Abstract
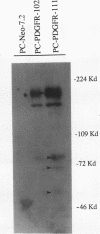
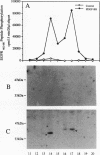
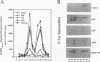
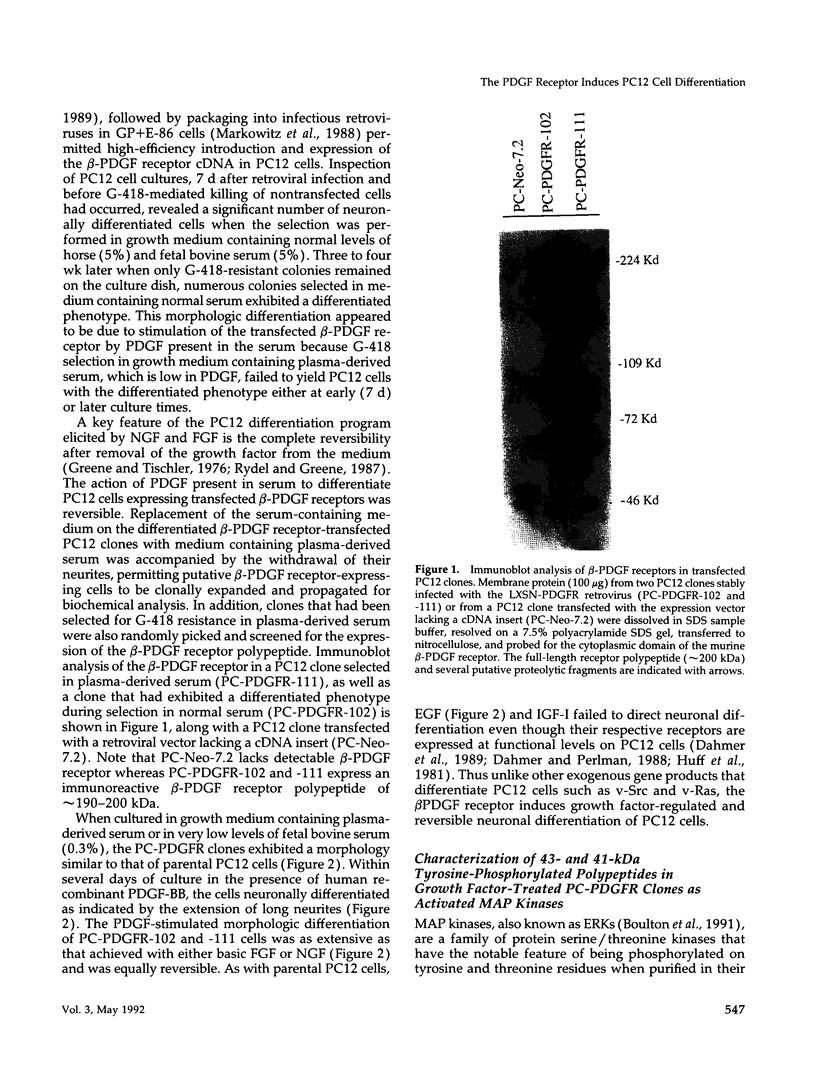
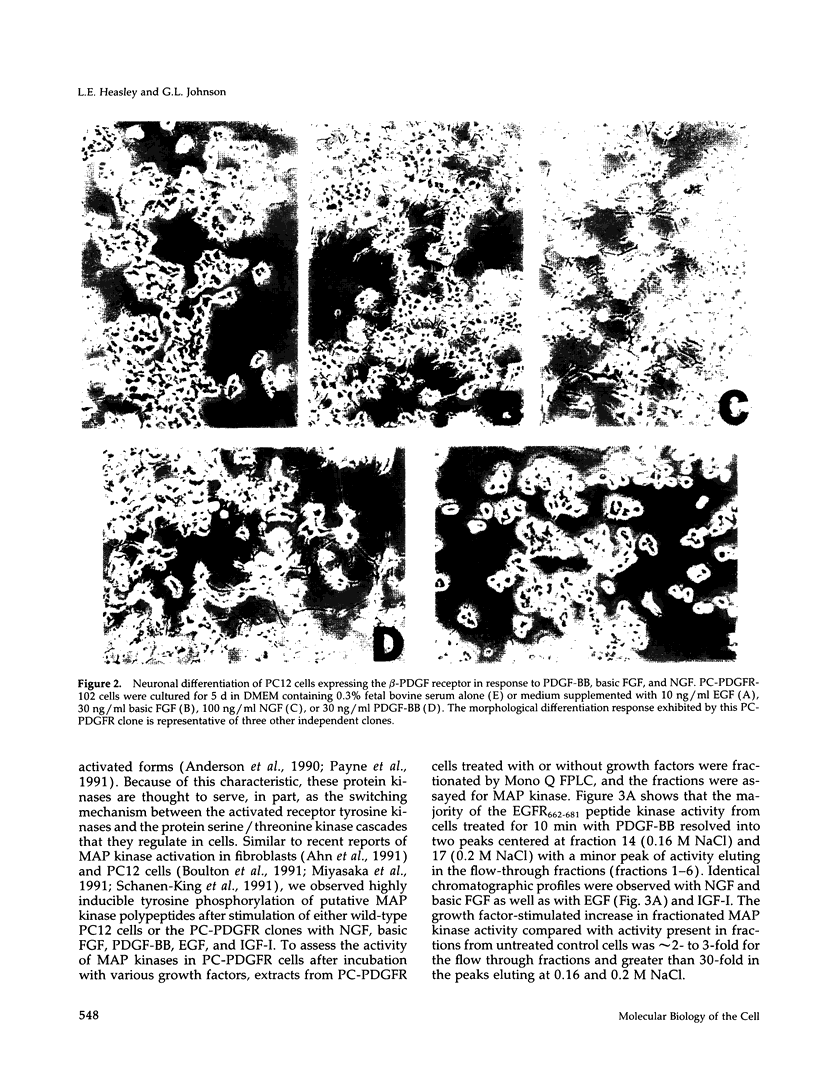
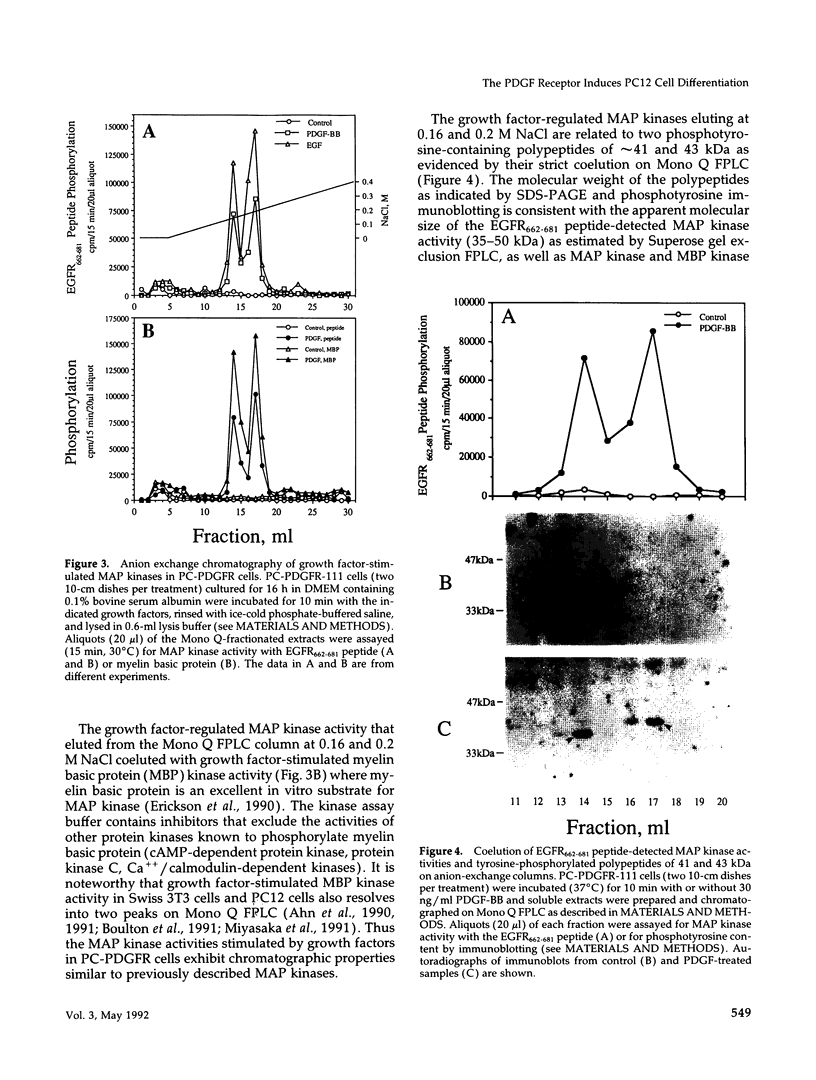
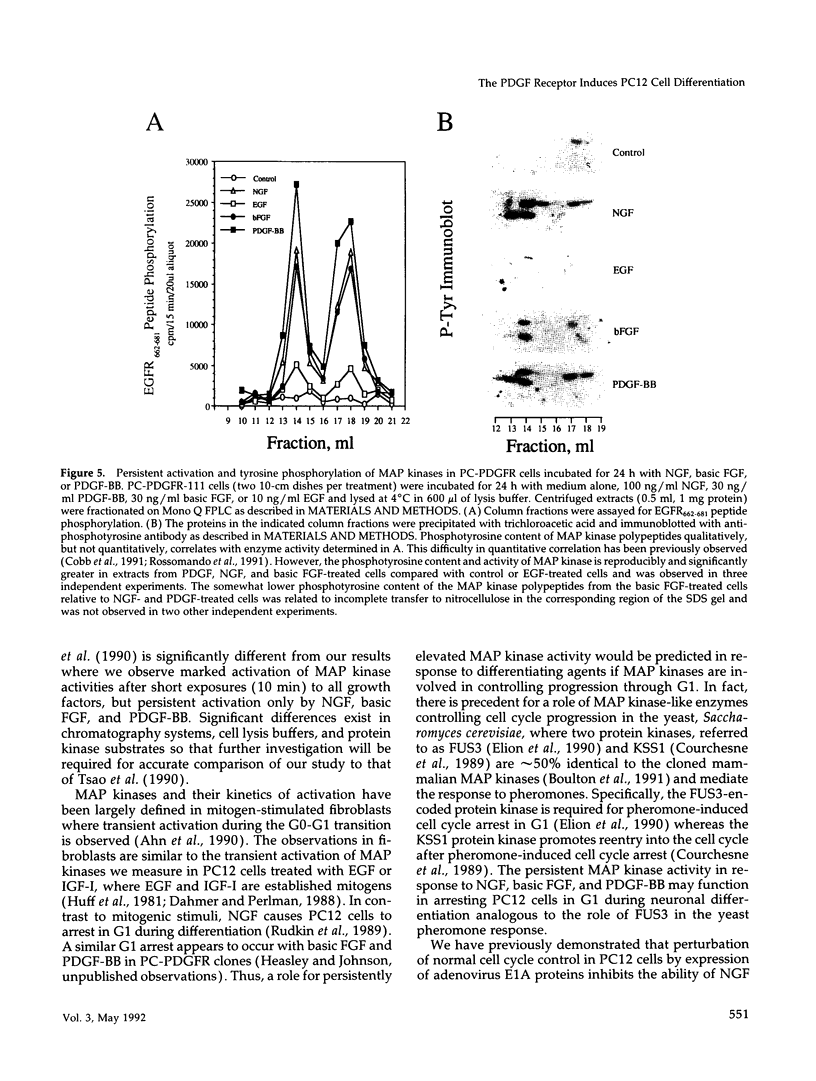
Expression of the mouse beta-PDGF receptor by gene transfer confers PDGF-dependent and reversible neuronal differentiation of PC12 pheochromocytoma cells similar to that observed in response to NGF and basic FGF. A common property of the PDGF, NGF, and basic FGF-induced differentiation response is the requirement for constant exposure of cells to the growth factor. To test the hypothesis that a persistent level of growth factor receptor signaling is required for the maintenance of the neuronal phenotype, we examined the regulation of the serine/threonine-specific MAP kinases after either short- (10 min) or long-term (24 h) stimulation with growth factors. Mono Q FPLC resolved two peaks of growth factor-stimulated MAP kinase activity that coeluted with tyrosine phosphorylated 41- and 43-kDa polypeptides. MAP kinase activity was markedly stimulated (approximately 30-fold) within 5 min of exposure to several growth factors (PDGF, NGF, basic FGF, EGF, and IGF-I), but was persistently maintained at 10-fold above basal activity after 24 h only by the growth factors that also induce PC12 cell differentiation (PDGF, NGF, and basic FGF). Thus the beta-PDGF receptor is in a subset of tyrosine kinase-encoded growth factor receptors that are capable of maintaining continuous signals required for differentiation of PC12 cells. These signals include the constitutive activation of cytoplasmic serine/threonine protein kinases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne W. E., Kunisawa R., Thorner J. A putative protein kinase overcomes pheromone-induced arrest of cell cycling in S. cerevisiae. Cell. 1989 Sep 22;58(6):1107–1119. doi: 10.1016/0092-8674(89)90509-6. [DOI] [PubMed] [Google Scholar]
