Abstract
The current definition of complete response in multiple myeloma includes a requirement for a bone marrow (BM) examination showing less than 5% plasma cells in addition to negative serum and urine immunofixation. There have been suggestions to eliminate the need for BM examinations when defining complete response. We evaluated 92 patients with multiple myeloma who achieved negative immunofixation in the serum and urine after therapy and found that 14% had BM plasma cells more than or equal to 5%. Adding a requirement for normalization of the serum-free light chain ratio to negative immunofixation studies did not negate the need for BM studies; 10% with a normal serum-free light chain ratio had BM plasma cells more than or equal to 5%. We also found that, on achieving immunofixation-negative status, patients with less than 5% plasma cells in the BM had improved overall survival compared with those with 5% or more BM plasma cells (6.2 years vs 2.3 years, respectively; P = .01).
Introduction
In multiple myeloma (MM), a complete response (CR) is defined by the European Group for Blood and Marrow Transplant (EBMT) and the International Myeloma Working Group (IMWG) uniform response criteria as absence of serum and urine monoclonal (M) protein by immunofixation (IFE) and less than 5% plasma cells (PCs) in the bone marrow (BM).1,2 Because CR is an important goal in MM therapy, stringent adherence to this criterion is required to ensure standardized comparisons between clinical trial data. However, BM examinations can be cumbersome in clinical practice and uncomfortable for patients, causing a significant proportion of noncompliance among physicians. It has been argued that increased PCs in the BM are very unlikely if patients achieve negative serum and urine IFE. The goal of this study was to determine the value of BM examinations in patients who can otherwise be considered to be in CR by virtue of a negative IFE in the serum and urine.
Methods
Patients with MM who had measurable M protein levels at baseline (defined as serum M protein > 1 g/dL and urine M protein > 0.2 g/day) who since 1995 had a negative serum and urine IFE with concomitant unilateral BM aspirate/biopsy, all performed within 30 days of each other, were included in this study. Baseline demographics and clinical characteristics; date of diagnosis and last follow-up; current follow-up status; treatment history; serum lactate dehydrogenase, β-2 microglobulin, and albumin at diagnosis; serum and urine M protein levels at diagnosis; results of serum and urine IFE, serum free light chain (FLC) ratio, and BM aspirate/biopsy within 30 days of CR; and the date of the first serum M-spike more than 0.5 g/dL or urine M-spike more than 0.2 g/day after the negative IFE data were collected from existing databases. StatView (SAS Institute) was used for all statistical analyses. All P values were 2-tailed, and statistical significance was set at the level of P values less than .05. Overall survival was estimated using the Kaplan-Meier method, taking the interval from the date of diagnosis to death or last contact. The study was approved by the Mayo Clinic Institutional Review Board, and patient informed consent was obtained in accordance with the Declaration of Helsinki.
Results and discussion
Ninety-two patients (median age, 59.4 years; range, 29.7-81.4 years) fulfilled the study criteria, all of whom had measurable disease at baseline and subsequently achieved negative serum and urine IFE. At diagnosis, median serum and urine M spike were 2.3 g/dL (range, 0.0-6.9 g/dL) and 0.3 g/day (range, 0.0-22.6 g/day), respectively.
Per entry criteria, all patients achieved negative IFE status. IFE negativity was achieved with high-dose therapy and transplantation in 51 patients, initial chemotherapy in 26 patients (immunomodulatory agent induction in 12, with other induction regimens in 14), after second-line therapy in 10 patients, and unknown in 5 patients.
We found only 79 patients (86%) who met criteria for CR by the EBMT/IMWG criteria with less than 5% PCs in the BM. Importantly, 13 patients (14%) had 5% or more PCs in the BM, and of these 3 patients (3%) had 10% or more PCs despite the negative IFE on serum and urine. In 11 of the 13 patients (85%), monoclonality of residual PCs was confirmed by immunofluorescent studies, including all 3 patients who had 10% or more PCs.
The utility of a normal serum FLC ratio in addition to negative serum and urine IFE in negating the need for BM examination was also investigated. Among 29 patients who had negative serum and urine IFE plus a normal serum FLC ratio, 26 patients (90%) met CR definition with less than 5% BM plasma cells. Three patients (10%) had 5% or more PCs in the BM. The addition of normal serum FLC ratio to negative serum and urine IFE appeared insufficient in confirming CR accurately in the absence of a BM using standard EBMT/IMWG criteria. In all 3 of these patients, residual PCs appeared polyclonal by immunofluorescence, but these samples were done before institution of multiparametric flow cytometry and need further study. It is possible to have 5% or more residual clonal PCs in the BM and yet have no evidence of monoclonal protein in the blood if the cells become nonsecretory or if the level of secretion is below the detection threshold of currently available assays.
