Abstract
Aging is associated with reduced endothelial function. There is indirect evidence for reduced prostacyclin mediated vasodilation with aging, but it is unknown if this is due to reduced dilation to prostacyclin or altered production. Additionally, the contribution of endothelial nitric oxide to prostacyclin-mediated dilation is unknown. Using plethysmography to determine forearm blood flow, we studied the effect of prostacyclin in ten older (61–73 years) and ten younger (19–45 years) subjects using three escalating intra-arterial doses of prostacyclin (epoprostenol). Prostacyclin was also administered after nitric oxide synthase inhibition with NG-Monomethyl-L-arginine acetate. Percent change in forearm vascular conductance (mean ± standard error of the mean) from baseline after prostacyclin was significantly lower (p=0.002) in the aging individuals (52±11, 164±23, 221±27 vs. 115±20, 249±19, 370±35 percent). Additionally, the group-by-dose interaction was also significant (p=0.018). Following nitric oxide synthase inhibition, the dose response curve to prostacyclin was blunted in the young subjects but unchanged in the older subjects; the difference between the groups was no longer significant. Our data suggest that the reduced dilator effects of prostacyclin in older individuals are due to a reduction in the contribution of endothelial-derived nitric oxide vs. alterations in the direct effects of prostacyclin on vascular smooth muscle.
Keywords: Aging, prostaglandins, nitric oxide, blood flow
Introduction
Aging is associated with reduced endothelial function independent of disease.1, 2 While much is known about how aging affects nitric oxide (NO)-mediated vasodilation there is less information available about its influence on prostacyclin (PGI2)-mediated vasodilation.3 In this context, indirect evidence obtained using cyclooxygenase (COX)-inhibitors suggests that aging causes either reduced production of vasodilating prostanoids or perhaps reduced vasodilator responses to them.4 However, cyclooxygenase is also involved in the production of several other prostaglandins (PG) including PGE, PGF, PGD, and thromboxane (TXA), all with varying vascular effects,5 and there is some evidence that aging causes an increase in the production of vasoconstricting prostanoids.6 It is unknown if the direct dilation caused by PGI2, is maintained in older humans and how NO may contribute to this prostacyclin-mediated dilation.
These questions are also clinically relevant in a general sense because essential hypertension appears to cause changes in prostanoid-mediated endothelial vascular regulation similar to aging. Additionally, suppression of PGI2 caused by selective inhibition of COX-2 has been implicated in predisposing patients to myocardial infarction or thrombotic stroke.7 It also appears this risk is associated with older patients who receive COX-2 inhibitors.8, 9 This suggests population differences in PGI2 might influence these events. With this information as a background, we sought to: 1) test the hypothesis that forearm blood flow responses to PGI2 in healthy older adults would be reduced compared with matched young adults; and, 2) determine whether age-related differences in PGI2-mediated vasodilation are dependent on the production of endothelial NO.
Materials and Methods
Subjects
All procedures and protocols for this study received prior approval by the Institutional Review Board (IRB) of the Mayo Clinic. Each subject provided written informed consent before participation. Initially, five younger subjects with a mean age of 32 yrs (range 24–42 yrs) participated in a dose response sub-study to determine PGI2 doses. The main protocol included ten older subjects with a mean age of 68 yrs (61–73 yrs) and ten younger controls, mean age of 29 yrs (19–45 yrs). (Table 1) The subjects were matched for body mass index (BMI ± 3 kg/m2) and gender and consisted of six male and four female pairs. Participants did not require regular prescription medications (other than oral contraceptives); non-prescription medicines were stopped for five half-lives prior to the study. Subjects were physically active (but not exercise trained), non-smokers, and presented without diagnosis of preexisting disease (i.e. cardiovascular, pulmonary or endocrine). Younger females were studied during the low estrogen phase of menstrual cycle or placebo phase of oral contraceptive use.10 Subjects refrained from foods and beverages that contain methylxanthines (caffeine, theobromine) and ethanol for 48 hours prior to the start of the study, and they were fasting after midnight before study day.
Table 1.
