Abstract
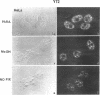
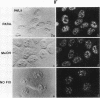
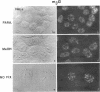
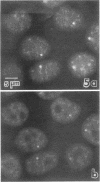
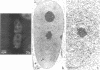
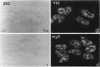
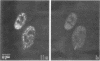
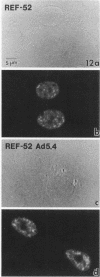
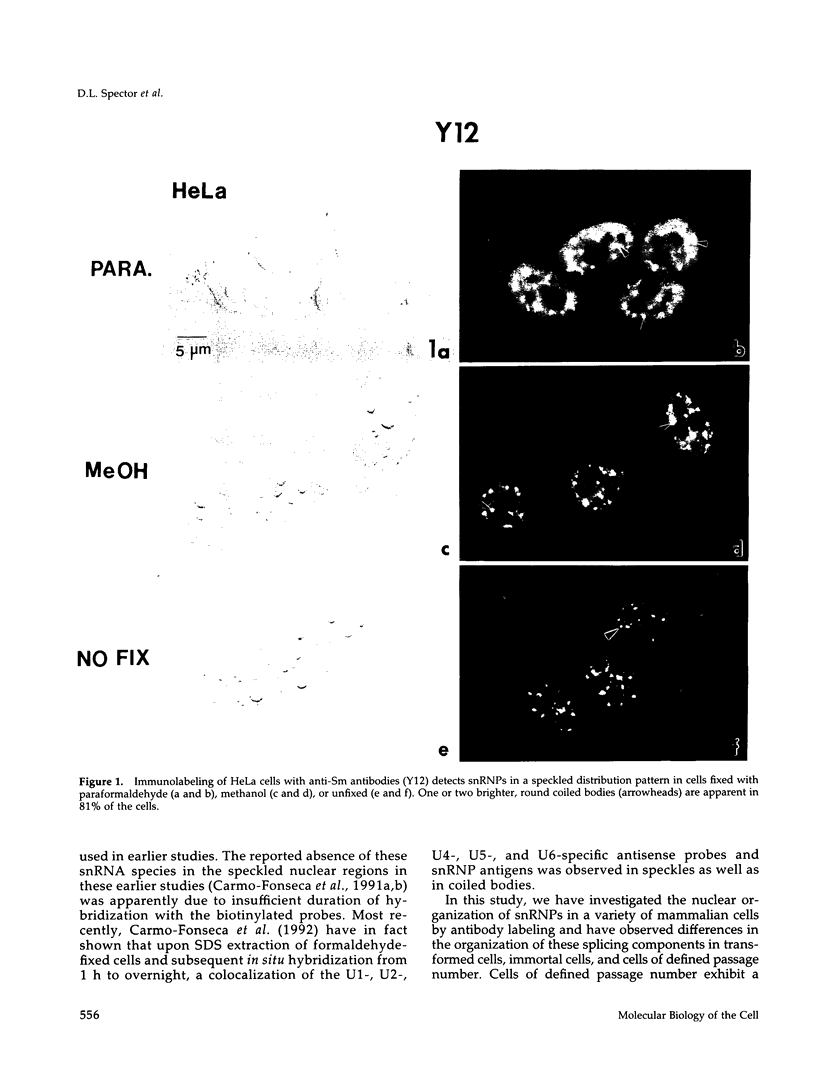
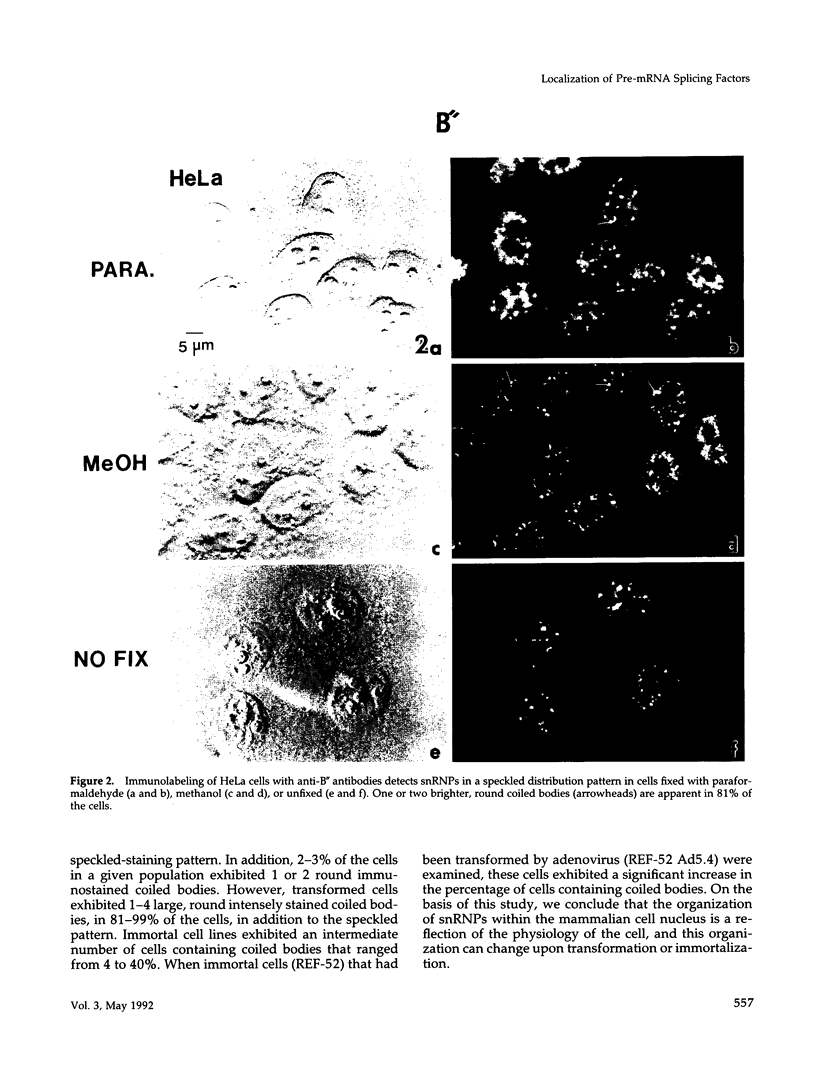
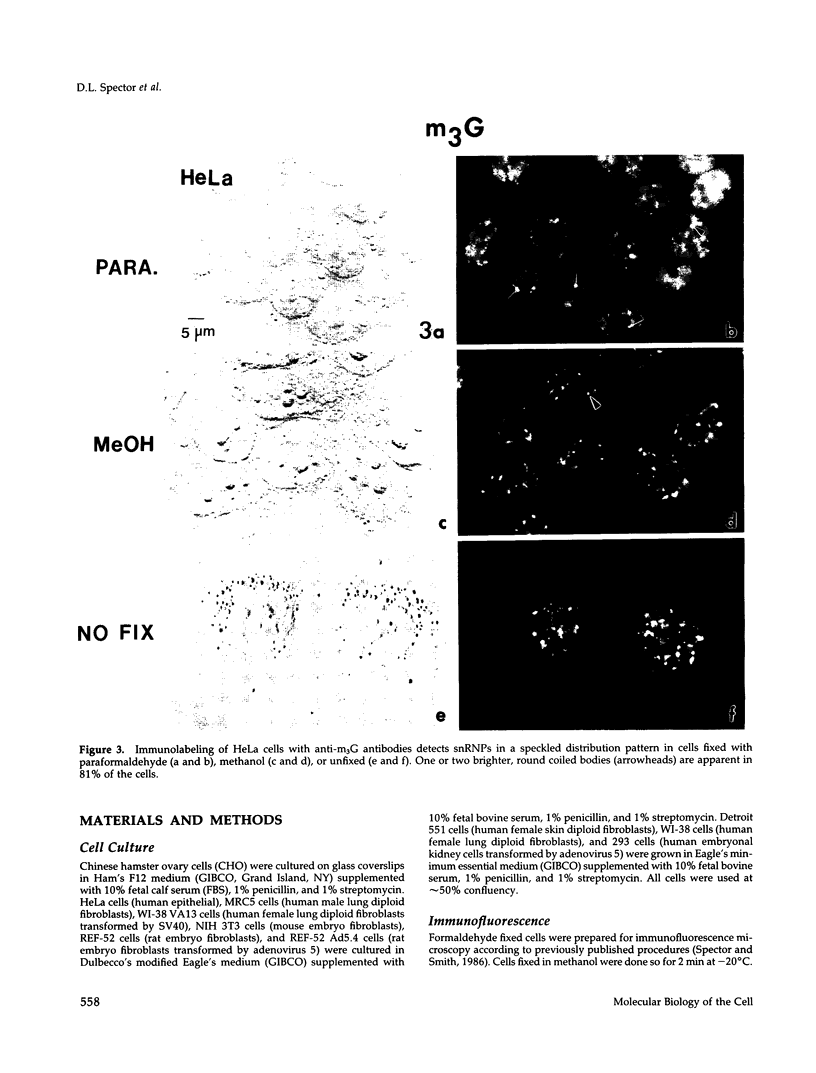
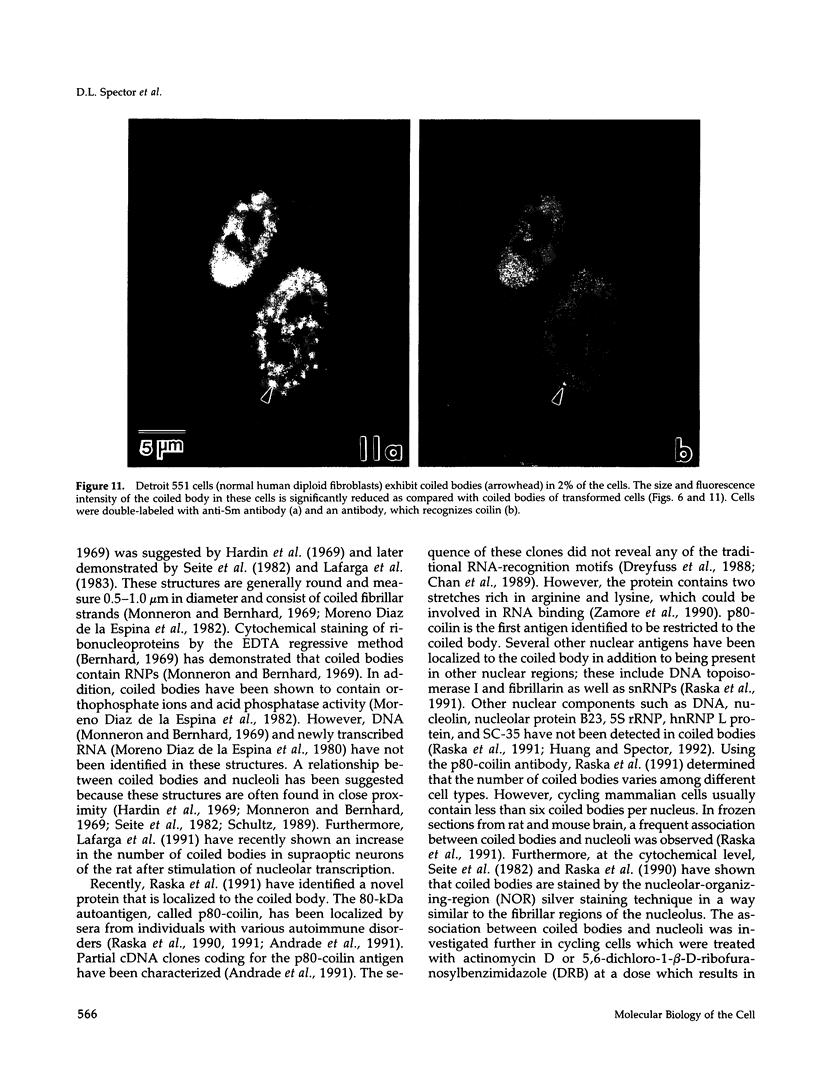
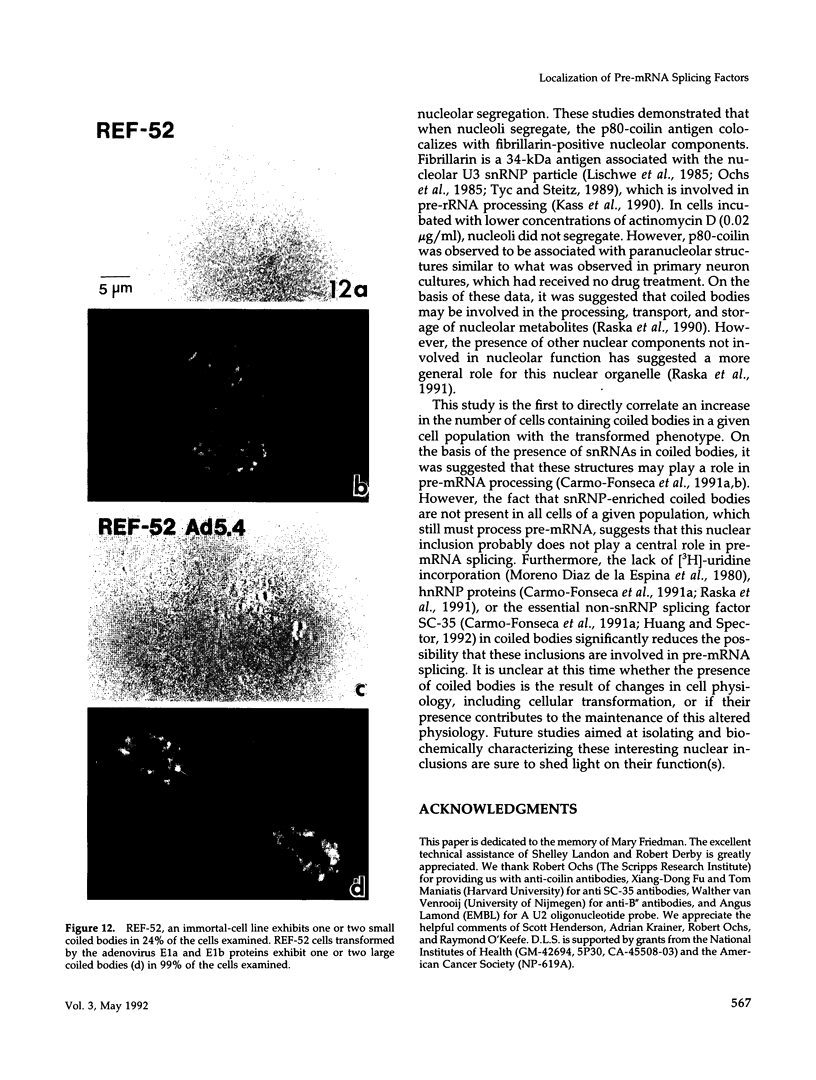
We have examined the localization of snRNPs in a variety of mammalian cells and have observed differences in the organization of these factors in transformed cells, immortal cells, and cells of defined passage number. Cells of defined passage number exhibit a speckled staining pattern after immunolabeling with anti-Sm, anti-B'', or anti-m3G antibodies. Furthermore, 2-3% of the cells, in a given population, exhibit labeling of 1 or 2 round coiled bodies in addition to the speckled-labeling pattern. However, transformed cells exhibited 1-4 intensely stained coiled bodies, in 81-99% of the cells, in addition to the speckled-labeling pattern. Immortal cells exhibited 1-4 intensely stained smaller coiled bodies in 4-40% of the cells, in addition to the speckled-labeling pattern. When immortal cells (REF-52) that had been transformed by adenovirus (REF-52Ad5.4) were examined, these