- Dahmer M. K., Ji L., Perlman R. L. Characterization of insulin-like growth factor-I receptors in PC12 pheochromocytoma cells and bovine adrenal medulla. J Neurochem. 1989 Oct;53(4):1036–1042. doi: 10.1111/j.1471-4159.1989.tb07392.x. [DOI] [PubMed] [Google Scholar]
- Dahmer M. K., Perlman R. L. Insulin and insulin-like growth factors stimulate deoxyribonucleic acid synthesis in PC12 pheochromocytoma cells. Endocrinology. 1988 May;122(5):2109–2113. doi: 10.1210/endo-122-5-2109. [DOI] [PubMed] [Google Scholar]
- Elion E. A., Grisafi P. L., Fink G. R. FUS3 encodes a cdc2+/CDC28-related kinase required for the transition from mitosis into conjugation. Cell. 1990 Feb 23;60(4):649–664. doi: 10.1016/0092-8674(90)90668-5. [DOI] [PubMed] [Google Scholar]
- Erickson A. K., Payne D. M., Martino P. A., Rossomando A. J., Shabanowitz J., Weber M. J., Hunt D. F., Sturgill T. W. Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J Biol Chem. 1990 Nov 15;265(32):19728–19735. [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Yamashita T., Hoshi M., Kawakami M., Sakai H. Microtubule-associated-protein (MAP) kinase activated by nerve growth factor and epidermal growth factor in PC12 cells. Identity with the mitogen-activated MAP kinase of fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):661–669. doi: 10.1111/j.1432-1033.1990.tb19384.x. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez N., Tonks N. K., Morrison C., Harmar T., Cohen P. Evidence for communication between nerve growth factor and protein tyrosine phosphorylation. FEBS Lett. 1990 Oct 1;271(1-2):119–122. doi: 10.1016/0014-5793(90)80386-w. [DOI] [PubMed] [Google Scholar]
