Abstract
We report outcomes of 932 recipients of unrelated donor peripheral blood stem cell hematopoietic cell transplantation (URD-PBSC HCT) for acute myeloid leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia, and myelodysplastic syndrome enrolled on a prospective National Marrow Donor Program trial from 1999 through 2003. Preparative regimens included myeloablative (MA; N = 611), reduced-intensity (RI; N = 160), and nonmyeloablative (NMA; N = 161). For MA recipients, CD34+ counts greater than 3.8 × 106/kg improved neutrophil and platelet engraftment, whereas improved overall survival (OS) and reduced transplant-related mortality (TRM) were seen for all preparative regimens when CD34+ cell doses exceeded 4.5 × 106/kg. Higher infused doses of CD34+ cell dose did not result in increased rates of either acute or chronic graft-versus-host disease (GVHD). Three-year OS and disease-free survival (DFS) of recipients of MA, RI, and NMA approaches were similar (33%, 35%, and 32% OS; 33%, 30%, and 29% DFS, respectively). In summary, recipients of URD-PBSC HCT receiving preparative regimens differing in intensity experienced similar survival. Higher CD34+ cell doses resulted in more rapid engraftment, less TRM, and better 3-year OS (39% versus 25%, MA, P = .004; 38% versus 21% RI/NMA, P = .004) but did not increase the risk of GVHD. This trial was registered at www.clinicaltrials.gov as #NCT00785525.
Introduction
In the early 1990s hematopoietic cell transplantation programs began using cytokine-mobilized peripheral blood stem cells (PBSCs) from sibling donors in lieu of bone marrow (BM) as a primary stem cell source.1–4 Unrelated donor (URD) transplantation networks followed suit at the end of the 1990s,5 and the use of URD-PBSC grafts has grown rapidly. In 2007, 59% of National Marrow Donor Program (NMDP)–facilitated URD transplantations involved PBSCs (versus bone marrow and cord blood) and adult recipients of non–cord blood donations received PBSC grafts 80% of the time. The marked increase in the use of URD PBSCs was fueled by early reports showing more rapid engraftment, good survival, and similar rates of graft-versus-host disease (GVHD) compared with URD BM.6,7 The trend toward the use of URD PBSCs was further influenced by a report of lower rates of rejection and disease progression compared with the use of BM after a nonmyeloablative preparative regimen,8 resulting in PBSCs being the preferred choice in many reduced toxicity regimen approaches. Finally, ease of acquisition (apheresis versus marrow harvest) and donor choice probably added to the increased use of URD PBSCs. This high rate of URD-PBSC usage continues despite recent studies raising concern about late chronic GVHD-related morbidity.9–13
Large studies have defined specific donor, graft, and transplant characteristics that lead to better outcome after URD BM transplantation.14–17 Aside from a recent analysis of CD34+ cell dose,18 the effect of other factors such as donor sex, HLA match, preparative regimen intensity, GVHD prophylactic regimen, and so forth, on survival and GVHD outcomes after URD-PBSC transplantation have not been studied in a large cohort.
Since 1999, all NMDP PBSC transplantations have been performed under a US Food and Drug Administration–accepted Investigational New Drug application protocol designed to assess URD-PBSC safety, collection efficacy, and recipient outcomes. To correlate transplant characteristics with URD-PBSC outcomes, we limited our cohort to recipients who received a transplant for the 4 most common hematologic malignancies (acute myeloid leukemia [AML], acute lymphoblastic leukemia [ALL], chronic myelogenous leukemia [CML], and myelodysplastic syndrome [MDS]) enrolled in the NMDP PBSC trial. We included key donor, product, and transplant-related variables.
Methods
Study cohort and data collection
The study cohort consisted of all recipients of primary PBSC transplants for AML, ALL, CML, or MDS facilitated by the NMDP from August 1999 through December 2003. Recipients of products that were manipulated for T-cell depletion or CD34+ cell selection were excluded from the analysis. This analysis was conducted on recipients who gave informed consent for submission of their outcome data to the NMDP for studies, in accordance with the Declaration of Helsinki. This study was approved by the NMDP central Institutional Review Board. This was done prospectively for all recipients since May 2002 but inconsistently for patients who received transplants at some centers before then. In 2002, the NMDP asked surviving recipients who received a transplant before May 2002 to document their consent for study participation. To address bias introduced by the inclusion of only a proportion of surviving recipients (those documenting consent) but all deceased recipients of transplants before May 2002, random exclusion of recipients who died before initiation of the corrective action plan was performed to generate a “corrective action plan–corrected” dataset as previously described.19 The final study population included 932 recipients from 99 transplantation centers. The analysis used the data collected on the NMDP Donor and Recipient Baseline and Follow-up Data Collection Forms and contract laboratory reports.
End points
Transplantation outcomes examined were neutrophil engraftment, platelet engraftment, overall survival, grades II-IV and grades III-IV acute GVHD, chronic GVHD, relapse, and transplant-related mortality (TRM). Neutrophil engraftment was defined as an achievement of an absolute neutrophil count of at least 500 neutrophils/mm3 sustained for 3 consecutive laboratory measurements on different days. Platelet engraftment was defined as an achievement of a platelet count recovery of at least 50000 platelets/mm3 sustained for 3 consecutive laboratory measurements on different days with no platelet transfusions in the previous 7 days. A severity grade for acute GVHD was calculated according to the reported stages of skin, liver, and intestinal involvement with the use of the Glucksberg grading system.20 Relapse was defined as hematologic recurrence; patients who failed to achieve remission after transplantation were considered to have had a recurrence at day 1. Treatment-related mortality was defined as death in continuous complete remission. Death from any cause was considered an event for overall survival.
Statistical methods
Patient-, disease-, transplant-, product-, and donor-related characteristics were compared for recipients of myeloablative (MA), reduced-intensity (RI), and nonmyeloablative (NMA) regimens with the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. A conditioning regimen was considered MA if it included one of the following: total body irradiation (TBI) greater than 500 cGy as a single fraction; TBI greater than 800 cGy regardless of the number of fractions; busulfan 9.5 mg/kg or more; melphalan greater than 150 mg/m2; any combination of busulfan and melphalan; or any combination of cyclophosphamide, etoposide (VP-16), and TBI. RI conditioning regimens included TBI between 200 and 500 cGy; TBI between 500 and 800 cGy as multiple fractions; busulfan less than 9.5 mg/kg; melphalan no greater than 150 mg/m2; 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), etoposide, cytarabine, and melphalan (BEAM regimens) or cyclophosphamide, BCNU, and VP-16 (CBV regimens); or any combination of VP-16 and cyclophosphamide. NMA conditioning regimens included TBI dose of 200 cGy, fludarabine with 200 cGy TBI, any combination of fludarabine and cyclophosphamide, or any combination of fludarabine and cytarabine. Univariate probabilities of overall survival were calculated with the Kaplan-Meier estimator; the log-rank test was used for univariate comparisons of survival curves; the chi-square test was used for pointwise comparisons.21 Probabilities of neutrophil and platelet recovery, acute and chronic GVHD, relapse, and TRM were calculated with the cumulative incidence function estimator with a subsequent transplantation as a censoring event.22,23 For neutrophil and platelet engraftment and acute and chronic GVHD, death without an event is the competing risk. For TRM, relapse was the competing risk; for relapse, TRM was the competing risk. The analyses of neutrophil and platelet engraftment were restricted to patients receiving MA regimens.
Assessment of potential risk factors for day 25 neutrophil engraftment and day 60 platelet engraftment was evaluated with the use of logistic regression. The estimated effects of each significant risk factor were given by odds ratios. Multivariate analyses of acute and chronic GVHD, relapse, TRM, and overall mortality were performed with the use of Cox proportional hazards regression.24 The estimated effects of each significant risk factor are given as relative risks (RRs). The following risk factors were considered as candidate effects in the model building process of each regression analysis.
Recipient- and disease-related factors.
