Abstract
We have previously shown that neonatal administration of (+/−)3,4-methylenedioxymethamphetamine and (+)fenfluramine produce deficits in spatial and path integration learning, whereas (+)methamphetamine causes deficits in spatial learning. Conversely, cocaine and (+/−)methylphenidate have no effect on either form of learning following neonatal administration. The purpose of the present study was to determine whether corticosterone and/or monoamine levels were changed following subcutaneous administration of 10 mg/kg (+)methamphetamine, (+/−)3,4-methylenedioxymethamphetamine, (+)fenfluramine, (+/−)methylphenidate or cocaine every 2 h (total of four injections) on postnatal day 11. Twenty-four hours after the first dose, plasma, striatum and hippocampus were collected. Corticosterone levels were increased in methamphetamine-, fenfluramine-, methylenedioxymethamphetamine- and methylphenidate-treated rats relative to levels in saline-treated rats, whereas cocaine-treated rats were unaffected. In the striatum and hippocampus, serotonin and 5-hydroxyindolacetic acid were reduced in animals treated with methylenedioxymethamphetamine or fenfluramine, compared with levels in saline controls. Dopamine levels were not changed by any of the drugs, although 3,4-dihydroxyphenylacetic acid was decreased following methylenedioxymethamphetamine or methamphetamine. Minimal effects were seen in neurotransmitter levels following injection of cocaine or methylphenidate. These data suggest that drugs that affect corticosterone and hippocampal serotonin are associated with both spatial learning and path integration deficits, and those that affect corticosterone and 3,4-dihydroxyphenylacetic acid are associated with spatial learning deficits only.
Keywords: cocaine, corticosterone, ecstasy, fenfluramine, methamphetamine, methylphenidate
The abuse of substituted amphetamines has been reported in women of childbearing age (EMCDDA 2004; Johnston et al. 2004). (+/−)3,4-Methylenedioxymethamphetamine (MDMA), (+)methamphetamine (MA), (+/−)methylphenidate (MPH) and cocaine (COC) can sometimes increase the likelihood of sexual activity (Buffum 1988) and are often abused in social gatherings. However, the anorectic properties of these drugs and others such as (+)fenfluramine (FEN) (even though it has been associated with valvulopathy; Khan et al. 1998) may be appealing because it has been suggested that some women who become pregnant still strive to be thin (Franko and Walton 1993). These factors increase the likelihood of fetal exposure and it is known that MDMA (Ho et al. 2001), MA (Smith et al. 2003; Chomchai et al. 2004), MPH (Debooy et al. 1993), COC (Richardson et al. 1999) and FEN (Jones et al. 2002) have been used during pregnancy, including the third trimester. Despite this, the ramifications of late fetal exposure are largely unknown.
Developmental psychostimulant exposure in humans is suspected to alter normal brain function that can last into adulthood (Billing et al. 1985; Cernerud et al. 1996; Plessinger 1998; Chang et al. 2004; Noland et al. 2005), but the hormonal and biochemical changes that occur following exposure to these drugs remain to be elucidated. In rats, we have shown that developmental exposure to MA (Vorhees et al. 2000a; Williams et al. 2002, 2003b,d), MDMA (Broening et al. 2001; Williams et al. 2003c; Vorhees et al. 2004) or FEN (Morford et al. 2002) produces deficits in latency, path length and cumulative distance as well as probe trial performance in the Morris water maze, a hippocampally mediated spatial learning task (Morris et al. 1982). Neonatal administration of MDMA (Broening et al. 2001; Williams et al. 2003c) and FEN (Morford et al. 2002) also produces an increased number of errors of commission and latency to locate an escape platform in the Cincinnati water maze which assesses path integration learning. Conversely, COC (Vorhees et al. 2000b) or MPH (Vorhees C. V., Moran M. S., Williams M. T., unpublished results) administration does not affect learning assessed by the Morris or Cincinnati water mazes (only COC-treated animals were tested in the Cincinnati water maze). Few studies have detailed the immediate changes induced by neonatal drug exposure during the sensitive period when long-term learning and memory changes originate, i.e. from postnatal day (P)11–20 (Broening et al. 1994; Williams et al. 2000, 2005; Koprich et al. 2003). The limited existing studies suggest there are changes in adrenal hormone production as well as monoamine levels in the brain following exposure to some of these drugs. We previously showed that administration of MDMA or MA to P11 rats caused an increase in corticosterone (CORT) levels that remained significantly increased for 24 h following the first dose of MDMA and at least 1 h after MA exposure (Williams et al. 2000; 2005). We and others have shown that administration of a divided daily dose of 40 mg/kg MDMA on P10 or P11 or P11–20 also produces decreases in serotonin (5-HT) in the hippocampus and striatum the day after the last administration (Broening et al. 1995; Koprich et al. 2003; Williams et al. 2005).
It has been proposed that alterations in glucocorticoids and monoamine levels during early developmental stages may disrupt normal brain development and alter later learning and memory. For example, fluctuations in CORT interfere with the proliferation of neurons during development, especially in the hippocampus (Woolley et al. 1990; Gould et al. 1991; Liu et al. 2003). The hippocampus is known to be involved in learning and memory functions, and is still going through neurogenesis in the late gestational period in humans and comparably in early postnatal weeks in the rat (Bayer et al. 1993). 5-HT is abundant in the brain during neurogenesis and is known to be important in neuronal development. 5-HT is important for the differentiation of neurons and receptive target cells, and is involved in organizing the developing brain (Lauder and Krebs 1978; Lauder 1993). Furthermore, disruptions to 5-HT in rats during early development produce learning and memory deficits when the animals are tested as adults (Mazer et al. 1997).
The main purpose of this study was to compare the effect of the drugs noted above following a single day of administration on plasma CORT and on monoamine content in the striatum and hippocampus. This is the first study to directly compare the changes that occur following administration of MDMA, MA, MPH, COC or FEN on P11, the first day of the critical period for the induction of later cognitive deficits following substituted amphetamine exposure.
Materials and methods
Animals and housing
Nulliparous female Sprague–Dawley CD (International Genetic Strain) rats (Charles River Laboratories, Raleigh, NC, USA) were mated with males of the same strain and supplier. Rats were housed in a 22 ± 1°C environment at 50% humidity with a 14/10 h light/ dark cycle (lights on at 06.00 hours). Before mating, animals were allowed at least 2 weeks to acclimate to the vivarium. Each female spent 2 weeks housed with one male in hanging wire cages before being housed singly in a polycarbonate cage (45.7 × 23.8 × 20.3 cm) containing wood chip bedding and ad libitum food and water. Pups from the matings were used as subjects. The Cincinnati Children’s Research Foundation’s Institutional Animal Care and Use Committee approved all procedures, and the vivarium was fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
The day of birth was considered P0, and on P1 litters were culled to eight pups with six males and two females. Only males were used for this study because we have previously shown no or minor effects of sex and neonatal drug treatment on the spatial learning abilities of these animals in adulthood. Furthermore, no significant differences between males and females in adrenal–pituitary hormone levels were noted following P11 methamphetamine (Williams et al. 2000) or MDMA administration (Williams et al. 2005). On P7, pups were weighed and individually identified by ear punch.
