Abstract
Purpose
To improve the performance of 7 T head coils over the rostral head regions. Due to RF field/ tissue interactions, the RF magnetic field profile produced by 7 T volume head coils is very inhomogeneous, with enhanced sensitivity near the center of the human brain and substantially reduced in the periphery.
Materials and Methods
Two head-sized quadrature volume coils of similar diameters but substantially different lengths (17 and 10 cm) were constructed and tested using 7 T Varian Inova system.
Results
Experimental data demonstrated that by using a shorter volume head-sized coil or simply by partially moving a head out of the coil, coil efficiency near the top of a head can be improved by 20%. The homogeneity also improved, largely resulting from an increase in peripheral B1 values. This resulted in 10–20% variation in axial slices located near the top of a head.
Conclusion
We have demonstrated a less deeply positioned head or substantially shorter volume coil can significantly improve coil performance and homogeneity for the rostral head at ultra-high magnetic fields (7 T and above). For studies that target superior brain regions, this coil arrangement can be highly effective.
Keywords: TEM, RF volume head coil, homogeneity improvement, high field MRI
INTRODUCTION
Recently, very high field (7 T) magnetic resonance imaging (MRI) systems have become available for human use and are supported by a variety of clinical and research vendors including General Electric, Siemens, Philips, Varian and Bruker. Increasing magnetic field strength up to 7 T offers significant advantages for increased SNR (at least linear), increased contrast for fMRI (quadratic increases depending upon approach, spin echo versus gradient echo) and spectral and spatial resolution for spectroscopic imaging (CSI). However, due to RF field/ tissue interactions at such high field, the RF magnetic field profile produced by head-sized volume coils displays a distinctive pattern of inhomogeneity, with enhanced sensitivity in the center of the human brain (more than 40% at 7 T as compared to the periphery) (1). Previously, methods for improving RF field inhomogeneity at high magnetic fields (above 4 T) have been reported e.g., multi-port driven head volume coils (2) or phased arrays (3,4). Multi-port driving provides flexibility in adjusting the homogeneity of the B1 field by varying phases and amplitudes of driving voltages. However, its practical implementation requires using independent sources with adjustable and controllable phases and magnitudes as well as independent high-power RF amplifiers for each element of the array (5). This design is still quite expensive and complex.
A critical feature of ultra-high (7 T and above) coil design is that the B1 distribution is dominated by the sample loading rather than by the coil geometry. For example, as reported by Nabetani et al (6) two 7 T head-sized birdcage coils with the same diameters but substantially different lengths (15 and 23 cm) produced very similar longitudinal RF distributions along the coil axis (6). In this comparison of open coils, the volunteer was positioned such that the head center approximately matched the coil center. This demonstrates that at high field the coil length can be substantially shortened without compromising coil performance. This in turn allows substantial decrease of the coil’s inductance, which is often problematic during the design of head-sized high field volume coils.
In this work we evaluate the effect of head position on B1 distributions with closed and back shielded transverse electromagnetic (TEM) coils, another commonly used type of high field volume coil (7). Having a reflecting mirror back wall improves B1 homogeneity in the longitudinal direction (7) but limits the positioning the head near the center of a volume coil. We demonstrate that by using a shorter volume coil or simply by partially moving the head out of the coil, coil homogeneity and efficiency can be substantially improved at least for the superior portion of a head. While clearly limited in whole brain coverage, this coil can be very effective for ultra-high field studies that focus on pathologies of the frontal-parietal lobes, of interest in psychiatric studies or degenerative disorders (8–10).
MATERIALS AND METHODS
TEM volume coil
Two head-sized quadrature TEM volume coils were built using tuneable capacitive coaxial elements with Teflon insulators similar to that previously described (1,7,11). Both coils consisted of 16 resonant elements constructed of copper tubes with the diameter of 12.5 mm and 6.4 mm utilized for the outer shells and the central conductors, respectively. Both coils had the same RF shield diameter of 29.8 cm and the shielded back wall for improved homogeneity of the RF field in longitudinal direction (7). The shield for both coils was constructed from a 50 µm polyamide film with a 5 µm copper layer laminated on top of it (Gould Electronics, Eastlake, Ohio). Two coils differ substantially in length with the longer coil measuring 17 cm and the shorter coil measuring 10 cm. Also an element ID (diameter measured at the element centers) was slightly different and measured 26.7 cm and 25.5 cm for the longer and shorter coils, respectively. Both TEM coils were driven in quadrature using variable capacitive matching and a four-port drive with the driven elements located at 45° in respect to vertical and horizontal axes similar to that previously described (12). By utilizing variable capacitors we were able to match volume coils under different loading conditions. Unloaded Q-factors, QU, measured about 500 for both TEM coils. Q-factors loaded with a human head, QL, measured 45 and 60 for the longer and shorter coils, respectively. QL of the longer 17-cm TEM coil with the head moved out by 7 cm (see text below) measured 70.
