Abstract
BACKGROUND
JAL (RH48) is a low-prevalence antigen in the Rh blood group system and anti-JAL has caused hemolytic disease of the newborn. JAL is associated with either a haplotype carrying depressed C and e antigens or one carrying depressed c and e antigens. Blood samples from JAL+ people were tested, published serologic findings were confirmed, serologic studies were extended to include expression of other Rh antigens, and the antibody specificities produced by three sensitized JAL+ probands are reported.
STUDY DESIGN AND METHODS
Red blood cell (RBC) samples from 17 (12 probands) JAL+ persons were tested by hemagglutination using standard methods.
RESULTS
RBCs from both the Caucasian JAL+ probands had the (C)(e) haplotype and weakened C, e, hrB, and hrS antigens. JAL+ samples from black persons had the (c)(e) haplotype and expressed weakened c, e, f, V, VS, hrB, and hrS antigens. Plasma from three sensitized c+e+ JAL+ probands contained alloanti-c, alloanti-e, or alloantibody of apparent anti-Rh17 specificity. This study shows that this alloanti-Rh17–like antibody recognizes the high-prevalence antigen antithetical to JAL that has been named CEST.
CONCLUSIONS
The presence of the JAL antigen has a quantitative (weakening) effect on the expression of C, e, hrB, and hrS antigens in Caucasian persons and of c, e, f, V, VS, hrB, and hrS antigens in people of black African ancestry. A qualitative effect also was demonstrated by the presence of alloanti-c or alloanti-e in the plasma of two transfused c+e+ patients and by an antibody (anti-CEST) that recognizes the high-prevalence antigen antithetical to JAL.
Of antibodies to protein-based blood groups, those to antigens in the Rh blood group system are unquestionably the most relevant in transfusion medicine. The Rh blood system is the most polymorphic and many antigens in this system are highly immunogenic and the corresponding antibodies can cause transfusion reactions and hemolytic disease of the fetus and newborn (HDFN).1 Antigens in the Rh blood group system are encoded by variant genes arising either as a consequence of a single nucleotide change in RHD or RHCE or from various rearrangements between these two homologous genes.1,2
Quantitative and qualitative alteration in expression of primary Rh blood group system antigens (D, C, E, c, e) is often associated with expression of a low-prevalence antigen. Furthermore, production of alloanti-D in a D+ person and alloanti-e in an e+ person is not uncommon. In contrast, partial C and partial c antigens are rarely encountered. Alloanti-C has been made by people with (C)ceS (r′S), CW+, CX+, or D(C)(e) phenotypes.1 Only two examples of alloanti-c in c+ people have been described. One was in a person with a presumed R1r phenotype3 and the other was actually anti-Rh26, which can appear as anti-c, made by a Rh26−, c+ person.4 Anti-Rh26 is more usually made by Rh26−, c− people.5 Molecular studies have shown that Rh26 is antithetical to the low-prevalence antigen LOCR.6 Other altered c antigens have been reported, [(c)(e)Be(a+), (c)(e)JAL+, (c)(e) (rL and rt), and (c)(E)]1 but to date, people with these altered c antigens have not been reported to have made alloanti-c.
