Abstract
We sought to determine if sex impacts the cognitive and neuropathological phenotype of the 3xTg-AD mice. We find that male and female 3xTg-AD mice show comparable impairments on Morris water maze (MWM) and inhibitory avoidance (IA) at 4 months. Shortly thereafter, however, the cognitive performance varies among the sexes, with females performing worse than males. These behavioral differences are not attributable to differences in Aβ or tau levels. The behavioral effect is transient as from 12 months onward, the disparity is no longer apparent. Because females perform worse than males on stressful tasks, we explored their corticosterone responses, and find that young female 3xTg-AD mice show markedly heightened corticosterone response after 5 days of MWM training compared to age-matched male 3xTg-AD mice; this difference is no longer apparent in older mice. Thus, the enhanced corticosterone response of the young female mice likely underlies their poorer performance on stressful tasks.
Introduction
Alzheimer disease (AD) is the leading cause of dementia and progressive memory loss in the elderly population. This debilitating disorder is marked by the aggregation of two proteins: β-amyloid (Aβ), which accumulates in plaques, and tau, which can become hyperphosphorylated and aggregates in neurofibrillary tangles (Selkoe, 2001). Postmortem analysis indicates these lesions are found in equal proportions in male and female AD patients (Sandberg et al., 2001). Epidemiological studies over the past several years report conflicting results regarding gender-related vulnerability to AD. Some studies claim that women are disproportionately susceptible to AD, even after accounting for their longer life span (Molsa et al., 1982; Jorm et al., 1987; Fratiglioni et al., 1997; Gao et al., 1998) while others find that both genders are equally vulnerable to AD (Barnes et al., 2003). Furthermore, although the prevalence of AD is higher in women (Bachman et al., 1992), the incidence, which is often considered a more reliable measure, does not appear to be significantly different (Bachman et al., 1993). Although AD-related gender discrepancies remain an unresolved issue, the preponderance of evidence suggests that its effect is likely to be relatively minor.
Stress may be a complicating factor that influences the development and progression of AD. It is notable that AD patients also exhibit changes in the hypothalamic-pituitary-adrenal (HPA) axis function (Davis et al., 1986; Hatzinger et al., 1995; Peskind et al., 1996). AD patients appear to be especially sensitive to changes in cortisol levels as AD patients with higher cortisol levels perform worse than AD patients with lower cortisol levels on memory tasks (Carlson et al., 1999). It has been suggested that AD patients are unable to appropriately terminate their stress response which leads to chronic HPA axis hyperactivity and deleterious effects on the aging brain (Deshmukh and Deshmukh, 1990).
Our lab generated a mouse model that harbors three dementia-relevant transgenes: PS1M146V, tauP301L, and APPSWE (Oddo et al., 2003). These mice are triple transgenic (3xTg-AD), and develop progressive cognitive and neuropathological deficits (Oddo et al., 2003; Billings et al., 2005). The mice develop intracellular Aβ accumulation at 4 months (mos) and plaques are apparent by 15 mos. Somato-dendritic tau accumulation is evident at 6 mos and PHF-1 positive tangles are visible at 15-18 mos. Notably, we previously reported that stress significantly modulates the pathology in these mice, as seven days of treatment with a synthetic glucocorticoid, dexamethasone, markedly increases AD-related pathology (Green et al., 2006). There is an intimate relationship between stress and memory (for review see (Roozendaal et al., 1997)), and there are often sex-related differences in these processes as well (for review see (Cahill, 2003)).
In the following experiments, we evaluated male and female 3xTg-AD mice on cognitive tests including Morris water maze (MWM), inhibitory avoidance (IA), and novel object recognition. We also compared age-dependent neuropathological changes in Aβ and tau pathology in male and female 3xTg-AD mice. The sum of these findings indicates that the 3xTg-AD mice develop an age- and sex-dependent modulation of cognitive phenotype.
Materials and Methods
Mice
A total of 219 mice were used in this study. The generation of the 3xTg-AD mice was previously described (Oddo et al., 2003). Briefly, human APP with the Swedish mutation (KM670/671NL) and human tau with the P301L mutation were microinjected into single-cell embryos from homozygous PS1M146V knockin mice. The background of the PS1 knockin mouse is a hybrid 129/C57BL6. NonTg mice used were from the same strain and genetic background as the PS1 knockin mice, but they harbor the endogenous wild-type mouse PS1. Male and female mice were individually housed and kept on a 12 hr light:12 hr dark schedule. All mice were given ad libitum access to food and water. The MWM apparatus, spatial reference water maze training, cued MWM training, and inhibitory avoidance were performed as described in (Billings et al., 2005).
