Abstract
Although perforator flaps mark an important conceptual change in reconstructive surgery, individual perforator vessels show a high degree of variability with respect to anatomic landmarks. We have developed an intraoperative imaging system that simultaneously displays surgical anatomy and otherwise invisible near-infrared (NIR) images. In 22 adult pigs, perforating vessels were identified within seconds using this optical imaging system and systemic injection of indocyanine green. Perforator flaps were then designed based on these results, and vessel location confirmed by direct visualization and anatomic dissection. Since x-ray angiography remains the gold standard for identification of underlying vessels, conventional x-ray angiography was also performed in 8 pigs to verify the location of perforators. There was full correlation of all the perforators identified among NIR fluorescence angiography, x-ray angiography, and anatomic dissection. The technology we describe provides high-sensitivity real-time image guidance throughout perforator dissection, and permits patient-specific flap design.
Keywords: Perforator Flaps, Flap Design, Near-infrared Fluorescence Imaging, X-Ray Angiography, Indocyanine Green
INTRODUCTION
With the introduction of perforator flaps, harvest of vessels through the intramuscular course is possible as the muscle is spared. Identification of suitable vessels is the critical component in perforator flap design. Conventional x-ray angiography using an iodinated dye and x-ray fluoroscopy is the gold standard for identification of the vascular system; however, it is not routinely used for preoperative planning in perforator flap surgery.
A number of different imaging modalities have been used to evaluate perforators. Duplex ultrasound was one of the first modalities described for perforator identification; however, the technology can be highly operator dependent (1). Newer techniques such as CT and MRI have been described for pre-operative perforator identification and initial studies have shown potential for decreased operative times and complications (2-5). Stereotactic image-guided perforator imaging has also been described as a more accurate planning modality; however, it is still based on a pre-operative CT scan and its practical utility is limited (6,7). These techniques all evaluate perforators in the pre-operative planning phase as a majority of surgeons still rely on intraoperative clinical assessment of vessel size and location as the final determining factor. The importance of intraoperative evaluation has been highlighted in the recent literature and algorithms have been proposed for perforator selection in DIEP and MS-2 free TRAM flaps (8). Proponents of intraoperative evaluation discuss the importance of perforator assessment after release of the fascial collar, an element that cannot be ascertained with preoperative imaging.
Near-infrared (NIR) fluorescence has been previously used in reconstructive surgery as a method of demonstrating adequacy of perfusion in cutaneous flaps (9). This technology employs near-infrared light (700-900 nm), which can penetrate relatively deeply into living tissue, in order to evaluate tissue perfusion. NIR light is invisible to the human eye, thus can provide vessel information without altering the look of the surgical field. Indocyanine green (ICG), a NIR fluorophore already FDA-approved for systemic injection to assess liver function, is the fluorophore of choice for this application. Previously described NIR fluorescence imaging systems required laser irradiation (9, 10). In addition, only a static evaluation was performed for perioperative flap monitoring with crude black-and-white images (11). After isolated reports, this technology never gained popularity and remained available to very few surgeons.
Our laboratory has developed a real-time, light emitting diode (LED)-based NIR imaging system (12). The system exploits invisible near-infrared (NIR) light to assess the physiology of tissue while providing simultaneous color video imaging of the surgical field. Recently, we demonstrated the feasibility of intraoperative NIR fluorescence angiography for perforator identification in large animal models (13). This technology can measure flap perfusion as well as the location of associated perforators. Dynamic evaluation can be performed throughout the course of surgery with subsequent injections, and recorded images can be reviewed for patient-specific planning. In this study, we compare the reliability of our system for perforator identification and flap design with conventional x-ray angiography.
