Abstract
Herein, we demonstrate a role of AMP-activated protein kinase (AMPK) as a potent counter-regulator of inflammatory signaling pathways in macrophages. Stimulation of macrophages with anti-inflammatory cytokines (i.e., IL-10 and TGFβ) resulted in the rapid Phosphorylation/activation of AMPK, whereas stimulation of macrophages with a proinflammatory stimulus (LPS) resulted in AMPK dephosphorylation/inactivation. Inhibition of AMPKα expression by RNA interference dramatically increased the mRNA levels of LPS-induced TNFα, IL-6 and cyclooxygenase- 2 (COX-2). Likewise, expression of a dominant negative AMPKα1 in macrophages enhanced TNFα and IL-6 protein synthesis in response to LPS stimulation, while diminishing the production of IL-10. In contrast, transfection of macrophages with a constitutively active form of AMPKα1 resulted in decreased LPS-induced TNFα and IL-6 production, and heightened production of IL-10. In addition, we found that AMPK negatively regulated LPS-induced IκB-α degradation and positively regulated Akt activation, accompanied by inhibition of GSK3-β and activation of CREB. Thus, AMPK directs signaling pathways in macrophages in a manner that suppresses proinflammatory responses and promotes macrophage polarization to an anti-inflammatory functional phenotype.
Keywords: Monocytes/Macrophages, Signal Transduction, Protein Kinases/Phosphatases, Inflammation
Introduction
AMP-activated protein kinase (AMPK) is an evolutionary conserved serine/threonine kinase that regulates energy homeostasis and metabolic stress. When the cellular AMP/ATP ratio is high, AMPK is activated, switching off ATP-consuming anabolic pathways and switching on ATP-producing catabolic pathways (1). Mammalian AMPK is a heterotrimeric complex comprised of a catalytic α subunit and regulatory β and γ subunits. Each subunit has two or three isoforms (α1, α2, β1, β2, γ1, γ2, γ3) encoded by different genes (2). Phosphorylation of the threonine-172 residue of the α subunit is crucial for the AMPK activity (3). There are two kinases that have been established as upstream activators of AMPK; the protein kinase LKB1/STRAD/MO25 complex (4) and the calmodulin-dependent protein kinase kinase β (CaMKKβ) (5). While the LKB1 complex phosphorylates AMPK in response to changes in the AMP/ATP ratio (6), CAMKKβ phosphorylates AMPK in response to an increase in intracellular Ca2+ level (5). In liver, active AMPK inhibits fatty acid synthesis and cholesterol synthesis (7) while in heart and skeletal muscle it stimulates fatty acid oxidation and glycolysis (8,9). AMPK has also been shown to inactivate the mammalian target of rapamycin (mTOR) pathway via phosphorylation and activation of the mTOR inhibitor, tuberous sclerosis complex-2 TSC2 (10). This would typically occur when AMPK is activated as a result of energy deprivation, the net result being suppression of protein synthesis and cell growth. Studies demonstrating the role of AMPK in improvement of insulin sensitivity and glucose homeostasis have identified AMPK as target for the treatment of Type 2 diabetes and obesity (11).
A potential role of AMPK in suppression of inflammatory responses has been suggested by studies using the pharmacological activator of AMPK, 5-aminoimidazole-4-carboxamide ribose (AICAR). For example, treatment of mice with AICAR was found to reduce the severity of experimental autoimmune encephalomyelitis (EAE) (12). AICAR has also been shown to reduce iNOS synthesis by adipocytes, macrophages, myocytes, and glial cells (13,14). However, AICAR is taken up by cells and converted to AMP analogue ZMP which mimics the effect of AMP, thus AICAR is a nonspecific activator of AMPK and has the potential to activate other AMP sensitive enzymes (15). Indeed, there are recent reports in which the anti-inflammatory effects of AICAR were determined to be independent of AMPK activity, thus clouding the interpretation of studies using this reagent (16,17). As part of our ongoing studies of the signaling pathways that govern macrophage behavior, we investigated the role of AMPK in the regulation of macrophage inflammatory activity in response to physiological stimuli. We demonstrate that AMPK in macrophages can be rapidly activated or inactivated by anti-inflammatory or proinflammatory stimuli, respectively, and we provide evidence that AMPK acts as a central regulator of macrophage inflammatory function.
Materials and Methods
Reagents
LPS (Escherichia coli serotype O111:B4) was purchased from Sigma-Aldrich. Mouse recombinant IL-10 and human recombinant TGFβ were purchased from R&D Systems. Western blot detection of specific proteins utilized the following primary antibodies: anti-phospho-AMPKα (Thr172), anti-AMPK-α, anti-phospho-ACC (Ser79), anti-ACC, anti-phospho-GSK3-β (Ser9), anti-GSK3-β, anti-phospho-Akt (Ser473), anti-Akt, anti-phospho-CREB (Ser133), anti-CREB, anti-IκB-α (Cell Signaling Technology), anti-AMPKα1, anti-AMPKα2 (Abcam), anti-β -actin (Sigma) and HRP-conjugated secondary antibody (Jackson ImmunoResearch).
Western blot analysis
Murine bone marrow-derived macrophages and human monocyte-derived macrophages were generated as previously described (18,19). Macrophages were lysed in a lysis buffer containing 125 mM Tris, pH 6.8, 2% SDS, 20% glycerol, 200µM PMSF, protease inhibitor cocktail (Promega) and phosphatase inhibitor cocktail (Pierce). Total protein content of the samples was assessed by BCA protein assay (Pierce). Equal amounts of protein were separated on 10 % Criterion gels (Bio-Rad) by SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Hybond; Amersham) using a Trans-Blot SD Semi-dry electrophoretic transfer cell (Bio-Rad) (to detect phospho-ACC and ACC, 6% gels and a wet transfer system were used.) Ab-bound proteins were detected using an ECL Western blotting analysis system (Amersham), and the membranes were exposed to Kodak Biomax XL X-ray film (Eastman). Densitometric analysis was performed using the Bio-Rad Quantity One software associated with Bio-Rad Fluor-S Multi-Imager and FX phosphoimager systems.