Baseline clinical characteristics
| Subject group |
N | M/F | Age years |
BMI | SBP mmHg |
DBP mmHg |
MAP mmHg |
|---|---|---|---|---|---|---|---|
| Young | 10 | 6/4 | 29.0 ± 2.8 | 24.0 ± 0.9 | 116 ± 5 | 61.5 ± 2.6 | 79.7 ± 2.5 |
| Older | 10 | 6/4 | 67.8 ± 1.3 | 24.5 ± 0.7 | 122 ± 4 | 69.3 ± 3.7 | 87.0 ± 3.5 |
Values are mean ± SEM. M/F =Males/Females, BMI=Body mass index, SBP=systolic blood pressure, DBP=diastolic blood pressure, MAP=mean arterial pressure.
Brachial arterial catherization
Following local anesthesia with 2% lidocaine hydrochloride and using aseptic technique, each subject underwent catheterization of the brachial artery. This was performed in the non-dominant arm using a 5-centimeter long, 20 gauge Teflon® arterial catheter. This catheter was connected to a three-port connector to allow for continuous measurement of arterial pressure (one port) and for the administration of study drugs (two ports).11
Blood flow measurement
Forearm blood flow (FBF) was determined four times each minute using venous occlusion plethysmography with mercury-in-silicone strain gauges placed around the non-dominant forearm at its greatest circumference.12 During measurement of FBF, blood flow to the hand was excluded by inflation of a wrist cuff to 250 mmHg. In addition to FBF, heart rate and mean arterial blood pressure were measured continuously.
Data acquisition
Arterial blood pressure, heart rate and FBF were monitored, stored, and analyzed offline using an automated electronic data acquisition system. FBF was determined from the derivative of the forearm plethysmographic tracing. To adjust for changes in systemic pressures, forearm vascular conductance (FVC) was calculated by using FBF and mean arterial pressure (MAP) with the equation FVC= (FBF/MAP X 100) and expressed as arbitrary units (a.u.). In general, the last two minutes of each dose was used to determine FBF (an average of four measurements) and FVC.
Brachial artery infusions
In order to standardize drug concentrations, forearm volume (FAV) was determined in each subject by water displacement and drugs were administered based on FAV. Normal saline (0.9% sodium chloride) was infused during baseline measurements to maintain similar total infusion rates during each trial.
Study Drugs
Epoprostenol (PGI2) (Flolan®, GlaxoSmithKline) was prepared by serial dilution of the 500,000 nanogram (ng) commercial vial and supplied diluent. The final infusion concentration was 100 ng•ml−1. The final doses of epoprostenol and infusion duration were validated in the dose response study in five younger participants. This permitted us to study three doses of prostacyclin that would elicit FBF responses (low, medium, high) comparable to the three established doses of sodium nitroprusside (SNP) that have long been used in our laboratory to evoke endothelial-independent vasodilation.
Sodium Nitroprusside (SNP) (Nitropress®, Abbott) was prepared using the 50 mg commercial vial with 0.9% sodium chloride to a final dilution concentration of 10 microgram (mcg) •ml−1. The SNP dose was based on previous studies from our lab.13
NG-monomethyl-L-arginine acetate (L-NMMA) (Clinalfa) was used to inhibit nitric oxide synthase (IND #41,190). L-NMMA was prepared by dilution with 0.9% sodium chloride to a final concentration for administration of 1 mg•ml−1. The L-NMMA dose was based on previous studies.14
Infusion protocol
After instrumentation, a control infusion of 0.9% sodium chloride was initiated during baseline measurements and to compensate for total flow rate throughout the protocol as new infusions were added. A three-point PGI2 dose response consisting of 2.5, 5, 10 ng•dl−1 FAV•minute−1 (low, medium, high) was performed followed by a twenty minute washout period and three doses of the endothelium-independent vasodilator SNP (0.25, 0.5, 1 mcg•dl−1 FAV•min−1). The objective of this portion of the protocol was to determine if aging was associated with a blunted vasodilator response to PGI2.