Behavioral assessment
All mice examined on the following behavior measures (MWM, IA, and object recognition) were tested in a cross-sectional design at 2, 4, 6, 9, 12, and 15 months of age (n=9-11 per group).
Morris Water Maze
The apparatus used for all water maze tasks was a circular aluminum tank (1.5m diameter) painted white and filled with water maintained at 26-29°C. The maze was located in a room containing several simple visual, extra-maze cues. All mice were placed on the platform in both the hidden and cued versions of the task for 10s prior to the first training trial to orient them to the existence of an escape platform.
Spatial reference water maze training
Mice were trained to swim to a 14-cm diameter circular, clear Plexiglas platform submerged 1.5-cm beneath the surface of the water and invisible to the mice. The location of the platform was selected randomly for each mouse, but was kept constant for each individual mouse throughout training. On each trial, the mouse was placed into the tank at one of four designated start points in a pseudorandom order. Mice were allowed to find and escape onto the submerged platform. If a mouse failed to find the platform within 60s, it was manually guided to the platform and allowed to remain there for 10s. After this, each mouse was placed into a holding cage under a warming lamp for 25s until the start of the next trial. To ensure that memory differences were not due to lack of task learning, mice were given four trials a day for as many days as were required to train the 3xTg-AD mice to criterion (< 20s mean group escape latency before the first probe trial was run). To control for overtraining, probe trials were run for each group both as soon as they reached group criterion (mean escape latency for group <20s).
Retention of the spatial training was assessed 1.5h and again 24h after the last training trial. Both probe trials consisted of a 60s free swim in the pool with the platform removed. Mice were monitored by a camera mounted in the ceiling directly above the pool, and all trials were stored on videotape for subsequent analysis. There were no significant differences between any genotypes in the swim speeds. The parameters measured during the probe trial included (1) initial latency to cross the platform location, (2) number of platform location crosses, and (3) time spent in the quadrant opposite to the one containing the platform during training. For the 6-month training, the target quadrant was changed to avoid “savings” from previous water maze experience. Target quadrants varied between animals within a group to control for potential differences in the salience of extramaze cues (Billings et al., 2005).
Inhibitory Avoidance
For inhibitory avoidance, testing began with a training trial in which the mouse was placed in a lighted chamber; when the mouse crossed over to the dark chamber it received a mild (0.25mA/1 sec) footshock. The footshock level was selected during a pilot study to determine an effective intensity level. This initial latency to enter the dark (shock) compartment served as the baseline measure. During the probe trials, 1.5- or 24hr after training, the mouse was again placed in the light compartment and the latency to return to the dark compartment (previously associated with shock) was measured as an index of inhibitory avoidance.
Object recognition
Mice were habituated to a 60 × 30 cm container for 5 minutes. Two identical objects were placed in the bottom of the cage as mirror images of each other. The animal was trained for 5 minutes followed by a 1.5- and 24h retention interval in the home cage. During this time, the experimenter replaced one of the objects with a novel object. During the 3-minute probe trial, the mouse was placed in the cage and time spent investigating both objects was measured. Retention was measured by recording how long the animal explored the novel object versus the familiar object. The following measures were collected (Tn = time spent exploring novel object (sec); Tf = time spent exploring familiar object). A recognition index (RI) score was calculated as follows: Tn * 100/(Tn + Tf).
Morris water maze training for corticosterone assay
Male and female 3xTg-AD and NonTg mice (n=5 for each group) were trained for five days on the MWM exactly as described for the behavior testing. Blood was collected retro-orbitally 30 minutes after the last trial on the fifth day of water maze training.
Retro-orbital blood collection
Fine-walled Pasteur pipettes (outer diameter of 1-2 mm) were inserted into the corner of the eye to collect blood as previously described in (Green et al., 2006).