ELISA
Following stimulation in 96-well plates, supernatants were collected and assayed by ELISA using OptEIA sets (BD Biosciences Pharmingen) according to the manufacturer’s instructions. Analysis was performed using an E-max Precision micro plate reader (Molecular Devices).
RNA interference
Murine bone marrow-derived macrophages were transfected with 0.5µg AMPK α1/2 siRNA or nonspecific control siRNA (Dharmacon) using Amaxa Biosystem’s Nucleofection technology according to the manufacturer’s instructions. Following nucleofection, the macrophages were plated in 12-well plates in RPMI 1640 (HyClone) medium containing 20% FBS (Atlanta Biologicals), 100 mM HEPES, 50 µg/ml gentamicin, 0.5 mM L-glutamine and 1.5 mM GlutaMAX (Invitrogen). The cells were analyzed 72 h post-transfection.
Real-time RT-PCR analysis
mMACs™ One-step cDNA Kits (Miltenyi Biotech) were used for RNA isolation and cDNA synthesis. cDNAs were amplified in a 20 µl reaction volume containing SYBR Green (New England Biolabs) and analyzed using a DNA Opticon 2 Monitor (MJ Research, currently Bio-Rad). IL-6, TNFα and COX-2 expression was analyzed by Quantitect Primer Assays (Qiagen). cDNA concentrations in each sample were normalized using transcripts for β-actin. The relative expression software tool (REST) was used to quantify mRNA expression of each gene (20).
Generation of stable transfectants
Dominant negative (DN-AMPKα1) and constitutively active (CA-AMPKα1) forms of AMPK were generated in the Carling laboratory as described previously (21). DN-AMPKα1 and CA-AMPKα1 coding regions were sub cloned into pcDNA-Zeo (Invitrogen) and endotoxin-free pcDNA-Zeo-DN-AMPKα1 (2 µg) and pcDNA-Zeo-CA-AMPKα1 (2 µg) were transfected into the B6J2 macrophage cell line (22) by using Nucleofection technology (Amaxa Biosystems) according to the manufacturer’s instructions. Selection of the transfectants was achieved via addition of zeocin to the cultures.
Statistical analysis
Statistical significance between groups was calculated with an unpaired Student’s t-test, with a P value < .05 considered statistically significant.
Results
AMPKα1 is the predominant AMPKα isoform expressed by macrophages
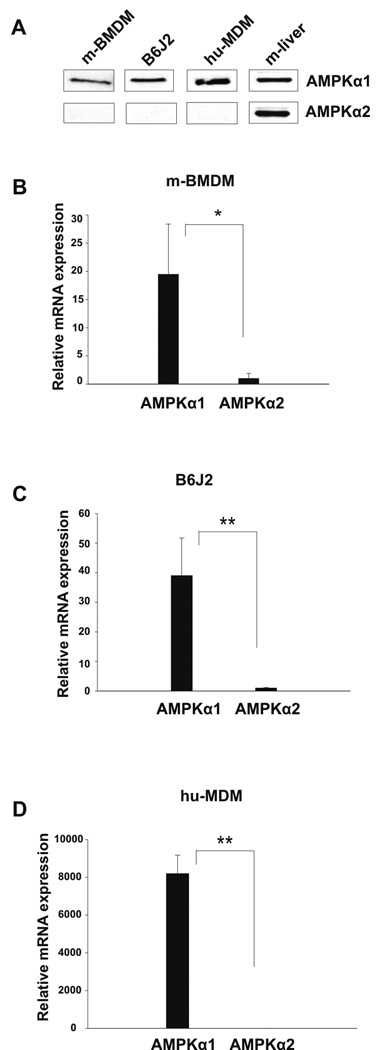
Expression of AMPKα isoforms by murine bone marrow-derived macrophages, the murine macrophage cell line B6J2, and human monocyte-derived macrophages was evaluated by Western blot and real-time RT-PCR. As shown in Figure 1A, Western blot analysis revealed expression of the 62 kDa AMPKα1 protein in each of the macrophage samples tested, whereas no detectable expression of AMPKα2 protein was observed. Analysis of AMPKα mRNA expression by murine bone marrow-derived macrophages (Fig. 1B) did show detectable levels of AMPKα2, however AMPKα1 was expressed at a 19-fold higher level. Likewise, AMPKα1 was expressed at a 39-fold higher level in murine macrophage cell line B6J2 (Fig. 1C). In human monocyte-derived macrophages AMPKα2 expression level was negligible (8,200 fold less than AMPKα1) (Fig. 1D). These data indicated that AMPKα1 is the dominant AMPKα isoform expressed in macrophages and our subsequent investigation focused on the manipulation of AMPKα1 expression and activity as a means to elucidate the role of AMPK in macrophages.
Figure 1. Mouse and human macrophages express predominantly the AMPKα1 isoform.
A, Lysates of mouse bone marrow derived macrophages (m-BMDM), the B6J2 macrophage cell line and human monocyte-derived macrophages (hu-MDM) were analyzed by Western blot using antibodies against the AMPKα1 and AMPKα2 proteins. Tissue lysate prepared from mouse liver was used as a positive control for expression of AMPKα2 protein. Data shown are representative of 3 independent experiments with similar results. B–D, Real-time RT-PCR analysis was performed for analysis of AMPKα1 and AMPKα2 mRNA expression. Data shown are mean ± S.D. of triplicate determinations (* p< 0.05, ** p< 0.01) and representative of 3 independent experiments with similar results.