Upon completion of the first set of dose response curves to PGI2 and SNP, the nitric oxide synthase L-NMMA was infused at 5 mg/min for 10 min (50 mg loading dose), followed by an infusion of 1 mg/min for the remainder of the experiment (maintenance dose). A second set of dose responses (low, medium, high doses) to PGI2 and SNP were then performed. The objective of this portion of the protocol was to determine if the NO contribution to the vasodilation caused by PGI2 differed in the older subjects compared to the younger control subjects.
Plasma Cholesterol Measurement
In an attempt to examine the affect of plasma cholesterol on the prostacyclin-mediated dilation, subject cholesterol data was either gathered from the patient’s medical record (closest available total cholesterol level to study day) or subjects without a recent value were requested to return to have lipids drawn.
Statistical analysis
Hemodynamic variables (heart rate, mean arterial pressure) were determined from the electronic records. Changes in FBF and FVC were determined with each subject serving as their own control using a paired analysis. Repeated measures of analysis of variance (ANOVA) were completed using SAS software version 9. The dependent variable was percent change from baseline and the repeated factor was dose. The independent cross-classification variable was age group. This model also included a group-by-dose interaction effects to assess whether differences between the comparative groups were dose dependent. In cases where dose-by-group interactions were indentified, supplemental group comparisons were performed at each dose level without adjusting for multiple comparisons. Data are expressed as means ± standard error of the mean (SEM). Statistical significance is set at p<0.05.
Results
Table 1 shows the baseline data for the younger and older subjects. Systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure were not significantly different between groups.
Mean FBF responses to the interventions are shown in Table 2 and the calculated FVC responses are shown in Table 3. There were no statistical differences in the baseline data between the two groups prior to the administration of each of the PGI2 or SNP trials.
Table 2.
FBF responses to infusion of PGI2 and SNP with and without co-infusion of L-NMMA
| FBF (ml•dl−1 FAV•min−1) | |||
|---|---|---|---|
| Drug | Young | Older | Co-infusion |
| PGI (ng•dl−1 FAV•min−1) | Normal Saline | ||
| Baseline | 1.86 ± 0.27 | 2.12 ± 0.23 | |
| 2.5 | 3.93 ± 0.72 | 3.04 ± 0.30 | |
| 5.0 | 6.19 ± 0.93 | 5.12 ± 0.47 | |
| 10.0 | 7.81 ± 0.86 | 6.23 ± 0.57 | |
| SNP (mcg•dl−1 FAV•min−1) | |||
| Baseline | 2.69 ± 0.35 | 2.75 ± 0.31 | |
| 0.25 | 6.63 ± 0.85 | 5.11 ± 0.65 | |
| 0.50 | 9.82 ± 0.83 | 9.07 ± 0.79 | |
| 1.0 | 12.72 ± 0.93 | 11.55 ± 0.98 | |
| PGI (ng•dl−1 FAV•min−1) | L-NMMA | ||
| Baseline | 1.71 ± 0.16 | 1.67 ± 0.13 | |
| 2.5 | 2.47 ± 0.29 | 2.26 ± 0.19 | |
| 5.0 | 4.14 ± 0.49 | 4.06 ± 0.53 | |
| 10.0 | 5.69 ± 0.54 | 5.34 ± 0.72 | |
| SNP (mcg•dl−1 FAV•min−1) | |||
| Baseline | 1.93 ± 0.19 | 1.67 ± 0.16 | |
| 0.25 | 5.34 ± 0.80 | 4.87 ± 0.56 | |
| 0.50 | 8.38 ± 0.82 | 8.51 ± 0.72 | |
| 1.0 | 9.96 ± 0.92 | 11.53 ± 1.02 | |
Values are mean ± SEM. FBF=forearm blood flow, SNP=sodium nitroprusside, PGI=epoprostenol.
Table 3.