MATERIALS AND METHODS
Intraoperative NIR Fluorescence Imaging System
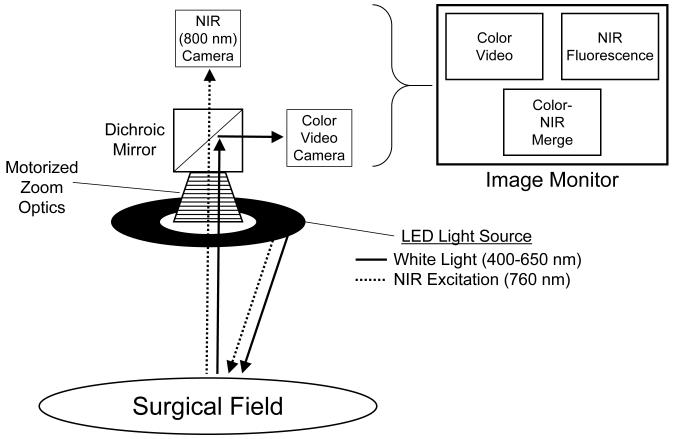
A schematic of the intraoperative imaging system used in these experiments is shown in Figure 1. The intraoperative NIR fluorescence imaging system has been described in detail previously (12). White light and invisible NIR fluorescence excitation light illuminate the surgical field. Reflected white light (i.e., surgical anatomy) is directed to a color video camera, while invisible NIR fluorescent light (from the introduced NIR fluorophore) is directed simultaneously to a NIR camera. The long working distance of the imaging system (18”) provided unobtrusive imaging during surgery.
Figure 1. Intraoperative NIR Fluorescence Imaging System.
The independent light paths for white light (solid arrows) and invisible NIR light (dotted arrows) are shown.
White (400-650 nm) light and NIR fluorescence excitation (745-779 nm) light are produced using LEDs. This system allows the surgeon to view color video, NIR fluorescence, and pseudo-colored merged images of the operative field in real-time. The merged image displays a non-anatomic pseudo-color (lime green) overlaid on images of the surgical field for accurate delineation. Images are acquired using custom optics and software, from which video can be reviewed and quantitative assessment performed. All images are refreshed up to 15 times per second. Hands-free operation utilizing motorized zoom and focus lenses with a foot-switch have been described in detail previously (14).
Animals and NIR Fluorescence Angiography
Animals were studied under the supervision of an approved institutional protocol. Anesthetized 35-kg Yorkshire pigs (n = 22) were injected with ICG (Sigma-Aldrich, St Louis, MO; 2.5 mg/ml stock solution in saline) as a rapid bolus via a central venous line. NIR fluorescence exposure time was 67 msec, with images stored to a hard disk every 500 msec pre- and post-injection. After the initial injection, the dominant perforators were identified and a flap was designed based on the perforators from the deep superior epigastric artery (DSEA). These flaps were unilaterally based and averaged 8 × 8 cm in size to facilitate visualization by the imaging system. Flap size was standardized throughout the study since perforator choice and location varied between animals. Another ICG injection was given after flap elevation to confirm perfusion. Fluorescence intensity of the perforating arteries (PA) was selected as a region-of-interest (ROI) and quantified in 12-bit pixel intensity. The contrast-to-noise-ratio (CBR) was calculated as CBR = (mean emitted fluorescence of ROI - mean fluorescence of background)/mean fluorescence of background.
C-Arm X-Ray Fluoroscopy and Angiography
In 8 of 22 DSEA perforator flaps, conventional x-ray angiography was used for further evaluation. Flap creation was first performed with guidance by the NIR imaging system, followed by cannulation of the DSEA with an indwelling catheter. Radiographic images were recorded by C-arm fluoroscopy (Series 9400 X-ray Imaging System, GE Healthcare, Princeton, NJ) with a 5 ml bolus of iodine contrast (Renografin®-60, Bracco Diagnostics, Princeton, NJ). The number of preserved PAs were identified through anatomic dissection and compared to NIR fluorescence optical imaging and x-ray angiography.