Dosing procedures
(+)Methamphetamine HCl (National Institutes of Health, NIH, National Institute on Drug Abuse, NIDA), (+/−)3,4-methylenedioxymethamphetamine HCl (NIH, NIDA), (+)-fenfluramine HCl (Sigma, St Louis, MO), cocaine HCl (Sigma, St. Louis, MO, USA) and MPH (Mallinckrodt, St. Louis, MO) were dissolved in isotonic saline at a concentration of 10 mg/3 mL (each expressed as the free base; purity > 95%). All drugs were administered at a dose of 10 mg/kg. This was a within-litter design such that the six treatments were represented within each of the eight litters, n = 8, and therefore treatment was considered a repeated factor (Kirk 1995; Winer 1978). Therefore, one male from each litter was randomly assigned to one of the following treatment groups: MA, MDMA, FEN, MPH, COC or saline (SAL). Each dose was administered through a subcutaneous injection in the dorsum. On P11, each animal received one injection every 2 h for a total of four injections. Each animal’s weight was recorded just before drug administration and injection sites were varied to prevent irritation to the dermis.
Blood and tissue collection
Each animal was decapitated 24 h after the first injection (i.e. 18 h following the last injection) because in a previous study we demonstrated that the greatest reduction in neurotransmitter content was induced with MDMA at this time point (Williams et al. 2005). All animals were decapitated between 0.900 and 11.00 hours in order to control for ultradian and circadian rhythm. Trunk blood was collected in tubes containing 2% EDTA (0.05 mL) and then centrifuged (1399 rotational centrifugal force) for 25 min at 4°C. Plasma was collected and stored at −80°C until assayed for CORT.
The brain was removed and a chilled brain block (Zivic-Miller, Pittsburgh, PA, USA) was used to aid in dissection of the neostriatum and hippocampus. For the neostriatum, a coronal cut was made at the optic chiasm and then another cut made 2 mm rostral to the first. The neostriatum was dissected from this 2-mm slab. Hippocampi were removed from the remaining tissue. All dissections were done on a chilled dissection plate and tissues frozen immediately on dry ice. Tissue weights were determined by placing the tissues in preweighed tubes and then re-weighing the tubes. All tissues were stored at −80°C until monoamines were quantified by HPLC with electrochemical detection.
Plasma CORT assessment
Plasma was diluted 3 : 1 for CORT in a kit-specific assay buffer before determination of hormone. CORT was assayed in duplicate using a commercially available enzymatic immunoassay (ALPCO Diagnostics, Windham, NH, USA). This assay has little cross-reactivity with other hormones or precursors (< 0.05%).
Monoamine determination
The tissue concentrations of dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), 5-HT and 5-hydroxyindolacetic acid (5-HIAA) in the neostriatum, and 5-HT and 5-HIAA in the hippocampus, were quantified using HPLC with electrochemical detection. Tissues were homogenized in 50 volumes of 0.2 n perchloric acid and centrifuged for 6 min at 10 000 g. Aliquots of 20 μL were injected on to a C18 column (MD-150, 3 × 150mm; ESA, Chelmsford, MA, USA) connected to either a LC-4B amperometric detector (Bioanalytical Systems, West Lafayette, IN, USA) or a Coulochem (ESA, Chelmsford, MA, USA) detector and an integrator recorded the peak heights that followed each injection. The potential for the LC-4B was 0.6 V and the potentials of E1 and E2 on the analytical cell (model 5014B) of the Coulochem were −150 and 160 mV respectively. The mobile phases consisted of 35 mm citric acid, 54 mm sodium acetate, 50 mg/L disodium ethylenedeamine tetraacetate, 70 mg/L octanesulfonic acid sodium salt, 6% (v/v) methanol and 6% (v/v) acetonitrile, pH 4.0, and pumped at a flow rate of 0.4 mL/min. Quantities of the analytes were calculated on the basis of known standards. Retention times for DOPAC, DA, 5-HIAA and 5-HT were approximately 6, 8, 11, and 17 min respectively.
Statistical analysis
Data were analyzed with repeated measures ANOVA using the general linear modeling procedure (Proc GLM; SAS, Cary, NC, USA). The within-subject factors were treatment and, for body weights, time of day. Litter membership was treated as a matching factor and so treatment was handled in the ANOVA as a repeated measures factor, in accordance with both Kirk (1995) and Winer (1978). The experimental unit was therefore the litter (n = 8) (Holson and Pearce 1992). The Greenhouse–Geisser correction was used in instances in which symmetry of the variance–co-variance matrices were significantly non-spherical. Significant main effects were analyzed further to determine differences using the step-down F-test procedure (Kirk 1995). Simple effect F-tests were used to analyze significant interactions followed by step-down F-tests for individual group comparisons. In a few cases, where previous data from this laboratory on the same dependent variable were available, pre-planned comparisons between specific groups were made where indicated. Significance was set at P = 0.05 and trends at P = 0.10.
Results
Body weight
No main effect of time or treatment was observed for the analysis of body weight during P11 dosing, but there was a time–treatment interaction (F5,35 = 4.88, p < 0.04). Simple effect analyses revealed no significant differences between treatments across time points. The interaction was apparently the result of a reduction in body weight gain during dosing in the FEN- and MDMA-treated animals that caused the growth curves to be non-parallel even though these groups did not differ from any of the other groups at any single time point (Table 1).
Table 1.
Body weights measured during the first and last dose
| Treatment | n | Weight at dose 1 (g) | Weight at dose 4 (g) |
|---|---|---|---|
| SAL | 8 | 25.59 ± 0.95 | 25.75 ± 1.02 |
| FEN | 8 | 26.14 ± 0.91 | 25.64 ± 1.01 |
| MDMA | 8 | 26.89 ± 1.26 | 26.10 ± 1.35 |
| MA | 8 | 26.89 ± 0.94 | 25.96 ± 0.92 |
| MPH | 8 | 26.15 ± 1.09 | 26.25 ± 1.10 |
| COC | 8 | 26.81 ± 1.04 | 26.73 ± 1.14 |
Values are mean ± SEM.
CORT
CORT samples obtained 24 h after the first dose demonstrated a significant treatment effect (F5,35 = 6.43, p < 0.01). Step-down analysis demonstrated increases in CORT in the animals treated with MA, FEN and MPH compared with SAL-treated animals. Because we previously demonstrated an increase in CORT following MDMA on P11 (Williams et al. 2005), a planned comparison was used and the CORT level was significantly higher than that in SAL-treated animals (p < 0.05) (Fig. 1). COC-treated animals did not differ from SAL-treated animals. MA treatment produced the greatest increase and was significantly different from all other treatments except FEN. The second largest increase was found in the FEN group, whereas the MDMA and MPH increases were the smallest and were similar to one another. Other than the MA group, none of the other drug-treated groups differed among themselves.