FVC responses to infusion of PGI2 and SNP with and without co-infusion of L-NMMA
| FVC (a.u.) | |||
|---|---|---|---|
| Drug | Young | Older | Co-infusion |
| PGI (ng•dl−1 FAV•min−1) | Normal Saline | ||
| Baseline | 2.11 ± 0.30 | 2.33 ± 0.27 | |
| 2.5 | 4.64 ± 0.86 | 3.42 ± 0.38 | |
| 5.0 | 7.22 ± 1.17 | 5.88 ± 0.67 | |
| 10.0 | 9.34 ± 1.35 | 7.17 ± 0.80 | |
| SNP (mcg•dl−1 FAV•min−1) | |||
| Baseline | 3.04 ± 0.45 | 3.11 ± 0.38 | |
| 0.25 | 7.56 ± 1.10 | 5.77 ± 0.76 | |
| 0.50 | 11.29 ± 1.00 | 10.41 ± 0.99 | |
| 1.0 | 14.85 ± 1.14 | 13.47 ± 1.21 | |
| PGI (ng•dl−1 FAV•min−1) | L-NMMA | ||
| Baseline | 1.86 ± 0.18 | 1.83 ± 0.18 | |
| 2.5 | 2.68 ± 0.30 | 2.44 ± 0.23 | |
| 5.0 | 4.50 ± 0.53 | 4.53 ± 0.71 | |
| 10.0 | 6.27 ± 0.61 | 5.99 ± 0.93 | |
| SNP (mcg•dl−1 FAV•min−1) | |||
| Baseline | 2.01 ± 0.18 | 1.81 ± 0.21 | |
| 0.25 | 5.80 ± 0.92 | 5.21 ± 0.58 | |
| 0.50 | 9.10 ± 0.90 | 9.33 ± 0.98 | |
| 1.0 | 11.06 ± 1.15 | 12.89 ± 1.33 | |
Values are mean ± SEM. FVC=forearm vascular conductance, SNP=sodium nitroprusside, PGI=epoprostenol.
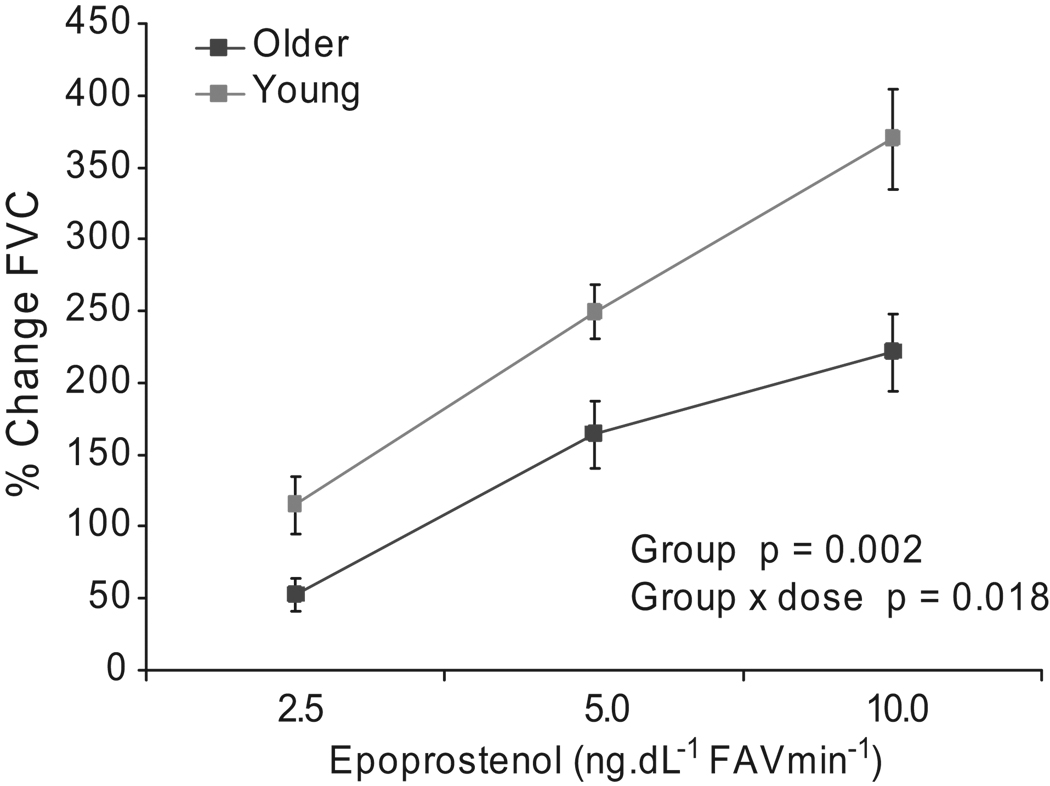
The percent change in FVC from baseline after PGI2 was significantly lower (p=0.002) in the older individuals (52±11, 164±23, 221±27 percent) compared to younger controls (115±20, 249±19, 370±35 percent). Additionally, the group-by-dose interaction was also significant (p=0.018). (Figure 1) SNP responses were not significantly different between these groups.