RESULTS
NIR Fluorescence-Guided Flap Design and Image-Guided Surgery
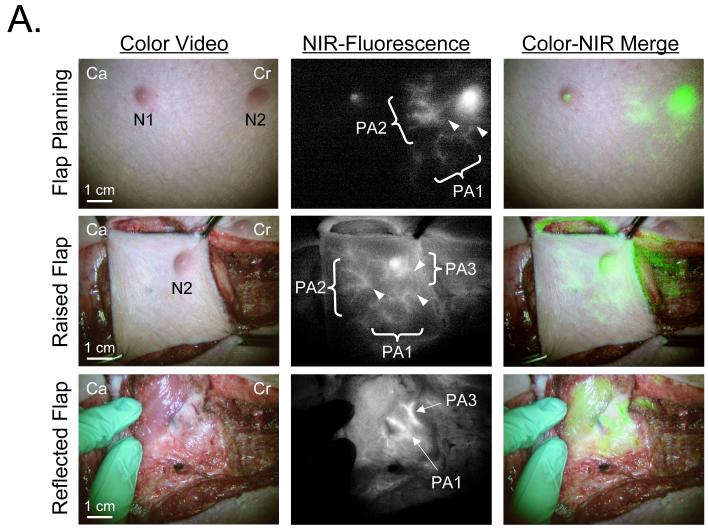
Perforator flaps were evaluated in all 22 pigs, since the location and distribution of PAs were identified by our system before and after flap creation in all cases (Fig. 2A). Dissection of the perforating vessels was performed for direct visualization and accurate confirmation. The average effective dose of ICG was 0.06 mg/kg per injection (range of 0.007 - 0.07 mg/kg), which generated a perforator CBR of ≥ 1.5. The time required for identification was less than one minute. The time interval between repeat injections was approximately 10 min, which minimized dose stacking. Accurate and precise perforator identification could be performed prior to surgery, and assessment of flap perfusion could be performed after flap elevation (Fig. 2A).
Figure 2. Real-Time Identification of the Perforating Arteries In Vivo.
A. Real-time intraoperative NIR fluorescence angiography. ICG (0.08 mg/kg; 0.1 mmol/kg; 2.5 mg total) was injected intravenously for flap planning (top row). Perforating arteries (PA1 and PA2; PA3 not shown) were identified around one of two nipples (N2) 12-sec post-injection. Arrowheads mark the origin of each PA. After raising the flap, all three PAs were identified by re-injection of ICG (11-sec post-injection; middle row). Two vascular pedicles, including PA1 and PA3 (bottom row; arrows), are shown at the underside of the flap. Images shown include color video (left), NIR fluorescence (middle), and a pseudo-colored (lime green) merged image of the two (right). Ca: Caudal, Cr: Cranial.
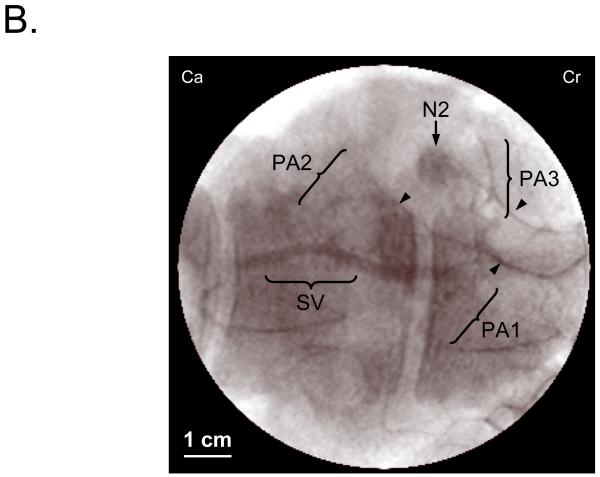
B. Conventional x-ray angiography of the same flap shown in (A). 5 ml of iodine contrast (Renografin®-60, Bracco Diagnostics, Princeton, NJ) was injected directly into the divided DSEA via an indwelling catheter. Arrowheads mark the penetrating point of each PA. The 3 PAs shown were all identified using NIR fluorescence as shown in (A). SV: Superficial vein.
Validation of NIR Fluorescence Imaging with Conventional C-Arm X-Ray Angiography
In the final 8 pigs, acquired NIR fluorescence images were compared before and after flap creation to images obtained using x-ray angiography. There was full correlation with the PAs identified using our technology and conventional x-ray angiography (Fig. 2B). The number of perforating vessels identified within each flap was retrospectively assessed, showing 100% correlation between the two imaging modalities (Table 1). Further confirmation was achieved by anatomic dissection of the perforating vessels, since all were located during surgery and fully matched the results from both NIR imaging and x-ray angiography (Table 1). As x-ray angiography was performed after flap creation and pedicle identification, this required cannulation of the source vessels and was used as a validation of NIR perforator identification and flap design. X-ray angiography was not used independently for flap design in our series.