Fig. 1.
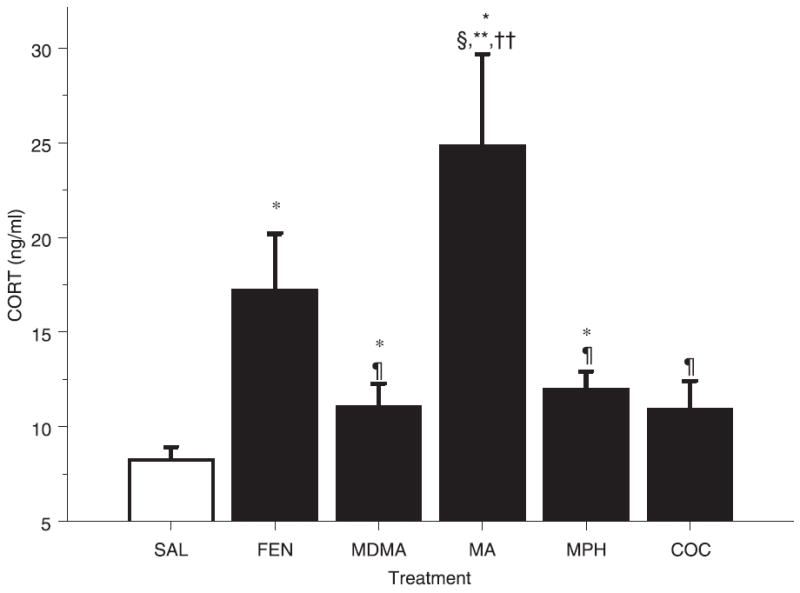
Corticosterone concentrations in plasma 24 h after the first of four doses (every 2 h) of FEN, MDMA, MA MPH and COC (10 mg/kg) that began on P11. Values are mean ± SEM. MA produced the greatest increase in CORT, giving significantly different levels from those in animals treated with SAL, MDMA, MPH and COC. FEN also produced an increase in these P12 animals. MDMA and MPH produced similar increases compared with SAL. *p < 0.05 versus SAL control; §p < 0.05 versus MDMA; ¶p < 0.05 versus MA; **p < 0.05 versus MPH; ††p < 0.05 versus COC. ANOVA with post-hoc step-down F-tests.
Monoamines
Striatum
5-HT in the striatum showed a significant effect of treatment (F5,35 = 7.86, p < 0.004). Post hoc analysis demonstrated that FEN, MDMA and MPH administration significantly decreased 5-HT compared with SAL (Fig. 2a), whereas no differences were noted following MA or COC administration. FEN produced the greatest 5-HT decrease. The rank order for magnitude of effect on 5-HT was FEN ≥ MDMA ≥ MPH ≥ COC = MA = SAL.
Fig. 2.
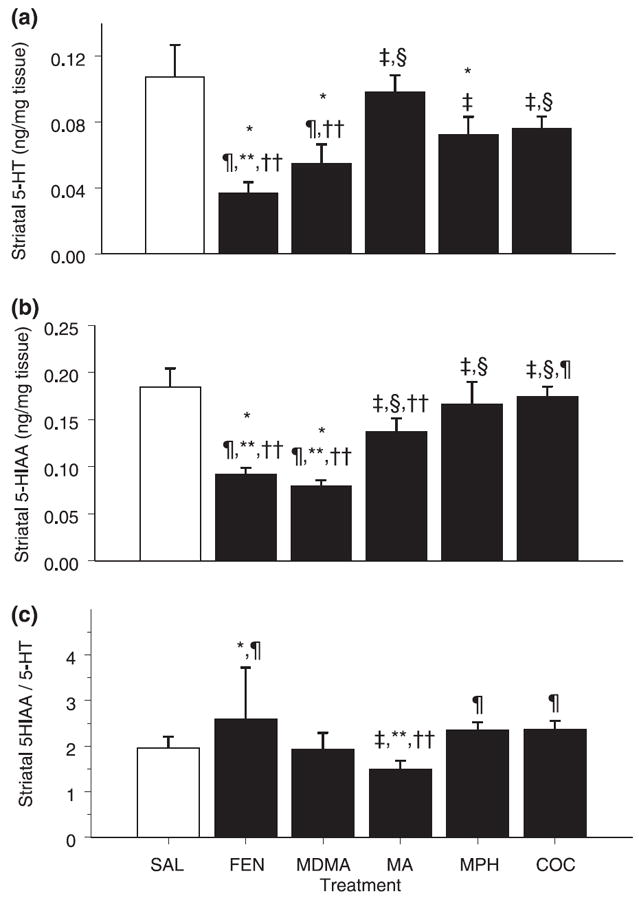
Serotoninergic markers in striatum. Values are mean ± SEM (n = 8/group). Concentrations of (a) 5-HT, (b) 5-HIAA and (c) 5-HIAA/ 5-HT ratio in the striatum of animals exposed to four doses of FEN, MDMA, MA, MPH or COC on P11 and examined on P12. Decreases in 5-HT were observed in FEN-, MDMA- and MPH-treated animals, and FEN and MDMA also decreased 5-HIAA. FEN produced a significant increase in the 5-HIAA/5-HT ratio compared with the SAL group. *p < 0.05 versus SAL control; ‡p < 0.05 versus FEN; §p < 0.05 versus MDMA; ¶p < 0.05 versus MA; **p < 0.05 versus MPH; ††p < 0.05 versus COC. ANOVA with post-hoc step-down F-tests.
As with 5-HT, 5-HIAA in the striatum was altered by treatment (F5,35 = 11.86, p < 0.0004). Post hoc analysis showed that only FEN and MDMA administration produced significant decreases in 5-HIAA compared with SAL administration (Fig. 2b). For 5-HIAA the rank order of drug effect was MDMA ≥ FEN > MA = MPH = COC = SAL.
There was a significant treatment effect on striatal turnover, i.e. the 5-HIAA/5-HT ratio (F5,35 = 3.89, p < 0.02). Only FEN-treated animals demonstrated a significant increase in the 5-HIAA/5-HT ratio compared with SAL-treated animals (Fig. 2c). The turnover of FEN-treated animals was also significantly different from that of MA-treated animals, which produced the lowest turnover ratio. The 5-HIAA/5-HT ratio observed in MA-treated animals was significantly lower than that of MPH- and COC-treated animals, although it did not differ from that in SAL-treated animals.
No significant treatment effects were observed on DA levels in the striatum (Fig. 3a). Treatment effects were exhibited for DOPAC (F5,35 = 13.45, p < 0.0001) (Fig. 3b). Post hoc analysis revealed that animals treated with MDMA or MA had decreased levels of DOPAC compared with SAL-treated animals, and there was a trend for an increase in the MPH-treated animals relative to levels after SAL treatment.