In a multilaboratory investigation, the low-prevalence JAL antigen was reported to be associated with two unusual Rh complexes, one in Caucasian persons with depressed expression of C and e antigens [(C)(e)] and the other in black persons with depressed expression of c and e antigens [(c)(e)].7 This antigen was first encountered in 1977 when the serum sample of a mother (S. Allen) had an antibody that reacted strongly with red blood cells (RBCs) from her baby and husband (J. Allen) but failed to react with many examples of RBC samples known to carry low-prevalence antigens. This, the third child of S. Allen, suffered from HDFN.7 Another example of anti-JAL, in the serum sample of J. Pas., also caused HDFN in her third child. This antibody reacted strongly with the RBCs of her husband (D. Pas.) whose RBCs had a weak C antigen. The only other known example of anti-JAL was in a serum also containing anti-Wra, -Pta, and -Swa (from J. McD).7
In a parallel study, Poole and coworkers8 used serum of J. Pas. to test 90,000 Swiss donor blood samples and found four additional JAL+ probands. These four donors were French-speaking and taking into account that only approximately 7.5% of the donors tested were French-speaking (91.3% were German-speaking and 1.2% were Italian-speaking), the prevalence of the JAL antigen in the French-speaking population was estimated to be 0.06%. Testing blood samples from family members of the four JAL+ probands showed that the JAL antigen is indeed in the Rh blood group system. When the C+JAL+ phenotype was associated with a c in trans, the C antigen was detected by only about 45% of serum samples containing polyclonal anti-C and the reactions were weaker than with DCe/ce control RBC samples. Also, when the gene complex producing C and JAL was aligned with DcE in trans, expression of the e antigen was weaker than that of DcE/ce control RBC samples. Approximately 50% of serum sample containing polyclonal anti-e agglutinated E+e+JAL+ RBC samples with reactivity that ranged from extremely weak to a strength that was equivalent to E+e+ control RBC samples. The JAL antigen was assigned an Rh number (RH48) in 1990.9
Since the two original studies,7,8 additional people with JAL+ RBCs have been identified. In the study we present here, we tested blood samples from three of the original seven JAL+ probands tested by Lomas and coworkers,7 in parallel with those identified in the subsequent years in several laboratories and referred to our reference laboratories.
We confirmed the published serologic findings and, in addition, show that both haplotypes that encode JAL have altered expression of hrS and hrB antigens and that the c and e antigens are qualitatively as well as quantitatively altered because two transfused patients have made, respectively, alloanti-c and alloanti-e. We also describe a proband, homozygous for the gene encoding JAL, who has an alloantibody to the high-prevalence antigen that is antithetical to JAL and that we have named CEST [ISBT number RH57 (004057)].
MATERIALS AND METHODS
Samples
Blood samples were from various sources, referred because of detection of an unexpected alloantibody, discrepant typing results, or the presence of a low-prevalence antigen. The referral reasons of the samples in this study are given in Table 1 and the ethnicity is given in Table 2. Sample 1 (E. H.), Sample 3 (J. Allen), and Sample 4 (SA6) had been tested in the original JAL study by Lomas and coworkers.7 Sample 3 is the husband of the original antibody producer for whom the antigen is named. Sample 4 presented as a discrepant c typing in a Caribbean black person in England in 1985. The remainder of the samples were referred to our reference laboratories over the years or detected during our investigation to determine the molecular basis of JAL. The probands were Caucasian (n = 2), black African American (n = 6), black African Caribbean (n = 1), black African Puerto Rican (n = 1), and black African Brazilian (n = 2).
TABLE 1.
Summary of JAL+ samples and referral reason
| Sample | Year | Referral reason |
|---|---|---|
| 1 EH | 1983 | Donor with low-prevalence antigen. |
| 2 | 1992 | Donor with low-prevalence antigen. |
| 3 J. Allen | 1977 | Husband of S. Allen; HDN index case. |
| 4 SA6 | 1985 | Donor with discrepant c typing. |
| 5 | 2004 | Patient with c+ RBCs and alloanti-c in the serum. |
| 6 | 2006 | Donor with discrepant c typing. |
| 7 | 2007 | Donor with discrepant c typing. |
| 8 | 1998 | Mother with discrepant e typing and disputed paternity case. |
| 9 | 1998 | Son of Sample 8. |
| 10 | 2007 | Donor with weak e, apparent hrB−. |
| 11 | 2007 | Patient with e+ RBCs and alloanti-e in the serum. |
| 12 | 1995 | Patient with apparent Dc– RBCs with alloanti-Rh17. Later shown to have weakened e antigen expression. |
| 13 | 2003 | Pregnancy (l-4); patient with apparent C−, c−, E+, e− RBCs. Later shown to have weakened c and e antigen expression. |
| 14 | 2003 | l-1, sister of l-4 (Sample 13). |
| 15 | 2003 | l-2, sister of l-4 (Sample 13). |
| 16 | 2003 | l-5, sister of l-4 (Sample 13). |
| 17 | 2003 | ll-1, child of l-4 (Sample 13). |
TABLE 2.