AMPK activity is rapidly modulated by anti-inflammatory and proinflammatory stimuli
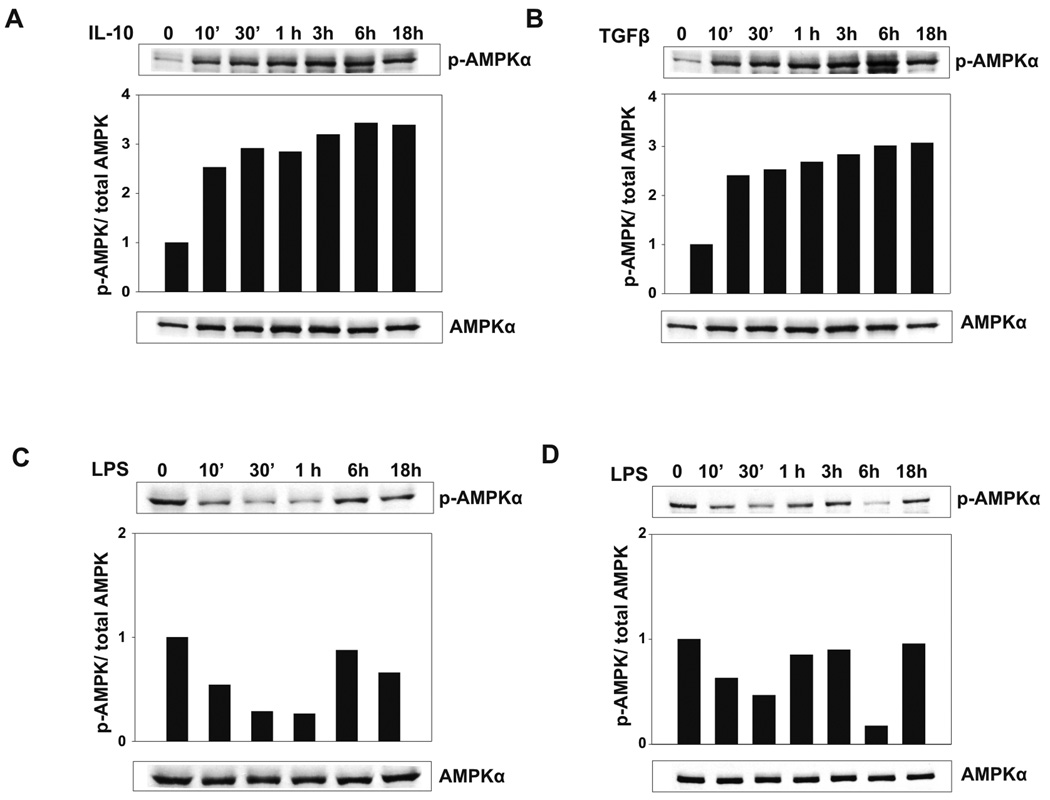
We evaluated the effect of anti-inflammatory and proinflammatory stimuli on the phosphorylation of the Thr-172 residue of the AMPKα catalytic domain, which is an indication of AMPK activation. Bone marrow-derived macrophages were stimulated with the typically anti-inflammatory cytokines IL-10 and TGFβ, or with the proinflammatory TLR4 agonist LPS. Stimulation of bone marrow-derived macrophages with IL-10 (Fig. 2A) and TGFβ (Fig. 2B) resulted in a rapid and marked increase in the phosphoryation level of AMPK, evident at 10 min post-stimulation, which was sustained over an 18 h time period. In contrast, LPS stimulation resulted in a significant reduction of AMPK phosphorylation, which was maintained through a 1 h time point, with a return to the phosphorylated state by the 6 h time point tested (Fig. 2C). We hypothesize that this return of AMPK phosphorylation may be due to an autocrine response to IL-10 produced by the macrophages in response to LPS stimulation. LPS stimulation also resulted in diminished AMPK phosphorylation in human macrophages over a 30 min time period, which returns to basal levels at the 1h time point (Fig. 2D). Interestingly, a second reduction in AMPK phosphorylation is apparent at 6h, which may be due to an autocrine influence of proinflammatory cytokine production. These data indicate that anti-inflammatory stimulus increases, while proinflammatory stimulus decreases AMPK activation in macrophages.
Figure 2. AMPK activity is rapidly modulated by anti-inflammatory and proinflammatory stimuli.
A and B, Anti-inflammatory cytokines IL-10 and TGFβ enhance the levels of phosphorylated AMPK in mouse macrophages. Bone marrow derived macrophages were stimulated with 20 ng/ml IL-10 or 5 ng/ml TGFβ for the time points indicated. C, LPS stimulation diminishes AMPK phosphorylation in mouse macrophages. Bone marrow derived macrophages were stimulated with 100 ng/ml LPS for the time points indicated. D, LPS stimulation decreases AMPK phosphorylation in human macrophages. Human macrophages were stimulated with 100 ng/ml LPS for the time points indicated. Western blot was performed using antibodies against phospho-AMPKα and total AMPKα. The p-AMPKα/AMPKα ratio for each was analyzed by densitometry and shown as bar graphs. Data shown are representative of 4 independent experiments with similar results.