Table 1. Number of Perforating Arteries Identified using NIR Fluorescence Angiography, X-Ray Angiography, and Anatomic Dissection.
| Animal | NIR Fluorescence | X-Ray Angiography |
Anatomic Dissection |
|
|---|---|---|---|---|
| Pre-Op | Post-Op | |||
| 1 | 4 | 4 | 4 | 4 |
| 2 | 5 | 5 | 5 | 5 |
| 3 | 5 | 5 | 5 | 5 |
| 4 | 5 | 5 | 5 | 5 |
| 5 | 3 | 3 | 3 | 3 |
| 6 | 4 | 4 | 4 | 4 |
| 7 | 6 | 6 | 6 | 6 |
| 8 | 5 | 5 | 5 | 5 |
DISCUSSION
In many areas of reconstructive surgery, perforator flaps are replacing conventional musculocutaneous flaps. Two difficulties arise with perforator flap reconstruction: perforator dissection and perforator selection. While perforator dissection is a technical exercise, perforator selection and mapping remains a challenge as current modalities all provide information based on pre-operative assessments.
Conventional x-ray angiography is routinely used to evaluate the vascular system and is the standard across many different fields including cardiology, cardiac surgery, interventional radiology and vascular surgery. The use of x-ray angiography, however, has not gained popularity for preoperative assessment in perforator flap reconstruction because of the increased risks involved with vessel cannulation, exposure to fluoroscopic radiation, and the toxicity profile of iodinated dyes. Less invasive techniques for evaluation of the perforators include handheld Doppler, Duplex ultrasound, CT, stereotactic CT, and MRI.
The handheld Doppler is currently used for perforator identification and post-operative monitoring in most microsurgical centers. Benefits include its ease of use and widespread availability; however, it provides limited data (1). Recent studies have shown that perforator mapping with the hand-held Doppler has limited accuracy in anterolateral flap design (15). Color Duplex ultrasound has the added advantage of determining blood flow and has been used extensively at many centers for pre-operative perforator mapping. The major disadvantage of the Duplex ultrasound is that it is highly operator dependent and therefore can be unreliable (16). Comparisons between Duplex ultrasound and pre-operative CT scan for perforator mapping have shown a clear advantage for the latter as this technology eliminates operator variability (5). The use of preoperative CT has also been shown to significantly reduce operative times in select studies (17). Accuracy can be further improved with stereotactic localization, however, this technology is not widely available and is still based on a CT scan taken pre-operatively (6, 7).
In this study, we performed real-time intraoperative NIR fluorescence angiography for evaluation of perforator location as well as flap perfusion. We validated the reliability of our imaging system by comparison to conventional x-ray angiography. In addition, direct identification of the perforators during flap elevation provided a further level of reliability through direct correlation of the vessel location. The NIR system was able to provide dynamic, real-time intraoperative guidance throughout the procedure, using a simple systemic injection. This is in sharp contrast to x-ray angiography, which required cannulation of the vessels under study and direct injection of the contrast agent.
ICG is currently FDA approved for use in determining cardiac output, hepatic function, liver blood flow, and for ophthalmic angiography. ICG angiography with NIR fluorescence has been previously used in reconstructive surgery for assessment of global flap perfusion (9, 10). These studies validated the clinical relevance of ICG fluorescence intensity to tissue perfusion and the excellent pharmacokinetic profile of ICG. Moreover, the use of NIR wavelengths for fluorescence emission minimizes tissue autofluorescence and photon scatter, resulting in relatively high sensitivity. Despite these advantages, this technique is infrequently used since the previously described imaging systems are laser-based, illuminate a small area of skin, and provide only a black-and-white image without color surgical landmarks (9, 10). The technology we describe utilizes safe LEDs, which permit high sensitivity imaging over a 15-cm field-of-view, without risk to the patient or surgeon. The custom optics permit simultaneous acquisition of NIR fluorescence and color video images, while software provides a “merged” image of the two. Additionally, fluorescence intensity from any desired region can be displayed for quantitative assessment of flap circulation.