Summary of hemagglutination tests on JAL+ samples
| Sample | Origin/ethnicity | C | c | E | e | f | V | VS | hrB | hrS | JAL | Predicted Rh complex† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 EH | UK/Caucasian | +W | + | 0 | + | + | 0 | 0 | + | + | + | D(C)(e)/ce |
| 2 | Switzerland/Caucasian | +W | + | + | +/0 | 0 | 0 | 0 | +/wk | + | + | D(C)(e)/DcE |
| 3 J. Allen | United States/black | 0 | + | 0 | + | + | 0 | 0 | + | + | + | D(c)(e)JAL/Dce |
| 4 SA6 | Caribbean/black | + | +/0 | 0 | + | NT | NT | 0 | NT | NT | + | D(c)(e)JAL/DCe |
| 5 | United States/black | + | +/0 | 0 | + | 0 | wk | 0 | + | + | + | D(c)(e)JAL/DCe |
| 6 | United States/black | + | +/0 | 0 | + | 0 | 0 | +w/0 | + | + | + | D(c)(e)JAL/DCe |
| 7 | United States/black | + | +/0 | 0 | + | NT | NT | NT | NT | NT | + | D(c)(e)JAL/DCe |
| 8 | Brazil/black | 0 | + | 0 | +/0 | 0 | +/0 | 0 | + | + | + | D(c)(e)JAL/Dce |
| 9 Son of 8 | Brazil/black | 0 | + | + | +/0 | 0 | +/0 | 0 | 0 | micro | + | D(c)(e)JAL/DcE |
| 10 | Puerto Rico/black | 0 | + | + | +/wk | NT | NT | NT | 0‡ | NT | + | D(c)(e)JAL/DcE |
| 11§ | United States/black | 0 | + | 0 | +/0 | NT | NT | NT | NT | NT | + | D(c)(e)JAL/DceS |
| 12 | Untied States/black | 0 | +/0 | 0 | +/0 | 0 | +w/0 | wk | 0/micro | wk | + | D(c)(e)JAL/D(c)(e) JAL |
| 13 (l-4) | Brazil/Black | 0 | +/0 | 0 | +/0 | 0 | 0 | 0 | 0 | 0 | + | D(c)(e)JAL/D(c)(e) JAL |
| 14 (l-1 sister of I-4, Sample13) | Brazil/Black | 0 | + | 0 | + | +/0 | + | + | + | +/0 | + | D(c)(e)JAL/DceS |
| 15 (l-2 sister of l-4, Sample 13) | Brazil/Black | 0 | +/0 | 0 | +/0 | 0 | 0 | 0/micro | 0 | 0/micro | + | D(c)(e)JAL/D(c)(e) JAL |
| 16 (l-5 sister of l-4, Sample 13) | Brazil/Black | 0 | + | 0 | + | +/0 | + | + | + | +/0 | + | D(c)(e)JAL/DceS |
| 17 (ll-1 son of l-4, Sample 13) | Brazil/Black | + | 0 | 0 | + | NT | NT | NT | NT | NT | NT | D(c)(e)JAL/DCe |
Many of the conclusions we made were possible because of the molecular results given in the companion paper by Westhoff and colleagues.13
Interpretation is based on serology and DNA based assays.13
RBCs from Sample 10 were tested only with one anti-hrB.
Antigen typing for Sample 11 was performed using a reticulocyte rich fraction.
+/0 = positive (albeit usually weak) with some reagents, negative with others; micro = microscopic reactivity; NT = not tested and no RBCs available; wk = weak expression.
Hemagglutination
Hemagglutination studies were performed in test tubes or gel cards (MTS, Ortho Clinical Diagnostics, Raritan, NJ) using various media by standard methods appropriate for the antibodies being used.10,11 Reagent RBCs, polyclonal antibodies, and monoclonal antibodies (MoAbs) were obtained from multiple sources, including our frozen inventories.
RESULTS
Antigen typing of JAL+ samples
The results of hemagglutination tests with selected antibodies to Rh antigens are shown in Table 2. For each specificity, unless noted, at least two examples were used. RBCs from all individuals expressed the D antigen. Due to hemolysis during shipment, RBCs from Samples 10 and 17 (as well as the sample from the husband of I-4, Sample 13) were not available for testing with anti-JAL or other relevant antibodies to Rh antigens. The JAL+ status of the RBCs from all samples was determined with the three known anti-JAL (J. Pas., S. Allen, J. McD) with the exception of Sample 7, whose RBCs were tested with only one example of anti-JAL (J. Pas.). Table 3 summarizes the results of testing RBCs from the two Caucasian C+c+ JAL+ probands with anti-C reagents, RBCs from the C+c+ JAL+ black persons and homozygous JAL+ with anti-c reagents, and E+e+JAL+ and homozygous JAL+ with anti-e reagents.