- Heasley L. E., Benedict S., Gleavy J., Johnson G. L. Requirement of the adenovirus E1A transformation domain 1 for inhibition of PC12 cell neuronal differentiation. Cell Regul. 1991 Jun;2(6):479–489. doi: 10.1091/mbc.2.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Detection of nerve growth factor and epidermal growth factor-regulated protein kinases in PC12 cells with synthetic peptide substrates. Mol Pharmacol. 1989 Mar;35(3):331–338. [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989 May 25;264(15):8646–8652. [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Huff K., End D., Guroff G. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J Cell Biol. 1981 Jan;88(1):189–198. doi: 10.1083/jcb.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Martin-Zanca D., Parada L. F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14;350(6314):158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Durden D. L., Cooper J. A. Functions of the major tyrosine phosphorylation site of the PDGF receptor beta subunit. Cell Regul. 1991 Jun;2(6):413–425. doi: 10.1091/mbc.2.6.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmeier P. T., Housey G. M., Johnson M. D., Perkins A. S., Weinstein I. B. Construction and characterization of a retroviral vector demonstrating efficient expression of cloned cDNA sequences. DNA. 1988 Apr;7(3):219–225. doi: 10.1089/dna.1988.7.219. [DOI] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Lev S., Givol D., Yarden Y. A specific combination of substrates is involved in signal transduction by the kit-encoded receptor. EMBO J. 1991 Mar;10(3):647–654. doi: 10.1002/j.1460-2075.1991.tb07993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P. A. Nerve growth factor induces protein-tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6788–6791. doi: 10.1073/pnas.85.18.