These factors included recipient age, sex, race or ethnicity, body mass index (BMI), Karnofsky/Lansky performance score at conditioning, diagnosis and stage, disease risk, time from diagnosis to transplantation, coexisting disease, and CMV status. Disease risk was classified into 3 categories. Early disease included acute leukemia in first complete remission, chronic leukemia in first chronic phase, refractory anemia, or refractory anemia with ringed sideroblasts. Intermediate diseases included acute leukemia in second or higher complete remission or chronic leukemia in accelerated or second chronic phase. Advanced diseases included acute leukemia in relapse, chronic leukemia in blastic phase, refractory anemia with excess blasts, or refractory anemia with excess blasts in transformation.
Transplantation-related factors.
These factors included donor/recipient HLA match, ABO match, sex match, race match, CMV status match, conditioning regimen type, use of TBI, GVHD prophylaxis, use of planned growth factors for engraftment defined as G-CSF or GM-CSF between day −3 and day 7, and year of transplantation. On the basis of the best available typing data at the time of analysis, HLA match was classified into 3 categories: well-matched, partially matched, and mismatched, according to a recently developed algorithm that considers level of typing resolution and matching at HLA-A, -B, -C, and -DRB1 loci as described by Weisdorf et al.25 Well matched was defined as no known disparity between donor and recipient at HLA-A, -B, -C, and -DRB1, partially matched as one known or one likely disparity, and mismatched as 2 or more disparities.
Product-related factor.
This included CD34+ cells per kilogram of recipient weight in infused product.
Donor-related factors.
These factors included donor age, sex, race or ethnicity, BMI, CMV status, donor parity, 1-day versus 2-day collection, and the preapheresis day 5 values of donor white blood cell counts, platelet counts, and CD34+ cell counts.
A stepwise selection technique with a significance level of .05 was used in all regression analyses. Separate analyses were performed for MA transplants and RI/NMA transplants. For the Cox regression models, all possible risk factors were checked for proportional hazards with a time-dependent covariate approach, and there were no violations to the proportionality assumption. No significant first-order interactions were observed. For the cell dose variables, the optimal cutpoint was determined by examining the Martingale residual plots. P values are 2-sided. All analyses were done with the use of SAS Version 9.1 (SAS Institute Inc).
Results
Donor and recipient populations
No significant differences were noted between recipients of MA, RI, and NMA conditioning regimens in donor/recipient HLA, ABO, sex, and race matching, as well as CMV status and performance scores (Tables 1 and 2). Age of recipients varied significantly, with a median age of 38 years for patients receiving MA conditioning, compared with 56 and 57 years for recipients of RI and NMA regimens, respectively (P < .001). Fifty-two percent of recipients in the entire cohort had at least one coexisting medical comorbidity. The most common conditions included cardiac disease/hypertension (20%), followed by pulmonary, endocrine, and gastrointestinal disorders in 10%, 10%, and 8% of recipients, respectively (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As would be expected in an older cohort, patients receiving RI and NMA regimens were more likely to have comorbid conditions (61% and 73% vs 45% of recipients of RI, NMA, and MA conditioning had comorbidities, respectively). The donor population in this study was predominantly younger than 40 years of age (69%). Donation for persons older than age 50 was rare (7%). Donor ages, sex, weight, parity, and other characteristics were similar among the 3 preparative regimen cohorts.
Table 1.
Recipient and transplantation characteristics according to preparative regimen (N = 932 donor/recipient pairs undergoing harvest/transplantation)
| Variable | Myeloablative | Reduced intensity | Nonmyeloablative | P |
|---|---|---|---|---|
| Recipient characteristics | ||||
| No. of patients | 611 | 160 | 161 | |
| No. of centers | 79 | 52 | 36 | |
| Median follow-up time among survivors, d (range) | 1224 (228-2612) | 1123 (364-2200) | 1417 (593-2295) | |
| Male recipients, n (%) | 345 (56) | 92 (58) | 90 (56) | .957 |
| Recipient race/ethnicity | .011* | |||
| White, n (%) | 525 (86) | 142 (89) | 153 (95) | |
| Hispanic, n (%) | 39 (6) | 6 (4) | 4 (2) | |
| Asian/Pacific Islander, n (%) | 19 (3) | 6 (4) | 1 (<1) | |
| Black/African American, n (%) | 18 (3) | 5 (3) | 2 (1) | |
| Other/declines, n (%) | 10 (2) | 1 (<1) | 1 (< 1) | |
| Recipient age, median (range) | 38 (<1 to 65) | 56 (1-75) | 57 (17-73) | < .001 |
| 0-9 y, n (%) | 28 (5) | 2 (1) | 0 (0) | < .001 |
| 10-19 y, n (%) | 60 (10) | 6 (4) | 2 (1) | |
| 20-29 y, n (%) | 110 (18) | 7 (4) | 8 (5) | |
| 30-39 y, n (%) | 122 (20) | 17 (11) | 6 (4) | |
| 40-49 y, n (%) | 174 (28) | 19 (12) | 22 (14) | |
| 50-59 y, n (%) | 105 (17) | 66 (41) | 67 (42) | |
| 60 y or older, n (%) | 12 (2) | 43 (27) | 56 (35) | |
| Karnofsky performance score | .258 | |||
| 90-100, n (%) | 371 (61) | 91 (57) | 87 (54) | |
| 10-80, n (%) | 184 (30) | 54 (34) | 63 (39) | |
| Unknown, n (%) | 56 (9) | 15 (9) | 11 (7) | |
| Disease and stage | < .001† | |||
| Acute myelogenous leukemia, n (%) | 249 (41) | 99 (62) | 71 (44) | |
| First CR, n (%) | 87 (14) | 33 (21) | 34 (21) | |
| Second CR, n (%) | 56 (9) | 21 (13) | 17 (11) | |
| Third CR, n (%) | 4 (1) | 2 (1) | 2 (1) | |
| Not in remission, n (%) | 102 (17) | 43 (27) | 18 (11) | |
| Acute lymphoblastic leukemia, n (%) | 159 (26) | 10 (6) | 16 (10) | |
| First CR, n (%) | 49 (8) | 4 (3) | 9 (6) | |
| Second CR, n (%) | 46 (8) | 3 (2) | 4 (2) | |
| Third CR, n (%) | 21 (3) | 1 (< 1) | 1 (< 1) | |
| Not in remission, n (%) | 43 (7) | 2 (1) | 2 (1) | |
| Chronic myelogenous leukemia, n (%) | 95 (16) | 15 (9) | 24 (15) | |
| First CP, n (%) | 40 (7) | 7 (4) | 13 (8) | |
| Accelerated phase/second CP, n (%) | 44 (7) | 5 (3) | 10 (6) | |
| Blast phase, n (%) | 11 (2) | 3 (2) | 1 (< 1) | |
| Myelodysplastic syndromes (MDS), n (%) | 108 (18) | 36 (23) | 50 (31) | |
| Refractory anemia, n (%) | 31 (5) | 9 (6) | 6 (4) | |
| RAEB/RAEB-T, n (%) | 35 (6) | 14 (9) | 13 (8) | |
| Other MDS, n (%) | 42 (7) | 13 (8) | 31 (19) | |
| Disease risk | .013 | |||
| Early, n (%) | 207 (34) | 53 (33) | 62 (39) | |
| Intermediate, n (%) | 213 (35) | 45 (28) | 65 (40) | |
| Advanced, n (%) | 191 (31) | 62 (39) | 34 (21) | |
| Transplantation characteristics | ||||
| HLA match | .021 | |||
| Well-matched, n (%) | 359 (59) | 88 (55) | 108 (67) | |
| Partially matched, n (%) | 173 (28) | 53 (33) | 46 (29) | |
| Mismatched, n (%) | 79 (13) | 19 (12) | 7 (4) | |
| Donor/recipient sex match | .295 | |||
| Male/male, n (%) | 212 (35) | 65 (41) | 52 (32) | |
| Male/female, n (%) | 143 (23) | 42 (26) | 46 (29) | |
| Female/male, n (%) | 133 (22) | 27 (17) | 38 (24) | |
| Female/female, n (%) | 123 (20) | 26 (16) | 25 (16) | |
| Donor/recipient CMV status | .200 | |||
| Negative/negative, n (%) | 187 (31) | 35 (22) | 52 (33) | |
| Negative/positive, n (%) | 199 (33) | 63 (39) | 57 (36) | |
| Positive/negative, n (%) | 88 (15) | 20 (13) | 21 (13) | |
| Positive/positive, n (%) | 131 (22) | 42 (26) | 29 (18) | |
| Unknown, n (%) | 6 (N/A) | 0 (N/A) | 2 (N/A) | |
| TBI, n (%) | 399 (65) | 25 (16) | 129 (80) | < .001 |
| GVHD prophylaxis | < .001‡ | |||
| CsA + MTX ± other, n (%) | 327 (54) | 25 (16) | 4 (2) | |
| CsA ± other (no MTX), n (%) | 18 (3) | 55 (34) | 132 (82) | |
| FK506 + MTX ± other, n (%) | 220 (36) | 33 (21) | 12 (7) | |
| FK506 ± other (no MTX), n (%) | 40 (7) | 46 (29) | 8 (5) | |
| Other, n (%)§ | 6 (<1) | 1 (<1) | 5 (3) | |
| Use of planned growth factors, n (%) | 187 (31) | 66 (41) | 27 (17) | < .001 |
CR indicates complete remission; CP, chronic phase; RAEB, refractory anemia with excess of blasts; RAEB-T, RAEB in transformation; N/A, not applicable; and MTX, methotrexate.