TABLE 3.
Characteristics of JAL+ RBCs
| (C)(e) JAL+ |
(c)(e) JAL+ |
|
|---|---|---|
| Characteristics | Caucasian | Black persons of African descent |
| C | +W/0 | 0 |
| c | 0 | +/+W/0 |
| E | 0 | 0 |
| e | +/+W/0 | +/+W/0 |
| f | 0 | 0 |
| V | 0 | +W/0 |
| VS | 0 | +W/0 |
| hrB | +W | +W/0 |
| hrS | +W | +W/0 |
| Gene13,15 | RHCE*CeMA | RHCE*ceS(340) |
| Alloantibody | None described | Anti-c; anti-e; anti-CEST; anti-Ce15 |
| Reactivity with anti-C reagents | ||
| MS24 (Gamma-Clone, Immuncor Series 1, Ortho, SNF) MS273(SNF), polyclonal (Immucor) (DCe/ce control 3+ to 4+) | + to 2+ | 0 |
| Reactivity with anti-c reagents | ||
| S42 (Ortho), BS240 (SNF) (DCe/ce control 4+) | 0 | 3+ |
| MS33 (Immucor), RaE11 (SNF), polyclonal (Gamma, Immucor) (DCe/ce control 4+) | 0 | 1+ to 2+ |
| 951 (Gamma-Clone) (DCe/ce control 4+) | 0 | 0 |
| Reactivity with anti-e reagents | ||
| MS62 (SNF), MS63 (SNF), Gamma-Clone (MS16, MS21 and MS63) (DcE/ce control 4+) | 4+ | 2+ to 3+ |
| MS21 (SNF), MS69 (SNF), HIRO38 (SNF), HIRO41 (SNF), HIRO43 (SNF) (DcE/ce control 2+ to 3+) | 1+ | 0 |
| MS16 (Ortho Bioclone) (DcE/ce control 4+) | 1+ | 0 |
| MS16 (Immucor Series 1) (DcE/ce control 2+) | 0 | 0 |
| Polyclonal (Immucor) (DcE/ce control 4+) | 2+ | 0 to 1+W |
SNF = supernatant culture fluid containing single-clone MoAb.
Table 3 also summarizes the characteristics of the two JAL+ haplotypes based on reactivity obtained with informative samples and various antibodies (i.e., for tests with anti-C, when c was on the other haplotype; for tests with anti-c, when C was on the other haplotype, and for tests with anti-e, when E was on the other haplotype; or RBCs with a double dose of JAL). RBCs carrying the (C)(e) JAL+ haplotype were, when in trans to a f− haplotype (Ce, cE), expectedly, f− and did not express V or VS antigens. RBCs carrying the (c)(e) JAL+ phenotype, if in trans to a f− haplotype, were also f−. RBCs with the (c)(e) JAL+ phenotype (when in trans to a V− VS− haplotype) express V and VS very weakly, and depending on the reagent used, the antigens may not be detectable. Similarly, tests on informative (c)(e)JAL+ RBCs showed that reactivity with anti-hrB or anti-hrS reagents was weak or absent. The hrB and hrS antigen expression is weaker on the ce form of JAL than on the Ce form. In summary (see Table 3), RBCs from Caucasian (C)(e)JAL+ people express C, e, hrB, and hrS weakly and lack f, V, and VS antigens. RBCs from black (c)(e)JAL+ people express c, e, V, VS, hrS, and hrB antigens weakly and lack f.