Figure 1.
Percent change of forearm vascular conductance (FVC) from baseline after administration of epoprostenol (PGI2) in young compared to older subjects (n=10/group). Values are mean ± SEM.
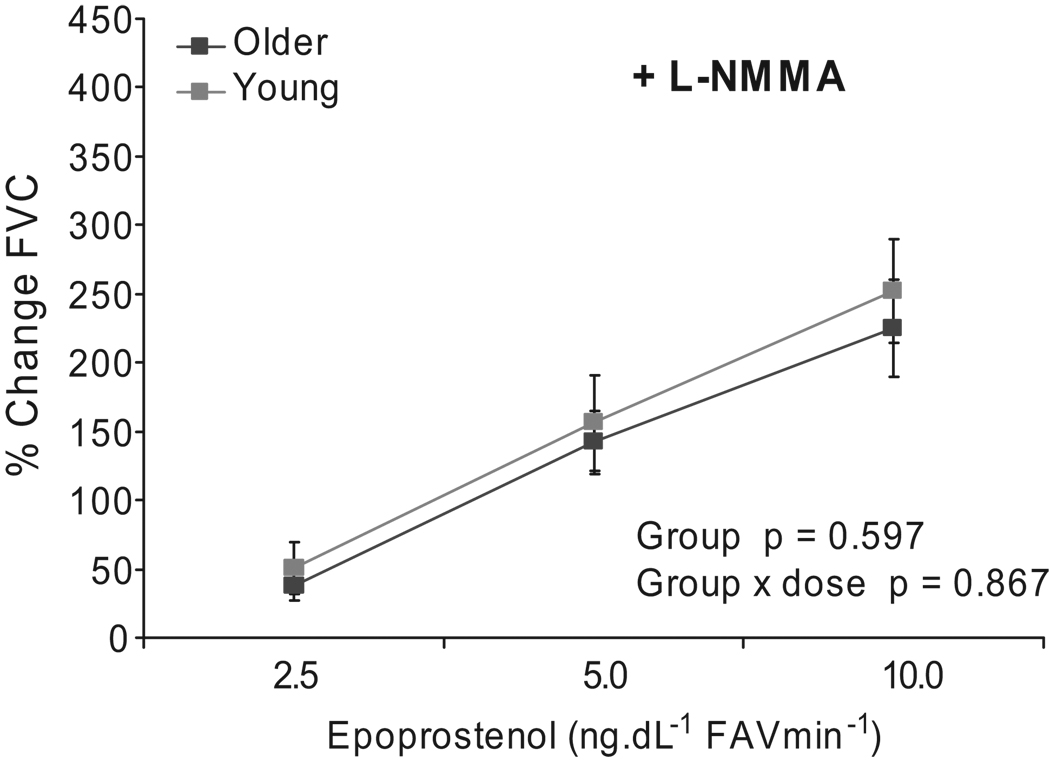
After the L-NMMA loading dose baseline FVC was reduced similarly in both groups; however, the dilator response to PGI2 was reduced in the younger individuals and no longer significantly different between younger and older subjects (p=0.60). In addition, the group-by-dose interaction was no longer significant (p=0.87) with the combination of PGI2 and L-NMMA. (Figure 2)
Figure 2.