Inhibition of AMPK expression or activity elevates LPS-induced macrophage inflammatory function
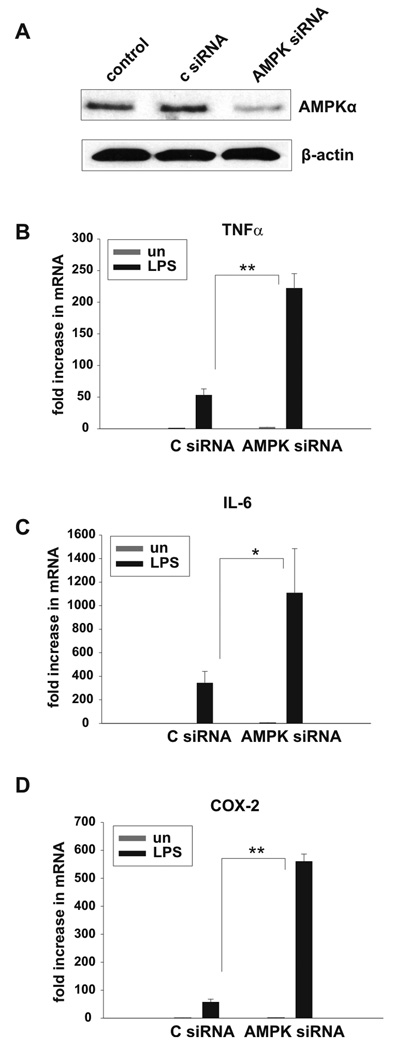
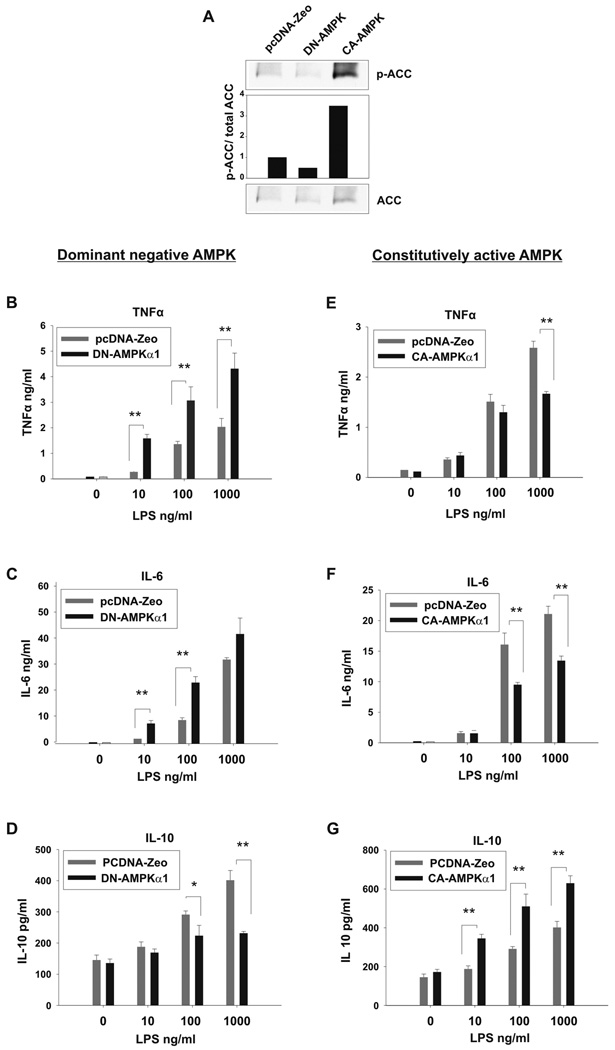
AMPK function in macrophages was evaluated by inhibition of AMPK expression and activity with use of siRNA silencing and a dominant-negative AMPKα1 mutant. Bone-marrow derived macrophages were transfected with AMPKα1/α2 siRNA or a scrambled control siRNA. Following transfection, LPS-induced TNFα, IL-6, and cyclooxygenase (COX-2) mRNA was evaluated. As shown in the Western blot analysis depicted in Figure 3A, macrophages transfected with AMPKα siRNA displayed a substantial reduction in AMPKα protein as compared to control siRNA transfected or untransfected macrophages. Typically, including in the experiment shown in Figure 3 A, siRNA suppression of AMPKα achieved an approximate 70% reduction in protein as assessed by densitometry of Western blot analyses. Suppression of the AMPKα catalytic domain expression in macrophages resulted in a dramatic increase in TNFα (4-fold), IL-6 (3-fold) and COX-2 (10-fold) mRNA expression in response to LPS stimulation as compared to control siRNA-treated macrophages (Fig. 3 B–D). Similar results were obtained with blockade of AMPKα activity. In these experiments, the B6J2 macrophage cell line was stably transfected with a dominant negative mutant of AMPKα1 (DN-AMPKα1). Empty vector transfected macrophages (pcDNA-Zeo) were used as control. To demonstrate that the macrophages expressing DN-AMPKα1 have impaired AMPK activity, phosphorylation (Ser 79) status of the AMPK substrate Acetyl CoA Carboxylase (ACC) was evaluated by Western blot. As shown in figure 4 A, DN-AMPKα1 macrophages display a 50% reduction in basal level of ACC Ser 79 phosphorylation, and therefore impaired AMPK activity, as compared to control cells. LPS-induced TNFα, IL-6 and IL-10 production of DN-AMPKα1 macrophages was analyzed by ELISA (Fig. 4 B–D). The B6J2 cell line expressing DN-AMPKα1 produced significantly more proinflammatory TNFα and IL-6, whereas production of the anti-inflammatory IL-10 was significantly reduced, as compared to the control cell line (pcDNA-Zeo empty vector transfectants).
Figure 3. Suppression of AMPK expression by RNA interference increases LPS induced pro-inflammatory activity of macrophages.
A, Bone marrow derived macrophages were transfected with AMPKα1/α2 siRNA or control siRNA or left untransfected. 72 h post-transfection, the cells were lysed and Western blot was performed with antibodies against AMPKα and β-actin. Data shown are representative of 3 independent experiments with similar results. B–D, 72 h post-siRNA transfection, macrophages were stimulated with 100 ng/ml LPS for 4hrs (TNFα, IL-6) or 2 hrs (COX-2) or left unstimulated. Real-time RT-PCR was performed for analysis of TNFα, IL-6 and COX-2 mRNA expression. Data shown are mean ± S.D. of triplicate determinations (* p< 0.05, ** p< 0.01) and representative of 3 independent experiments with similar results.
Figure 4. Expression of dominant negative or constitutively active AMPKα1 modulates the inflammatory response of macrophages.