Alloantibodies to c and e antigens
Two probands (Samples 5 and 11), who had received transfusions, made alloantibodies. Sample 5, whose RBCs were predicted to have a D(c)(e)JAL/DCe haplotype, made alloanti-c and has been described elsewhere.12 Serum from Sample 11 appeared to contain alloanti-e; this proband was a 34-year-old with lupus erythematosis, never pregnant, but had received transfusion 3 months previous. Based on reactivity with the following RBCs, her serum appeared to contain an alloanti-e: c+e+ (weak), C+e+(±), e+ hrB− (weak), c+E+ (negative), autologous (negative). Her RBCs had the D(c)(e)JAL/DceS haplotype. No plasma or serum from Sample 11 was available for testing against informative JAL+ RBC samples, which would allow confirmation that the e antigen in the (c)(e)JAL haplotype encodes a partial antigen. The presence of alloanti-c in Sample 5 and alloanti-e in Sample 11 demonstrates that the c and e antigens on the JAL+ haplotype are altered qualitatively.
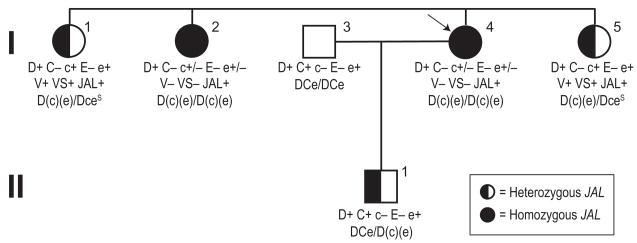
Family with two JAL homozygotes
Unusual initial typings of RBCs from an African Brazilian mother (Sample 13) after the birth of her first child led to a family study of the proband, her child, husband, and three sisters (Fig. 1). The initial typings, performed at the birth of the baby were mother (I-4) D+C–c–E–e+w, father D+C+c–E–e+, and baby D+C+c–E–e+. Further testing showed that the mother’s RBCs were agglutinated by some but not all anti-c and anti-e reagents and were JAL+. Results of selected antigen typing are shown in Table 2, and the serologic results and interpretations of the presumed Rh haplotypes are summarized in the pedigree (Fig. 1). All three sisters were JAL+. RBCs from the husband and child were not available for testing with anti-JAL or other relevant antibodies. RBCs from the proband of this pedigree (I-4) and her sister (I-2) initially did not react with anti-V, -VS, -hrB, and -hrS, but were later shown to be extremely weakly reactive with some examples of anti-VS and -hrS. Reactivity obtained in testing RBCs from one of the probands (Sample 12; see Table 2), who is homozygous for the gene encoding the (c)(e)JAL+ complex,13 indicates that indeed, this complex expresses V, VS, hrB, and hrS antigens extremely weakly. The nonreactivity of reagents with these specificities with Sample 13 (Proband I-4) and her sister (I-2) is likely due to the age and condition of the RBCs when tested. Serum from Sample 13 did not contain any alloantibodies and serum from her homozygous JAL+ sister (I-2) was not available for testing. This family has been reported in part in Lomas-Francis and coworkers.14
Fig. 1.
Family pedigree for Sample 13. The arrow indicates the proband. The JAL status of II-1 is predicted from DNA test results.13
Antibody to the high-prevalence antigen antithetical to JAL
Serum from Sample 12 had been shown 13 years previously to have alloanti-Rh17 (i.e., it failed to react only with D – –and Rhnull RBCs) and the RBCs had altered c and e antigens and were JAL+. Molecular analyses revealed this proband to be homozygous for the gene encoding (c)(e) JAL.13 Based on this finding, it is apparent that this “anti-Rh17” recognizes the high-prevalence antigen antithetical to JAL that we have named CEST (and assigned the number RH57 by the ISBTWorking Party for Terminology of Red Cell Surface Markers at the 2008 meeting in Macao). Thus, the antibody made by people homozygous for the allele encoding JAL is actually anti-CEST. This patient had been pregnant twice but not transfused with allogeneic blood.