White compared with others.
Comparing broad diseases.
CsA compared with FK506.
Other GVHD prophylaxis included MTX, mycophenolate mofetil, corticosteroids, and G-CSF.
Table 2.
Donor and product characteristics according to preparative regimen
| Variable | Myeloablative | Reduced intensity | Nonmyeloablative | P |
|---|---|---|---|---|
| Product characteristics | ||||
| Median CD34+ cell dose, × 106/kg (range)* | 6.2 (0.4-56.0) | 5.4 (0.7-55.4) | 6.3 (0.3-29.0) | .415 |
| Donor characteristics | ||||
| Male donors, n (%) | 355 (58) | 107 (67) | 98 (61) | .128 |
| Donor race/ethnicity | .004† | |||
| White, n (%) | 484 (79) | 130 (81) | 146 (91) | |
| Hispanic, n (%) | 47 (8) | 13 (8) | 5 (3) | |
| Multiple, n (%) | 24 (4) | 7 (4) | 4 (2) | |
| Asian/Pacific Islander, n (%) | 26 (4) | 5 (3) | 1 (< 1) | |
| Black/African American, n (%) | 16 (3) | 3 (2) | 1 (< 1) | |
| Other/declines/unknown, n (%) | 14 (2) | 2 (1) | 4 (2) | |
| Median donor age at donation, y (range) | 35 (19-60) | 37 (19-58) | 36 (18-60) | .286 |
| 18-30 y, n (%) | 213 (35) | 42 (26) | 51 (32) | .237 |
| 31-40 y, n (%) | 217 (36) | 70 (44) | 54 (34) | |
| 41-50 y, n (%) | 135 (22) | 39 (24) | 44 (27) | |
| 51-60 y, n (%) | 46 (8) | 9 (6) | 12 (7) | |
| Donor parity (female only) | .252 | |||
| 0, n (%) | 100 (39) | 11 (21) | 27 (43) | |
| 1-2, n (%) | 77 (30) | 21 (40) | 17 (27) | |
| 3 or more, n (%) | 60 (23) | 15 (28) | 15 (24) | |
| Unknown, n (%) | 19 (7) | 6 (11) | 4 (6) |
CD34+ cell dose is missing in 176 myeloablative cases, 46 reduced-intensity cases, and 39 nonmyeloablative cases.
White compared with others.
Transplant characteristics, engraftment, and overall survival
Table 1 reviews characteristics of the transplants included in the analysis. Sixty percent of the transplants were from well-matched donors. Most recipients received MA conditioning procedures (66%). Most recipients received cyclosporine-based GVHD prophylaxis, but nearly 40% of the recipients received FK506. Forty-six percent of recipients underwent sex-mismatched procedures, and 56% of recipients were CMV positive.
The median time to neutrophil engraftment for patients receiving MA regimens was 14 days with a 92% and 95% cumulative incidence of engraftment at 25 and 100 days, respectively. The median time for platelets to reach 50000 mm3 was 21 days with a cumulative incidence of 70% at 60 days and 77% at 1 year. Data were not available to assess the timing or cumulative incidence of lymphocyte recovery. The probability of overall survival of the entire cohort at 100 days, 1 year, 2 years, and 3 years was 75%, 47%, 39%, and 33%, respectively.
Multivariate analysis of transplantation outcomes in patients receiving MA conditioning
Because the time course of some transplantation outcomes differs after MA versus RI/NMA regimens, multivariate analyses attempting to define key factors contributing to outcomes was performed separately for MA versus RI/NMA approaches. The risk factors considered as candidate effects for the model building process of each regression analysis are described in “Statistical methods.”
Neutrophil and platelet engraftment.
Table 3 shows logistic regression results for neutrophil engraftment at day 25 and platelet recovery to 50000 mm3 at day 60 in the MA cohort. Recipients were more likely to engraft neutrophils at day 25 and platelets at day 60 if the Karnofsky score at transplantation was at least 90, if they received planned doses of growth factors (filgrastim or sargramostim), or if the CD34+ cell dose exceeded 3.8 × 106/kg recipient weight. Recipients whose BMI was below 25 kg/m2 were less likely to engraft neutrophils. Recipients who were CMV positive and those who received HLA-mismatched grafts were less likely to achieve platelet engraftment.
Table 3.
Multivariate analysis of factors associated with engraftment in patients undergoing myeloablative unrelated donor PBSC transplantation
| Variable | n | Engrafted, n | OR (95% CI) | P |
|---|---|---|---|---|
| Day 25 neutrophil engraftment | ||||
| Karnofsky score | .002 | |||
| 90-100 | 364 | 344 | 1.00 | |
| 10-80 | 176 | 151 | 0.37 (0.18-0.64) | < .001 |
| Missing | 56 | 53 | 1.24 (0.34-4.49) | .738 |
| CD34+ cells dose, ×106/kg | .001 | |||
| 3.8 or less | 107 | 94 | 1.00 | |
| More than 3.8 | 319 | 305 | 3.70 (1.61-8.47) | .002 |
| Missing | 170 | 149 | 0.97 (0.45-2.11) | .941 |
| Recipient BMI, kg/m2 | .034 | |||
| Less than 18.5 | 47 | 41 | 0.8 (0.32-2.41) | .798 |
| 18.5-24.9 | 220 | 196 | 1.00 | |
| 25-29.9 | 185 | 175 | 2.36 (1.07-5.22) | .033 |
| 30 or greater | 144 | 136 | 2.72 (1.14-6.46) | .024 |
| Use of planned growth factors | ||||
| No | 412 | 373 | 1.00 | |
| Yes | 184 | 175 | 2.37 (1.08-5.17) | .031 |
| Day 60 platelet 50000 engraftment | ||||
| Karnofsky score | .007 | |||
| 90-100 | 362 | 272 | 1.00 | |
| 10-80 | 180 | 110 | 0.54 (0.36-0.81) | .003 |
| Missing | 52 | 35 | 0.61 (0.32-1.17) | .135 |
| CD34+ cell dose, ×106/kg | < .001 | |||
| 3.8 or less | 102 | 55 | 1.00 | |
| More than 3.8 | 321 | 238 | 2.69 (1.68-4.33) | < .001 |
| Missing | 171 | 125 | 2.48 (1.46-4.21) | < .001 |
| HLA matching status | .016 | |||
| Well-matched | 352 | 261 | 1.00 | |
| Partially matched | 166 | 111 | 0.74 (0.48-1.12) | .150 |
| Mismatched | 76 | 45 | 0.47 (0.27-0.80) | .005 |
| Recipient CMV status | ||||
| Negative | 268 | 202 | 1.00 | |
| Positive | 326 | 215 | 0.68 (0.47-0.99) | .042 |
Grades II-IV and grades III-IV acute and chronic GVHD.