Fig. 3.
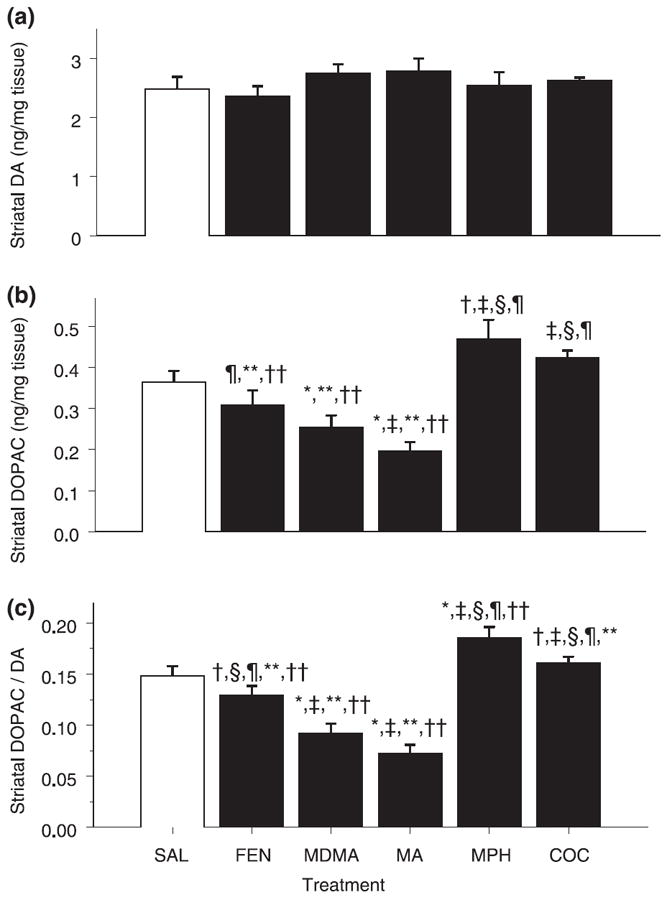
Dopaminergic markers in striatum. Values are mean ± SEM (n = 8/group). Concentrations of (a) DA, (b) DOPAC and (c) DOPCA/ DA ratio in the striatum on P12 following administration of FEN, MDMA, MA, MPH or COC on P11. No changes were observed in DA. MA and MDMA produced a significant decrease in DOPAC. The ratio of DOPAC/DA was significantly decreased following MDMA and MA treatments, and increased in the MPH group. There was also a trend for the DOPAC/DA ratio to be decreased following FEN and increased following COC. *p < 0.05, †p < 0.10 versus SAL control; ‡p < 0.05 versus FEN; §p < 0.05 versus MDMA; ¶p < 0.05 versus MA; **p < 0.05 versus MPH; ††p < 0.05 versus COC.
Although there were no differences observed in DA concentrations in the striatum, the DOPAC/DA ratio demonstrated a significant treatment effect (F5,35 = 27.81, p < 0.0001). MDMA and MA treatment produced a decrease in the DOPAC/DA ratio, whereas MPH treatment significantly increased and COC tended to increase the DOPAC/ DA ratio compared with SAL treatment (Fig. 3c). The rank order for magnitude of decreased DA turnover was MA ≥ MDMA > FEN > SAL; the rank order for increased turnover was MPH > COC > SAL.
Hippocampus
In the hippocampus, both 5-HT (F5,35 = 18.60, p < 0.0001) and 5-HIAA (F5,35 = 21.53, p < 0.0001) were significantly affected by treatment (Figs 4a and b respectively). FEN- and MDMA-treated animals showed significantly decreased 5-HT and 5-HIAA levels compared with SAL-treated animals. There was also a trend for 5-HIAA to be decreased in the MA-treated animals and increased in the MPH-treated animals relative to SAL-treated animals. The rank order effect of the drugs at reducing 5-HT was FEN > MDMA > MA = MPH = COC = SAL. For 5-HIAA the rank order effect was similar and was FEN = MDMA > MA > MPH = COC = SAL.
Fig. 4.
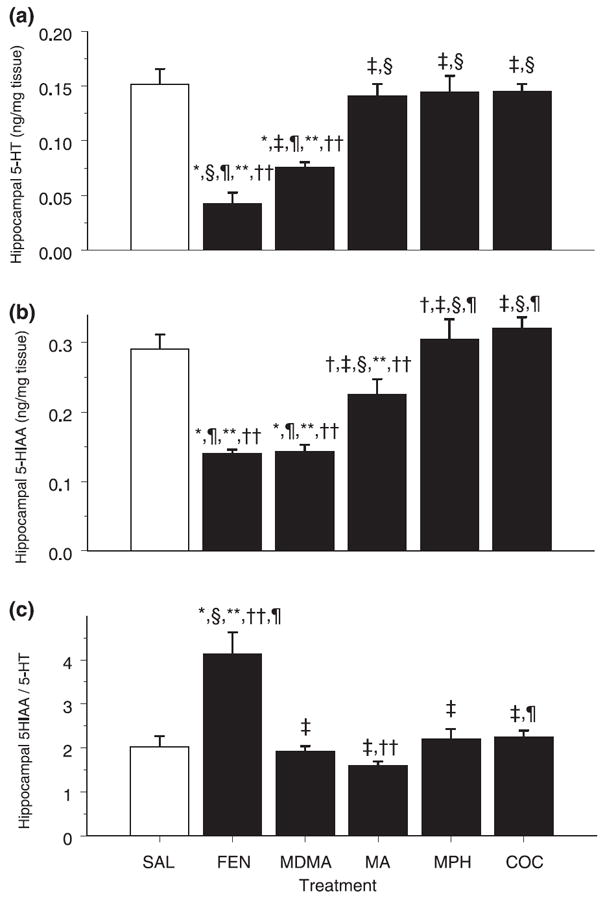
Serotonergic markers in the hippocampus. Values are mean ± SEM (n = 8/group). Concentrations of (a) 5-HT, (b) 5-HIAA and (c) 5-HIAA/5-HT ratio in the hippocampus of animals on P12 after four doses of FEN, MDMA, MA, MPH or COC on P11. Decreases in 5-HT were observed in FEN- and MDMA-treated animals. FEN and MDMA treatment also produced a significant decrease in 5-HIAA. There was a trend for a decrease in 5-HIAA in the MA group and an increase following MPH. Only FEN produced a significant increase in the 5-HIAA/5-HT ratio compared with the SAL group. *p < 0.05, †p < 0.10 versus SAL control; ‡p < 0.05 versus FEN; §p < 0.05 versus MDMA; ¶p < 0.05 versus MA; **p < 0.05 versus MPH; ††p < 0.05 versus COC.
