Abstract
The immunologic basis for the potential enhanced HIV-1 acquisition in Ad5 seropositive individuals who received the Merck rAd5 HIV-1 vaccine in the STEP study remains unclear. Here we show that baseline Ad5-specific neutralizing antibodies are not correlated with Ad5-specific T lymphocyte responses and that Ad5 seropositive subjects do not develop higher vector-specific cellular immune responses as compared with Ad5 seronegative subjects following vaccination. These findings challenge the hypothesis that activated Ad5-specific T lymphocytes were the cause of the potential enhanced HIV-1 susceptibility in the STEP study.
In the phase 2b efficacy study (the STEP study) evaluating the Merck recombinant adenovirus serotype 5 (rAd5) vector-based HIV-1 vaccine, vaccinees with baseline Ad5-specific neutralizing antibodies (NAbs) exhibited a 2.3-fold increased rate of HIV-1 acquisition as compared with controls1,2. This potential increased HIV-1 susceptibility appeared to be durable for >52 weeks of follow-up and remains unexplained. These findings largely paralyzed the HIV-1 vaccine field and emphasized the importance of evaluating vector-specific immunity in the context of HIV-1 vaccine studies. A hypothesis has been proposed in which baseline Ad5-specific NAbs may have been surrogate markers for Ad5-specific T lymphocyte responses, and anamnestic Ad5-specific CD4+ T lymphocytes following vaccination in Ad5 seropositive subjects may have served as increased targets for HIV-1 infection.
To explore the immunologic basis of this hypothesis, we analyzed Ad vector-specific immunity in 116 subjects vaccinated with 1010 or 1011 viral particles (vp) of the Merck rAd5-Gag vaccine in the phase 1 studies that preceded the STEP study3. Serum and peripheral blood mononuclear cells (PBMC) at week 0 (baseline) and week 8 (4 weeks following the second vaccination) were utilized to assess vector-specific humoral and cellular immunity before and after vaccination.
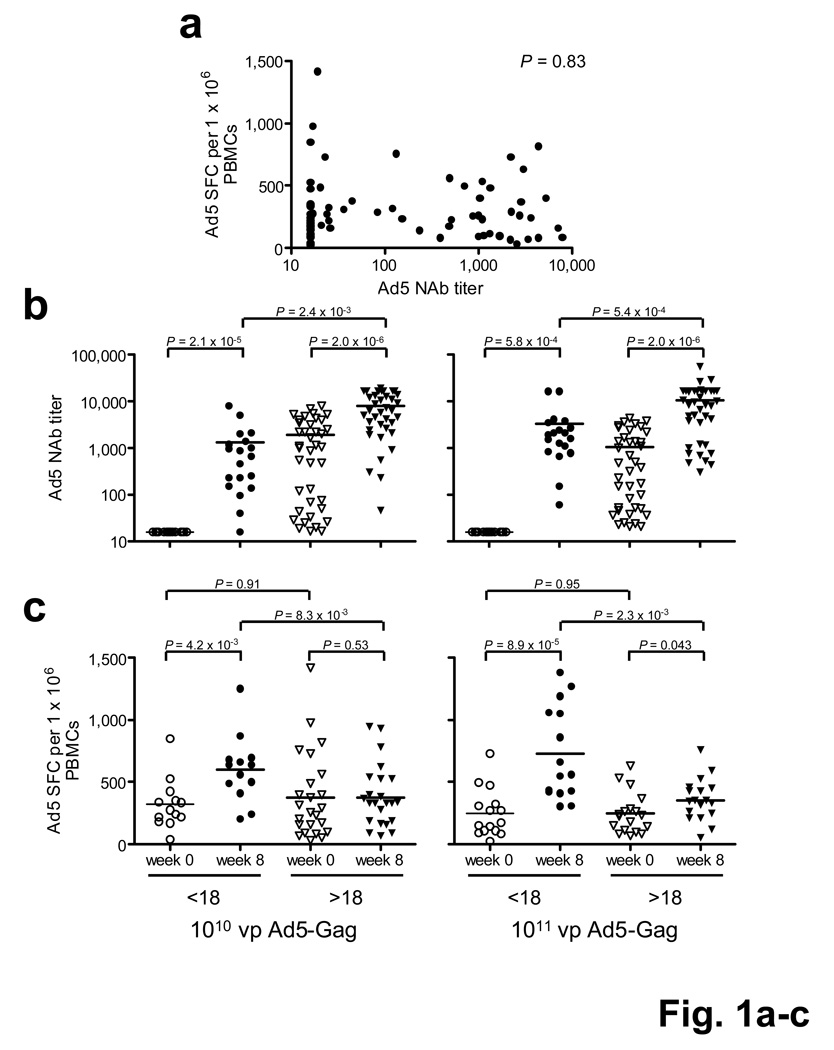
We assessed baseline Ad5-specific NAb titers by virus neutralization assays4 and and Ad5-specific T lymphocyte responses by IFN-γ ELISPOT assays5 following Ad5 virus stimulation (Supplementary Methods). Ad5-specific cellular immune responses were detected in >90% of subjects at baseline (Fig. 1a), and these data were confirmed by ELISPOT assays using pooled Ad5 hexon peptides (data not shown). We speculate that the remarkably high frequency of Ad5-specific T lymphocyte responses reflect cross-reactive hexon-specific responses among multiple common subgroup C Ads (data not shown). We observed no correlation between baseline Ad5-specific ELISPOT responses and NAb titers (Fig. 1a, P = 0.83, Spearman rank-correlation test), demonstrating that Ad5-specific NAbs are not simply surrogate markers for Ad5-specific T lymphocyte responses.