Table 4 shows Cox proportional hazards regression results for grades II-IV and grades III-IV acute GVHD and chronic GVHD in the MA cohort. As anticipated, HLA mismatching increased the risk of grades III-IV acute GVHD. Of note, higher CD34+ dose was not associated with an increase in acute or chronic GVHD. The risk of grades II-IV acute GVHD was noted to be less in based prophylaxis regimens compared with cyclosporine-based regimens (RR = 0.68, P < .001). The risk of chronic GVHD was also less with based GVHD prophylaxis (RR = 0.59, P < .001) or when TBI was used (RR = 0.72, P = .007).
Table 4.
Multivariate analysis of factors associated with GVHD, relapse, and TRM in patients undergoing myeloablative unrelated donor PBSC transplantation
| Variable | n | RR (95% CI) | P |
|---|---|---|---|
| Grades II-IV acute GVHD | |||
| GVHD prophylaxis | |||
| CsA-based | 337 | 1.00 | |
| FK506-based | 260 | 0.68 (0.54-0.85) | < .001 |
| Grades III-IV acute GVHD | |||
| HLA matching status | .001 | ||
| Well-matched | 345 | 1.00 | |
| Partially matched | 173 | 1.57 (1.13-2.19) | .007 |
| Mismatched | 79 | 1.93 (1.29-2.88) | .001 |
| Chronic GVHD | |||
| GVHD prophylaxis | |||
| CsA-based | 342 | 1.00 | |
| FK506-based | 260 | 0.59 (0.46-0.75) | < .001 |
| Conditioning regimen | |||
| Non-TBI | 209 | 1.00 | |
| TBI | 393 | 0.72 (0.57-0.91) | .007 |
| Year of transplantation | |||
| 1999-2000 | 95 | 1.00 | |
| 2001 | 126 | 1.28 (0.88-1.87) | .193 |
| 2002 | 158 | 1.55 (1.08-2.23) | .018 |
| 2003 | 223 | 1.70 (1.21-2.40) | .002 |
| Relapse | |||
| Disease | < .001 | ||
| AML | 238 | 1.00 | |
| ALL | 157 | 0.83 (0.56-1.23) | .348 |
| CML | 93 | 0.18 (0.07-0.46) | < .001 |
| MDS | 107 | 0.43 (0.25-0.73) | .002 |
| Disease risk | < .001 | ||
| Early | 203 | 1.00 | |
| Intermediate | 210 | 2.17 (1.33-3.54) | .002 |
| Advanced | 182 | 3.72 (2.33-5.93) | < .001 |
| Transplant-related mortality | |||
| HLA matching status | < .001 | ||
| Well-matched | 348 | 1.00 | |
| Partially matched | 169 | 1.47 (1.12-1.92) | .005 |
| Mismatched | 76 | 2.30 (1.63-3.26) | < .001 |
| CD34+ cells dose, ×106/kg | .031 | ||
| 4.5 or less | 138 | 1.00 | |
| More than 4.5 | 287 | 0.68 (0.50-0.92) | .013 |
| Missing | 170 | 0.89 (0.64-1.23) | .472 |
| Karnofsky score | < .001 | ||
| 90-100 | 362 | 1.00 | |
| 10-80 | 177 | 1.83 (1.41-2.37) | < .001 |
| Missing | 56 | 0.94 (0.57-1.54) | .799 |
| GVHD prophylaxis | |||
| CsA-based | 339 | 1.00 | |
| FK506-based | 256 | 1.51 (1.18-1.93) | .001 |
Relapse and TRM.
Recipients in the MA cohort with AML or ALL were more likely to relapse, with markedly lower rates in patients who received transplants for MDS or CML (Table 4). Disease risk was a significant determinant of relapse outcomes in the MA group, with a RR of 3.72 and 2.17 for patients with advanced disease and intermediate disease, respectively, compared with those with early disease (P < .001 and P = .002, respectively). Of note, TRM in the MA cohort was not associated with advanced disease as it has been in previous URD BM studies; instead, TRM was associated with HLA mismatching, CD34+ dose 4.5 × 106/kg or less, lower Karnofsky scores, and the use of FK506–based GVHD prophylaxis regimens.
Multivariate analysis of transplantation outcomes in patients receiving RI/NMA conditioning
Acute GVHD, relapse, and TRM.
Because many recipients of RI/NMA conditioning did not become neutropenic or require platelet transfusions, engraftment was not assessed by multivariate analysis. In addition, insufficient data were available for chimerism analysis in this cohort.
FK506-based prophylaxis was associated with lower risk of acute GVHD in the RI/NMA group (grades II-IV: RR = 0.62, P = 0.040; grades III-IV: RR = 0.52, P = .033; Table 5). The risk of acute GVHD tended to decrease through the years. The risk of significant acute GVHD (grades III-IV) was also lower in patients receiving NMA versus RI conditioning (RR = 0.37, P < .001). The only factor associated with relapse in the RI/NMA cohort was disease risk: patients who received a transplant for advanced disease were twice as likely to recur compared with those who received a transplant for early disease (RR = 2.00, P = .003). Advanced disease was also associated with TRM of patients receiving RI/NMA conditioning, with a RR of 1.91 for patients with advanced disease compared with those with early disease (P = .008). Finally, TRM was decreased in the RI/NMA group when CD34+ cell doses exceeded 4.5 × 106/kg recipient weight (RR = 0.58, P = .017).
Table 5.
Multivariate analysis of factors associated with GVHD, relapse, and TRM in patients undergoing reduced-intensity or nonmyeloablative unrelated donor PBSC transplantation
| Variable | n | RR (95% CI) | P |
|---|---|---|---|
| Grades II-IV acute GVHD | |||
| GVHD prophylaxis | |||
| CsA-based | 215 | 1.00 | |
| FK506-based | 99 | 0.62 (0.40-0.98) | .040 |
| Year of transplantation | .028 | ||
| 1999-2000 | 43 | 1.00 | |
| 2001 | 53 | 0.74 (0.42-1.31) | .307 |
| 2002 | 93 | 0.56 (0.33-0.95) | .033 |
| 2003 | 125 | 0.47 (0.28-0.79) | .004 |
| Grades III-IV acute GVHD | |||
| GVHD prophylaxis | |||
| CsA-based | 215 | 1.00 | |
| FK506-based | 99 | 0.52 (0.28-0.95) | .033 |
| Year of transplantation | .016 | ||
| 1999-2000 | 43 | 1.00 | |
| 2001 | 53 | 1.06 (0.51-2.18) | .877 |
| 2002 | 93 | 0.56 (0.27-1.15) | .114 |
| 2003 | 125 | 0.41 (0.20-0.85) | .016 |
| Conditioning intensity | |||
| Reduced intensity | 159 | 1.00 | |
| Nonmyeloablative | 155 | 0.37 (0.22-0.64) | < .001 |
| Relapse | |||
| Disease risk | .005 | ||
| Early | 114 | 1.00 | |
| Intermediate | 107 | 1.15 (0.71-1.84) | .572 |
| Advanced | 95 | 2.00 (1.27-3.14) | .003 |
| Transplant-related mortality | |||
| CD34+ cells dose, × 106/kg) | .039 | ||
| No more than 4.5 | 79 | 1.00 | |
| More than 4.5 | 154 | 0.56 (0.36-0.88) | .013 |
| Missing | 83 | 0.63 (0.38-1.04) | .073 |
| Disease risk | .029 | ||
| Early | 114 | 1.00 | |
| Intermediate | 107 | 1.38 (0.87-2.18) | .175 |
| Advanced | 95 | 1.91 (1.18-3.09) | .008 |
Multivariate analysis of mortality of patients receiving MA and RI/NMA conditioning
We analyzed both cohorts for overall mortality and for treatment failure (defined as TRM or relapse). Because extended survival after relapse was rare, outcomes from both analyses were interchangeable, and we present only the mortality analysis. Table 6 outlines key transplant characteristics associated with increased risk of mortality in the study cohorts. For recipients of an MA transplant, intermediate and advanced disease significantly increased a patient's risk of death, as did low Karnofsky score and HLA mismatching. Other important factors increasing risk of mortality included the use of FK506-based regimens (RR = 1.37, P = .002) and CD34+ doses less than 4.5 × 106/kg (RR = 0.75, P = .021). Finally, there was a statistically significant increase in mortality when both donor and recipient were CMV positive compared with when both were negative (RR = 1.61, P < .001).