The 5-HIAA/5-HT ratio in the hippocampus showed a significant treatment effect on 5-HT turnover (F5,35 = 11.81, p < 0.0008). FEN-treated animals had a higher ratio compared with all other treatments (Fig. 4c). No other drug-treated animals differed from SAL-treated animals. MA and COC treatments were significantly different from one another.
Discussion
This study is the first to directly compare the developmental effects of several psychostimulants and fenfluramine on early hormonal and monoamine changes 24 h after administration on P11. There were several major findings. First, MA and FEN, and to a lesser extent MDMA and MPH, administration produced prolonged adrenal activation as demonstrated by increases in plasma CORT long after the last treatment. The extent of this increase varied, with MA producing a greater than 3-fold increase, FEN a greater than 2-fold increase, and MDMA and MPH about a 50% increase in CORT. Second, FEN and MDMA treatment produced dramatic decreases in 5-HT and 5-HIAA in both the striatum and hippocampus, whereas MA administration produced only a trend toward decreased 5-HIAA in the hippocampus. Third, unlike adult administration of several of these drugs (especially MA), DA levels were not affected. Because it has been established previously that treatment for 10 days with FEN (20–60 mg/ kg/day) (Morford et al. 2002), MA (20–60 mg/kg/day) (Williams et al. 2003a,d) or MDMA (10–40 mg/kg/day) (Broening et al. 2001; Williams et al. 2003c) beginning on P11 results in long-term spatial learning and memory deficits, whereas treatment with MPH (20–120 mg/kg/day) (Vorhees C. V., Moran M. S., Williams M. T., unpublished observation) or COC (60 mg/kg/day) (Vorhees et al. 2000b) does not, and the substituted amphetamines when administered for 1 day dramatically affect 5-HT and/or CORT, but little or no effect of MPH or COC is observed, it appears that these early drug-induced perturbations may be antecedent to and possibly associated with later cognitive outcomes. However, it must remain clear that behavioral deficits were seen after a 10-day dosing regimen of FEN, MA or MDMA (40 mg/kg/ day for each drug) and the monoamine and CORT data in this study were obtained following only a single day of drug administration, although the fact that there is a pattern of associations with later learning and memory deficits raises the possibility that some or all may be causally linked.
In previous studies, we have examined the effect of neonatal drug exposure on learning and memory ability in the offspring as adults. We have found that administration of FEN (Morford et al. 2002), MDMA (Broening et al. 2001; Williams et al. 2003c; Vorhees et al. 2004), and MA (Vorhees et al. 2000a; Williams et al. 2002, 2003d) at similar doses when given on P11–20 all cause deficits in Morris water maze performance. Moreover, FEN (Morford et al. 2002) and MDMA (Broening et al. 2001; Williams et al. 2003c; Cohen et al. 2005) also produce path integration deficits in the Cincinnati water maze. Neither COC (Vorhees et al. 2000b) nor MPH (Vorhees C. V., Moran M. S., Williams M. T., unpublished observations) administered on these same days, even at higher doses, produced similar deficits. The efficacious drugs are all similar in that they all are substituted amphetamines whereas the ineffective drugs are not. It is likely that the long-term effects of the substituted amphetamines originate from changes induced by these drugs during the period of exposure. For example, the behavioral differences we observed might involve changes in CORT and/or monoamine levels in the neonate that lead to altered stress responsiveness or miswiring of critical learning circuits. This is supported by the finding that increases in CORT or depletions of 5-HT during the neonatal period produce cognitive deficits (Olton et al. 1975; Mazer et al. 1997).
In adult animals, MDMA, MA, COC, MPH and FEN are known to affect monoaminergic systems, but reports describing the ramifications of fetal or neonatal exposure are virtually non-existent, with the exception of COC. In adult subjects these drugs produce effects on serotonergic and/or dopaminergic neurons. For example, FEN primarily affects 5-HT (Rothman et al. 2001; Rowland and Carlton 1986) and MDMA has also been reported to preferentially affect the serotonergic system, but to some extent it also affects the dopaminergic system (Lyles and Cadet 2003). In addition to decreasing monoamine levels, MDMA, MA and FEN have been shown to cause short-term release of various neurotransmitters from axon terminals in adults (Rothman et al. 2001). However, we felt that the protracted changes in neurotransmitter content would be more likely to affect brain development and possibly alter function later in life than the immediate release of neurotransmitters. Similar to effects in adults, in developing animals we found decreases in striatal and hippocampal 5-HT levels that were reduced by 72 and 66% respectively following FEN administration, and 50 and 49% respectively following MDMA administration. Adult MA exposure produces depletion in both dopaminergic and serotonergic neurons (Cappon et al. 1997; Frost and Cadet 2000). However, in this study, four doses of 10 mg/kg MA did not produce any reductions in either 5-HT or DA at the 24-h time point. Therefore, there appears to be a different action for MA on monoaminergic pathways during brain development relative to that seen in the mature brain. This is evident because MA has been shown to produce a 40–60% decrease in DA and 5-HT even 3 days after drug administration in adult animals (Wallace et al. 1999; Cappon et al. 2000; O’Callaghan and Miller 2002), whereas no effect was observed in the present study after developmental exposure. Cocaine, like MPH, primarily blocks the DA transporter (Kollins et al. 2001; Muller et al. 2003), but does not affect levels of brain DA. Although we found no effects of COC in this study, MPH produced a slight decrease in 5-HT in the striatum, but without changes in 5-HIAA, and trends for increased DOPAC in the striatum and 5-HIAA in the hippocampus. The dissimilarities between COC and MPH are difficult to reconcile, but these small monoamine changes might imply that MPH may produce modest alterations in brain development that the Morris water maze, as used previously, could not detect.
In the developing brain, 5-HT not only functions as a neurotransmitter but is also thought to influence neuronal differentiation and synaptogenesis (Whitaker-Azmitia et al. 1996). Depletion of 5-HT is thought to cause disruptions in brain development (i.e. reductions in neuronal number and disruption of neuronal differentiation and synaptogenesis) that may manifest later in life as cognitive deficits. For example, transient depletion of 5-HT using p-chloroamphetamine or permanent reduction by 5,7-dihydroxytryptamine has been shown to produce fewer spines on the dendrites of neurons in the dentate gyrus without affecting dendritic length (Yan et al. 1997). We have found a similar effect in spine densities following MA administration from P11–20 (Williams et al. 2004). Although we did not observe a decrease in 5-HT levels at the 24-h time point following only 1 day of MA administration, it is possible that multiple days of treatment may affect brain 5-HT concentrations. It has also been found that the depletion of 5-HT from P10–20 using the tryptophan hydroxylase inhibitor p-chlorophenylalanine causes spatial learning and memory deficits (Mazer et al. 1997). Taken together, it is evident that 5-HT is important in the development of circuits involved in cognitive ability, and that FEN and MDMA can alter this developmental pattern and lead to later cognitive dysfunction.