With the safety profile of ICG and NIR angiography, we foresee its clinical use in the operating room as an adjunct during the planning phases of surgery. Ideally, prior to flap design, imaging is performed of the anatomic area to localize the underlying perforator vessels. The flap is then designed based on vessel location and size as imaging can be repeated during dissection to assess flap perfusion. For example, one critical phase may be after the fascial collar has been released around the perforator, where it may be beneficial to evaluate the vessel and flap again. Another example may be in perforator selection where the desired perforators are preserved and the remaining perforators can be temporarily clamped; imaging can be performed to assess flap perfusion at this time and, if deemed inadequate, clamps are removed and alternate vessels selected. In a microsurgical procedure, imaging can also be done to ascertain perfusion after flap transfer as well as evaluate the anastomotic site. Finally, in the post-operative phase, imaging can be performed to confirm proper flap perfusion. We currently have the ability to quantify perfusion based on the intensity of the NIR fluorescence in the flap and this may give us information in situations of flap loss and vessel compromise. These clinical studies are currently underway.
Although there is still no standardized method for evaluation of flap perfusion, most reconstructive surgeons still rely on clinical assessment such as flap color, capillary refill, and hand-held Doppler for flap monitoring. While our study does not evaluate free flap transfer and success after microsurgery, it addresses one of the more important aspects specific to perforator flap surgery, namely, localization of the underlying perforators. The intraoperative evaluation of perfusion for flap design could be equally applied to flap assessment after microsurgery.
Our imaging system does have some limitations for use during surgery. Although the imaging system is positioned 18” from the field, this can be logistically challenging in certain anatomic locations where access to the flap is difficult, such as a body cavity or an intra-oral reconstruction. The system, however, can be easily repositioned in and out of the field with the articulating arm. Another drawback is that our system may lengthen the operative time, unlike pre-operative imaging. Our system requires coordination between the surgeon, anesthesiologist, and operating room staff as the ICG IV bolus needs to be timed to the system setup. With familiarity, this additional time can be reduced, and the perfusion data from NIR imaging can be gathered within only 2 minutes.
Our study design also has some limitations. The swine model has few deep inferior epigastric perforating (DIEP) vessels compared to humans, but it does have a well-developed DSEA system and perforators (18); our flap model focused on the latter. While this DSEA system is not a perfect match for a DIEP flap commonly used in breast reconstruction, our model was designed to evaluate perforating vessels and flap design; this could be easily adapted to other perforator flap procedures. To permit standardization of our animal model, we purposely did not vary the flap dimensions. Further studies, beyond the scope of the present study, will be needed to determine the required perforator size for a desired flap size. Finally, pig skin is thicker than human skin and the subcutaneous fat layer in a pig is thinner than in a human. The results obtained, however, should be reproducible in humans and further studies are currently underway.
The addition of NIR imaging provides another tool for the reconstructive surgeon in perforator identification and flap design. An intraoperative assessment provides information that is difficult to obtain from other modes of pre-operative imaging. We are able to demonstrate that perforators identified based on NIR angiography correlate with traditional X-ray angiography.
ACKNOWLEDGMENTS
We thank Rita G. Laurence and Carrie S. Vooght for surgical assistance and Barbara L. Clough and Alice Gugelmann for editing. This work was funded by NIH grants #R01-EB-005805 and #R01-CA-115296 to J.V.F.
Sources of Funding: This work was funded by NIH grants #R01-EB-005805 and #R01-CA-115296 to J.V.F.