Table 6.
Multivariate analysis of factors associated with overall mortality in patients undergoing myeloablative or reduced intensity/nonmyeloablative unrelated donor PBSC transplantation
| Variable | n | RR (95% CI) | P |
|---|---|---|---|
| Myeloablative | |||
| HLA matching status | < .001 | ||
| Well-matched | 352 | 1.00 | |
| Partially matched | 169 | 1.11 (0.89-1.40) | .351 |
| Mismatched | 78 | 1.84 (1.38-2.44) | < .001 |
| CD34+ cells dose, ×106/kg | .024 | ||
| 4.5 or less | 138 | 1.00 | |
| More than 4.5 | 289 | 0.75 (0.58-0.96) | .021 |
| Missing | 172 | 0.97 (0.74-1.27) | .808 |
| Karnofsky score | < .001 | ||
| 90-100 | 363 | 1.00 | |
| 10-80 | 180 | 1.79 (1.43-2.23) | < .001 |
| Missing | 56 | 0.94 (0.64-1.39) | .775 |
| GVHD prophylaxis | |||
| CsA-based | 342 | 1.00 | |
| FK506-based | 257 | 1.37 (1.12-1.67) | .002 |
| Donor/recipient CMV status | .007 | ||
| Negative/negative | 186 | 1.00 | |
| Negatuve/positive | 196 | 1.19 (0.93-1.53) | .173 |
| Positive/negative | 87 | 1.17 (0.84-1.62) | .357 |
| Positive/positive | 130 | 1.61 (1.23-2.12) | < .001 |
| Disease risk | < .001 | ||
| Early | 203 | 1.00 | |
| Intermediate | 210 | 1.32 (1.03-1.69) | .029 |
| Advanced | 186 | 1.74 (1.35-2.24) | < .001 |
| Reduced intensity/nonmyeloablative | |||
| CD34+ cells dose, ×106/kg | .024 | ||
| 4.5 or less | 80 | 1.00 | |
| More than 4.5 | 156 | 0.66 (0.48-0.91) | .010 |
| Missing | 85 | 0.67 (0.47-0.96) | .031 |
| Disease risk | < .001 | ||
| Early | 115 | 1.00 | |
| Intermediate | 110 | 1.17 (0.84-1.63) | .355 |
| Advanced | 96 | 1.85 (1.33-2.56) | < .001 |
Only 2 variables reached significance in the regression analysis of mortality in the RI/NMA cohort. As expected, patients with advanced disease did poorly compared with those with early disease (RR = 1.85, P < .001). As with recipients of MA conditioning, cell dose was important. CD34+ cell doses exceeding 4.5 × 106/kg recipient weight were associated with a decrease in overall mortality (RR = 0.66, P = .010).
Effect of higher CD34+ cell doses on engraftment, GVHD, and survival
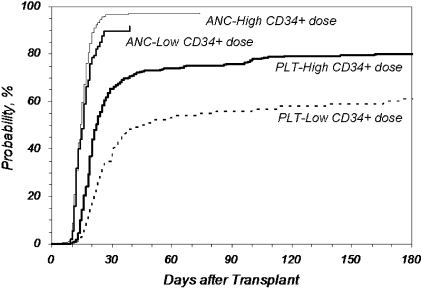
We further analyzed the effect of infused CD34+ dose on key outcomes (Figures 1–3). Figure 1 shows the cumulative incidence of neutrophil and platelet engraftment after MA transplantation in recipients who received 3.8 × 106 CD34+ cells/kg recipient weight or less (Low) compared with those who received more than the cutoff value (High; P = .025 for neutrophil engraftment at 25 days; P < .001 for platelet engraftment at 60 days). The difference in both the rapidity and eventual ability to achieve platelet engraftment with higher doses of CD34+ cells is marked.
Figure 1.
Cumulative incidence of neutrophil and platelet engraftment after MA URD-PBSC transplantation by CD34+ dose. CD34+ cell doses higher than 3.8 × 106/kg recipient weight improved neutrophil and platelet engraftment compared with lower doses (P = .025 for neutrophil engraftment at 25 days; P < .001 for platelet engraftment > 50000/μL at 60 days). ANC indicates neutrophil engraftment; PLT, platelet engraftment; Low, no more than 3.8 × 106 CD34+/kg (n = 107, ANC; n = 106, PLT); High, greater than 3.8 × 106 CD34+/kg (n = 327, ANC; n = 324, PLT).
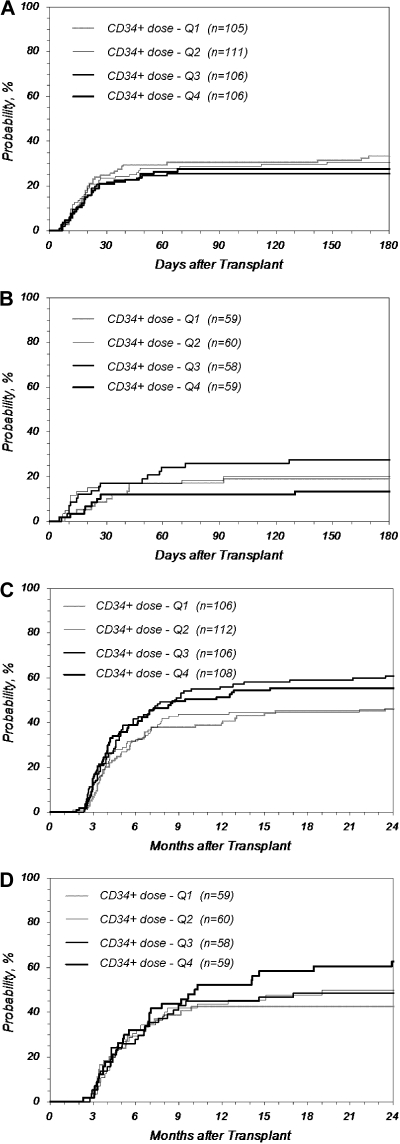
Figure 2.
Cumulative incidence of GVHD after URD-PBSC transplantation by quartile (Q) of CD34+ dose. Higher CD34+ cell doses did not increase the incidence of GVHD. (A) Grades III-IV acute GVHD after MA transplantation (P = .599 at 180 days); (B) grades III-IV acute GVHD after RI/NMA transplantation (P = .305 at 180 days); (C) chronic GVHD after MA transplantation (P = .068 at 2 years); (D) chronic GVHD after RI/NMA transplantation (P = .189 at 2 years). MA: Q1 indicates no greater than 3.8; Q2, 3.8 to 6.2; Q3,6.2 to 9.5; Q4, greater than 9.5; RI/NMA: Q1, no greater than 3.6; Q, 3.6 to 5.9; Q3, 5.9 to 9.4; Q4, greater than 9.4 (× 106 CD34+/kg).
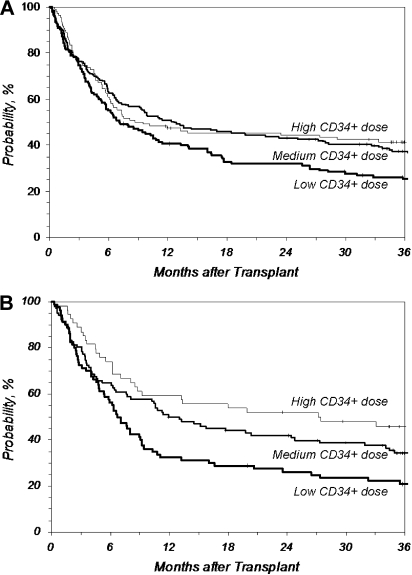
Figure 3.