Immunohistochemistry
6-, 9-, 12- and 15-month-old mice (n=8-10 per sex per age) were sacrificed by CO2 asphyxiation, and the brains were rapidly removed and hemi-sectioned. Half of the brain was placed directly in 4% paraformaldehyde buffered with PBS, pH 7.4, for 48 hours. Free-floating sections (50 μm thick) were processed for immunohistochemistry as described previously (Oddo et al., 2003). The anti-Aβ antibody 6E10 (Signet; Dedham, MA; diluted 1:1000), anti-tau antibody HT7 (Innogenetics; Alpharetta, GA; diluted 1:1000) anti-tau antibody AT8 (phosphoserine 202 and 205; Innogenetics; Alpharetta, GA; diluted 1:200), and anti-tau antibody 12E8 (phosphoserine 262; diluted 1:1000; generous gift from Dr. Peter Seubert) were applied overnight at 4°C and labeling was visualized with AlexaFlour 488 anti-mouse secondary antibody (Molecular Probes, Eugene, OR). Quantification of Aβ and tau in cortical and hippocampal regions was performed as follows. The images were captured using a Zeiss digital camera. The photomicrographs were imported to Scion Image and converted to black and white images and inverted. The threshold intensity was set manually and kept constant for all photomicrographs as the number of pixels was counted. Quantification of neuropathology was performed in a blinded fashion with regard to the sex of the animal.
Aβ ELISA
Aβ40 and Aβ42 levels were measured by sandwich ELISA using techniques as described in (Oddo et al., 2005). Briefly, whole brains were solubilized with TPER (Pierce, Rockford, IL) and spun at 35,000 RPM. The supernatant was removed (detergent-soluble fraction) and the pellets were solubilized with 70% formic acid and spun again at 35,000 RPM, and the supernatant was removed (detergent-insoluble fraction). n=8-10 per sex per age measured.
Corticosterone assay
Basal and 30 min post-water maze training corticosterone levels were measured in blood plasma using the Corticosterone EIA assay kit (Assay Designs, Ann Arbor, Michigan) as per the manufacturer's instructions. n=10-12 per sex per age and genotype analyzed.
Statistics
For behavior tests, multifactor ANOVA including age, genotype, sex and/or MWM or IA probe trial (1.5 hr and 24 hr). Student's t-tests were used to determine sex differences on MWM-induced corticosterone response, ELISA, and immunofluorescent quantification. Data were considered to be significant if p <0.05.
Results
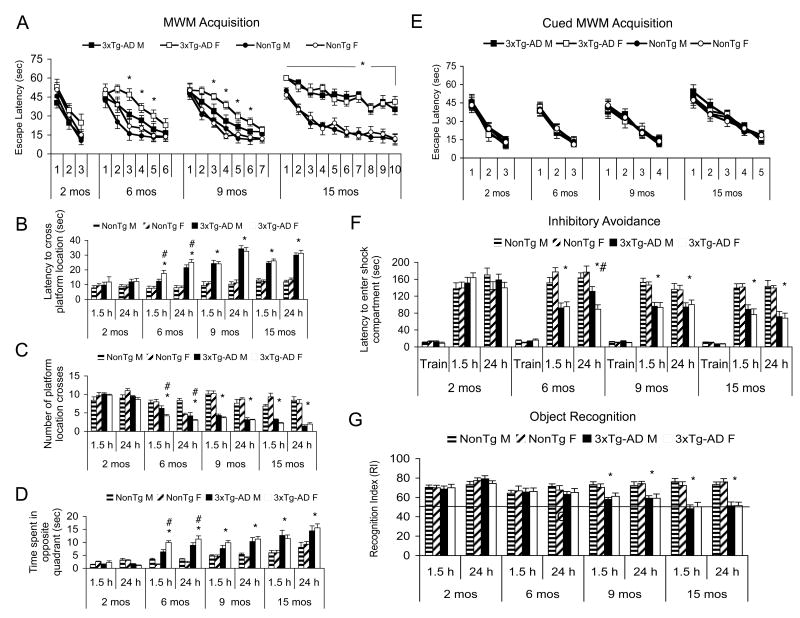
The 3xTg-AD mice begin to show impairments on MWM and IA at 4 months, coincident with the onset of intracellular Aβ (Billings et al., 2005). However, the 3xTg-AD mice are unimpaired on the cued version of the MWM as previously reported (Billings et al., 2005), and there are no trends or significant differences in swim speed (data not shown). At 2- and 4-months of age, male and female 3xTg-AD mice perform comparably on hippocampal-, cortical-, and amygdala-dependent tasks as measured by MWM, IA, and object recognition. However, we noted a sex difference in cognition, which emerged at 6-months of age, whereby female 3xTg-AD mice exhibit greater cognitive deficits compared to age-matched male 3xTg-AD mice. The observed discrepancy in cognitive performance was apparent on both acquisition and on the 1.5- and 24-hr retention probe trials of the MWM (Fig. 1A, B, C, D) as well as the 24-hr retention probe trail of IA (Fig. 1F; p<0.05). Notably, the disparity in cognitive performance is still evident at 9 months of age in MWM acquisition, but by 12 months and onward, female and male mice again perform comparably (Fig. 1A; p>0.05). Male and female 3xTg-AD and NonTg mice perform comparably at all ages on the cued MWM, indicating there is no difference in motivation or ability to perform the task (Fig. 1E; p>0.05). In contrast to MWM and IA, cognitive differences in the object recognition task initially manifest later, at 9-months of age, when the 3xTg-AD mice begin to show impairments compared to age-matched NonTg mice (Fig. 1G; p<0.05). Curiously, there are no overt discrepancies with respect to sex at any age on object recognition (Fig. 1G; p>0.05). Thus, there is a transient, age-dependent discrepancy on cognitive performance on MWM and IA, two tasks generally considered to be stressful, between the male and female 3xTg-AD mice that is discernible at younger ages but not in older mice.