cells exhibited an increase in the percentage of cells containing 1 or 2 intensely stained coiled bodies, in addition to the speckled labeling, from 24 to 99%. On the basis of this study, we conclude that the organization of snRNPs within the mammalian cell nucleus is a reflection of the physiology of the cell that may change upon transformation or immortalization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade L. E., Chan E. K., Raska I., Peebles C. L., Roos G., Tan E. M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med. 1991 Jun 1;173(6):1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard W. A new staining procedure for electron microscopical cytology. J Ultrastruct Res. 1969 May;27(3):250–265. doi: 10.1016/s0022-5320(69)80016-x. [DOI] [PubMed] [Google Scholar]
- Bindereif A., Green M. R. Identification and functional analysis of mammalian splicing factors. Genet Eng (N Y) 1990;12:201–224. doi: 10.1007/978-1-4613-0641-2_11. [DOI] [PubMed] [Google Scholar]
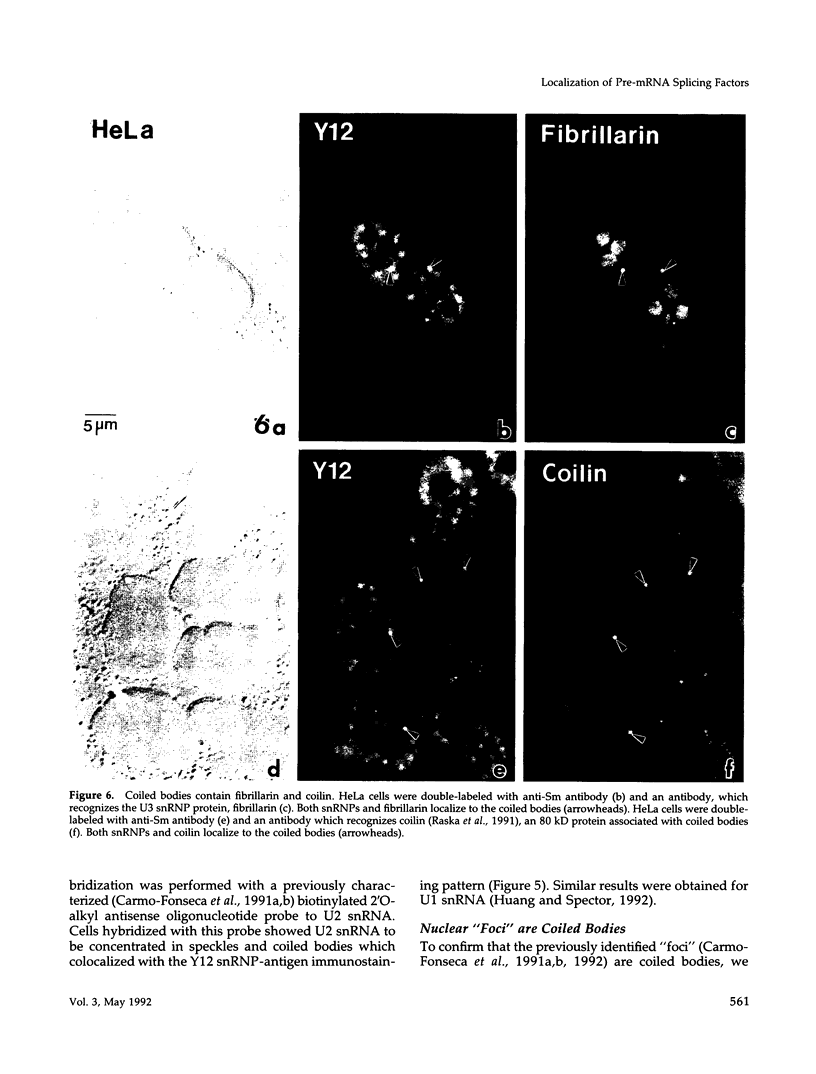
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Sproat B. S., Ansorge W., Swanson M. S., Lamond A. I. In vivo detection of snRNP-rich organelles in the nuclei of mammalian cells. EMBO J. 1991 Jul;10(7):1863–1873. doi: 10.1002/j.1460-2075.1991.tb07712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Fonseca M., Tollervey D., Pepperkok R., Barabino S. M., Merdes A., Brunner C., Zamore