Figure 1.
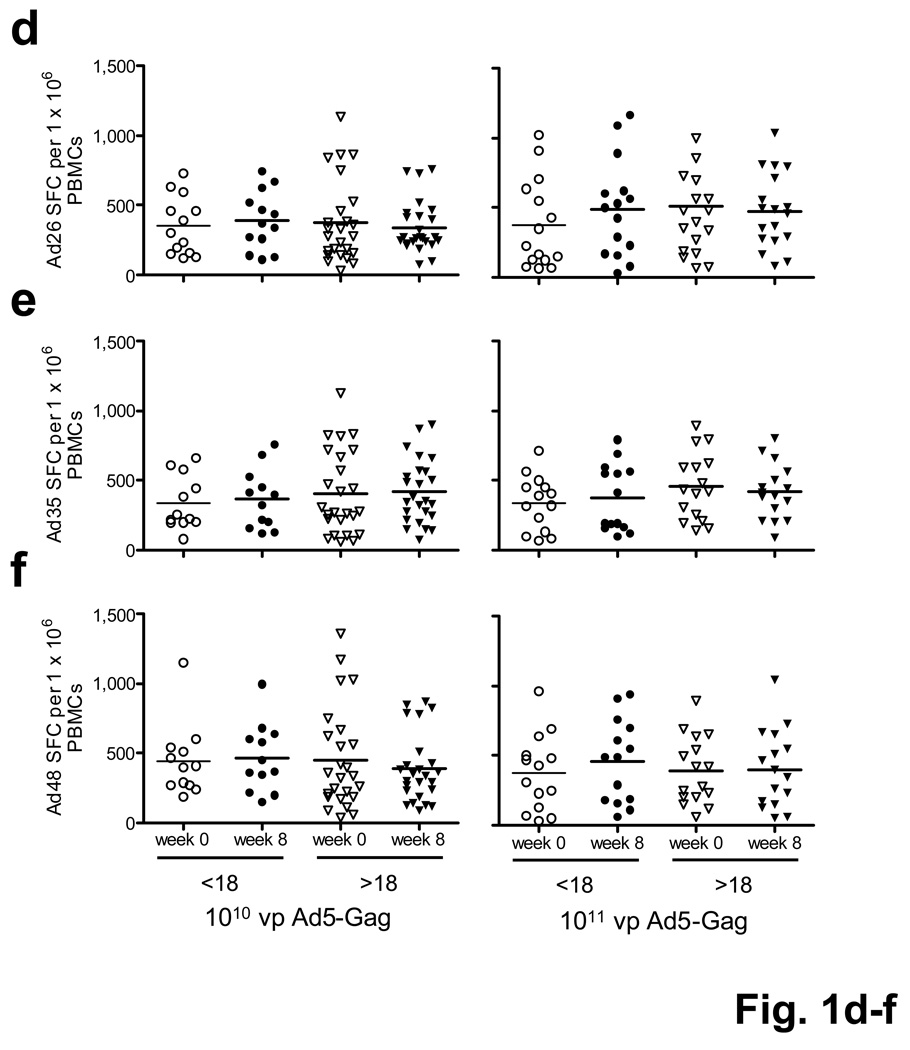
Ad-specific humoral and cellular immune responses before and after rAd5-Gag vaccination. (a) Correlation between Ad5-specific IFN-γ ELISPOT responses and Ad5-specific NAb titers at baseline. (b) Ad5-specific NAb titers and (c) Ad5-specific IFN-γ ELISPOT responses as stratified by vaccine dose (1010 vp, 1011 vp), baseline Ad5 titer (<18, >18), and study timepoint (week 0, week 8). (d) Ad26-, (e) Ad35-, and (f) Ad48-specific IFN-γ ELISPOT responses.