Overall survival after URD-PBSC transplantation by CD34+ dose. CD34+ cell doses higher than 4.5 × 106/kg recipient weight improved overall survival compared with lower doses. However, doses much higher than 4.5 × 106/kg did not further improve the survival rate compared with doses just above 4.5 × 106/kg. (A) Overall survival after MA transplantation (P = .020 at 3 years for Medium vs Low; P = .489 at 3 years for Medium vs High). (B) Overall survival after RI/NMA transplantation (P = .045 at 3 years for Medium vs Low; P = .157 at 3 years for Medium vs High). Low indicates no greater than 4.5 (n = 142, MA; n = 80, RI/NMA); Medium, 4.5 to 9.5 (n = 183, MA; n = 102, RI/NMA); High, greater than 9.5 (n = 110, MA; n = 54, RI/NMA) (× 106 CD34+/kg).
We explored in more depth whether higher CD34+ cell doses were associated with increased rates of acute or chronic GVHD or both. Figure 2A and B shows the cumulative incidence of grades III-IV acute GVHD based on CD34+ doses by quartiles for recipients of MA and RI/NMA conditioning, respectively. No difference was noted between the quartiles (P = .599 and .305 at 180 days for MA and RI/NMA, respectively). For recipients of MA conditioning, the incidence of grades III-IV acute GVHD in the top quartile of CD34+ cells doses (> 9.5 × 106/kg) compared with doses in the second quartile (between 3.8 and 6.2 × 106/kg) had a RR of 0.81 (P = .393); doses above the 90th percentile (> 14.9 × 106/kg) had a similarly nonsignificant RR of 1.13 (P = .696). For recipients of RI/NMA conditioning, the RR of grades III-IV acute GVHD in the top quartile (> 9.4 × 106/kg) and the top 10% (> 14.6 × 106/kg) compared with the second quartile (between 3.6 and 5.9 × 106/kg) were 0.62 (P = .301) and 0.64 (P = .488), respectively. An analysis of grades II-IV acute GVHD similarly showed no increase in incidence with higher cell doses (data not shown).
Figure 2C and D shows the incidence of chronic GVHD by quartiles, demonstrating no increase in incidence with higher cell doses for recipients of MA and RI/NMA conditioning, respectively (P = .068 and .189 at 2 years, respectively). Further analysis of patients receiving cell doses above the top quartile and the 90th percentile compared with the second quartile similarly shows no evidence of an increase in chronic GHVD for recipients of MA and RI/NMA conditioning (MA: RR = 1.17, P = .405 for top quartile vs second quartile; RR = 1.24, P = .389 for top 10% vs second quartile; RI/NMA: RR = 1.35, P = .262 for top quartile vs second quartile; RR = 1.24, P = .508 for top 10% vs second quartile).
Higher cell doses were independent predictors of better survival regardless of preparative regimen approaches. CD34+ doses between 4.5 and 9.5 × 106/kg recipient weight resulted in a 12% improvement in 3-year survival in recipients of MA conditioning compared with lower doses (37% vs 25%; P = .020, Medium vs Low; Figure 3A). However, doses greater than 9.5 × 106/kg did not further improve the survival rate (P = .489 at 3 years, Medium vs High). Three-year survival after RI/NMA preparative regimens also significantly improved in patients with CD34+ doses between 4.5 and 9.5 × 106/kg recipient weight compared with patients with lower doses (34% vs 21%; P = .045, Medium vs Low; Figure 3B). Similar to the MA cohort, CD34+ cell doses greater than 9.5 × 106/kg did not further improve outcome in this cohort (P = .157 at 3 years; Medium vs High).
We also analyzed CD34 doses based on ideal body weight as opposed to actual body weight. This analysis was restricted to adults only because of the differences in computing ideal body weight for children. Compared with CD34+ cells/kg based on actual body weight, CD34+ cells/kg based on ideal body weight was similarly associated with neutrophil and platelet engraftment, but it was not significantly associated with TRM or survival in MA transplantations. Among NMA/RI transplants, CD34 dose based on ideal body weight was only significantly associated with survival but not TRM (data not shown). This indicates that cell dose based on ideal body weight may be a less sensitive predictor of transplantation outcomes in URD-PBSC transplantations, compared with cell dose based on actual body weight.
Comparison of outcomes between preparative regimen cohorts
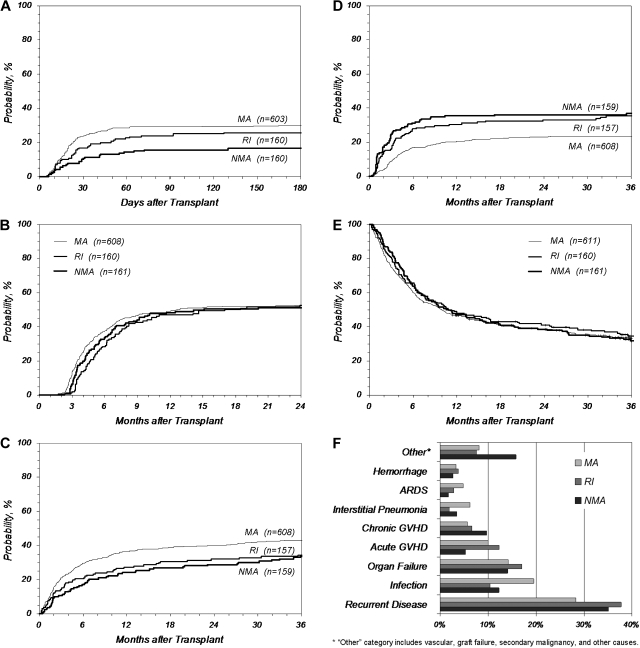
Although the preparative regimen cohorts differed by age and the presence of coexisting conditions, these variables did not come out as significant in multivariate outcome analysis; therefore, comparisons of the cohorts may be instructive. NMA regimens resulted in significantly less severe (grades III-IV) acute GVHD compared with RI or MA regimens (cumulative incidence at 180 days: 16% vs 26% vs 30%, NMA vs RI vs MA, P < .001; Figure 4A), but chronic GVHD was statistically identical between the regimens, with an incidence just above 50% at 2 years (Figure 4B). TRM was higher after MA procedures (cumulative incidence at 3 years: 34% vs 34% vs 43%, NMA vs RI vs MA, P = .027; Figure 4C), but any gains in survival from decreased TRM in NMA and RI conditioning were offset by increased relapse (cumulative incidence at 3 years: 37% vs 35% vs 24%, NMA vs RI vs MA, P < .001; Figure 4D). This resulted in overall survival that was indistinguishable among the 3 cohorts, ranging from 32% to 35% at 3 years (Figure 4E). Three subanalyses were performed: (1) excluding ALL, (2) AML first complete remission alone, and (3) patients aged 40 to 60 years with AML/MDS. Survival in the 3 subanalyses was the same in each of the 3 preparative regimen cohorts (data not shown).
Figure 4.
Key outcomes after URD-PBSC transplantation by preparative regimen. (A) Grades III-IV acute GVHD, (B) chronic GVHD, (C) TRM, (D) relapse, (E) overall survival, and (F) primary cause of death. ARDS indicates acute respiratory distress syndrome.
The most common single cause of death was relapse (28%-38%) followed by infection, organ failure, and acute and chronic GVHD (Figure 4F). Recipients receiving MA procedures died more frequently of infection, acute respiratory distress syndrome, and interstitial pneumonia compared with patients who received RI/NMA conditioning, whereas patients who received RI/NMA conditioning died more frequently of recurrent disease, chronic GVHD (recipients of NMA conditioning), and other causes (recipients of NMA conditioning). Causes of death did not vary by CD34+ cell dose.
Discussion
We have shown in this large, multi-institutional prospective study that transplantation with NMDP-facilitated URD PBSCs results in rapid engraftment and survival comparable to published transplantation experiences with URD BM.17,26 Because PBSCs from URDs has become the most commonly used URD stem cell source, the multivariate risk factor analysis presented here is useful in defining prognosis and identifying populations at risk to design strategies aimed at improving outcome.