Data collection
Data were collected using a 7 Tesla Varian Inova system (Varian Associates, Inc., Palo Alto, CA). To test the coil performance, gradient echo images of a human head (256 × 256 × 13 slices) were collected using 2/8 mm slice thickness/gap, 19.2 × 19.2 cm field of view (FOV), TE = 50 ms, TR = 400 ms, nominal flip angle 15°. B1 maps (13 slices) were collected using a rapid gradient echo dual angle approach (13) with 64 × 64 resolution, TR = 1 s, 5/5 mm slice thickness/gap centered on the matched gradient echo images. To determine the B1 amplitude produced by the coils, two nominal 60° excitation pulses at a target 1 kHz were applied sequentially prior to incrementing the phase encoding value. After correcting for slice profile, the ratio of the amplitudes of the two images (S2/S1) is given by Cos(Θ) where Θ = ∫B1(t)dt, (B1 in units of Hz) (13). All the B1 maps are presented below as distributions of the flip angle, with 1 kHz scaled to 60°. For proper comparison of the coil performance all the reported images and B1 maps were obtained with the same volunteer, thus providing very similar loading conditions.
RESULTS
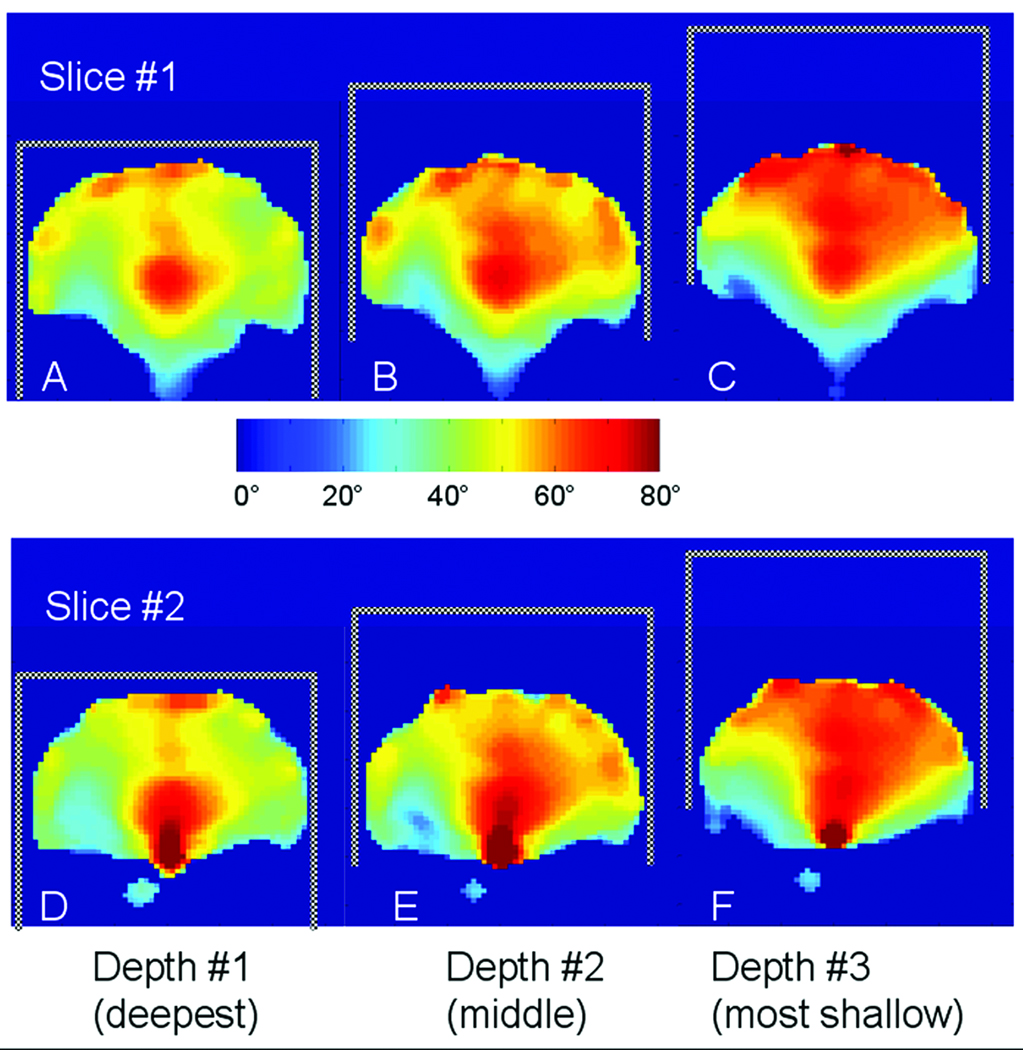
We examined how the positioning of a head inside of the longer (17 cm) TEM volume coil affects the RF field distribution. The length and diameter of this coil are very similar to that of the 7 T TEM head coil previously