Figure 1. Young female 3xTg-AD mice exhibit enhanced cognitive deficits compared to age-matched male 3xTg-AD mice.
(A) MWM acquisition. There is no difference between 2-month-old 3xTg-AD and NonTg mice. (#) indicates sex differences where 6- and 9-month-old 3xTg-AD females performed worse than the 3xTg-AD males (p<0.05). (*) indicates all 15-month-old 3xTg-AD mice performed significantly worse than NonTg mice, although no sex difference was apparent. (B) MWM probe trial for latency to cross platform location. The following notation for significance applies to B, C, & D: (*) indicates genotype interaction where 3xTg-AD mice performed worse than NonTg mice. (#) indicates where 6-month-old female 3xTg-AD performed worse than age-matched male 3xTg-AD mice (p<0.05). (C) MWM probe trial for number of platform location crosses. (D). MWM probe trial for time spent in the quadrant opposite of the target quadrant. (E). Cued MWM acquisition indicates male and female mice are indistinguishable at 2-, 6-, 9-, and 15-months of age (p>0.05). (F). Inhibitory avoidance. There is no effect of sex or genotype at 2 months of age. (*) indicates 6-, 9-, and 15-month-old 3xTg-AD mice performed worse than NonTg mice at both 1.5-h and 24-h post training (p<0.05). (#) indicates 6-month-old female 3xTg-AD mice performed worse than age-matched male 3xTg-AD mice at the 24-h retention interval (p<0.05). (G). Novel object recognition. (*) indicates a genotype difference whereby 3xTg-AD mice perform significantly worse than age-matched NonTg mice at 9 and 12 months of age. No sex differences were observed.
To determine if neuropathological differences underlie the observed disparity in cognitive performance, we evaluated Aβ and tau levels in male and female 3xTg-AD mice. Based on sandwich ELISA, we found that levels of Aβ40 and Aβ42 were comparable between both sexes in both the detergent-insoluble (Fig. 2A) and detergent-soluble (Fig. 2B) fractions at 2-, 6-, 12-, and 15-months of age. There were also no differences in the Aβ40/42 ratio (data not shown). Furthermore, qualitative assessment of Aβ-like immunoreactivity (6E10) at the aforementioned ages indicated no difference in spatial localization of Aβ, APP holo-protein, or APP degradation products (data not shown). Based on our semi-quantitative analysis of tau (HT7) in the hippocampus of 6-month old 3xTg-AD mice (representative image in Fig. 2C, quantification of hippocampal tau in Fig. 2D), amygdala (data not shown) and various cortical regions including the entorhinal cortex (data not shown), we failed to detect any differences between the male and female 3xTg-AD mice. We quantified tau load in 6- (Fig. 2D) and 9-month old (data not shown) mice, as these were the time points when sex differences were most apparent in MWM and IA performance. Qualitative tau immunoreactivities, including staining with phosphotau specific markers (AT8 and 12E8) was also comparable between male and female mice in the hippocampus, cortex, and amygdala at 12- and 15-months of age (data not shown). Therefore, our analysis indicates that it is unlikely that marked differences in Aβ or tau pathology underlie the age- and sex-dependent disparity in cognitive performance at 6- and 9-months of age.
Figure 2. Differences in AD-related pathology do not account for the observed sex-related differences in cognition.