We next evaluated the evolution of Ad5-specific NAbs following vaccination (Fig. 1b). Individuals who were Ad5 seronegative at baseline developed high titers of Ad5-specific NAbs at week 8. Subjects who were Ad5 seropositive at baseline developed supraphysiologic titers of Ad5-specific NAbs at week 8 that were significantly higher than those that developed in Ad5 seronegative subjects (P = 5.4×10−4, Wilcoxon rank-sum test, 1011 vp dose). In contrast, rAd5 vaccination did not elicit NAbs to the rare serotype Ad26, Ad35, and Ad48 viruses6–8, indicating minimal cross-reactive NAbs among these Ad serotypes (Supplementary Fig. 1, P = NS).
Ad5-specific cellular immune responses at baseline were comparable between Ad5 seronegative and Ad5 seropositive subjects (Fig. 1c, open symbols). In Ad5 seronegative subjects, rAd5 vaccination resulted in a 2-fold (1010 vp dose, P = 4.2×10−3) or a 3-fold (1011 vp dose, P = 8.9×10−5) increase of Ad5-specific ELISPOT responses at week 8 (Fig. 1c, circles). In Ad5 seropositive subjects, vaccination resulted in only marginal increases in Ad5-specific ELISPOT responses (Fig. 1c, inverted triangles; 1010 vp dose, P = 0.53; 1011 vp dose, P = 0.043), which were significantly lower than those observed in Ad5 seronegative subjects (1010 vp dose, P = 8.3×10−3; 1011 vp dose, P = 2.3×10−3). These data suggest that baseline Ad5-specific NAbs partially neutralized the rAd5 vaccine vector following vaccination and resulted in a lower effective dose of the vaccine in Ad5 seropositive subjects. Gag-specific antibody and T lymphocyte responses were similarly blunted by baseline Ad5-specific NAbs as expected2,3,9 (Supplementary Fig. 2). Baseline Ad5-specific NAbs did not impact the cytokine profile (IFN-γ, IL-2 ≫ IL-4, IL-10) or IgG subtype (IgG1, IgG3 ≫ IgG2, IgG4) of Ad5-specific cellular or humoral immune responses following vaccination (data not shown).