P. D., Green M. R., Hurt E., Lamond A. I. Mammalian nuclei contain foci which are highly enriched in components of the pre-mRNA splicing machinery. EMBO J. 1991 Jan;10(1):195–206. doi: 10.1002/j.1460-2075.1991.tb07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. K., Sullivan K. F., Tan E. M. Ribonucleoprotein SS-B/La belongs to a protein family with consensus sequences for RNA-binding. Nucleic Acids Res. 1989 Mar 25;17(6):2233–2244. doi: 10.1093/nar/17.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Swanson M. S., Piñol-Roma S. Heterogeneous nuclear ribonucleoprotein particles and the pathway of mRNA formation. Trends Biochem Sci. 1988 Mar;13(3):86–91. doi: 10.1016/0968-0004(88)90046-1. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L., Ryerse J. S. Detection of intranuclear clusters of Sm antigens with monoclonal anti-Sm antibodies by immunoelectron microscopy. J Cell Physiol. 1984 Nov;121(2):449–451. doi: 10.1002/jcp.1041210226. [DOI] [PubMed] [Google Scholar]
- Fakan S., Leser G., Martin T. E. Ultrastructural distribution of nuclear ribonucleoproteins as visualized by immunocytochemistry on thin sections. J Cell Biol. 1984 Jan;98(1):358–363. doi: 10.1083/jcb.98.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990 Feb 1;343(6257):437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- Habets W. J., Hoet M. H., De Jong B. A., Van der Kemp A., Van Venrooij W. J. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally-induced mouse monoclonal antibodies. J Immunol. 1989 Oct 15;143(8):2560–2566. [PubMed] [Google Scholar]