Percent change of forearm vascular conductance (FVC) from baseline after administration of epoprostenol (PGI2) during continuous infusion of L-NMMA 1 mg·min−1 in young compared to older subjects (n=10/group). Values are mean ± SEM.
Following L-NMMA, and SNP there was some evidence suggesting that differences between groups were dose-dependent (dose-by-group interaction p=0.047). Subsequent post-hoc analyses that did not adjust for multiple comparisons suggested that the percent change from baseline caused by both the SNP 0.25 and 0.5 mcg•dl−1 FAV•min−1 doses did not differ significantly between age groups (p=0.817 and p=0.340 respectively). However, the older group experienced a greater change (p=0.036) at the highest dose (SNP 1 mcg•dl−1 FAV•min−1).
In subsequent examination of total cholesterol, the mean value was 179±6 mg/dl for nine younger subjects and 206±16 mg/dl for ten of the older subjects (pt-test=0.154) showing no significant difference between groups. In addition, there was no correlation between total cholesterol and maximum PGI-mediated dilation (10 ng•dl−1 FAV•minute−1) in the younger individuals (r2=0.0041) or older individuals (r2=0.0062).
Discussion
The main findings of this study are 1) that forearm vasodilator responses to PGI2 are reduced with aging, and 2) that these differences in PGI2-mediated vasodilation are likely due to a reduction in the contribution of endothelial-derived NO. While previous studies have shown that both prostacyclin and NO contribute to endothelial-mediated forearm vasodilation in both young and older subjects,4, 15 the influence of aging on the vascular effects of prostacyclin and the contribution of endothelial-derived NO to prostacyclin-mediated dilation were unknown. These findings suggest that in addition to an age-related shift toward endothelial production of vasoconstricting prostanoids, there is a loss of dilator responsiveness to vasodilating prostanoids as well.
Endothelial contributions
Aging is associated with progressive endothelial dysfunction in normal humans.16 Taddei et al.17 demonstrated an age-related reduction of NO-mediated forearm vasodilation that was largely due to reduced bioavailability of NO. The dilation evoked by acetylcholine decreased with increasing age and the greatest reduction was seen in the oldest group (60–80 yrs). In addition, the effects of NOS inhibition on acetylcholine-mediated dilation decreased in aging, demonstrating that the NO component of the acetylcholine–mediated dilation progressively decreased as the individual aged.
Aging effects have also been studied using other endothelial-dependent dilators. In a study by DeSouza et al.,18 acetylcholine-induced vasodilation was impaired in aging individuals; however, the forearm endothelial vasodilation in response to bradykinin, substance P, and isoproterenol were well preserved in older men (50–76 yrs) when compared to younger men (23–35 yrs). These findings suggested that agonist-stimulated endothelium-dependent vasodilation is not universally impaired with age and depends on the agonist. However, similar to acetylcholine, our data indicate that prostacyclin-mediated dilation is impaired with age. While age-related decreases in endothelial-dependent NO release or NO bioavailability appear to be agonist specific, vascular smooth muscle responsiveness to the direct effects of NO remains relatively unchanged in otherwise healthy subjects. Additionally, the current data suggest that vascular smooth muscle responsiveness to the direct effects of PGI2 is unchanged with aging.
PGI2
To the best of our knowledge, PGI2 has not been used in forearm studies focused on vascular aging in escalating doses. However, in a previous forearm trial Kamper et al.19 demonstrated that the NOS-inhibitor L-NMMA blunted the vasodilation evoked by the prostacyclin analog iloprost. This early finding is consistent with our observations that NO mediates a portion of prostacyclin-induced vasodilation.
Based on previous studies with COX-inhibition, we hypothesized that the direct vasodilatory effects of prostacyclin would be reduced in the older compared to the younger subjects. This idea came from studies that used an indirect approach to identify the role of prostaglandins in regulating vascular tone with aging. In these studies the effects of non-selective inhibitors of COX (i.e. ketorolac, aspirin) 4, 20 on blood flow were determined. In the study by Singh and colleagues4, the COX inhibitor aspirin (3, 9, and 30µmol/min) was administered to 18 young and 15 older healthy subjects. Aspirin caused a greater dose-related reduction in FBF in the younger vs. older subjects. This reduction was interpreted to suggest that the role played by prostaglandins in the regulation of vascular tone diminishes with age.