The B6J2 macrophage cell line was stably transfected with DN-AMPKα1, (A–D) or CA-AMPKα1, (A,E–G). Empty vector (pcDNA-Zeo) B6J2 transfectants served as a control (A–G). Lysates of transfected macrophages were analyzed by Western blot using antibodies against p-ACC and ACC. The p-ACC/total ACC ratio was analyzed by densitometry and shown as a bar histogram. Data shown are representative of 2 independent experiments with similar results (A). Transfected macrophages were stimulated with LPS at the concentrations shown for 18 hrs or left unstimulated. TNFα, IL-6 and IL-10 levels were detected by ELISA. Data shown are mean ± S.D. of triplicate determinations (* p< 0.05, ** p< 0.01) and representative of 3 independent experiments with similar results.
Expression of a constitutively active AMPKα1 results in reduced macrophage inflammatory cytokine production and enhanced production of IL-10
We next evaluated the impact of elevated AMPK activity on macrophage inflammatory activity with use of a constitutively active AMPKα mutant (CA-AMPKα1). Stable transfectants of B6J2 expressing CA-AMPKα1 were generated and constitutive AMPK activation in these cells was confirmed by evaluation of the Ser 79 phosphorylation of the AMPK substrate ACC by Western blot. CA-AMPKα1 macrophages displayed 3.5 fold increased basal level of ACC Ser 79 phosphorylation, and therefore AMPK activity, as compared to empty vector transfected macrophages (Fig. 4A). Cytokine production by CA-AMPKα1 macrophages in response to LPS stimulation was evaluated by ELISA (Fig. 4 E–G). B6J2 macrophages transfected with CA-AMPKα1 displayed significantly lower levels of LPS-induced TNFα and IL-6 production as compared to the control cell line (pcDNA-Zeo transfectants), whereas IL-10 production was significantly higher in the CA-AMPKα1-expressing cells as compared to controls (Fig. E–G). These data provide further evidence that AMPK counter-regulates the inflammatory function of macrophages.
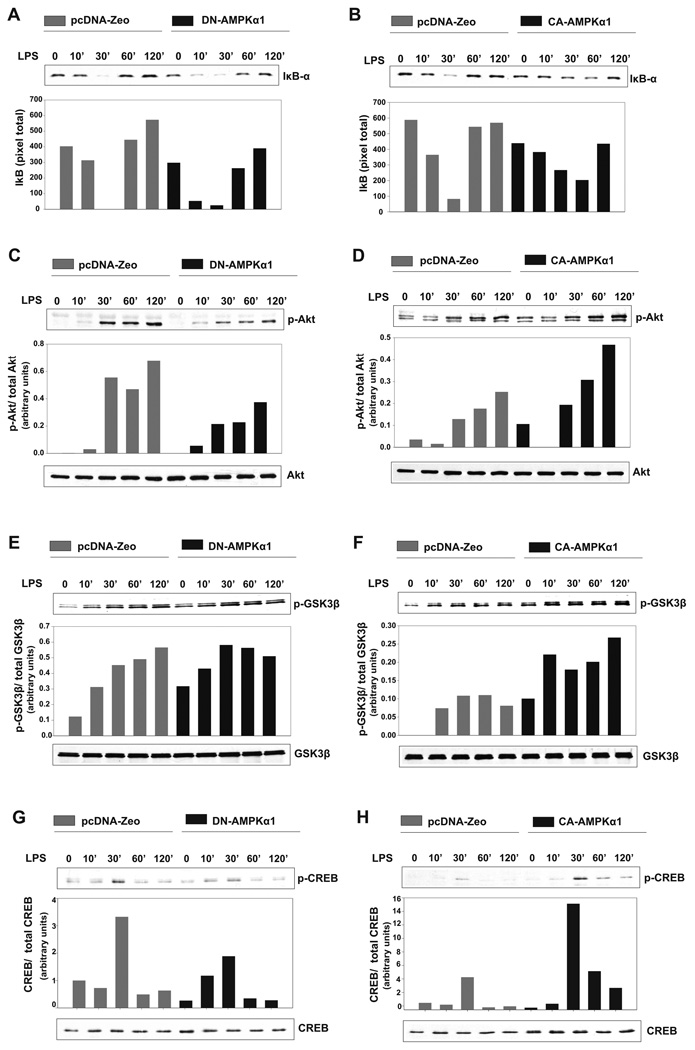
Evidence for AMPKα1 regulation of IKK/NF-κB, Akt, GSK3β and CREB mediated signaling pathways
We considered a number of downstream targets of AMPK as possible mediators of the counter-inflammatory activity we observed. For example, in endothelial cells AMPK activity has been implicated as a negative regulator of the transcription factor NF-κB (23,24), which is well-established as playing a critical role in the induction of pro-inflammatory gene expression (25). In unstimulated cells, the NF-κB p65/p50 heterodimer is held inactive in the cytoplasm by the inhibitory protein IκB. Pro-inflammatory stimuli activate IκB kinase (IKK), which in turn phosphorylates IκB, resulting in its ubiquitination-mediated degradation, allowing liberated NF-κB to enter the nucleus and activate gene expression (25). Thus, degradation of IκB is widely used as an indication of NF-κB activation. We evaluated the levels of LPS-induced IκB degradation in macrophages expressing either the dominant negative, DN-AMPKα1, or the constitutively active, CA-AMPKα1, mutants. As shown in Figure 5 A, macrophages containing DN-AMPKα1 have increased IκB degradation after LPS stimulation as compared to empty vector-transfected macrophages. IκB is nearly absent at 10 min in macrophages expressing DN-AMPKα1 and remains low through the 30 min time point, whereas in the control cells maximum degradation occurs at 30 min, and the overall levels of IκBα protein are substantially higher than in the cells expressing DN-AMPKα1. In contrast, expression of CA-AMPKα1 both delays and decreases LPS-induced IκB degradation (Fig. 5B). These results suggest that AMPK acts as a negative regulator of the IKK/IκB/NF-κB pathway in macrophages.
Figure 5. Expression of dominant negative or constitutively active AMPKα1 modulates LPS induced IκB-α, Akt, GSK3β and CREB activity.