reported by Vaughan (1). This length allows practically full physical coverage of the brain longitudinally when a head is completely moved into the coil. Figure 1 shows B1 maps from two coronal images (separated by 2 cm) obtained near the center of a head for three different head depths within the coil. In Figs. 1A and 1D (depth #1, deepest), the head is completely moved in; Figs. 1B and 1E (depth #2, middle) the head is shifted out by 3.5 cm; in Figs. 1C and 1F (depth #3, most shallow) the head is shifted out by 7 cm. All the maps shown in Fig. 1 were obtained at the same RF power level.
Figure 1.
B1 maps of two coronal slices obtained using 17-cm-long TEM volume coil near the center of a human brain and separated by 2 cm. Maps were acquired for three positions of the coil in respect to a head. A),D) Depth #1 corresponds to a head fully inserted into the coil (deepest). Depth #2 (B,F, middle) and Depth #3 (C,D, most shallow) correspond to a head moved out of the coil by 3.5 cm and 7 cm, respectively. Positions of the TEM coil (crosshatched) with respect to the head are shown in each figure.
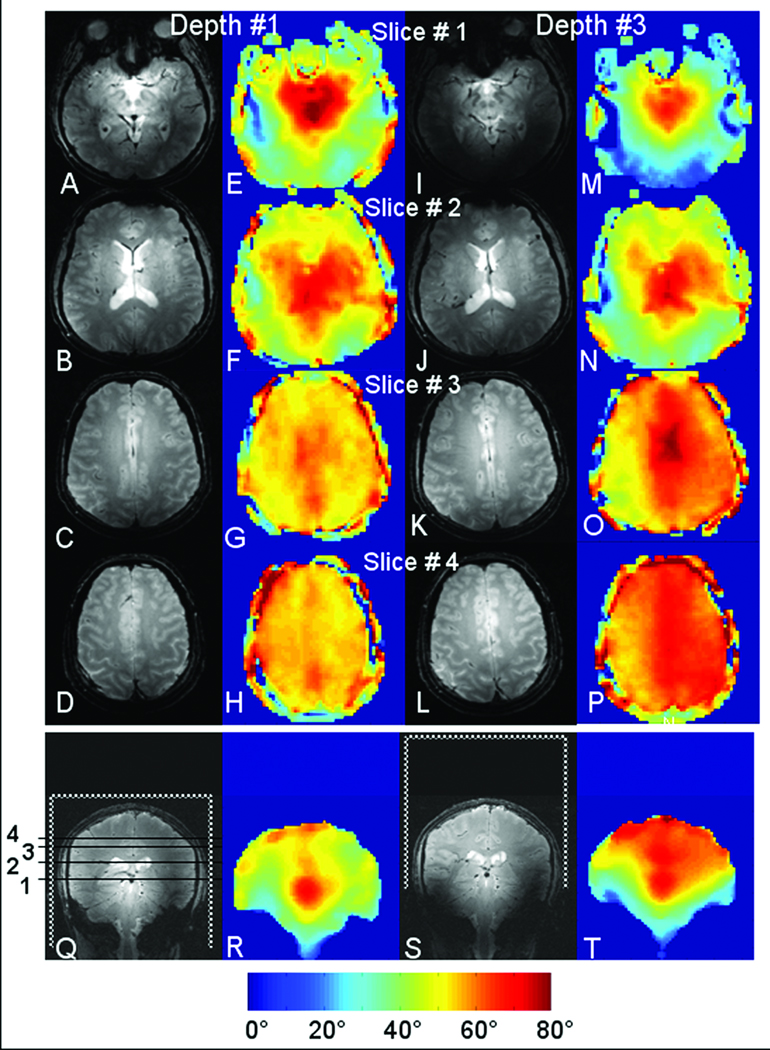
Using the long 17 cm TEM coil, Fig. 2 shows a stack of axial gradient echo images (Figs. 2A–D and I–L) and their corresponding B1 maps (Figs. 2E–H and M–P) obtained at two different depths. The two depths shown match that shown in Figs. 1A and C (depths #1 and #3). For clarity, Figs. 2Q–T shows the coronals (gradient echo and B1 maps) that indicate the positions of the coil and axial slices 1–4. Axial slices 1 through 3 are separated by 2 cm and the distance between slices 3 and 4 is 1 cm. In the shallow position (depth #3 Figs. 2I–L, M–P), image quality is maintained through the superior brain.
Figure 2.
Axial gradient echo images (A–D; I–L) and corresponding B1 (E–H; M–P) maps obtained using the 17-cm-long TEM coil and two positions of the coil (depths #1 and #3). Positions of the TEM coil in respect to a head are shown in the coronal plane, Figs 2Q–T. Axial slices #1–3 separated by 2 cm, and slices #3 and #4 – by 1 cm. Locations of two axial slices (#2 and #4) are also used to evaluate coil efficiency and homogeneity as shown in Table 1.
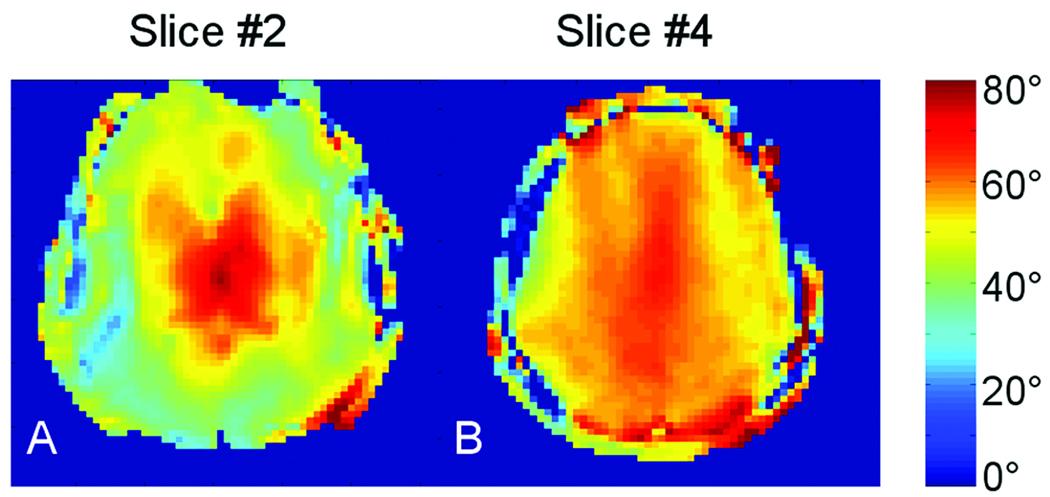
Data from Fig. 3 (B1 maps) shows performance from the short 10 cm TEM coil for two axial slices matching positions of slices #2 and #4 shown in Fig. 2. With respect to head coverage, “full” head insertion used with this short coil matched depth position #3 (used with the long coil) as shown in Figs. 1C and 1F. The B1 map of the short TEM agrees well with that from depth #3 (most shallow) positioning of the long TEM coil.
Figure 3.
Axial B1 maps obtained for A) slice #2 and B) slice #4 (see Fig.2Q) using the shorter 10-cm-long TEM volume coil.