- Hardin J. H., Spicer S. S., Greene W. B. The paranucleolar structure, accessory body of Cajal, sex chromatin, and related structures in nuclei of rat trigeminal neurons: a cytochemical and ultrastructural study. Anat Rec. 1969 Aug;164(4):403–431. doi: 10.1002/ar.1091640403. [DOI] [PubMed] [Google Scholar]
- Huang S., Spector D. L. U1 and U2 small nuclear RNAs are present in nuclear speckles. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):305–308. doi: 10.1073/pnas.89.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass S., Tyc K., Steitz J. A., Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990 Mar 23;60(6):897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- Krainer A. R. Pre-mRNA splicing by complementation with purified human U1, U2, U4/U6 and U5 snRNPs. Nucleic Acids Res. 1988 Oct 25;16(20):9415–9429. doi: 10.1093/nar/16.20.9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafarga M., Andres M. A., Berciano M. T., Maquiera E. Organization of nucleoli and nuclear bodies in osmotically stimulated supraoptic neurons of the rat. J Comp Neurol. 1991 Jun 15;308(3):329–339. doi: 10.1002/cne.903080302. [DOI] [PubMed] [Google Scholar]
- Lafarga M., Hervás J. P., Santa-Cruz M. C., Villegas J., Crespo D. The "accessory body" of Cajal in the neuronal nucleus. A light and electron microscopic approach. Anat Embryol (Berl) 1983;166(1):19–30. doi: 10.1007/BF00317942. [DOI] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leser G. P., Fakan S., Martin T. E. Ultrastructural distribution of ribonucleoprotein complexes during mitosis. snRNP antigens are contained in mitotic granule clusters. Eur J Cell Biol. 1989 Dec;50(2):376–389. [PubMed] [Google Scholar]
- Lischwe M. A., Ochs R. L., Reddy R., Cook R. G., Yeoman L. C., Tan E. M., Reichlin M., Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985 Nov 15;260(26):14304–14310. [PubMed] [Google Scholar]
- Monneron A., Bernhard W. Fine structural organization of the interphase nucleus in some mammalian cells. J Ultrastruct Res. 1969 May;27(3):266–288. doi: 10.1016/s0022-5320(69)80017-1. [DOI] [PubMed] [Google Scholar]
- Nyman U., Hallman H., Hadlaczky G., Pettersson I., Sharp G., Ringertz N. R. Intranuclear localization of snRNP antigens. J Cell Biol. 1986 Jan;102(1):137–144. doi: 10.1083/jcb.102.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs R. L., Lischwe M. A., Spohn W. H., Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54(2):123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Raska I., Andrade L. E., Ochs R. L., Chan E. K., Chang C. M., Roos G., Tan E. M. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res. 1991 Jul;195(1):27–37. doi: 10.1016/0014-4827(91)90496-h. [DOI] [PubMed] [Google Scholar]