A, B6J2 macrophages stably transfected with DN-AMPKα1 or empty vector were stimulated with 100 ng/ml LPS for the time points indicated. After cell lysis, Western blot was performed using an anti-IκB-α antibody. Bands were analyzed by densitometry, displayed as a bar histogram. B, B6J2 macrophages stably transfected with CA-AMPKα1 or empty vector were treated and analyzed as in A. C, Western blot was performed with antibodies against p-Akt (Ser473) and total Akt. The p-Akt/total Akt ratio was analyzed by densitometry and shown as a bar histogram. D, B6J2 macrophages stably transfected with CA-AMPKα1 were stimulated and assayed as in C. E, DN-AMPKα1 transfectants were stimulated as in A and Western blot was performed with antibodies against p-GSK3-β (Ser9) and total GSK3-β. The p-GSK3-β/total GSK3-β ratio was analyzed by densitometry and shown as a bar histogram. F, CA-AMPKα1 transfectants were stimulated and assayed as in E. G, DN-AMPKα1 transfectants were stimulated as in A and Western blot was performed with antibodies against p-CREB (Ser133) and total CREB. The p-CREB/total CREB ratio was analyzed by densitometry and shown as a bar histogram. H, CA-AMPKα1 transfectants were stimulated and assayed as in G. Data shown are representative of 2 (B,D,E,G) and 3 (A,C,F,H) independent experiments with similar results.
AMPK has also been shown to modulate downstream events mediated by phosphoinositide-3 kinase (PI3K) (26). PI3K indirectly activates the serine/threonine kinase Akt, which in turn can phosphorylate glycogen synthase kinase β (GSK3-β) at Ser9, resulting GSK3-β inhibition. The PI3K/Akt pathway has been shown to regulate the production of inflammatory cytokines in monocytes and macrophages (27) and inhibition of GSK3-β has been shown to inhibit TLR-mediated production of pro-inflammatory cytokines, while enhancing IL-10 production by human monocytes (27,28). As shown in Figure 5C, macrophages expressing DN-AMPKα1 display greatly reduced Akt Ser473 phosphorylation after LPS stimulation as compared to control, empty vector transfectants, whereas macrophages expressing CA-AMPKα1 display enhanced Akt Ser473 phosphorylation, indicative of Akt activation (Fig. 5D).
Having shown that AMPK activity is associated with enhanced Akt activation, we examined the influence of AMPK on the Akt substrate GSK3-β. Interestingly, despite the dampening effect of DN-AMPKα1 expression in macrophages on Akt phosphorylation, DN-AMPKα1 expression did not impact GSK3-β phosphorylation after LPS stimulation (Fig. 5E). However, expression of CA-AMPKα1 resulted in a substantial increase in the level of LPS induced GSK3-β phosphorylation, indicating that elevated AMPK activity promotes GSK3β inactivation in macrophages (Fig. 5F). GSK3-β is known to negatively regulate the activation of the transcription factor cyclic AMP responsive element binding protein (CREB) (29). CREB activation has been shown to be essential in IL-10 production by monocytes (30). Since we found that activation of AMPK resulted in enhanced IL-10 production as well as GSK3-β inactivation following LPS stimulation, we evaluated the impact of AMPK activity on CREB Ser133 phosphorylation/activation. Moreover, AMPK has been recently shown to directly phosphorylate CREB at Ser 133 causing its activation (31). As shown in Figure 5G, expression of DN-AMPKα1 resulted in reduced CREB Ser133 phosphorylation 30 min after LPS stimulation as compared to empty vector transfected macrophages. In contrast, expression of CA-AMPKα1 in macrophages enhanced LPS-induced CREB phosphorylation/activation (Fig. 5H). These data suggest a pathway whereby enhanced AMPK leads to Akt activation resulting in GSK3-β phosphorylation/inactivation and CREB phopshorylation/activation and fit well with the published evidence for a role of GSK3-β in modulating inflammatory function (27,28).
Discussion
Macrophages are capable of displaying a wide range of functional phenotypes and can produce different arrays of both pro- and anti-inflammatory mediators in response to various stimuli, or due to their tissue environment (32). Stimulation of macrophages with the Th1 cytokine IFNγ is often referred to as “classical activation” whereas stimulation with the Th2 cytokines IL-4 and IL-13 has been assigned the designation “alternative activation” (33). The designations M1 and M2 have also been used to categorize macrophages expressing a pro-inflammatory versus an anti-inflammatory functional profile, respectively (34,35). Although these terminologies are used to broadly define macrophages as either inflammatory or anti-inflammatory state, in most cases the patterns of gene expression of macrophages in response to various stimuli, or in response to their tissue environment, are heterogeneous and do not precisely fit the published patterns associated with these M1/M2 designations (36–38). Indeed, the array of possible functional outcomes of macrophage activation is quite broad (32) and we and others have demonstrated that macrophages can rapidly change their functional profile, both in vitro and in vivo, in response to changes in the microenvironment (39–41). Therefore, the M1/M2 nomenclature defines only the most polarized macrophage functional profiles amongst a wide array, and these polarized states are mutable. The observation that macrophage functional phenotypes can be manipulated has drawn attention to macrophages as a potential therapeutic target (40,42). Thus, elucidation of the signaling pathways that regulate macrophage functional polarization will aid in the design of strategies for modification of macrophage behavior.