DISCUSSION
The B1 maps in Figs. 1A and 1D demonstrate that the general features of the RF field distribution are very similar to that reported previously (1) particularly when the head is deeply positioned. It shows a very characteristic bright spot in the center of the brain and decreased field in peripheral locations. Both B1 maps (Figs. 1A and 1D) reveal slightly increased RF field at the very top of a head due to the presence of the shielded back wall (7). As further demonstrated in Fig. 1, partially moving a head out of the volume coil improves the B1 field homogeneity at the top of a head. This improvement appears largely continuous as the head is gradually withdrawn from the coil (Fig. 1, left to right). Given that all the maps shown in Fig.1 were obtained at the same RF power level, this argues that there is more efficient and homogeneous RF generation superiorly as seen with Figs. 1C and 1F in comparison to Figs. 1A and 1D.
Also very similar features are demonstrated in Fig. 2, which shows a stack of axial images and B1 maps for two positions of the coil in respect to a head, i.e. the deepest (#1) and most shallow (#3) depths. Again dielectric brightening is clearly seen in the slices near the head center for both positions of the coil (most inferior axial slice #1). It is noteworthy that for the shallowest depth (#3) the axial slice #1 is very close to the coil edge. This demonstrates propagation of the RF field through the head tissue out of the coil and supports conclusion of previous work that at high field, the sample rather than the coil, heavily influences the RF field distribution (6). Other features demonstrated in Fig.2 include: 1) the RF field distribution superiorly (axial slices #3 and #4) is substantially more homogeneous for both head depths but with the shallow head depth the B1 appears to be more intense; 2) the homogeneity in the shallow position is decreased inferiorly (axial slices #1 and #2) and the RF field distribution reveals more pronounced dielectric brightening effect.
Table 1 presents quantitative data obtained for two axial slices #2 and #4 (Fig. 2Q, these slices separated by 3 cm) including B1 values at the slice centers (averaged over elliptical area 50 × 40 mm) and homogeneity evaluated as a ratio of the central B1 to that measured at the periphery. The long TEM coil with a head positioned deeply produces a central B1 of 0.6 kHz per 1 kW (Fig. 2Q, slice #2) which is analogous to that previously reported for the 7 T TEM of similar size (1). The B1 homogeneity measured about 1.5 fold over the inferior slice #2 which is also in agreement with data reported previously (1). With the head shallowly positioned, the B1 field measured at the center of the axial slice #4 (Fig. 2Q, slice #4) was about 20 % better than that measured with the deeply positioned head. The ratio of central to peripheral B1 was about 1.1–1.15 fold which is similar to that measured at 3 T. However, for the shallowly positioned head, the B1 homogeneity measured for slice #2, which is now located near the coil entrance, is decreased (Table 1). The loss, however, is not an issue if the study targets supraventricular regions.
Table 1.
Volume Coil Efficiency
| Description | Slice #2 | Slice #4 | |||||
|---|---|---|---|---|---|---|---|
| B1 (cent.)*, kHz/ 1kW |
|
B1 (aver)#, kHz/ 1kW (SD, %) | B1 (cent.)*, kHz/ 1kW |
|
B1 (aver)#, kHz/1kW (SD, %) | ||
| Long TEM Position 1 | 0.60±0.04 | 1.47 | 0.48±0.077 (16%) | 0.48±0.02 | 1.08 | 0.46±0.028 (6%) | |
| Long TEM Position 3 | 0.61±0.06 | 1.60 | 0.45±0.086 (19%) | 0.59±0.03 | 1.12 | 0.56±0.039 (7%) | |
| Short TEM | 0.60±0.04 | 1.63 | 0.44±0.088 (20%) | 0.58±0.025 | 1.13 | 0.54±0.041 (7.5%) | |
The value was measured from an axial B1 map and averaged over 50 × 40 mm ellipse
Peripheral B1 was averaged over 145 × 120 × 20 mm elliptical ring
Peripheral B1 was averaged over 135 × 110 × 20 mm elliptical ring
B1 was averaged over 145 × 120 mm elliptical area
The logical conclusion from the above data is that at 7 T, good B1 homogeneity in the frontal-parietal regions may be achieved with a long volume coil given shallow positioning of the head. Alternatively, a short volume coil can be used. Decreasing the coil’s length helps minimize the inductance of the coil elements, which is often a problem in designing head-sized volume coils at 7 T. Specifically, for the TEM design, the inductance is determined by the element length and the distance between the shield and the elements. Therefore, to decrease the coil inductance while raising the resonance frequency for a given coil length this shield-to-element distance needs to be decreased. Making this distance smaller has several consequences, including smaller separation between coil modes and decreased QU/QL ratio, resulting in potentially greater shading artifact (14) and decreased efficiency. The problem of an excessively high coil inductance is even more severe for the 7 T head-sized birdcage coils where inductance is determined by the size of the meshes, i.e. by the coil diameter and number of the rungs. It is clear that a shorter coil would allow using a fewer number of rungs and simplifies the design.