- Raska I., Ochs R. L., Andrade L. E., Chan E. K., Burlingame R., Peebles C., Gruol D., Tan E. M. Association between the nucleolus and the coiled body. J Struct Biol. 1990 Jul-Sep;104(1-3):120–127. doi: 10.1016/1047-8477(90)90066-l. [DOI] [PubMed] [Google Scholar]
- Reuter R., Appel B., Bringmann P., Rinke J., Lührmann R. 5'-Terminal caps of snRNAs are reactive with antibodies specific for 2,2,7-trimethylguanosine in whole cells and nuclear matrices. Double-label immunofluorescent studies with anti-m3G antibodies and with anti-RNP and anti-Sm autoantibodies. Exp Cell Res. 1984 Oct;154(2):548–560. doi: 10.1016/0014-4827(84)90179-4. [DOI] [PubMed] [Google Scholar]
- Schultz M. C. Ultrastructural study of the coiled body and a new inclusion, the "mykaryon," in the nucleus of the adult rat Sertoli cell. Anat Rec. 1989 Sep;225(1):21–25. doi: 10.1002/ar.1092250104. [DOI] [PubMed] [Google Scholar]
- Spector D. L. Colocalization of U1 and U2 small nuclear RNPs by immunocytochemistry. Biol Cell. 1984;51(1):109–112. doi: 10.1111/j.1768-322x.1984.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Spector D. L., Fu X. D., Maniatis T. Associations between distinct pre-mRNA splicing components and the cell nucleus. EMBO J. 1991 Nov;10(11):3467–3481. doi: 10.1002/j.1460-2075.1991.tb04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Schrier W. H., Busch H. Immunoelectron microscopic localization of snRNPs. Biol Cell. 1983;49(1):1–10. doi: 10.1111/j.1768-322x.1984.