008;68:580–8. doi: 10.1158/0008-5472.CAN-07-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nafz J, et al. Interference with energy metabolism by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside induces HPV suppression in cervical carcinoma cells and apoptosis in the absence of LKB1. Biochem J. 2007;403:501–10. doi: 10.1042/BJ20061053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Buzzai M, et al. The glucose dependence of Akt-transformed cells can be reversed by pharmacologic activation of fatty acid beta-oxidation. Oncogene. 2005 doi: 10.1038/sj.onc.1208622. [DOI] [PubMed] [Google Scholar]
- 151.Shell SA, et al. Activation of AMPK is necessary for killing cancer cells and sparing cardiac cells. Cell Cycle. 2008;7:1769–75. doi: 10.4161/cc.7.12.6016. [DOI] [PubMed] [Google Scholar]
- 152.Laderoute KR, et al. 5′-AMP-Activated Protein Kinase (AMPK) Is Induced by Low-Oxygen and Glucose Deprivation Conditions Found in Solid-Tumor Microenvironments. Mol Cell Biol. 2006;26:5336–47. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O’Connor MJ, Martin NM, Smith GC. Targeted cancer therapies based on the inhibition of DNA strand break repair. Oncogene. 2007;26:7816–24. doi: 10.1038/sj.onc.1210879. [DOI] [PubMed] [Google Scholar]
- 154.Podsypanina K, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/-mice. Proc Natl Acad Sci U S A. 2001;98:10320–5. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Johannessen CM, et al. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18:56–62. doi: 10.1016/j.cub.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 156.Lee L, et al. Efficacy of a rapamycin analog (CCI-779) and IFN-gamma in tuberous sclerosis mouse models. Genes Chromosomes Cancer. 2005;42:213–27. doi: 10.1002/gcc.20118. [DOI] [PubMed] [Google Scholar]
- 157.Wei C, et al. Suppression of Peutz-Jeghers polyposis by targeting mammalian target of rapamycin signaling. Clin Cancer Res. 2008;14:1167–71. doi: 10.1158/1078-0432.CCR-07-4007. [DOI] [PubMed] [Google Scholar]
- 158.Robinson J, et al. Oral rapamycin reduces tumour burden and vascularization in Lkb1(+/-) mice. J Pathol. 2009 doi: 10.1002/path.2562. [DOI] [PubMed] [Google Scholar]
- 159.Hudes G, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 160.Cloughesy TF, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bissler JJ, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Davies DM, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med. 2008;358:200–3. doi: 10.1056/NEJMc072500. [DOI] [PubMed] [Google Scholar]
- 163.Martinez ME, Marshall JR, Giovannucci E. Diet and cancer prevention: the roles of observation and experimentation. Nat Rev Cancer. 2008;8:694–703. doi: 10.1038/nrc2441. [DOI] [PubMed] [Google Scholar]
- 164.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–11. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 165.Jiang W, Zhu Z, Thompson HJ. Dietary energy restriction modulates the activity of AMP-activated protein kinase, Akt, and mammalian target of rapamycin in mammary carcinomas, mammary gland, and liver. Cancer Res. 2008;68:5492–9. doi: 10.1158/0008-5472.CAN-07-6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moore T, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila Pa) 2008;1:65–76. doi: 10.1158/1940-6207.CAPR-08-0022. [DOI] [PubMed] [Google Scholar]
- 167.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–5. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 169.Sugiyama M, et al. Adiponectin inhibits colorectal cancer cell growth through the AMPK/mTOR pathway. Int J Oncol. 2009;34:339–44. [PubMed] [Google Scholar]
- 170.Zheng B, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–47. doi: 10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hallstrom TC, Mori S, Nevins JR. An E2F1-dependent gene expression program that determines the balance between proliferation and cell death. Cancer Cell. 2008;13:11–22. doi: 10.1016/j.ccr.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lee M, Vasioukhin V. Cell polarity and cancer--cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121:1141–50. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- 173.Saadat I, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–3. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 174.Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007;3:381–3. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- 175.Wang W, Yang X, Lopez de Silanes I, Carling D, Gorospe M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem. 2003;278:27016–23. doi: 10.1074/jbc.M300318200. [DOI] [PubMed] [Google Scholar]