One of the key findings of this study is the independent predictive value of higher CD34+ dose for improvements in major transplantation outcomes. Cell dose has long been recognized to be important in allogeneic transplantation. Early studies showed less rejection and better survival in patients undergoing transplantation for severe aplastic anemia who received higher mononuclear cell doses in their BM grafts.27,28 More recent studies involving MA approaches have shown faster count recovery, less TRM, and better survival in recipients of matched sibling BM or PBSCs containing higher numbers of CD34+ cells.29–35 One study of matched sibling donor MA transplantation correlated clinical chronic extensive GVHD with high CD34+ doses, but survival was not affected.36 Studies of RI regimens have correlated higher cell doses with better survival, but at a cost of more chronic GVHD.37–40 Two of these studies associated better outcomes with higher CD8+ cell doses, whereas the other 2 studies correlated higher CD34+ doses with chronic GVHD. High CD4+, CD8+, total T-cell, monocyte, natural killer cell, and CD34+ counts have been associated with more rapid achievement of full-donor chimerism and a trend toward decreased rejection in NMA approaches.41,42
Studies correlating cell dose with outcomes in URD-PBSC transplantation are few and limited. Nakamura et al18 reviewed a single center experience of URD-PBSC transplantation with the use of either MA or RI regimens for a variety of hematologic malignancies and myeloproliferative disorders. They showed by multivariate analysis that higher CD34+ doses were associated with faster recovery of absolute lymphocyte counts on day 30 and a reduced rate of relapse. The group also noted a greater reduction in relapse associated with RI regimens when a high dose of CD34+ cells was given compared with MA regimens. Small numbers of URD-PBSC products have been included in a few of the analyses described in the previous paragraph,38,41 but the study we present is the only large, multicenter trial describing the effect of URD-PBSC CD34+ cell dose on patients undergoing a variety of transplantation regimens.
The lack of an association between higher cell doses and increased rates of acute and/or chronic GVHD in our study may seem to be surprising; however, although a handful of studies has suggested an association of CD34+ cell dose with increased chronic GVHD after sibling donor transplantation,36,39,40,43 other studies of sibling donors and URDs find either no association of cell dose and acute or chronic GVHD29,35,38,41,44 or a decrease in grades III-IV acute34 or chronic GVHD18 in recipients of higher cell doses. We were unable to define any specific adverse outcome associated with high URD-PBSC cell doses. That said, as long as patients achieved the cell dose cutoff associated with better outcomes (4.5 × 106 CD34+ cells/kg), we were not able to discern specific advantages to receiving doses significantly higher than that threshold.
In summary, cell dose is a key factor after URD-PBSC transplantation. Collection practices leading to acquisition and infusion of at least 4.5 × 106 CD34+ cells/kg may improve survival and decrease morbidity in patients receiving this stem cell source for transplantation, regardless of regimen intensity. Other factors identified in this study may help individual patients understand risk and may assist investigators in targeting high-risk populations for studies aimed at improving outcome.
Supplementary Material
Acknowledgments
This work was supported by funding from the National Marrow Donor Program and the Health Resources and Services Administration (contract Nos. 240-97-0036 and 231-02-0007) to the National Marrow Donor Program. The CIBMTR is supported by the National Cancer Institute (Public Health Service grant U24-CA76518), the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research; Health Services Research Administration (DHHS); and grants from Abbott Laboratories; Aetna; American International Group Inc; American Red Cross; Amgen Inc; Anonymous donation to the Medical College of Wisconsin; AnorMED Inc; Astellas Pharma US Inc; Baxter International Inc; Berlex Laboratories Inc; Biogen IDEC Inc; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bristol-Myers Squibb Company; BRT Laboratories Inc; Cangene Corporation; Celgene Corporation; CellGenix Inc; Cell Therapeutics Inc; CelMed Biosciences; Cylex Inc; Cytonome Inc; CytoTherm; DOR BioPharma Inc; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals Inc; Gambro BCT Inc; Gamida Cell Ltd; Genzyme Corporation; Gift of Life Bone Marrow Foundation; GlaxoSmithKline Inc; Histogenetics Inc; HKS Medical Information Systems; Kirin Brewery Co Ltd; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals Inc; Miller Pharmacal Group; Milliman USA Inc; Miltenyi Biotec Inc; MultiPlan Inc; National Marrow Donor Program; Nature Publishing Group; Novartis Pharmaceuticals Inc; Osiris Therapeutics Inc; Pall Medical; Pfizer Inc; Pharmion Corporation; PDL BioPharma, Inc; Roche Laboratories; Sanofi-aventis; Schering Plough Corporation; StemCyte Inc; StemSoft Software Inc; SuperGen, Inc; Sysmex; The Marrow Foundation; THERAKOS Inc; University of Colorado Cord Blood Bank; ViaCell Inc; ViraCor Laboratories; Wellpoint Inc; and Zelos Therapeutics Inc. The German AML Intergroup is supported by the Bundesministerium für Bildung und Forschung (Kompetenznetz “Akute und chronische Leukämien”; grant 01GI9981), Germany.
The views expressed in this article do not reflect the official policy or position of the Health Resources and Services Administration, the National Marrow Donor Program, the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A.P. had primary responsibility for study design, data analysis, data interpretation, and manuscript writing and had primary responsibility for the entire paper as an accurate and verifiable report; P.C. had primary responsibility for study design, data file preparation, data analysis, interpretation of data, and manuscript writing; B.R.L. participated in study design, data analysis, interpretation of data, and manuscript writing; S.F.L. and P.A. participated in interpretation of data and manuscript writing; J.P.K. participated in study design, data analysis, and interpretation of data; M.M.H. participated in study design, interpretation of data, and manuscript writing; J.P.M. and R.J.K. participated in data file preparation, interpretation of data, and manuscript writing; and D.L.C. had responsibility for study design, data file preparation, data analysis, data interpretation, and manuscript writing and had responsibility for the entire paper as an accurate and verifiable report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Pulsipher, University of Utah School of Medicine, Division of Hematology/Blood and Marrow Transplant, 30 North 1900 East, Rm 5C402, Salt Lake City, UT 84132-2408; e-mail: michael.pulsipher@hsc.utah.edu.