DISCUSSION
JAL is a low-prevalence antigen in the Rh blood group system that was reported in 1990.7,8 Serologic testing showed that JAL is associated with two Rh complexes: one, in Caucasian persons, with (C)(e) and the other, in black persons, with (c)(e). The quantitative weakening of C, c, or e antigens was clearly apparent; indeed Poole and coworkers8 noted that only 45% of the anti-C reagents used in their 1990 study agglutinated C+c+ JAL+ RBCs. It is likely the anti-C were from a single donor source and more weakly reactive than current reagents. The nonreactivity with some anti-C might indicate that the C antigen on C+c+JAL+ RBCs is also qualitatively altered, but no evidence was presented to verify this possibility. In this study, all anti-C reagents agglutinated C+JAL+/c+ RBCs albeit to different strengths even with the same MoAb clone (MS24). Anti-c reagents gave variable reactions with c+JAL+/C+ RBCs: Ortho (MS42) reacts more strongly than Immucor (MS33) and the Gamma (951) reagent did not react. Anti-e also gave variable reactions even with the same MoAb clone (MS16). The variation in reaction strength obtained with the same MoAb clone (anti-C, MS24, and anti-e, MS16) is likely due to different formulations. Our findings are in agreement with the 1990 reports and clearly show a quantitative difference in the c and e antigens in cis to the JAL antigen.
We also show that the f antigen is not expressed on the (c)(e)JAL+ complex. In addition, we show that this complex expresses V and VS antigens extremely weakly and that both JAL+ complexes have a weak expression of hrS and hrB antigens. Indeed, some anti-f, -V, -VS, -hrS, and -hrB reagents did not react on informative RBC samples (in trans to R2 or double-dose JAL), which raises the possibility that these antigens may be qualitatively as well as quantitatively altered. However, as none of the patients have made anti-f, -V, -VS, -hrS, or -hrB the possibility that they are partial antigens remains to be determined. The variability of reaction strength with anti-C, -c, -e, -V, -VS, -hrB, or -hrS on JAL+ RBCs is somewhat dependent on the different antibodies, formulation, technique used, condition of RBCs, and which haplotype is in cis and/or in trans.
The e antigen on the (C)(e) complex was consistently stronger than on the (c)(e) complex. This, and the weaker expression of both hrB and hrS antigens on RBCs with the (c)(e) complex than with the (C)(e) complex, are presumably caused by the presence of 245 Val on the (c)(e) complex, which is described in detail in Westhoff and coworkers.13
The detection, in two patients who received transfusions, of alloanti-c in a (c)(e)JAL+/Ce person, and alloanti-e in a ceS/(c)(e)JAL+ person (based on the genotype13) indicates that the c and e antigens are qualitatively (and thus, respectively, are partial c and e) as well as quantitatively altered. The finding of a homozygous JAL+ proband with an alloantibody (anti-CEST) to the high-prevalence antigen (CEST) that is antithetical to JAL, in the absence of transfusion, shows the high immunogenic potential of this antigen.
Acknowledgments
We thank Beat Fry from Blood Transfusion Service SRC (Zürich, Switzerland) for sending a blood sample from Proband 2; Marilyn Moulds from Gamma Biologicals (Houston, TX) for frozen samples from several probands; Joseph Ong and Phyllis Walker (Blood Centers of the Pacific, San Francisco, CA) for blood from Sample 5; and Nanette Thomas (American Red Cross, Baltimore, MD) and Sakhone Leam for referring Sample 11. We also thank Karen Kirkley, from Medical Center of Louisiana (New Orleans, LA) for anti-JAL (S. Allen); Hein Hustinx, from Swiss Blood Transfusion Service (Bern, Switzerland) for anti-f and anti-JAL (J. Pas.); Malcolm Rhodes, from BioScot Limited (Livingston, UK) for the MS MoAbs; Makoto Uchikawa, from the Central Blood Center Japanese Red Cross (Tokyo, Japan) for the HIRO MoAbs; Dominique Goossens, from the Institut National de la Transfusion Sanguine (Paris, France) for RaE11; and Hans Sonneborn, from Biotest AG (Dreieich, Germany) for BS240. We thank numerous current and past technologists in the Laboratory of Immunohematology for their technical assistance and Robert Ratner for help in the preparation of the manuscript.