- 176.Brugarolas J, Kaelin WG., Jr. Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell. 2004;6:7–10. doi: 10.1016/j.ccr.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 177.Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG., Jr. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell. 2003;4:147–58. doi: 10.1016/s1535-6108(03)00187-9. [DOI] [PubMed] [Google Scholar]
- 178.Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol. 2004;14:736–41. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 179.Suzuki A, et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14:1425–35. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 180.Kusakabe M, Nishida E. The polarity-inducing kinase Par-1 controls Xenopus gastrulation in cooperation with 14-3-3 and aPKC. EMBO J. 2004;23:4190–201. doi: 10.1038/sj.emboj.7600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang Y, et al. PAR-1 kinase phosphorylates Dlg and regulates its postsynaptic targeting at the Drosophila neuromuscular junction. Neuron. 2007;53:201–15. doi: 10.1016/j.neuron.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- 183.Ossipova O, Bardeesy N, DePinho RA, Green JB. LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nat Cell Biol. 2003;5:889–94. doi: 10.1038/ncb1048. [DOI] [PubMed] [Google Scholar]
- 184.Asada N, Sanada K, Fukada Y. LKB1 regulates neuronal migration and neuronal differentiation in the developing neocortex through centrosomal positioning. J Neurosci. 2007;27:11769–75. doi: 10.1523/JNEUROSCI.1938-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang S, et al. The tumor suppressor LKB1 regulates lung cancer cell polarity by mediating cdc42 recruitment and activity. Cancer Res. 2008;68:740–8. doi: 10.1158/0008-5472.CAN-07-2989. [DOI] [PubMed] [Google Scholar]
- 186.Alessi DR, Sakamoto K, Bayascas JR. Lkb1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–63. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 187.Puffenberger EG, et al. Polyhydramnios, megalencephaly and symptomatic epilepsy caused by a homozygous 7-kilobase deletion in LYK5. Brain. 2007;130:1929–41. doi: 10.1093/brain/awm100. [DOI] [PubMed] [Google Scholar]
- 188.Towler MC, et al. A novel short splice variant of the tumour suppressor LKB1 is required for spermiogenesis. Biochem J. 2008 doi: 10.1042/BJ20081447. [DOI] [PubMed] [Google Scholar]
- 189.Denison FC, Hiscock NJ, Carling D, Woods A. Characterization of an alternative splice variant of LKB1. J Biol Chem. 2009;284:67–76. doi: 10.1074/jbc.M806153200. [DOI] [PubMed] [Google Scholar]
- 190.Marignani PA, et al. Novel splice isoforms of STRADalpha differentially affect LKB1 activity, complex assembly and subcellular localization. Cancer Biol Ther. 2007;6:1627–31. doi: 10.4161/cbt.6.10.4787. [DOI] [PubMed] [Google Scholar]
- 191.McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xiao B, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 193.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–48. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dolinsky VW, Dyck JR. Role of AMP-activated protein kinase in healthy and diseased hearts. Am J Physiol Heart Circ Physiol. 2006;291:H2557–69. doi: 10.1152/ajpheart.00329.2006. [DOI] [PubMed] [Google Scholar]
- 195.Robinson J, Nye E, Stamp G, Silver A. Osteogenic tumours in Lkb1-deficient mice. Exp Mol Pathol. 2008;85:223–6. doi: 10.1016/j.yexmp.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 196.Takeda H, Miyoshi H, Kojima Y, Oshima M, Taketo MM. Accelerated onsets of gastric hamartomas and hepatic adenomas/carcinomas in Lkb1+/-p53-/-compound mutant mice. Oncogene. 2006;25:1816–20. doi: 10.1038/sj.onc.1209207. [DOI] [PubMed] [Google Scholar]
- 197.Wei C, et al. Mutation of Lkb1 and p53 genes exert a cooperative effect on tumorigenesis. Cancer Res. 2005;65:11297–303. doi: 10.1158/0008-5472.CAN-05-0716. [DOI] [PubMed] [Google Scholar]
- 198.Shorning BY, et al. Lkb1 deficiency alters goblet and paneth cell differentiation in the small intestine. PLoS ONE. 2009;4:e4264. doi: 10.1371/journal.pone.0004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pearson HB, McCarthy A, Collins CM, Ashworth A, Clarke AR. Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 2008;68:2223–32. doi: 10.1158/0008-5472.CAN-07-5169. [DOI] [PubMed] [Google Scholar]
- 200.Hezel AF, et al. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol. 2008;28:2414–25. doi: 10.1128/MCB.01621-07. [DOI] [PMC free article] [PubMed] [Google Scholar]