We next evaluated the capacity of rAd5 vaccination to induce T lymphocyte responses to rare serotype Ads. Ad26-, Ad35-, and Ad48-specific ELISPOT responses were common (Fig. 1d–f) despite their low seroprevalence6,7,10. Nevertheless, these responses were not detectably augmented following rAd5 vaccination (Fig. 1d–f, P = NS). These data indicate that cellular immune responses to the homologous Ad5 virus were substantially more potent than cross-reactive responses to these rare Ad serotypes.
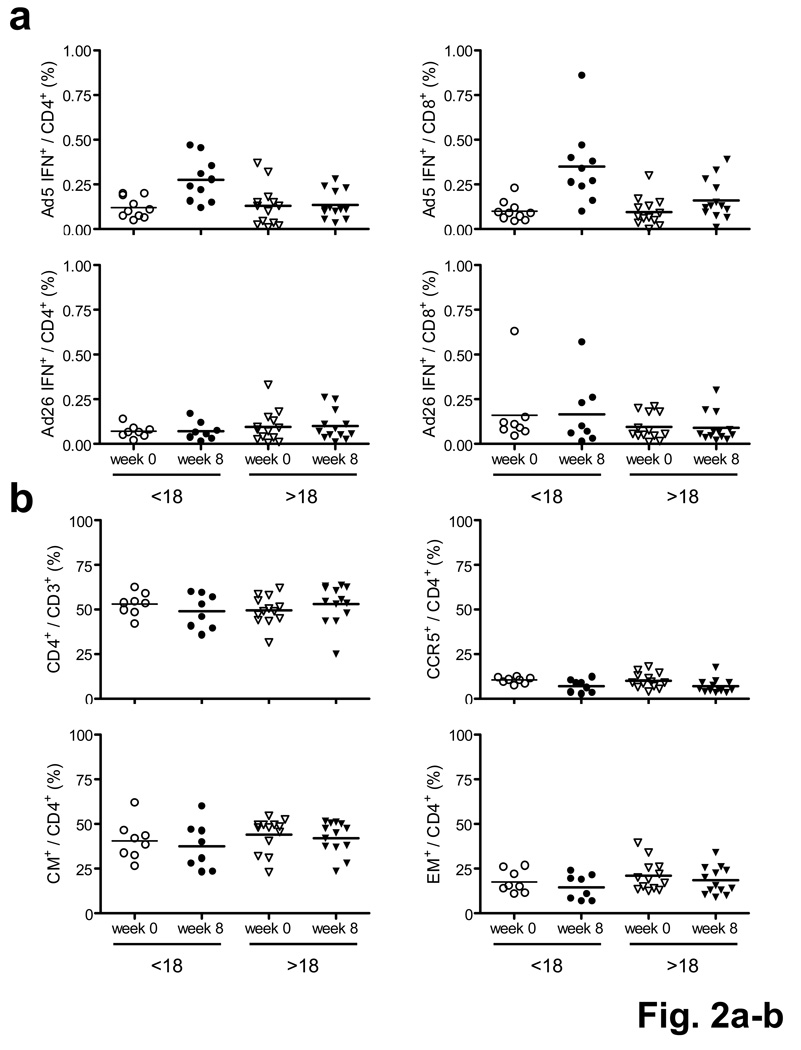
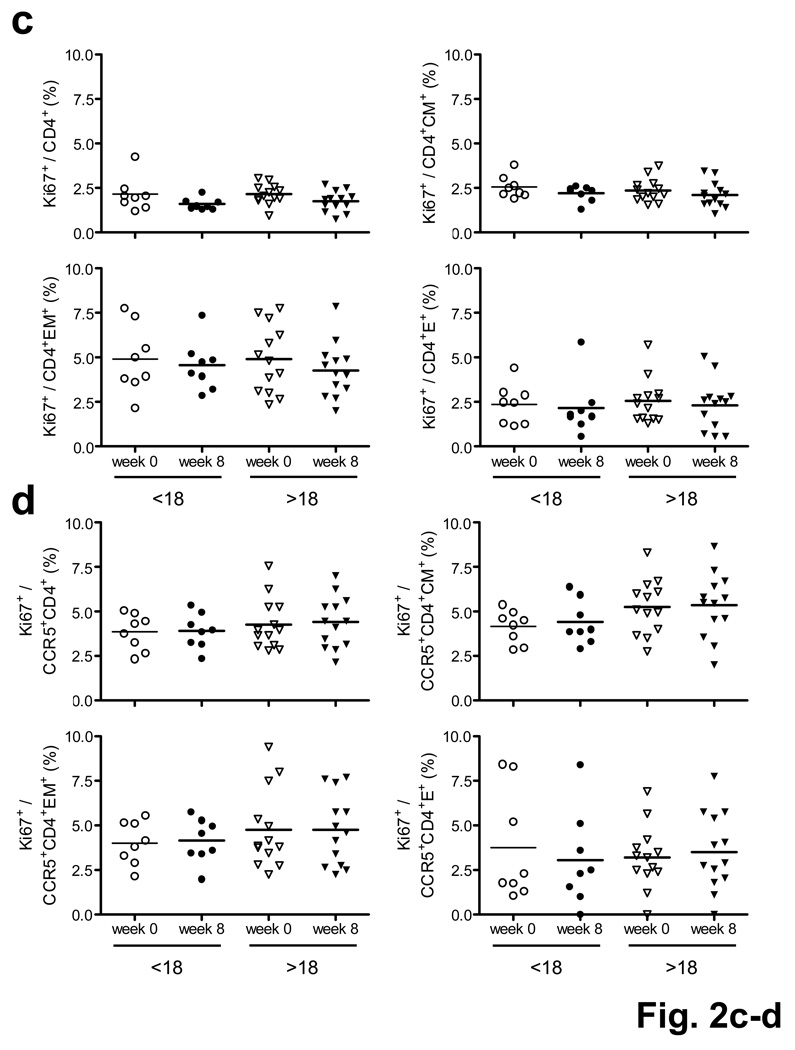
We confirmed these findings in CD4+ and CD8+ T lymphocyte subpopulations by multiparameter intracellular cytokine staining (ICS) assays5,11 (Supplementary Fig. 3; Supplementary Methods) in 23 subjects who received the 1011 vp dose of the vaccine. Vaccination resulted in clear increases in Ad5-specific IFN-γ+CD4+ and IFN-γ+CD8+ T lymphocyte responses in Ad5 seronegative subjects, but less convincing responses were observed in Ad5 seropositive individuals (Fig. 2a). Similar results were evident for Ad5-specific IL-2+ T lymphocyte responses (data not shown). In contrast, no clear changes in Ad26-specific CD4+ and CD8+ T lymphocytes were observed following rAd5 vaccination. We also found no perturbation of total, CCR5+, CD45RO+CD27+ central memory (CM), and CD45RO+CD27− effector memory (EM) CD4+ T lymphocyte subpopulations (Fig. 2b, P = NS) and no sustained Ki67+ activation of any of these CD4+ T lymphocyte subpopulations (Fig. 2c, d, P = NS) at week 8. We also did not detect any differences in Ki67 expression on Ad5-specific CD4+ T lymphocytes between Ad5 seronegative and Ad5 seropositive subjects (Supplementary Fig. 4a). Short-term cellular immune activation for 1–2 weeks following rAd5 vaccination has been reported12, but these transient effects do not likely explain the durable increase in HIV-1 susceptibility for >52 weeks in the STEP study1,2.