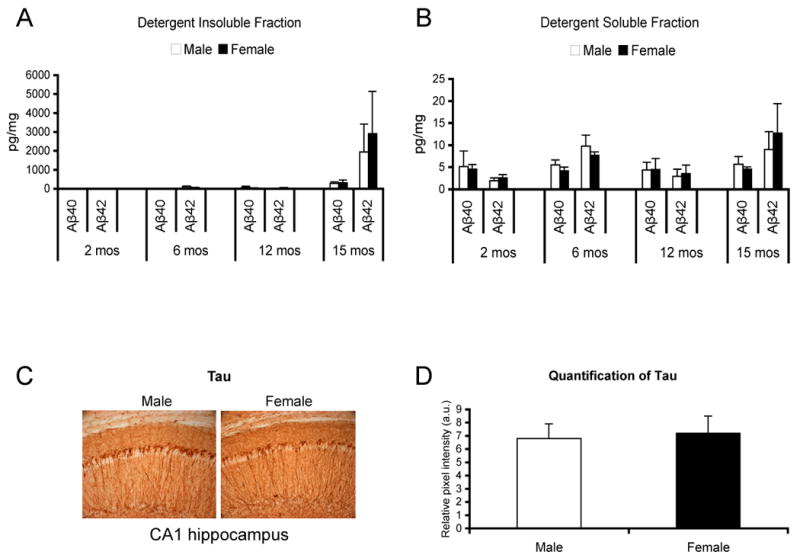
(A) Detergent-insoluble (the tissue was solubilized in 70% formic acid) Aβ40 and Aβ42 ELISA fractions for 2-, 6-, 12- and 15-month old 3xTg-AD mice. (B) Detergent-soluble Aβ40 and Aβ42 ELISA fractions for 2-, 6-, 12- and 15-month-old 3xTg-AD mice. No difference in either soluble or insoluble Aβ between male and female 3xTg-AD mice at any age from 2- to 20-months for either Aβ40 or Aβ42 was apparent (p>0.05). (C) Representative photomicrographs indicates no overt qualitative differences in tau (HT7) in the CA1 region of the hippocampus 6-month-old male (left) or female (right) 3xTg-AD mice. (D) Quantification of tau immunoreactivity in the CA1 region of the hippocampus suggests there are no quantitative differences between age-matched male and female 3xTg-AD mice, p>0.05.
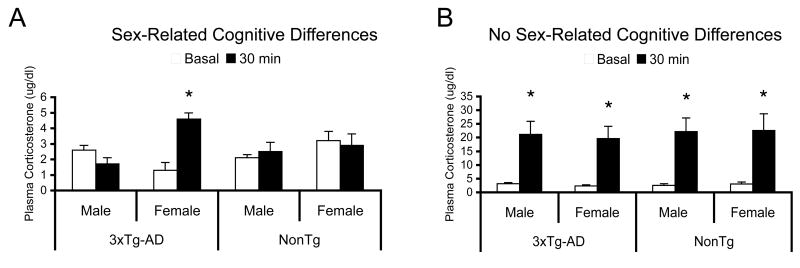
Because female 3xTg-AD mice perform worse than male mice on stressful tasks such as IA and MWM, we hypothesized that female 3xTg-AD mice may have an elevated stress response compared to age-matched males. To test this hypothesis, we compared corticosterone levels in the blood plasma at basal time-points and after five days of MWM training. Our results indicate that young (9-month old) female 3xTg-AD mice have significantly higher stress responses compared to age-matched male 3xTg-AD mice (Fig. 3A, p<0.05). Female 3xTg-AD mice also display a significantly higher stress response compared to NonTg females (Fig. 3A; p<0.05). Because the difference in cognitive performance in the MWM and IA in the 3xTg-AD mice is no longer apparent by 12-months of age, we evaluated an older cohort of mice following five days of MWM training to determine if the MWM-induced elevation in corticosterone still persisted and was higher in female 3xTg-AD mice. Our analysis indicates that the corticosterone response was not significantly different between male and female NonTg mice in the 15-month old cohort (Fig. 3B; p>0.05). Interestingly, coincident with the lack of disparity in the cognitive phenotype between males and females, 15-month old male and female 3xTg-AD exhibit a largely similar stress response following five days of MWM training (Fig. 3B; p>0.05). Therefore, the age- and sex-dependent changes in cognition and behavior are likely due to differences in stress-induced corticosterone release and subsequent effects on memory.
Figure 3. Differences in cognition between female and male 3xTg-AD mice may be due to altered corticosterone release.