Not only is 5-HT important in development, but adrenal hormones also play a role in neuronal differentiation and survival. Rodents exhibit an endogenously generated decreased responsiveness to stressors during the neonatal period that is hypothesized to ensure optimal glucocorticoid levels for normal brain development (Sapolsky and Meaney 1986). This period is termed the stress hyporesponsive period (SHRP) and lasts for approximately the first 2 weeks of life. The decreased hypothalamic-pituitary-adrenal axis responsiveness during the SHRP is thought to prevent stress-induced increases in glucocorticoids from interfering with neuronal proliferation, especially in regions that express abundant glucocorticoid receptors such as the dentate gyrus (Sapolsky and Meaney 1986; Vazquez 1998). During the sensitive period of drug administration for substituted amphetamine-induced cognitive deficits, which begins on P11, the hippocampus is growing and differentiating rapidly (Bayer et al. 1993; Liu et al. 2003) and is an essential mediator involved in spatial learning and memory (Morris et al. 1982). In the present study, we found that just 1 day of FEN, MDMA or MA treatment produced increases in CORT for up to 18 h following the last dose (24 h following the first dose), much longer than we previously documented using MA (e.g. 60 min after the last dose) (Williams et al. 2000). Moreover, MA induced a >3-fold increase in CORT that persisted over this time span during the SHRP. This effect has an early onset and can be seen by 30 min following the first dose of MA (Williams M. T., Furay A. F., Ehrman L. A., Schaefer T. L., Vorhees C. V. unpublished data). This is unlike any other chemical or stressor effect reported in the literature at this age while pups are with their mother, although it is known that maternal separation is a potent stressor during the SHRP. Maternal separations of ≤ 2 h can induce small, but significant, increases in CORT (Pihoker et al. 1993; Huot et al. 2002); however, it takes at least 8 h of separation before a substantial increase in CORT is achieved (Levine et al. 1991). MPH produced a small increase in CORT, but not of the magnitude observed following MA or FEN administration, and COC had no effect. Consistent with the CORT levels found for MPH and COC, no spatial learning deficits were found after exposure to these drugs. Hence, MA treatment appears unique among the psychostimulant/monoamine releasers tested in terms of the magnitude of adrenal output it induces at this age.
The central aim of this study was to identify neurochemical changes important in neurodevelopment, such as in levels of CORT and 5-HT that are perturbed by psychostimulants. In this study, we examined a single time point, 24 h after the first exposure, which was 18 h after the last drug exposure. This time point was selected based on previous time-course data following MDMA, because this was when the largest decrease in 5-HT occurred (Williams et al. 2005). Nevertheless, we do not know whether the other drugs administered in this study have a temporal profile of effects similar to those of MDMA, so there are limitations to the present results. For example, there might be transient changes in monoamines following administration of the other drugs studied here that were not detected 24 h after the first dose. However, we were most interested in lasting, rather than transient changes in this study. The age chosen (P11) was selected because this is the first day of the critical period of induction of later cognitive deficits after exposure to substituted amphetamines. As described previously, we found that FEN, MA and MDMA cause cognitive deficits following drug administration from P11–20, although evidence suggests that shorter exposure periods are likely to have the same effects. For example, MA administration from P11–15, but not P16–20, is sufficient to cause deficits in spatial learning (Williams et al. 2003b). In addition, a single exposure of MA on P14 in gerbils has been shown to cause alterations in behavior, neuroanatomical structure and neurotransmitter content (Dawirs et al. 1996; Blaesing et al. 2001; Busche et al. 2002; Neddens et al. 2002), suggesting that even a single day of exposure is enough to perturb some aspects of neurodevelopment.
If we compare studies in which MA, MDMA, FEN, MPH or COC were administered to rats from P11–20 and tested during adulthood in the Morris water maze acquisition phase, and express the data as a percentage of the control value, there is a clear rank ordering of the behavioral effects. It should be noted that each of the behavioral studies presented also used a within-litter design. In future studies it would be valuable to compare the behavioral effects of the drugs within the same study. As illustrated in Fig. 5, FEN administration (40 mg/kg/day) produced the greatest deficits in the Morris water maze (Morford et al. 2002) followed by MDMA (40 mg/kg/day) (Williams et al. 2003c) and MA (40 mg/kg/day) (Williams et al. 2002), whereas MPH (40 mg/kg/day) (Vorhees C. V., Moran M. S., Williams M. T. unpublished results) and COC (60 mg/kg/day) (Vorhees et al. 2000b) had no effects. In the present study, we have shown that FEN produces the second largest increase in CORT and the most dramatic decrease in striatal and hippocampal 5-HT levels. MDMA produced a decrease in 5-HT in both the striatum and hippocampus, and a modest increase in CORT. Therefore, both FEN and MDMA produce extended changes in early signaling molecules that are important for normal brain development and are likely to be related to later learning ability. MA administration caused significant deficits in the Morris water maze (Williams et al. 2002), but they were not as severe as after FEN or MDMA administration. However, MA produced the largest increases in CORT, but no changes in 5-HT, at least when measured at 24 h. This suggests that the CORT increases produce damage that is less severe than the combination of increased CORT and reduced 5-HT. COC (Vorhees et al. 2000b) or MPH (Vorhees C. V., Moran M. S., Williams M. T. unpublished results) treatment produced no learning or memory deficits after P11–20 exposure (Fig. 5) and COC did not affect CORT or 5-HT. MPH, on the other hand, produced small changes in CORT and 5-HT levels in the striatum but not enough to affect cognition. Although we did not see learning deficits, MPH during this sensitive period does cause developmental problems such as growth retardation and may produce other as yet uncharacterized behavioral effects (Gauron and Rowley 1974). It should be noted that in the behavioral studies reported, drug was administered for 10 days instead of a single day as in the present study. In addition, there is a possibility that some of the drugs have pharmacological profiles that are different from those of MDMAwith respect to early neurochemical changes and the prospect of these differences needs to be considered when evaluating the neurochemical/behavioral comparison. Although we used similar mg/kg doses in this study, it should be noted that these doses were not meant to be equivalent in relative potency but they represented doses of the substituted amphetamines known to produce long-term learning and memory differences when administered for 10 days. Nonetheless, higher levels of MPH (up to 120 mg/kg/day) or COC (60 mg/kg/day) did not produce learning deficits. Therefore, we used similar dosing concentrations of each drug for this comparison. Data from the present study, considered together with the degree of impairment observed following FEN, MDMA, MA, MPH or COC in the Morris water maze, suggest that developmental disruptions in CORT and 5-HT are important in the etiology of substituted amphetamine-induced learning and memory deficits. Therefore, closer examination of these effects on long-term learning and memory deficits warrants further investigation.
Fig. 5.