In the present study we have identified AMPK as a potent counter-regulator of macrophage inflammatory function and promoter of macrophage polarization towards an anti-inflammatory phenotype. Our data demonstrate an association of AMPK activity with reduced IκB degradation, enhanced Akt activity, GSK3β inhibition, and activation of CREB. The enhanced Akt activation associated with AMPK activity is of interest given the mixed reports of the relationship of AMPK and Akt. While some studies demonstrate a positive correlation of AMPK activity with that of Akt, as we report here, there are many reports of an association of AMPK activation with decreased Akt activation (reviewed in ref. 26). We found that macrophages expressing a constitutively active AMPKα1, CA-AMPKα1, displayed enhanced Akt Ser473 phosphorylation as compared to empty vector transfected macrophages, whereas macrophages expressing a dominant negative AMPKα1, DN-AMPKα1, display diminished Akt Ser473 phosphorylation following LPS stimulation (Fig. 5 C and D). It has been established that activation of Akt requires both phosphorylation of Thr308 by the phosphoinositide-dependent kinase 1 (PDK1) (43,44) and phosphorylation of the Ser473 by the mammalian target of rapamycin complex 2 (mTORC2) (45). The serine/threonine kinase mTOR is a key regulator of the protein synthesis and cell growth. Due to energy depletion, AMPK inhibits mTOR complex 1 (mTORC1) by phosphorylating and activating the negative regulator of mTOR, tuberous sclerosis complex 2 (TSC2) (10). In contrast to AMPK, Akt can activate mTORC1 by phosphorylating and inhibiting TSC2 (46,47). mTOR is the only critical subunit shared by mTORC1 and mTORC2, and competition for mTOR interaction can occur between the mTORC1 and mTORC2 complexes (48). In macrophages expressing CA-AMPKα1, it is possible that inhibition of mTORC1 by AMPK could increase availability of mTOR to the mTORC2 complex. The enhanced phosphorylation of Akt in these cells could therefore be due to the ability of mTORC2 to phosphorylate Akt at Ser473. Akt would then be available to activate mTORC1 to balance mTORC1 activity in the cell.
The increased LPS-mediated Akt activation and GSK3β inhibition in macrophages expressing CA-AMPKα1 indicates that active AMPK may exert its anti-inflammatory effects through an Akt/GSK3β pathway (28). Interestingly, although we see an increase in LPS-mediated GSK3β phosphorylation/inhibition in CA-AMPKα1-exressing macrophages, we do not observe any decrease in GSK3β phosphorylation in macrophages expressing DN-AMPKα1 (Fig. 5E ). This observation may be also explained by the influence of AMPK on the mTOR pathway. mTORC1 controls mRNA translation, in part, by phosphorylating and activating S6K1 which phosphorylates the S6 protein of the 40S ribosomal protein (49). S6K1 has been found to phosphorylate GSK3β at Ser9, resulting in GSK3β inactivation under conditions where mTORC1 is over-active (50). Therefore, it is likely that the absence of a decrease in GSK3β Ser9 phosphorylation in DN-AMPKα1-expressing macrophages may be due to the over-activation of mTORC1, resulting from decreased AMPK activity. This would lead to enhanced S6K1 activity and, therefore, the maintenance of GSK3β phosphorylation.
Since GSK3-β is a negative regulator of the transcription factor CREB, GSK3-β inactivation enhances CREB activity (28,29). It is hypothesized that GSK3-β inhibition allows CREB to compete for the nuclear coactivator protein CBP (CREB-binding protein), also required for NF-κB function. This results in reduced NF-κB activation of proinflammatory gene expression, and enhanced expression of CREB-activated IL-10 synthesis (28). This scenario fits well with our data, as we observe elevated CREB activation in macrophages expressing CA-AMPK (Fig. 5H), as well as elevated IL-10 production (Fig. 4F). In earlier published work, GSK3-β inhibition was shown to decrease the binding of NF-κB p65 with CBP, but did not affect the levels of nuclear NF-κB p65 (28). In contrast, our finding that IκBα degradation is attenuated in CA-AMPKα1 transfectants (Fig. 5B) suggests that NF-κB activation and translocation to the nucleus are likely to be reduced in the presence of high AMPK activity.
In addition to IL-10 and TGFβ, we have found that other anti-inflammatory mediators such as IL-4, the PPARγ agonists 15dPJG2 and ciglitazone, as well as the green tea polyphenol epigallocatechin-3-gallate (EGCG), activate AMPK in macrophages (unpublished data), suggesting that, regardless of the initial signaling events involved, anti-inflammatory signals converge on AMPK as a central regulator that promotes progression to an anti-inflammatory phenotype. As mentioned previously, the two known upstream activators of AMPK are the kinases LKB1 (4) and CAMKKβ (5), however, a potential role of these kinases as activators of AMPK activation in macrophages has not been established. Due to the diversity of molecules capable of AMPK activation in macrophages, the identity of upstream activators is of particular interest and is under investigation in our laboratory. AMPK has frequently been referred to as the “metabolic master switch” in discussion of its role in the regulation of energy homeostasis (51). The ability of AMPK to be rapidly activated by anti-inflammatory stimuli, or rapidly inactivated by proinflammatory stimuli, combined with the downstream consequences of AMPK activity that we observe herein, implicates AMPK as a “master switch” of macrophage functional polarization.
Acknowledgements
The authors thank Ms Lihua Zhang for her excellent technical assistance and Caglar Cekic for valuable comments and suggestions.
Abbreviations used in this paper
- AMPK
AMP-activated protein kinase
- ACC
acetyl-CoA carboxylase
- COX-2
cyclooxygenase- 2
- GSK3-β
glycogen synthase kinase β
- IκB-α
inhibitory κB-α
- IKK
inhibitory κB kinase
- AICAR
5-aminoimidazole-4-carboxamide ribose
- siRNA
small interfering RNA
- mRNA
messenger RNA
- DN-AMPKα1
dominant negative AMP-activated protein kinase α1
- CA-AMPKα1
constitutively active AMP-activated protein kinase α1
- Ser
serine
Footnotes
This work was supported by National Institutes of Health grant AI048850 (J. Suttles), by an American Heart Association Predoctoral Fellowship (D. Sag), and, in part, by the Commonwealth of Kentucky Research Challenge Trust Fund (J. Suttles and R. D. Stout).
Disclosures
The authors have no financial conflict of interest.