(A) Young (9-month old) female 3xTg-AD mice exhibit a heightened MWM-induced corticosterone response compared to NonTg female and 3xTg-AD male mice. Key: (*) indicates p<0.05. The y-axis corresponds to the basal and 30-post MWM training corticosterone levels. (B) Old (15-month old) male and female 3xTg-AD and NonTg mice all exhibit very high MWM-induced corticosterone release compared to basal corticosterone levels. Key: (*) indicates p<0.05.
Discussion
In this study, we report that young female 3xTg-AD mice exhibit learning and memory deficits on MWM and IA, but female mice are indistinguishable from age-matched males on novel object recognition. Quantitative and qualitative immunohistochemistry of Aβ and tau in age-matched male and female 3xTg-AD mice revealed no significant differences in AD-related pathology at any age. Our findings in the 3xTg-AD mice accurately models clinical findings as AD patients, regardless of gender, are equally afflicted with plaques and tangles (Sandberg et al., 2001). Although the amount of AD pathology may not differ between men and women, recent evidence suggests that the relationship between pathology and clinical status may be gender specific. A one unit (as defined by Barnes et al, 2005) increase of AD pathology was associated with a 3-fold increase in the odds of clinical AD in men compared with a more than 20-fold increase in the odds of clinical AD in women (Barnes et al., 2005). Thus, the finding that female 3xTg-AD mice have increased cognitive deficits compared to male 3xTg-AD mice without differences in AD-related pathology is in accord with the aforementioned findings in humans.
Other mouse models of AD exhibit sex-related differences in pathology, but the etiology of these differences is debatable. For example, the Tg2576 mice exhibit sex-related differences in pathology whereby old female mice (15- and 19-months old) have a significantly increased plaque burden and overall higher Aβ40 levels than age-matched male mice (Callahan et al., 2001). Tauopathy mouse models have also been shown to exhibit sex-related differences in pathology. For example, the female JNPL3 mice with human tauP301L have more severe neurofibrillary tangle (NFT) pathology than age-matched male mice. However, the apparently augmented pathology in the female mice appears to be a consequence of the higher transgene expression in the female mice (Lewis et al., 2000; Lewis et al., 2001). Indeed, the transgene expression in both the JNPL3 and Tg2576 (Hsiao et al., 1996) mice is under control of the mouse prion promoter (MoPrP), which drives higher levels of expression in female versus male mice. Thus, the sex difference in the Tg2576 mice may be due to differential levels of APP and the resultant Aβ peptide that accumulates in plaques. In summary, the sex differences reported in some models of human neurodegenerative disorders are due to differential levels of transgene expression.
Male and female mammals have different levels of circulating sex hormones such as estrogen and testosterone. Interestingly, after reproductive senescence is reached in the females (12-14 months), the cognitive discrepancy is no longer apparent on MWM and IA. Young male 3xTg-AD mice may exhibit enhanced cognitive ability compared to female mice due to the protective effects of testosterone. Interestingly, recent data suggest that gonadectomized male 3xTg-AD mice exhibit decreased hippocampal function and a robust increase in Aβ (Rosario et al., 2006). In clinical cases, low testosterone may predispose patients to AD and there are reports that men who are genetically predisposed to acquiring AD have lower testosterone (Hogervorst et al., 2005). Furthermore, gonadotropins may influence the progression of AD pathology as these sex hormones can cross the blood brain barrier (Lukacs et al., 1995) and there is a high density of gonadotropin receptors in the hippocampus (Lei et al., 1993; Al-Hader et al., 1997b; Al-Hader et al., 1997a). Notably, there is a significant increase in luteinizing hormone in the cytoplasm of pyramidal neurons and neurofibrillary tangles of AD brains compared with age-matched controls (Bowen et al., 2002). Although it is possible that there may be an influence of gonadotropins on the pathology and/or etiology of AD, the relationship still remains to be elucidated.