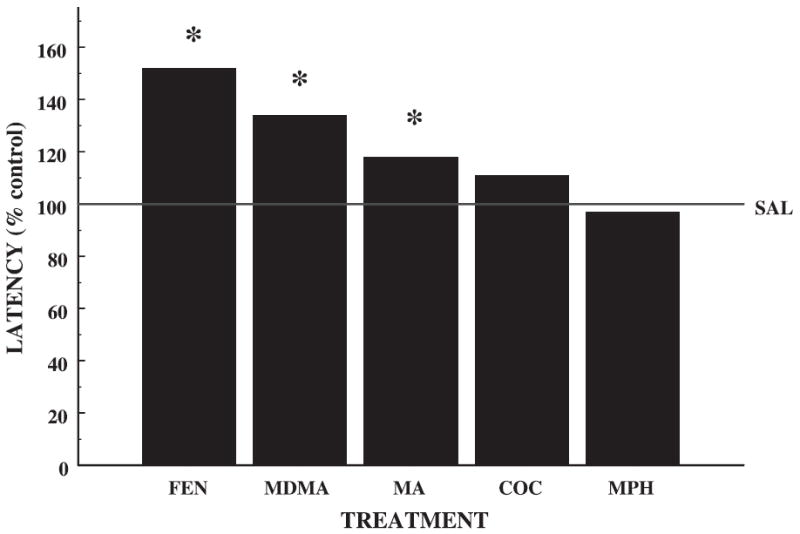
Comparison of FEN, MDMA, MA, COC and MPH effects on Morris water maze spatial learning from previous experiments. Each of the drugs was administered at a dose of 40 mg/kg/day from P11–20 with the exception of COC that was administered at 60 mg/kg/day. There is a clear rank order of effects (expressed as percent of SAL from each study) in the acquisition phase of the Morris water maze. FEN produced the longest latencies in finding the hidden platform (Morford et al. 2002). In the present study, FEN-treated animals showed the most dramatic decreases in both striatal (Fig. 2a) and hippocampal (Fig. 4a) 5-HT, and a significant increase in CORT (Fig. 1). MDMA produced the next greatest deficit in the Morris maze (Williams et al. 2003c), and next largest decreases in striatal (Fig. 2a) and hippocampal (Fig. 4a) 5-HT levels. MA produced significant increases in latency (Williams et al. 2002), but not to the degree of FEN or MDMA, and it did not produce any changes in 5-HT (Figs 2a and 4a), but produced the largest increase in CORT (Fig. 1). Neither MPH (Vorhees C. V., Moran M. S., Williams M. T. unpublished results) nor COC (Vorhees et al. 2000b) produced any changes in latency and only MPH slightly affected 5-HT in the striatum (Fig. 2a). *p < 0.05 versus SAL controls (100% reference line).
Acknowledgments
Supported by National Institutes of Health grants DA014269 (MTW) and DA006733 (CVV), DA007427 (GAG) and training grant ES007051 (TLS, LAE).
Abbreviations used
- COC
cocaine
- CORT
corticosterone
- DOPAC
3,4-dihydroxyphenylacetic acid
- DA
dopamine
- FEN
(+)fenfluramine
- 5-HIAA
5-hydroxyindolacetic acid
- 5-HT
serotonin
- MA
(+)methamphetamine
- MDMA
(+/−)3,4-methylenedioxymethamphetamine
- MPH
(+/−)methylphenidate
- P
postnatal day
- SAL
saline
- SHRP
stress hyporesponsive period
Footnotes
Some of these data were presented at the 12th annual meeting of the International Behavioral Neuroscience Society meeting in San Juan, Puerto Rico, 2003.
References
- Bayer SL, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurol Toxicol. 1993;14:83–144. [PubMed] [Google Scholar]
- Billing L, Eriksson M, Steneroth G, Zetterstrom R. Preschool children of amphetamine-addicted mothers. I. Somatic and psychomotor development. Acta Paediatr Scand. 1985;74:179–184. doi: 10.1111/j.1651-2227.1985.tb10946.x. [DOI] [PubMed] [Google Scholar]
- Blaesing B, Nossoll M, Teuchert-Noodt G, Dawirs RR. Postnatal maturation of prefrontal pyramidal neurones is sensitive to a single early dose of methamphetamine in gerbils (Meriones unguiculatus) J Neural Transm. 2001;108:101–113. doi: 10.1007/s007020170101. [DOI] [PubMed] [Google Scholar]
- Broening HW, Bacon L, Slikker W., Jr Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1994;271:285–293. [PubMed] [Google Scholar]
- Broening HW, Bowyer JF, Slikker W., Jr Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (+/−)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-Methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffum J. Substance abuse and high-risk sexual behavior: drugs and sex – the dark side. J Psychoactive Drugs. 1988;20:165–168. doi: 10.1080/02791072.1988.10524489. [DOI] [PubMed] [Google Scholar]
- Busche A, Neddens J, Dinter C, Dawirs RR, Teuchert-Noodt G. Differential influence of rearing conditions and methamphetamine on serotonin fibre maturation in the dentate gyrus of gerbils (Meriones unguiculatus) Dev Neurosci. 2002;24:512–521. doi: 10.1159/000069362. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Morford LL, Vorhees CV. Ontogeny of methamphetamine-induced neurotoxicity and associated hyperthermic response. Brain Res Dev Brain Res. 1997;103:155–162. doi: 10.1016/s0165-3806(97)81791-9. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate–putamen after single-dose treatment. Brain Res. 2000;863:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatr. 1996;85:204–208. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chomchai C, Na MN, Watanarungsan P, Yossuck P, Chomchai S. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2004;35:228–231. [PubMed] [Google Scholar]
- Cohen MA, Skelton MR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Learning and memory after neonatal exposure to 3,4-methylenedioxymethamphetamine (ecstasy) in rats: interaction with exposure in adulthood. Synapse. 2005;57:148–159. doi: 10.1002/syn.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawirs RR, Teuchert-Noodt G, Czaniera R. Ontogeny of PFC-related behaviours is sensitive to a single non-invasive dose of methamphetamine in neonatal gerbils (Meriones unguiculatus) J Neural Transm. 1996;103:1235–1245. doi: 10.1007/BF01271184. [DOI] [PubMed] [Google Scholar]
- Debooy VD, Seshia MM, Tenenbein M, Casiro OG. Intravenous pentazocine and methylphenidate abuse during pregnancy. Maternal lifestyle and infant outcome. Am J Dis Child. 1993;147:1062–1065. doi: 10.1001/archpedi.1993.02160340048012. [DOI] [PubMed] [Google Scholar]
- EMCDDA. Annual Report 2004: The state of the Drugs Problem in the European Union and Norway. European. Monitoring Centre for Drugs and Drug Addiction; Luxembourg: 2004. [Google Scholar]
- Franko DL, Walton BE. Pregnancy and eating disorders: a review and clinical implications. Int J Eat Disord. 1993;13:41–47. doi: 10.1002/1098-108x(199301)13:1<41::aid-eat2260130106>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Frost DO, Cadet JL. Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Res Brain Res Rev. 2000;34:103–118. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- Gauron EF, Rowley VN. Effects of chronic methylphenidate administration on learning and offspring behavior. J Gen Psychol. 1974;91:157–158. doi: 10.1080/00221309.1974.9920794. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus. II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991;313:486–493. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Ho E, Karimi-Tabesh L, Koren G. Characteristics of pregnant women who use ecstasy (3,4-methylenedioxymethamphetamine) Neurotoxicol Teratol. 2001;23:561–567. doi: 10.1016/s0892-0362(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Abuse, 1975–2003, Vol. II. College Students and Adult Ages 19–45. National Institute of Drug Abuse; Bethesda: 2004. NIH Publication vol. 04-5508. [Google Scholar]
- Jones KL, Johnson KA, Dick LM, Felix RJ, Kao KK, Chambers CD. Pregnancy outcomes after first trimester exposure to phentermine/fenfluramine. Teratology. 2002;65:125–130. doi: 10.1002/tera.10023. [DOI] [PubMed] [Google Scholar]