ABBREVIATION
- HDFN
hemolytic disease of the fetus and newborn
References
- 1.Reid ME, Lomas-Francis C. Blood group antigen factsbook. 2. San Diego: Academic Press; 2004. [Google Scholar]
- 2.Westhoff CM. The structure and function of the Rh antigen complex. Semin Hematol. 2007;44:42–50. doi: 10.1053/j.seminhematol.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moulds JJ, Case J, Anderson TD, Cooper ES. The first example of allo-anti-c produced by a c-positive individual. Recent advances in haematology, immunology and blood transfusion: Proceedings of the Plenary Sessions of the Joint Meeting of the 19th Congress of the International Society of Haematology and the 17th Congress of the International Society of Blood Transfusion; Budapest. August 1982; New York: John Wiley & Sons; 1983. [Google Scholar]
- 4.Faas BH, Ligthart PC, Lomas-Francis C, Overbeeke MA, von dem Borne AE, van der Schoot CE. Involvement of Gly96 in the formation of the Rh26 epitope. Transfusion. 1997;37:1123–30. doi: 10.1046/j.1537-2995.1997.37111298088040.x. [DOI] [PubMed] [Google Scholar]
- 5.Huestis DW, Catino ML, Busch S. A “new” Rh antibody (anti-Rh 26) which detects a factor usually accompanying hr’. Transfusion. 1964;4:414–8. doi: 10.1111/j.1537-2995.1964.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 6.Coghlan G, Moulds M, Nylen E, Zelinski T. Molecular basis of the LOCR (Rh55) antigen. Transfusion. 2006;46:1689–92. doi: 10.1111/j.1537-2995.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- 7.Lomas C, Poole J, Salaru N, Redman M, Kirkley K, Moulds M, McCreary J, Nicholson GS, Hustinx H, Green C. A low-incidence red cell antigen JAL associated with two unusual Rh gene complexes. Vox Sang. 1990;59:39–43. doi: 10.1111/j.1423-0410.1990.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 8.Poole J, Hustinx H, Gerber H, Lomas C, Liew YW, Tippett P. The red cell antigen JAL in the Swiss population: family studies showing that JAL is an Rh antigen (RH48) Vox Sang. 1990;59:44–7. doi: 10.1111/j.1423-0410.1990.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 9.Lewis M, Anstee DJ, Bird GW, Brodheim E, Cartron J-P, Contreras M, Crookston MC, Dahr W, Daniels GL, Engelfriet CP, Giles CM, Issitt PD, Jorgensen J, Kornstad L, Lubenko A, Marsh WL, McCreary J, Moore BP, Morel P, Moulds JJ, Nevanlinna H, Nordhagen R, Okubo Y, Rosen-field RE, Rouger P, Rubinstein P, Salmon C, Seidl S, Sistonene P, Tippett P, Walker RH, Woodfield G, Young S. Blood group terminology 1990. ISBT working party on terminology for red cell surface antigens. Vox Sang. 1990;58:152–69. [Google Scholar]
- 10.Brecher M. Technical manual. 15. Bethesda: American Association of Blood Banks; 2005. [Google Scholar]
- 11.Judd WJ. Methods in immunohematology. 2. Durham (NC): Montgomery Scientific Publications; 1994. [Google Scholar]
- 12.Reid M, Lomas-Francis C, Ong J, Walker PS, Schmulbach E, Storry J, Vege S, Westhoff C. A partial Rh4 phenotype [abstract] Vox Sang. 2008;95 (Suppl 1):70. doi: 10.1111/j.1423-0410.2008.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westhoff C, Vege S, Wylie D, Lomas-Francis C, Hue-Roye K, Reid ME. The JAL antigen (RH48) is the result of a change in RHCE that encodes Arg114Trp. Transfusion. doi: 10.1111/j.1537-2995.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lomas-Francis C, Valvasori M, Powell V, Vege S, Hue-Roye K, Reid ME, Westhoff C. Homozygosity for the gene encoding the Rh antigen, JAL, in two Brazilian sisters [abstract] Transfusion. 2008;48 (Suppl):185A. [Google Scholar]
- 15.Noizat-Pirenne F, Lee K, Le Pennec P-Y, Simon P, Kazup P, Bachir D, Rousaud A-M, Roussel M, Juszczak G, Ménanteau C, Rouger P, Kotb R, Cartron JP, Ansart-Pirenne H. Rare RHCE phenotypes in black individuals of Afro-Caribbean origin: identification and transfusion safety. Blood. 2002;100:4223–31. doi: 10.1182/blood-2002-01-0229. [DOI] [PubMed] [Google Scholar]