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Schiavi S. C., Huse W., Johnson G. L., Ruley H. E. myc and E1A oncogenes alter the responses of PC12 cells to nerve growth factor and block differentiation. Oncogene. 1987;1(4):361–367. [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miyasaka T., Chao M. V., Sherline P., Saltiel A. R. Nerve growth factor stimulates a protein kinase in PC-12 cells that phosphorylates microtubule-associated protein-2. J Biol Chem. 1990 Mar 15;265(8):4730–4735. [PubMed] [Google Scholar]
- Miyasaka T., Sternberg D. W., Miyasaka J., Sherline P., Saltiel A. R. Nerve growth factor stimulates protein tyrosine phosphorylation in PC-12 pheochromocytoma cells. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2653–2657. doi: 10.1073/pnas.88.7.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley W. C., Schenker A., Shooter E. M. Characterization and isolation of proteolytically modified nerve growth factor. Biochemistry. 1976 Dec 14;15(25):5543–5552. doi: 10.1021/bi00670a019. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B., Singer S. J. Identification of a developmentally regulated protein-tyrosine kinase by using anti-phosphotyrosine antibodies to screen a cDNA expression library. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5449–5453. doi: 10.1073/pnas.86.14.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Sanghera J. S., Marsden L. A., Weber M. J., Pelech S. L., Sturgill T. W. Biochemical characterization of a family of serine/threonine protein kinases regulated by tyrosine and serine/threonine phosphorylations. J Biol Chem. 1991 Oct 25;266(30):20270–20275. [PubMed] [Google Scholar]