To demonstrate this conclusion we tested a shorter TEM coil measuring only 10 cm in length. The coil produced a RF field distribution and the B1 field value very similar to the longer TEM coil with a shallowly positioned head (equivalent to depth position #3, Figs. 1C and 1F). As predicted, the RF field distributions measured for both coils were well matched. Fig. 3 demonstrates examples of axial B1 maps obtained for two slices matching slices #2 and #4 shown in Fig.2Q. Homogeneity evaluated in the same way as above measured 1.63 and 1.13 (Table 1) for the maps shown in Figs. 3A and 3B, respectively. Value of the B1 field in the center of the slices (averaged over 50 × 40 mm elliptical area) was also very similar.
A complete description of the observed phenomena is beyond the scope of the current work, which is rather intended to produce a practical solution of the B1 inhomogeneity problem at 7 T at least for certain applications. However, the following comments may give some insight into the observed effect. Two extreme cases of boundary conditions producing entirely different RF field distributions inside of a human head have been previously described (1,6,15). In the first case (1,6), a head is completely immersed into the RF field by positioning it near the center of a volume coil (similar as in Figs. 1A and 1D). As has been shown previously, in this case the RF field distribution is determined by the sample and practically does not change with increasing the coil length (6). This geometry results in the familiar inhomogeneous B1 distribution which is bright in the center and decaying towards periphery (1,6). In the other limit when the source (for example, a surface coil) is located at the head edge the RF field is maximized near the edge and decays inside of the head (15). Our situation of a shallowly positioned head (or a short volume coil) is in between these two limits. The combination of a short distance of RF immersion within a resonator helps to extend the RF field within the sample while simultaneously maximizing the B1 field near the sample edge. There are most likely several approaches to understanding this phenomenon, e.g., analysis of standing wave versus local constructive and destructive interference behavior, both of which are altered by the interaction and boundary conditions imposed by the human head (16,17). However, either approach (or their combination) requires more detailed analytical or numerical analysis, which was not the primary task of the current work.
Even though TEM volume coils were evaluated in this study, this effect is general and not limited to any specific type of coil. The same approach can be used for optimization of the performance of other types of head-sized high field volume coils near the top portion of a human head.
In conclusion, we have demonstrated that by using a shorter volume coil or simply by partially moving a head out of the coil, the coil efficiency and homogeneity near the top of a head can be substantially improved. In this region, the homogeneity evaluated for axial slices measured 10–20%, which is similar to values reported for 3 T images. For studies where scope of interest is limited to the rostral brain, this coil is very effective. Finally, short volume coils also results in a significant decrease in coil inductance, which substantially simplifies the design of head-sized 7 T volume coils.