However, using this approach it is not possible to determine if aging alters vascular responsiveness to PGI2, or shifts the production from dilating to constricting prostanoids. Additionally, there is the possibility that the direct smooth muscle effects of PGI2 might increase due to the chronically low circulating PGI2 levels in older individuals with a resultant vascular receptor up-regulation over time. Under any of these circumstances, determination of the contribution of dilating PGs indirectly via COX inhibition would be difficult. However, the effects of PGI2 on forearm vasodilation were reduced in our older subjects, compared to the younger individuals clearly demonstrating a loss of PGI2-mediated vasodilation with aging.
NOS inhibition
The older individuals demonstrated that the vasodilation caused by the PGI2 was similar before and following the L-NMMA administration. Since L-NMMA inhibits the endothelial production of NOS, this suggests that older subjects produce less NO in response to PGI2. This finding is consistent with the observation that the effects of L-NMMA diminish with advanced age, indicating a generalized blunting of basal endothelial function in older people.4 By contrast, the NO contribution to PGI2-mediated vasodilation was larger in younger individuals. In this context, NOS inhibition also facilitated the comparison of the smooth muscle effects of PGI2 and showed that the smooth muscle effects of prostacyclin appeared similar in both populations once the NO component of dilation was absent.
Although prostacyclin caused an NO-mediated dilation in the younger group only (as evidenced by the L-NMMA effect), administration of SNP also increased flow, but NOS inhibition with L-NMMA had little effect on the dilator responses to SNP. Along these lines, many agonists that increase FBF show a 20–40% reduction in flow after NOS inhibition and a question that always arises is whether this reduction is due to a non-specific blunting of shear/flow related NO release or is likely a receptor-mediated response. The current data indicate that a general flow-related effect on endothelium-dependent NO release is unlikely to explain the interactions of L-NMMA and PGI2 on vasodilator responses between the two groups.
Limitations
There are limitations to this study. First, the forearm vasculature is indicative but not necessarily representative of all human blood vessels, as other vascular beds may be more or less sensitive to the effects of these agents due to differences in receptor density or signaling. Second, even though all subjects denied a history of hypertension, we did find that the older subject group displayed a greater mean arterial pressure than the young group (87.0 ± 3.5 vs. 79.7 ± 2.5 mmHg) which may have affected our interpretation. Third, we later assessed plasma cholesterol. Although the lipid results were not contemporaneous with our blood flow study, we feel that these results are likely representative of the lipid status of our study subjects. Upon analysis, the mean total cholesterol was higher in the older subject group (206 ± 16 vs. 179 ± 6 mg/dl). Although these values did not reach significance, we acknowledge the possibility of a type II error due to the small sample size. However, no correlation between total cholesterol and maximum PGI-mediated dilation (10 ng•dl−1 FAV•minute−1) was observed in either group. Finally, there was some evidence suggesting that older individuals may dilate more compared to the younger subjects with the highest dose combination of SNP and L-NMMA. Only the SNP 1 mcg•dl−1 FAV•min−1 dose was significant between groups (p=0.036 unadjusted for multiple comparisons), unlike the other two doses following post hoc analysis. Since an improvement in the vasodilator effects of SNP after NOS inhibition was not expected, we believe that the overall dose-by-age group interaction differences (p=0.047) are not meaningful.
Perspectives
The importance of nitric oxide and prostacyclin as vasodilators are well known. We believe our findings have several areas of physiological and/or clinical significance. First, there is evidence that the aging endothelium appears to produce more vasoconstricting prostanoids. The current findings demonstrate that the blood vessels of healthy older subjects are also less responsive to the overall vasodilating effects of prostacyclin. Additionally, these age related changes in PGI-mediated dilation do not appear to be easily explained by differences in plasma cholesterol. Finally, the vasodilating effects of PGI appear to be greater when nitric oxide is available and NO release from the vascular endothelium by prostacyclin plays an essential role in our observations. Of potential clinical importance, prostacyclin inhibition has been implicated in the toxicity of the selective COX- 2 inhibitors, which might explain the increased rate of adverse cardiovascular effects with COX-2 inhibitors in older individuals.