REFERENCES
- 1.Hallock GG. Doppler sonography and color duplex imaging for planning a perforator flap. Clin Plast Surg. 2003;30:347–357. v–vi. doi: 10.1016/s0094-1298(03)00036-1. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Burgos A, Garcia-Tutor E, Bastarrika G, et al. Preoperative planning of deep inferior epigastric artery perforator flap reconstruction with multislice-CT angiography: imaging findings and initial experience. J Plast Reconstr Aesthet Surg. 2006;59:585–593. doi: 10.1016/j.bjps.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Masia J, Clavero JA, Larranaga JR, et al. Multidetector-row computed tomography in the planning of abdominal perforator flaps. J Plast Reconstr Aesthet Surg. 2006;59:594–599. doi: 10.1016/j.bjps.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Masia J, Larranaga J, Clavero JA, et al. The value of the multidetector row computed tomography for the preoperative planning of deep inferior epigastric artery perforator flap: our experience in 162 cases. Ann Plast Surg. 2008;60:29–36. doi: 10.1097/SAP.0b013e31805003c2. [DOI] [PubMed] [Google Scholar]
- 5.Rozen WM, Phillips TJ, Ashton MW, et al. Preoperative imaging for DIEA perforator flaps: a comparative study of computed tomographic angiography and doppler ultrasound. Plast Reconstr Surg. 2008;121:1–8. [PubMed] [Google Scholar]
- 6.Rozen WM, Ashton MW, Stella DL, et al. Developments in perforator imaging for the anterolateral thigh flap: CT angiography and CT-guided stereotaxy. Microsurgery. 2008;28:227–232. doi: 10.1002/micr.20485. [DOI] [PubMed] [Google Scholar]
- 7.Rozen WM, Ashton MW, Stella DL, et al. Stereotactic image-guided navigation in the preoperative imaging of perforators for DIEP flap breast reconstruction. Microsurgery. 2008 doi: 10.1002/micr.20511. [DOI] [PubMed] [Google Scholar]
- 8.Lindsey JT. Integrating the DIEP and muscle-sparing (MS-2) free TRAM techniques optimizes surgical outcomes: presentation of an algorithm for microsurgical breast reconstruction based on perforator anatomy. Plast Reconstr Surg. 2007;119:18–27. doi: 10.1097/01.prs.0000244743.90178.89. [DOI] [PubMed] [Google Scholar]
- 9.Rubben A, Eren S, Krein R, et al. Infrared videoangiofluorography of the skin with indocyanine green--rat random cutaneous flap model and results in man. Microvasc Res. 1994;47:240–251. doi: 10.1006/mvre.1994.1018. [DOI] [PubMed] [Google Scholar]
- 10.Holm C, Tegeler J, Mayr M, et al. Monitoring free flaps using laser-induced fluorescence of indocyanine green: a preliminary experience. Microsurgery. 2002;22:278–287. doi: 10.1002/micr.10052. [DOI] [PubMed] [Google Scholar]
- 11.Evans BC, Evans GR. Microvascular surgery. Plast Reconstr Surg. 2007;119:18e–30e. doi: 10.1097/01.prs.0000246717.81077.e4. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka E, Choi HS, Fujii H, et al. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol. 2006;13:1671–1681. doi: 10.1245/s10434-006-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsui A, Lee BT, Winer J, et al. Real-time intraoperative near-infrared fluorescence angiography for perforator identification and flap design. Plast Recontr Surg. 2008 doi: 10.1097/PRS.0b013e31819a3617. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Gioux S, De Grand AM, Lee DS, et al. Improved optical sub-systems for intraoperative near-infrared fluorescence imaging. Proc. of SPIE. 2005;6009:39–48. [Google Scholar]
- 15.Yu P, Youssef A. Efficacy of the handheld Doppler in preoperative identification of the cutaneous perforators in the anterolateral thigh flap. Plast Reconstr Surg. 2006;118:928–933. doi: 10.1097/01.prs.0000232216.34854.63. discussion 934-925. [DOI] [PubMed] [Google Scholar]
- 16.Blondeel PN, Beyens G, Verhaeghe R, et al. Doppler flowmetry in the planning of perforator flaps. Br J Plast Surg. 1998;51:202–209. doi: 10.1016/s0007-1226(98)80010-6. [DOI] [PubMed] [Google Scholar]
- 17.Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstr Aesthet Surg. 2008 doi: 10.1016/j.bjps.2007.12.090. [DOI] [PubMed] [Google Scholar]
- 18.Taylor GI, Minabe T. The angiosomes of the mammals and other vertebrates. Plast Reconstr Surg. 1992;89:181–215. doi: 10.1097/00006534-199202000-00001. [DOI] [PubMed] [Google Scholar]