One of the most striking findings to emerge from this study is the observation that the female 3xTg-AD mice performed worse than male mice on selective cognitive tasks. One of the common features of MWM and IA, the two tasks where the female 3xTg-AD mice were more severely impaired than the male 3xTg-AD mice at 6 and 9 months of age, is that they are stressful tasks that force the mice to experience the aversive stimuli of swimming or electric shock, respectively. Object recognition, on the other hand, takes advantage of a mouse's innate tendency toward exploring novel objects and environments in a largely non-stressful environment. Interestingly, the 3xTg-AD mice exhibit impairments later in life on object recognition at 9 months, whereas they are impaired at an earlier age on MWM and IA at 4 months. Although AD pathology does not affect the brain uniformly, the hippocampus, specifically CA1, is almost always impacted (Ball, 1977; Ball, 1978; Coleman and Flood, 1987). Notably, there is a high density of glucocorticoid receptors in the hippocampus (Herman et al., 1989). As humans age, there are age-dependent changes in the hypothalamic-pituitary-adrenal (HPA) axis (Heuser et al., 1994) and subsequent cortisol dysregulation. The combination of HPA axis changes and AD pathology may synergistically exacerbate each other, especially in the hippocampus, leading to the profound memory loss seen in clinical AD. Alternatively, the lack of sex differences in the object recognition task could be due to decreased sensitivity of the task when compared to MWM and IA. Indeed, overall cognitive deficits as measured by object recognition do not manifest until 9 months of age whereas deficits in water maze and inhibitory avoidance are evident by 6 months of age. It is also feasible that sex-related differences in IA could be due to differential responses to shock; age, sex, and genotype could each individually impact how an animal responds to a given shock intensity.
Transgenic mouse models of AD also show HPA axis hyperactivity in an age- and sex-dependent manner (Touma et al., 2004). Indeed, recent evidence from our lab suggests that there is an age-dependent increase in basal plasma corticosterone in the 3xTg-AD mice (Green et al., 2006). Here we show that 3xTg-AD mice exhibit HPA axis hyperactivity in response to stress compared to NonTg mice in an age- and sex-dependent manner. 9-month-old female 3xTg-AD mice have a heightened stress-induced corticosterone response compared to both age-matched NonTg females and 3xTg-AD male mice. This differential stress response is no longer apparent at 15 months of age along with the notable disappearance of the cognitive sex disparity. It appears that there is only a cognitive sex difference on stressful tasks only when female mice have a heightened stress response compared to males, regardless of genotype. Interestingly, even though the older NonTg mice exhibit a heightened stress response compared to young NonTg mice, they do not display cognitive deficits. Thus, it appears that there is a progressive synergist effect of pathology combined with a heightened stress response in the old 3xTg-AD mice. However, further investigation into both basal levels of corticosterone and stress-induced corticosterone response in the 3xTg-AD mice may be necessary. Indeed, there may be other currently unexplored contributing factors that account for the sex- and age-dependent changes in memory including the possible protective effects of testosterone.
Acknowledgments
This work was supported by grants from the Alzheimer's Association, by N.I.A. grant (AG0212982) and by P50 AG16573 to F.M.L, F32 AG24035-01 to L.M.B., MH-12526 to J.L.M. L.K.C. was supported by a training grant from N.I.A. (T32 AG00096). We thank David Cheng for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hader A, Lei ZM, Rao CV. Neurons from fetal rat brains contain functional luteinizing hormone/chorionic gonadotropin receptors. Biol Reprod. 1997a;56:1071–1076. doi: 10.1095/biolreprod56.5.1071. [DOI] [PubMed] [Google Scholar]
- Al-Hader A, Lei ZM, Rao CV. Novel expression of functional luteinizing hormone/chorionic gonadotropin receptors in cultured glial cells from neonatal rat brains. Biol Reprod. 1997b;56:501–507. doi: 10.1095/biolreprod56.2.501. [DOI] [PubMed] [Google Scholar]
- Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, D'Agostino RB, White LR. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- Bachman DL, Wolf PA, Linn RT, Knoefel JE, Cobb JL, Belanger AJ, White LR, D'Agostino RB. Incidence of dementia and probable Alzheimer's disease in a general population: the Framingham Study. Neurology. 1993;43:515–519. doi: 10.1212/wnl.43.3_part_1.515. [DOI] [PubMed] [Google Scholar]
- Ball MJ. Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. A quantitative study. Acta Neuropathol (Berl) 1977;37:111–118. doi: 10.1007/BF00692056. [DOI] [PubMed] [Google Scholar]