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Spector D. L., Smith H. C. Redistribution of U-snRNPs during mitosis. Exp Cell Res. 1986 Mar;163(1):87–94. doi: 10.1016/0014-4827(86)90560-4. [DOI] [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989 Oct;8(10):3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen R., Kuijpers H., Vooijs P., Van Venrooij W., Ramaekers F. Distribution of the 70K U1 RNA-associated protein during interphase and mitosis. Correlation with other U RNP particles and proteins of the nuclear matrix. J Cell Sci. 1986 Dec;86:173–190. doi: 10.1242/jcs.86.1.173. [DOI] [PubMed] [Google Scholar]
- Zamore P. D., Green M. R. Biochemical characterization of U2 snRNP auxiliary factor: an essential pre-mRNA splicing factor with a novel intranuclear distribution. EMBO J. 1991 Jan;10(1):207–214. doi: 10.1002/j.1460-2075.1991.tb07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Zapp M. L., Green M. R. Gene expression. RNA binding: beta s and basics. Nature. 1990 Dec 6;348(6301):485–486. doi: 10.1038/348485a0. [DOI] [PubMed] [Google Scholar]
- de la Espina S. M., Sánchez-Pina M. A., Risueño M. C. Localization of acid phosphatase activity, phosphate ions and inorganic cations in plant nuclear coiled bodies. Cell Biol Int Rep. 1982 Jun;6(6):601–607. doi: 10.1016/0309-1651(82)90184-9. [DOI] [PubMed] [Google Scholar]