Figure 2.
Magnitude and phenotype of Ad-specific and total CD4+ T lymphocyte subpopulations before and after 1011 vp rAd5-Gag vaccination. (a) Ad5- (top panels) and Ad26- (bottom panels) specific IFN-γ+CD4+ (left panels) and IFN-γ+CD8+ (right panels) ICS responses as stratified by baseline Ad5 titer (<18, >18) and study timepoint (week 0, week 8). (b) Total, CCR5+, CD45RO+CD27+ central memory (CM), and CD45RO+CD27− effector memory (EM) CD4+ T lymphocyte subpopulations. (c) Ki67+ activation of total, CM, EM, and CD45RO−CD27− effector (E) CD4+ T lymphocyte subpopulations. (d) Ki67+ activation of total, CM, EM, and E CCR5+CD4+ T lymphocyte subpopulations.
In summary, Ad5-specific NAbs at baseline are not surrogate markers for Ad5-specific cellular immune responses, and Ad5-specific T lymphocyte responses following rAd5 vaccination were lower in Ad5 seropositive as compared with Ad5 seronegative subjects. We also observed no evidence of sustained modulation or activation of CD4+ T lymphocyte subpopulations following rAd5 vaccination. These data challenge the hypothesis that anamnestic Ad5-specific CD4+ T lymphocyte responses following rAd5 vaccination were responsible for the potential enhancement of HIV-1 acquisition in Ad5 seropositive subjects in the STEP study. A caveat is that we were unable to assess mucosal immune responses in the present study, although we did not detect differences between Ad5 seronegative and Ad5 seropositive subjects in α4 and β7 integrin expression on total or Ad5-specific peripheral CD4+ T lymphocytes (Supplementary Fig. 4b). An alternative model has been suggested in which the potential enhancement of HIV-1 susceptibility in Ad5 seropositive subjects may have been due to immune complex formation following rAd5 vaccination13. If this model proves correct, then the use of vaccine vectors that evade baseline vector-specific NAbs may offer a potential solution to this problem.
Supplementary Material
Acknowledgements
We thank J. Custers, M. Pau, P. Abbink, A. La Porte, N. Letvin, F. Stephens, and T. Swanson for generous advice, assistance, and reagents. We acknowledge support from the Bill & Melinda Gates Foundation (#38614) and the National Institutes of Health (AI066305, AI066924, AI078526, AI074078, AI076066).
Footnotes
The authors declare no competing financial interests.
References
- 1.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McElrath MJ, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priddy FH, et al. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clinical infectious diseases. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 4.Sprangers MC, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–5052. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogels R, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbink P, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch DH, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 9.Catanzaro AT, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorner AR, et al. Age dependence of adenovirus-specific neutralizing antibody titers in individuals from sub-Saharan Africa. J Clin Microbiol. 2006;44:3781–3783. doi: 10.1128/JCM.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. Journal of virology. 2008;82:4844–4852. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElrath J. Vaccine-induced immunity in T-cell based candidate HIV vaccines. AIDS Vaccine 2008, Cape Town, South Africa. 2008 [Google Scholar]
- 13.Perreau M, Pantaleo G, Kremer EJ. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. The Journal of experimental medicine. 2008;205:2717–2725. doi: 10.1084/jem.20081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.