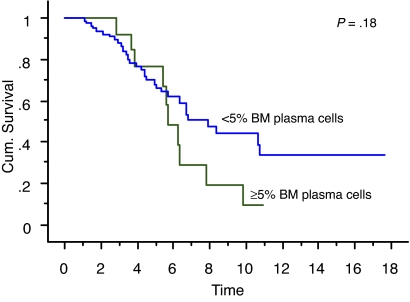
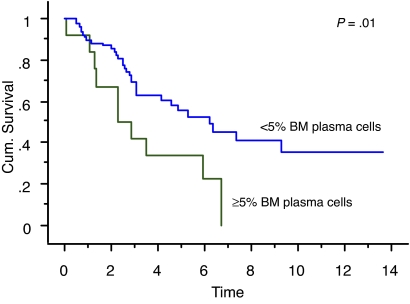
We also studied the survival of patients who achieved true CR (negative serum and urine IFE and < 5% BM plasma cells) compared with patients who had negative serum and urine IFE but 5% or more BM plasma cells. Overall survival measured from initial diagnosis between the 2 groups was 7.9 years versus 5.7 years, respectively (P = .18; Figure 1). Overall survival measured from onset of negative serum and urine IFE was significantly different: median survival was 6.2 years in patients with less than 5% PCs (true CR patients) compared with 2.3 years in patients with 5% or more PCs (P = .01; Figure 2). Median progression-free survival estimated by the EBMT/IMWG criteria was 3.2 years for the cohort and was not different between the 2 groups (P = .7).
Figure 1.
Kaplan-Meier curve showing survival (in years) from diagnosis of patients with true CR (negative serum and urine IFE and < 5% BM plasma cells) compared with patients with negative serum and urine IFE but 5% or more BM plasma cells.
Figure 2.
Kaplan-Meier curve showing survival (in years) after achieving negative serum and urine IFE of patients with less than 5% BM plasma cells (true CR patients) compared with patients with 5% or more BM plasma cells.
Based on this study, we recommend that the CR definition in the EBMT/IMWG criteria should not be revised to eliminate the need for BM biopsy and aspirate. This is to prevent the inclusion of a significant proportion of false-positive CR cases if the BM examination was not performed. We also found that the addition of normal FLC ratio did not improve the detection of true CR cases if the BM examination was removed. The use of unilateral BM examination may lead to sampling error, which underestimates the results of this study, further justifying the need to retain the BM examination for defining CR. The frequency of CR has dramatically increased over the past few years, reflecting significant improvement and efficacy of MM treatment options.3 If no reliable CR criteria were enforced, not only would comparison of outcomes be difficult between clinical trials but the definition of disease-free survival,4 which incorporates CR in its time-to-event endpoint, would also be affected in some clinical trials. Furthermore, as molecular genetics become more important in determining prognosis of patients with MM, BM examinations are essential to evaluate for immunophenotypic CR and molecular CR in MM.
This study also showed improved survival in patients with less than 5% BM PCs once negative serum and urine IFE were achieved, highlighting the crucial role of BM examination at CR to confirm normal hematopoietic recovery. Indeed, approximately 35% of patients in this cohort achieved more than 10-year survival from onset of CR. However, this may be the result of imbalance in baseline prognostic markers and needs a larger study for further validation. Based on our findings, considerations could be made for testing continuation or change in therapy if less than 5% PCs in the BM were not achieved despite negative serum and urine IFE in clinical trials. Such studies may lead to more patients achieving true CR with normal hematopoietic recovery and improving overall survival with the current selection of highly effective drugs in the treatment of MM.
Acknowledgments
This work was supported by National Cancer Institute grants CA62242 and CA107476.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.E.C. collected and analyzed data and wrote the manuscript; S.K. and S.V.R. designed and conducted research and analyzed data; R.A.K., A.D., and M.A.G. designed and conducted research; and D.R.L. and C.L.C. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. Vincent Rajkumar, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: rajks@mayo.edu.
References
- 1.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation: Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 2.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 3.Lonial S, Gertz MA. Eliminating the complete response penalty from myeloma response assessment. Blood. 2008;111(6):3297–3298. doi: 10.1182/blood-2008-01-132456. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22(2):231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]