References
- 1.Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J. Biochem. 1997;246:259. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 3.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J. Biol. Chem. 1996;271:27879. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 4.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 2007;403:139. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henin N, Vincent MF, Gruber HE, Van den BG. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995;9:541. doi: 10.1096/fasebj.9.7.7737463. [DOI] [PubMed] [Google Scholar]
- 8.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase--development of the energy sensor concept. J. Physiol. 2006;574:7. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 10.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 11.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem. Sci. 2004;29:18. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Nath N, Giri S, Prasad R, Salem ML, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J. Immunol. 2005;175:566. doi: 10.4049/jimmunol.175.1.566. [DOI] [PubMed] [Google Scholar]
- 13.Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J. Biol. Chem. 2004;279:20767. doi: 10.1074/jbc.M401390200. [DOI] [PubMed] [Google Scholar]
- 14.Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J. Neurosci. 2004;24:479. doi: 10.1523/JNEUROSCI.4288-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towler MC, Hardie DG. AMP-Activated Protein Kinase in Metabolic Control and Insulin Signaling. Circ Res. 2007;100:328. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 16.Jhun BS, Jin Q, Oh YT, Kim SS, Kong Y, Cho YH, Ha J, Baik HH, Kang I. 5-Aminoimidazole-4-carboxamide riboside suppresses lipopolysaccharide-induced TNF-alpha production through inhibition of phosphatidylinositol 3-kinase/Akt activation in RAW 264.7 murine macrophages. Biochem. Biophys. Res Commun. 2004;318:372. doi: 10.1016/j.bbrc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 17.Kuo CL, Ho FM, Chang MY, Prakash E, Lin WW. Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 gene expression by 5-aminoimidazole-4-carboxamide riboside is independent of AMP-activated protein kinase. J. Cell Biochem. 2008;103:931. doi: 10.1002/jcb.21466. [DOI] [PubMed] [Google Scholar]
- 18.Mukundan L, Bishop GA, Head KZ, Zhang L, Wahl LM, Suttles J. TNF receptor-associated factor 6 is an essential mediator of CD40-activated proinflammatory pathways in monocytes and macrophages. J. Immunol. 2005;174:1081. doi: 10.4049/jimmunol.174.2.1081. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds JM, Liu Q, Brittingham KC, Liu Y, Gruenthal M, Gorgun CZ, Hotamisligil GS, Stout RD, Suttles J. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:313. doi: 10.4049/jimmunol.179.1.313. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods A, zzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell Biol. 2000;20:6704. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemons-Miller AR, Cox GW, Suttles J, Stout RD. LPS stimulation of TNF-receptor deficient macrophages: a differential role for TNF-alpha autocrine signaling in the induction of cytokine and nitric oxide production. Immunobiology. 2000;202:477. doi: 10.1016/s0171-2985(00)80105-9. [DOI] [PubMed] [Google Scholar]
- 23.Okayasu T, Tomizawa A, Suzuki K, Manaka K, Hattori Y. PPARalpha activators upregulate eNOS activity and inhibit cytokine-induced NF-kappaB activation through AMP-activated protein kinase activation. Life Sci. 2008;82:884. doi: 10.1016/j.lfs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Hattori Y, Nakano Y, Hattori S, Tomizawa A, Inukai K, Kasai K. High molecular weight adiponectin activates AMPK and suppresses cytokine-induced NF-kappaB activation in vascular endothelial cells. FEBS Lett. 2008 doi: 10.1016/j.febslet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 25.Karin M, Delhase M. The I kappa B kinase (IKK ) and NF-kappa B: key elements of proinflammatory signalling. Seminars in Immunology. 2000;12:85. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 26.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J. Physiol. 2006;574:63. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guha M, Mackman N. The Phosphatidylinositol 3-Kinase-Akt Pathway Limits Lipopolysaccharide Activation of Signaling Pathways and Expression of Inflammatory Mediators in Human Monocytic Cells. Journal of Biological Chemistry. 2002;277:32124. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 28.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005;6:777. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimes CA, Jope RS. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J. Neurochem. 2001;78:1219. doi: 10.1046/j.1471-4159.2001.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platzer C, Fritsch E, Elsner T, Lehmann MH, Volk HD, Prosch S. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur. J. Immunol. 1999;29:3098. doi: 10.1002/(SICI)1521-4141(199910)29:10<3098::AID-IMMU3098>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 31.Thomson DM, Herway ST, Fillmore N, Kim H, Brown JD, Barrow JR, Winder WW. AMP-activated protein kinase phosphorylates transcription factors of the CREB family. J. Appl. Physiol. 2008;104:429. doi: 10.1152/japplphysiol.00900.2007. [DOI] [PubMed] [Google Scholar]
- 32.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004;76:509. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 34.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000;164:6166. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J. Leukoc. Biol. 2002;72:101. [PubMed] [Google Scholar]
- 37.Sironi M, Martinez FO, D'Ambrosio D, Gattorno M, Polentarutti N, Locati M, Gregorio A, Iellem A, Cassatella MA, Van DJ, Sozzani S, Martini A, Sinigaglia F, Vecchi A, Mantovani A. Differential regulation of chemokine production by Fcgamma receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2) J. Leukoc. Biol. 2006;80:342. doi: 10.1189/jlb.1005586. [DOI] [PubMed] [Google Scholar]
- 38.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, Shibata T, Takami T. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J. Leukoc. Biol. 2008;83:1136. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 39.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005;175:342. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 40.Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J. Immunol. 2007;178:1357. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 41.Gratchev A, Kzhyshkowska J, Kothe K, Muller-Molinet I, Kannookadan S, Utikal J, Goerdt S. Mphi1 and Mphi2 can be re-polarized by Th2 or Th1 cytokines, respectively, and respond to exogenous danger signals. Immunobiology. 2006;211:473. doi: 10.1016/j.imbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008;18:349. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 1997;7:776. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 44.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 45.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 46.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002;4:648. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 47.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 48.Bhaskar PT, Hay N. The two TORCs and Akt. Dev. Cell. 2007;12:487. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and −2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13571. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol. Cell. 2006;24:185. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol. 1999;277:E1. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]