- Ball MJ. Topographic distribution of neurofibrillary tangles and granulovacuolar degeneration in hippocampal cortex of aging and demented patients. A quantitative study. Acta Neuropathol (Berl) 1978;42:73–80. doi: 10.1007/BF00690970. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62:685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Gender, cognitive decline, and risk of AD in older persons. Neurology. 2003;60:1777–1781. doi: 10.1212/01.wnl.0000065892.67099.2a. [DOI] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Smith MA, Harris PL, Kubat Z, Martins RN, Castellani RJ, Perry G, Atwood CS. Elevated luteinizing hormone expression colocalizes with neurons vulnerable to Alzheimer's disease pathology. J Neurosci Res. 2002;70:514–518. doi: 10.1002/jnr.10452. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Ann N Y Acad Sci. 2003;985:163–173. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol. 2001;158:1173–1177. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Sherwin BB, Chertkow HM. Relationships between dehydroepiandrosterone sulfate (DHEAS) and cortisol (CRT) plasma levels and everyday memory in Alzheimer's disease patients compared to healthy controls. Horm Behav. 1999;35:254–263. doi: 10.1006/hbeh.1999.1518. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging. 1987;8:521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathe AA, Johns CA, Horvath TB. Cortisol and Alzheimer's disease, I: Basal studies. Am J Psychiatry. 1986;143:300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- Deshmukh VD, Deshmukh SV. Stress-adaptation failure hypothesis of Alzheimer's disease. Med Hypotheses. 1990;32:293–295. doi: 10.1016/0306-9877(90)90109-r. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer's disease: incidence data from the Kungsholmen Project. Stockholm Neurology. 1997;48:132–138. doi: 10.1212/wnl.48.1.132. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzinger M, Z'Brun A, Hemmeter U, Seifritz E, Baumann F, Holsboer-Trachsler E, Heuser IJ. Hypothalamic-pituitary-adrenal system function in patients with Alzheimer's disease. Neurobiol Aging. 1995;16:205–209. doi: 10.1016/0197-4580(94)00159-6. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser IJ, Gotthardt U, Schweiger U, Schmider J, Lammers CH, Dettling M, Holsboer F. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol Aging. 1994;15:227–231. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Moffat SD. Increasing testosterone levels and effects on cognitive functions in elderly men and women: a review. Curr Drug Targets CNS Neurol Disord. 2005;4:531–540. doi: 10.2174/156800705774322049. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–479. doi: 10.1111/j.1600-0447.1987.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Rao CV, Kornyei JL, Licht P, Hiatt ES. Novel expression of human chorionic gonadotropin/luteinizing hormone receptor gene in brain. Endocrinology. 1993;132:2262–2270. doi: 10.1210/endo.132.5.8477671. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Lukacs H, Hiatt ES, Lei ZM, Rao CV. Peripheral and intracerebroventricular administration of human chorionic gonadotropin alters several hippocampus-associated behaviors in cycling female rats. Horm Behav. 1995;29:42–58. doi: 10.1006/hbeh.1995.1004. [DOI] [PubMed] [Google Scholar]
- Molsa PK, Marttila RJ, Rinne UK. Epidemiology of dementia in a Finnish population. Acta Neurol Scand. 1982;65:541–552. doi: 10.1111/j.1600-0404.1982.tb03109.x. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, Laferla FM. Temporal profile of Abeta oligomerization in an in vivo model of Alzheimer's disease: A link between Abeta and tau pathology. J Biol Chem. 2005 doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Raskind MA, Wingerson D, Pascualy M, Thal LJ, Dobie DJ, Wilkinson CW. Hypothalamic-pituitary-adrenocortical axis responses to physostigmine: effects of Alzheimer's disease and gender. Biol Psychiatry. 1996;40:61–68. doi: 10.1016/0006-3223(95)00318-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Stress-activated hormonal systems and the regulation of memory storage. Ann N Y Acad Sci. 1997;821:247–258. doi: 10.1111/j.1749-6632.1997.tb48284.x. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll JC, Oddo S, LaFerla FM, Pike CJ. Androgens regulate the development of neuropathology in a triple transgenic mouse model of Alzheimer's disease. J Neurosci. 2006;26:13384–13389. doi: 10.1523/JNEUROSCI.2514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg G, Stewart W, Smialek J, Troncoso JC. The prevalence of the neuropathological lesions of Alzheimer's disease is independent of race and gender. Neurobiol Aging. 2001;22:169–175. doi: 10.1016/s0197-4580(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Touma C, Ambree O, Gortz N, Keyvani K, Lewejohann L, Palme R, Paulus W, Schwarze-Eicker K, Sachser N. Age- and sex-dependent development of adrenocortical hyperactivity in a transgenic mouse model of Alzheimer's disease. Neurobiol Aging. 2004;25:893–904. doi: 10.1016/j.neurobiolaging.2003.09.004. [DOI] [PubMed] [Google Scholar]