Acknowledgments
Contract grant sponsor: National Institutes of Health
Contract grant numbers: R01-EB000473
REFERENCES
- 1.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, Andersen GP, Merkle H, Goebel R, Smith MB, Ugurbil K. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Res Med. 2001;46:24–30. doi: 10.1002/mrm.1156. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim TS, Lee R, Baertlein BA, Abduljalil AM, Zhu H, Robitaille P-ML. Effect of RF coil excitation on field inhomogeneity at ultra high fields: a field optimized TEM resonator. Magn Res Imag. 2001;19:1339–1347. doi: 10.1016/s0730-725x(01)00404-0. [DOI] [PubMed] [Google Scholar]
- 3.Collins CM, Liu W, Swift BJ, Smith MB. Combination of optimized transmit arrays and some receive array reconstruction methods can yield homogeneous images at very high frequencies. Magn Reson Med. 2005;54:1327–1332. doi: 10.1002/mrm.20729. [DOI] [PubMed] [Google Scholar]
- 4.Adriany G, Van de Moortele P-F, Wiesinger F, Moeller S, Strupp JP, Andersen P, Snyder C, Zhang X, Chen W, Pruessmann KP, Boesiger P, Vaughan JT, Uğurbil K. Transmit and receive transmission line arrays for 7 Tesla parallel imaging. Magn Reson Med. 2005;53:434–445. doi: 10.1002/mrm.20321. [DOI] [PubMed] [Google Scholar]
- 5.Vaughan JT, DelaBarre L, Snyder C, Tian J, Akgun C, Shrivastava D, Liu W, Olson C, Adriany G, Strupp J, Andersen P, Gopinath A, van de Moortele P-F, Garwood M, Ugurbil K. 9.4 T human MRI: Preliminary results. Magn Reson Med. 2006;56:1274–1282. doi: 10.1002/mrm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabetani A, McKinnon G, Nakada T. Performance comparison with 15 cm long and 23 cm long birdcage coil on 7 T. Proceedings of the 14th annual meeting of ISMRM; Seattle, USA. 2006. p. 2608. [Google Scholar]
- 7.Vaughan JT, Hetherington HP, Otu JO, Pan JW, Pohost GM. High frequency volume coils for clinical NMR imaging and spectroscopy. Magn Reson Med. 1994;32:206–218. doi: 10.1002/mrm.1910320209. [DOI] [PubMed] [Google Scholar]
- 8.Ke Y, Streeter C, Nassar L, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, Awad LA, Rendall MJ, Gruber SA, Nason A, Mudrick MJ, Blank SR, Meyer AA, Knapp C, Ciraulo DA, Renshaw PF. Frontal lobe GABA levels in cocaine dependence: a 2D J-resolved MRS study. Psychiatry Research: Neuroimaging. 2004;130(3):283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Unrath A, Ludolph A, Kassubek J. Brain metabolites in definite ALS. J Neurology. 2007;254(8):1099–10106. doi: 10.1007/s00415-006-0495-2. [DOI] [PubMed] [Google Scholar]
- 10.Kalra S, Hanstock C, Martin W, Allen PS, Johnston WS. Detection of cerebral degeneration in ALS using high field MRS. Arch Neurol. 2006;63(8):1144–1148. doi: 10.1001/archneur.63.8.1144. [DOI] [PubMed] [Google Scholar]
- 11.Avdievich NI, Hetherington HP. A 4T actively detuneable transmit/receive 1H transverse electromagnetic (TEM) and 4-channel receive-only phased array for 1H human brain studies. Magn Reson Med. 2004;52:1459–1464. doi: 10.1002/mrm.20264. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan JT, Adriany G, Garwood M, Yacoub T, Duong T, DelaBarre L, Andersen P, Ugurbil K. Detuneable Transverse Electromagnetic (TEM) volume coil for high field NMR. Magn Reson Med. 2000;47:990–1000. doi: 10.1002/mrm.10141. [DOI] [PubMed] [Google Scholar]
- 13.Pan JW, Twieg DB, Hetherington HP. Quantitative spectroscopic imaging of the human brain. Magn Reson Med. 1998;40:363–369. doi: 10.1002/mrm.1910400305. [DOI] [PubMed] [Google Scholar]
- 14.Tropp J. Proceedings of the Workshop on MRI hardware in Cleveland 2001. Cleveland: 2001. A model for MR image shading in multi-mode resonators; p. 11. [Google Scholar]
- 15.Yang QX, Wang J, Zhang X, Collins CM, Smith MB, Liu H, Zhu X-H, Vaughan JT, Ugurbil K, Chen W. Analysis of wave behavior in lossy dielectric samples at high field. Magn Reson Med. 2002;47:982–989. doi: 10.1002/mrm.10137. [DOI] [PubMed] [Google Scholar]
- 16.Van de Moortele P-F, Akgun C, Adriany G, Moeller S, Ritter J, Collins CM, Smith MB, Vaughan JT, Uğurbil K. B1 destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil. Magn Reson Med. 2005;54:1503–1518. doi: 10.1002/mrm.20708. [DOI] [PubMed] [Google Scholar]
- 17.Collins CM, Liu W, Schreiber W, Yang QY, Smith MB. Central brightening due to constructive interference with, without, and despite dielectric resonance. J Magn Reson Imag. 2005;21:192–196. doi: 10.1002/jmri.20245. [DOI] [PubMed] [Google Scholar]