- Rudkin B. B., Lazarovici P., Levi B. Z., Abe Y., Fujita K., Guroff G. Cell cycle-specific action of nerve growth factor in PC12 cells: differentiation without proliferation. EMBO J. 1989 Nov;8(11):3319–3325. doi: 10.1002/j.1460-2075.1989.tb08493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydel R. E., Greene L. A. Acidic and basic fibroblast growth factors promote stable neurite outgrowth and neuronal differentiation in cultures of PC12 cells. J Neurosci. 1987 Nov;7(11):3639–3653. doi: 10.1523/JNEUROSCI.07-11-03639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanen-King C., Nel A., Williams L. K., Landreth G. Nerve growth factor stimulates the tyrosine phosphorylation of MAP2 kinase in PC12 cells. Neuron. 1991 Jun;6(6):915–922. doi: 10.1016/0896-6273(91)90232-o. [DOI] [PubMed] [Google Scholar]
- Takishima K., Griswold-Prenner I., Ingebritsen T., Rosner M. R. Epidermal growth factor (EGF) receptor T669 peptide kinase from 3T3-L1 cells is an EGF-stimulated "MAP" kinase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2520–2524. doi: 10.1073/pnas.88.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H., Aletta J. M., Greene L. A. Nerve growth factor and fibroblast growth factor selectively activate a protein kinase that phosphorylates high molecular weight microtubule-associated proteins. Detection, partial purification, and characterization in PC12 cells. J Biol Chem. 1990 Sep 15;265(26):15471–15480. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Yaciuk P., Carter M. C., Pipas J. M., Moran E. Simian virus 40 large-T antigen expresses a biological activity complementary to the p300-associated transforming function of the adenovirus E1A gene products. Mol Cell Biol. 1991 Apr;11(4):2116–2124. doi: 10.1128/mcb.11.4.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Schlessinger J., Chao M. V. Chimeric NGF-EGF receptors define domains responsible for neuronal differentiation. Science. 1991 Apr 26;252(5005):561–563. doi: 10.1126/science.1850551. [DOI] [PubMed] [Google Scholar]