- Khan MA, Herzog CA, St Peter JV, Hartley GG, Madlon-Kay R, Dick CD, Asinger RW, Vessey JT. The prevalence of cardiac valvular insufficiency assessed by transthoracic echocardiography in obese patients treated with appetite-suppressant drugs. N Engl J Med. 1998;339:713–718. doi: 10.1056/NEJM199809103391101. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Science. Brooks/Cole Publishing Co; Pacific Grove: 1995. [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Campbell NG, Lipton JW. Neonatal 3,4-methylenedioxymethamphetamine (ecstasy) alters dopamine and serotonin neurochemistry and increases brain-derived neurotrophic factor in the forebrain and brainstem of the rat. Brain Res Dev Brain Res. 2003;147:177–182. doi: 10.1016/s0165-3806(03)00219-0. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Krebs H. Serotonin as a differentiation signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic–pituitary–adrenal axis in the infant rat. Dev Psychobiol. 1991;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- Liu H, Kaur J, Dashtipour K, Kinyamu R, Ribak CE, Friedman LK. Suppression of hippocampal neurogenesis is associated with developmental stage, number of perinatal seizure episodes, and glucocorticosteroid level. Exp Neurol. 2003;184:196–213. doi: 10.1016/s0014-4886(03)00207-3. [DOI] [PubMed] [Google Scholar]
- Lyles J, Cadet JL. Methylenedioxymethamphetamine (MDMA, Ecstasy) neurotoxicity: cellular and molecular mechanisms. Brain Res Brain Res Rev. 2003;42:155–168. doi: 10.1016/s0165-0173(03)00173-5. [DOI] [PubMed] [Google Scholar]
- Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV. Impaired spatial and sequential learning in rats treated neonatally with d-fenfluramine. Eur J Neurosci. 2002;16:491–500. doi: 10.1046/j.1460-9568.2002.02100.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Huston JP. Serotonin as an important mediator of cocaine’s behavioral effects. Drugs Today (Barc) 2003;39:497–511. doi: 10.1358/dot.2003.39.7.799442. [DOI] [PubMed] [Google Scholar]
- Neddens J, Lesting J, Dawirs RR, Teuchert-Noodt G. An early methamphetamine challenge suppresses the maturation of dopamine fibres in the nucleus accumbens of gerbils: on the significance of rearing conditions. J Neural Transm. 2002;109:141–155. doi: 10.1007/s007020200010. [DOI] [PubMed] [Google Scholar]
- Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Lester KH, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–438. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxic effects of substituted amphetamines in rats and mice. In: Massaro EJ, editor. Handbood of Neurotoxicology. Vol. 2. Humana Press Inc; Totowa: 2002. pp. 269–301. [Google Scholar]
- Olton DS, Johnson CT, Howard E. Impairment of conditioned active avoidance in adult rats given corticosterone in infancy. Dev Psychobiol. 1975;8:55–61. doi: 10.1002/dev.420080108. [DOI] [PubMed] [Google Scholar]
- Pihoker C, Owens MJ, Kuhn CM, Schanberg SM, Nemeroff CB. Maternal separation in neonatal rats elicits activation of the hypothalamic–pituitary–adrenocortical axis: a putative role for corticotropin-releasing factor. Psychoneuroendocrinology. 1993;18:485–493. doi: 10.1016/0306-4530(93)90042-j. [DOI] [PubMed] [Google Scholar]
- Plessinger MA. Prenatal exposure to amphetamines. Risks and adverse outcomes in pregnancy. Obstet Gynecol Clin North Am. 1998;25:119–138. doi: 10.1016/s0889-8545(05)70361-2. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Hamel SC, Goldschmidt L, Day NL. Growth of infants prenatally exposed to cocaine/crack: comparison of a prenatal care and a no prenatal care sample. Pediatrics. 1999;104:e18. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Carlton J. Neurobiology of an anorectic drug: fenfluramine. Prog Neurobiol. 1986;27:13–62. doi: 10.1016/0301-0082(86)90011-0. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J Dev Behav Pediatr. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Vazquez DM. Stress and the developing limbic–hypothalamic–pituitary–adrenal axis. Psychoneuroendocrinology. 1998;23:663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to d-methamphetamine: selective effects on spatial navigation and memory. J Neurosci. 2000a;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Inman-Wood SL, Morford LL, Reed TM, Moran MS, Pu C, Cappon GD. Evaluation of neonatal exposure to cocaine on learning, activity, startle, scent marking, immobility, and plasma cocaine concentrations. Neurotoxicol Teratol. 2000b;22:255–265. doi: 10.1016/s0892-0362(99)00071-9. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Skelton MR, Williams MT. Exposure to 3,4-methylenedioxymethamphetamine (MDMA) on postnatal days 11–20 induces reference but not working memory deficits in the Morris water maze in rats: implications of prior learning. Int J Dev Neurosci. 2004;22:247–259. doi: 10.1016/j.ijdevneu.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphet-amine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Pre-weaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal day 11–20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Brain Res Dev Brain Res. 2003a;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003b;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymeth-amphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects or injection stress. Brain Res. 2003c;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003d;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Brown RW, Vorhees CV. Neonatal methamphetamine administration induces region-specific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens, and parietal cortex. Eur J Neurosci. 2004;19:3165–3170. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Ehrman LA, Able JA, Gudelsky GA, Sah R, Vorhees CV. 3,4-Methylenedioxymeth-amphetamine administration on postnatal day 11 in rats increases pituitary-adrenal output and reduces striatal and hippocampal serotonin without altering SERT activity. Brain Res. 2005;1039:97–107. doi: 10.1016/j.brainres.2005.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistics and data analysis: trading bias for reduced mean squared error. Annu Rev Psychol. 1978;29:647–681. doi: 10.1146/annurev.ps.29.020178.003243. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Yan W, Wilson CC, Haring JH. Effects of neonatal serotonin depletion on the development of rat dentate granule cells. Brain Res Dev Brain Res. 1997;98:177–184. doi: 10.1016/s0165-3806(96)00176-9. [DOI] [PubMed] [Google Scholar]