Conclusion
Aging blunts the vasodilator effects of PGI2, and NOS inhibition significantly reduces prostacyclin-mediated vasodilation in young but not older subjects. This suggests that the reduced vasodilator effects of prostacyclin in aging individuals may be due to a reduction in the contribution of endothelial-derived NO when compared to younger individuals.
Acknowledgments
We thank Darrell R. Schroeder (biostatistics), Niki M. Dietz, Timothy B. Curry (anesthesiology), Lakshmi P.M. Somaraju, Tasha L. Pike, Karen P. Krucker and Pamela A. Engrav (anesthesia research) for outstanding assistance and support during this study. In addition, we thank our subjects who participated in this study.
Sources of Funding
This research was supported by National Institutes of Health (NIH) grants HL-46493 (M. Joyner), the NIH CTSA grant UL1-RR24150 (Mayo Clinic, Rochester, MN), and the Clinical Pharmacology Training grant GM-08685 (W. Nicholson). Additionally, the DFG grant (Germany) HE 4605/1-1 (C. Hesse).
Footnotes
Conflict of Interest
None
References
- 1.Tao J, Jin YF, Yang Z, Wang LC, Gao XR, Lui L, Ma H. Reduced arterial elasticity is associated with endothelial dysfunction in persons of advancing age: comparative study of noninvasive pulse wave analysis and laser Doppler blood flow measurement. Am J Hypertens. 2004;17:654–659. doi: 10.1016/j.amjhyper.2004.03.678. [DOI] [PubMed] [Google Scholar]
- 2.Matz RL, Andriantsitohaina R. Age-related endothelial dysfunction : potential implications for pharmacotherapy. Drugs Aging. 2003;20:527–550. doi: 10.2165/00002512-200320070-00005. [DOI] [PubMed] [Google Scholar]
- 3.Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann N Y Acad Sci. 2007;1100:353–360. doi: 10.1196/annals.1395.038. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Prasad S, Singer DR, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci (Lond) 2002;102:595–600. [PubMed] [Google Scholar]
- 5.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 8.Solomon DH, Schneeweiss S, Glynn RJ, Kiyota Y, Levin R, Mogun H, Avorn J. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation. 2004;109:2068–2073. doi: 10.1161/01.CIR.0000127578.21885.3E. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM, Arber N, Levin B, Meinert CL, Martin B, Pater JL, Goss PE, Lance P, Obara S, Chew EY, Kim J, Arndt G, Hawk E. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117:2104–2113. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torgrimson BN, Meendering JR, Kaplan PF, Minson CT. Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am J Physiol Heart Circ Physiol. 2007;292:H2874–H2880. doi: 10.1152/ajpheart.00762.2006. [DOI] [PubMed] [Google Scholar]
- 11.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol. 1994;480(Pt 2):361–368. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol. 2001;91:2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- 13.Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol. 2002;92:2019–2025. doi: 10.1152/japplphysiol.01025.2001. [DOI] [PubMed] [Google Scholar]
- 14.Schrage WG, Dietz NM, Eisenach JH, Joyner MJ. Agonist-dependent variablity of contributions of nitric oxide and prostaglandins in human skeletal muscle. J Appl Physiol. 2005;98:1251–1257. doi: 10.1152/japplphysiol.00966.2004. [DOI] [PubMed] [Google Scholar]
- 15.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol. 1996;81:1807–1814. doi: 10.1152/jappl.1996.81.4.1807. [DOI] [PubMed] [Google Scholar]
- 16.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 17.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 18.DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol. 2002;542:255–262. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamper AM, Paul LC, Blauw GJ. Prostaglandins are involved in acetylcholine-and 5-hydroxytryptamine-induced, nitric oxide-mediated vasodilatation in human forearm. J Cardiovasc Pharmacol. 2002;40:922–929. doi: 10.1097/00005344-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2007;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]