References
- 1.Bensinger WI, Weaver CH, Appelbaum FR, et al. Transplantation of allogeneic peripheral blood stem cells mobilized by recombinant human granulocyte colony-stimulating factor. Blood. 1995;85(6):1655–1658. [PubMed] [Google Scholar]
- 2.Goldman J. Peripheral blood stem cells for allografting. Blood. 1995;85(6):1413–1415. [PubMed] [Google Scholar]
- 3.Korbling M, Przepiorka D, Huh YO, et al. Allogeneic blood stem cell transplantation for refractory leukemia and lymphoma: potential advantage of blood over marrow allografts. Blood. 1995;85(6):1659–1665. [PubMed] [Google Scholar]
- 4.Schmitz N, Dreger P, Suttorp M, et al. Primary transplantation of allogeneic peripheral blood progenitor cells mobilized by filgrastim (granulocyte colony-stimulating factor). Blood. 1995;85(6):1666–1672. [PubMed] [Google Scholar]
- 5.Karanes C, Confer D, Walker T, Askren A, Keller C. Unrelated donor stem cell transplantation: the role of the National Marrow Donor Program. Oncology (Williston Park) 2003;17(8):1036–1038. 1043–1034, 1164–1037. [PubMed] [Google Scholar]
- 6.Remberger M, Ringden O, Blau IW, et al. No difference in graft-versus-host disease, relapse, and survival comparing peripheral stem cells to bone marrow using unrelated donors. Blood. 2001;98(6):1739–1745. doi: 10.1182/blood.v98.6.1739. [DOI] [PubMed] [Google Scholar]
- 7.Elmaagacli AH, Basoglu S, Peceny R, et al. Improved disease-free-survival after transplantation of peripheral blood stem cells as compared with bone marrow from HLA-identical unrelated donors in patients with first chronic phase chronic myeloid leukemia. Blood. 2002;99(4):1130–1135. [PubMed] [Google Scholar]
- 8.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102(6):2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 9.Anderson D, DeFor T, Burns L, et al. A comparison of related donor peripheral blood and bone marrow transplants: importance of late-onset chronic graft-versus-host disease and infections. Biol Blood Marrow Transplant. 2003(1):52–59. doi: 10.1053/bbmt.2003.50000. [DOI] [PubMed] [Google Scholar]
- 10.Eapen M, Horowitz MM, Klein JP, et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J Clin Oncol. 2004;22(24):4872–4880. doi: 10.1200/JCO.2004.02.189. [DOI] [PubMed] [Google Scholar]
- 11.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant. 2007;13(12):1461–1468. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remberger M, Beelen DW, Fauser A, Basara N, Basu O, Ringden O. Increased risk of extensive chronic graft-versus-host disease after allogeneic peripheral blood stem cell transplantation using unrelated donors. Blood. 2005;105(2):548–551. doi: 10.1182/blood-2004-03-1000. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz N, Eapen M, Horowitz MM, et al. Long-term outcome of patients given transplants of mobilized blood or bone marrow: a report from the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. Blood. 2006;108(13):4288–4290. doi: 10.1182/blood-2006-05-024042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. doi: 10.1182/blood.v98.7.2043. [DOI] [PubMed] [Google Scholar]
- 15.Hurley CK, Baxter Lowe LA, Logan B, et al. National Marrow Donor Program HLA-matching guidelines for unrelated marrow transplants. Biol Blood Marrow Transplant. 2003;9(10):610–615. doi: 10.1016/j.bbmt.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 16.McGlave PB, Shu XO, Wen W, et al. Unrelated donor marrow transplantation for chronic myelogenous leukemia: 9 years' experience of the national marrow donor program. Blood. 2000;95(7):2219–2225. [PubMed] [Google Scholar]
- 17.Kernan NA, Bartsch G, Ash RC, et al. Analysis of 462 transplantations from unrelated donors facilitated by the National Marrow Donor Program. N Engl J Med. 1993;328(9):593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura R, Auayporn N, Smith DD, et al. Impact of graft cell dose on transplant outcomes following unrelated donor allogeneic peripheral blood stem cell transplantation: higher CD34+ cell doses are associated with decreased relapse rates. Biol Blood Marrow Transplant. 2008;14(4):449–457. doi: 10.1016/j.bbmt.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12(8):876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Klein J, Moeschberger M. Survival Analysis: Statistical Methods for Censored and Truncated Data. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 24.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 25.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beatty PG, Hansen JA, Longton GM, et al. Marrow transplantation from HLA-matched unrelated donors for treatment of hematologic malignancies. Transplantation. 1991;51(2):443–447. doi: 10.1097/00007890-199102000-00034. [DOI] [PubMed] [Google Scholar]
- 27.Niederwieser D, Pepe M, Storb R, Loughran TP, Jr, Longton G. Improvement in rejection, engraftment rate and survival without increase in graft-versus-host disease by high marrow cell dose in patients transplanted for aplastic anaemia. Br J Haematol. 1988;69(1):23–28. doi: 10.1111/j.1365-2141.1988.tb07597.x. [DOI] [PubMed] [Google Scholar]
- 28.Storb R, Prentice RL, Thomas ED. Marrow transplantation for treatment of aplastic anemia. An analysis of factors associated with graft rejection. N Engl J Med. 1977;296(2):61–66. doi: 10.1056/NEJM197701132960201. [DOI] [PubMed] [Google Scholar]
- 29.Bittencourt H, Rocha V, Chevret S, et al. Association of CD34 cell dose with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;99(8):2726–2733. doi: 10.1182/blood.v99.8.2726. [DOI] [PubMed] [Google Scholar]
- 30.Bahceci E, Read EJ, Leitman S, et al. CD34+ cell dose predicts relapse and survival after T-cell-depleted HLA-identical haematopoietic stem cell transplantation (HSCT) for haematological malignancies. Br J Haematol. 2000;108(2):408–414. doi: 10.1046/j.1365-2141.2000.01838.x. [DOI] [PubMed] [Google Scholar]
- 31.Morariu-Zamfir R, Rocha V, Devergie A, et al. Influence of CD34(+) marrow cell dose on outcome of HLA-identical sibling allogeneic bone marrow transplants in patients with chronic myeloid leukaemia. Bone Marrow Transplant. 2001;27(6):575–580. doi: 10.1038/sj.bmt.1702852. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura R, Bahceci E, Read EJ, et al. Transplant dose of CD34(+) and CD3(+) cells predicts outcome in patients with haematological malignancies undergoing T cell-depleted peripheral blood stem cell transplants with delayed donor lymphocyte add-back. Br J Haematol. 2001;115(1):95–104. doi: 10.1046/j.1365-2141.2001.02983.x. [DOI] [PubMed] [Google Scholar]
- 33.Mavroudis D, Read E, Cottler-Fox M, et al. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood. 1996;88(8):3223–3229. [PubMed] [Google Scholar]
- 34.Dominietto A, Lamparelli T, Raiola AM, et al. Transplant-related mortality and long-term graft function are significantly influenced by cell dose in patients undergoing allogeneic marrow transplantation. Blood. 2002;100(12):3930–3934. doi: 10.1182/blood-2002-01-0339. [DOI] [PubMed] [Google Scholar]
- 35.Ringden O, Barrett AJ, Zhang MJ, et al. Decreased treatment failure in recipients of HLA-identical bone marrow or peripheral blood stem cell transplants with high CD34 cell doses. Br J Haematol. 2003;121(6):874–885. doi: 10.1046/j.1365-2141.2003.04364.x. [DOI] [PubMed] [Google Scholar]
- 36.Zaucha JM, Gooley T, Bensinger WI, et al. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood. 2001;98(12):3221–3227. doi: 10.1182/blood.v98.12.3221. [DOI] [PubMed] [Google Scholar]
- 37.Gorin NC, Labopin M, Boiron JM, et al. Results of genoidentical hemopoietic stem cell transplantation with reduced intensity conditioning for acute myelocytic leukemia: higher doses of stem cells infused benefit patients receiving transplants in second remission or beyond–the Acute Leukemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2006;24(24):3959–3966. doi: 10.1200/JCO.2006.05.5855. [DOI] [PubMed] [Google Scholar]
- 38.Cao TM, Shizuru JA, Wong RM, et al. Engraftment and survival following reduced-intensity allogeneic peripheral blood hematopoietic cell transplantation is affected by CD8+ T-cell dose. Blood. 2005;105(6):2300–2306. doi: 10.1182/blood-2004-04-1473. [DOI] [PubMed] [Google Scholar]
- 39.Mohty M, Bilger K, Jourdan E, et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia. 2003;17(5):869–875. doi: 10.1038/sj.leu.2402909. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Simon JA, Diez-Campelo M, Martino R, et al. Impact of CD34+ cell dose on the outcome of patients undergoing reduced-intensity-conditioning allogeneic peripheral blood stem cell transplantation. Blood. 2003;102(3):1108–1113. doi: 10.1182/blood-2002-11-3503. [DOI] [PubMed] [Google Scholar]
- 41.Baron F, Maris MB, Storer BE, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19(5):822–828. doi: 10.1038/sj.leu.2403718. [DOI] [PubMed] [Google Scholar]
- 42.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104(8):2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 43.Gorin NC, Labopin M, Rocha V, et al. Marrow versus peripheral blood for geno-identical allogeneic stem cell transplantation in acute myelocytic leukemia: influence of dose and stem cell source shows better outcome with rich marrow. Blood. 2003;102(8):3043–3051. doi: 10.1182/blood-2003-03-0665. [DOI] [PubMed] [Google Scholar]
- 44.Cao TM, Wong RM, Sheehan K, et al. CD34, CD4, and CD8 cell doses do not influence engraftment, graft-versus-host disease, or survival following myeloablative human leukocyte antigen-identical peripheral blood allografting for hematologic malignancies. Exp Hematol. 2005;33(3):279–285. doi: 10.1016/j.exphem.2004.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.