Abstract
Using an in vivo adeno-associated virus (AAV)–mediated gene transfer technique, this study evaluated the therapeutic effects of an Osteoprotegerin (OPG) transgene against orthopaedic wear debris–induced osteolysis in a long-term murine model. A titanium pin was surgically implanted into proximal tibia of Balb/c mice to mimic a weight-bearing knee arthroplasty, followed by an intra-articular challenge with Ti-particles to provoke periprosthetic inflammation and osteolysis. rAAV-hOPG or AAV-LacZ vectors were injected into the prosthetic joint at 3 weeks post-op. The tissues were harvested at 2, 4, 12 and 24 weeks after transfection for histological and molecular analyses. Successful transgene expression at the local site was confirmed by real-time PCR and ELISA. Inflammatory pseudo-membranes were ubiquitously presence at the interface between the Ti implant and the surrounding bone in both LacZ and virus-free control groups, while soft tissue was only observed sporadically at the bone-implant interface in the OPG group. A significant reduction in TRAP+ osteoclast numbers was observed in the OPG treatment group. MicroCT assessment indicated a marked reversal in the loss of peri-implant bone mineral density (BMD) in the OPG-transduced group, when compared with the LacZ and virus-free controls. Further, OPG gene modification appeared to reduce local bone collagen loss by a mean of 40%. Real time PCR examination confirmed that in vivo OPG gene transfer dramatically influenced the periprosthetic tissue gene expression profiles by diminishing the mRNA expression of TNF, IL-1, CPK and RANKL. There were no transgene-associated toxic effects apparent during the experiment, and the PCR detection of transgenes in remote organs such as lungs, kidneys, liver, and muscle of contralateral limb were consistently negative. Overall, rAAV-mediated OPG gene transfer effectively reversed Ti-particle induced bone resorption in this experimental model. The therapeutic effects may be due to the blockage of local osteoclastogenesis and possibly the down-regulation of RANKL expression.
Keywords: Aseptic loosening, gene therapy, wear debris, osteolysis, adeno-associate viral vectors
Introduction
Total joint replacement is a common procedure that has proven highly successful for the treatment of end stage arthritis. However, as many as 34% of total joint replacement components fail because of aseptic loosening within 10-25 years [1], which has become the major long-term complication of this procedure. Thus, the prevention or treatment of this complication remains an important issue in the field [2, 3].
Among the most important factors that contribute to loosening is the adverse tissue response to particulate wear debris [4]. Titanium-alloy components have been broadly used in total joint prosthesis, and the histological evaluation of tissues from failed primary arthroplasties often showed numerous particulate wear debris including polyethylene and metals in periprosthetic tissue [5, 6]. While polyethylene particles are the most common debris composition in retrieved uncemented periprosthetic tissue; particulate titanium debris is one of the frequent seen metal debris in tissues at the failed joint prosthesis, and appears to be associated with accelerated bone loss and implant loosening [5] It has been accepted that particulate biomaterials generated by mechanical wear of the prosthesis are phagocytosed by macrophages and other inflammatory cells, resulting in cellular activation and release of proinflammatory mediators and cytokines such as interleukin-1 (IL-1), tumor necrosis factor (TNF), and IL-6. These mediators, in turn, induce local chronic inflammation with activation and recruitment of osteoclasts to the bone–implant interface. This affects bone remodeling around the implant and leads to periprosthetic osteolysis and aseptic loosening [7, 8].
While many different cytokines contribute to this process, studies have shown that the receptor activator of nuclear factor-κB ligand (RANKL) is one of the only 2 essential mediators required to promote osteoclastogenesis. It binds to its membrane-bond signaling receptor, RANK, and stimulates osteoclast differentiation and maturation [9, 10]. A soluble protein osteoprotegerin (OPG) was identified in many types of cells and has been proven as a natural “decoy” receptor that competitively binds with RANKL and blunts its effects on osteoclastogenesis. Previous studies have reported that mice genetically deficient for RANKL or RANK often suffered from severe osteopetrosis, while OPG expressing transgenic mice exhibit the same pathology [11-13]; demonstrating that RANKL and RANK are essential for osteoclast development and OPG is a potent negative regulator for osteoclastogenesis.
Based on the anti-osteolytic nature of OPG, it may be a potential therapeutic agent to treat debris-associated periprosthetic bone resorption and aseptic loosening. Due to the short-half life of the biological agent, and chronic nature of the debris-associated periprosthetic osteolysis, it is difficult to utilize conventional therapy to administer sufficient OPG to osteolytic sites around the prosthetic joint. New techniques that deliver genes encoding therapeutic proteins enable their expression in the local tissues where the bone resorption occurs and offer an attractive alternative to continuous infusion of therapeutic proteins [14]. In this study, we examined the therapeutic effects and potential safety issues of AAV-OPG gene transfer in our recently characterized long-term murine model of debris-associated titanium-pin implantation failure [15].
Materials and Methods
1. Establishment of the mouse pin-implantation model
All the animal procedures were approved by Institutional Animal Care and Use Committees (IACUC, Wayne State University and Wichita State University). Seventy-eight Balb/c mice (Jackson Labs, Bar Harbor, ME) aged 10-12 weeks were quarantined in our local animal facility for 2 weeks prior to experimentation. Titanium-alloy pins of 0.8 mm diameter and 5 mm long with a flat large top of 1.4 mm diameter were specially manufactured by Stryker Orthopaedic, Inc. (Mahwah, NJ). The titanium particles (Ti-6Al-4V) used in this experiment were kindly provided by Zimmer, Inc. (Warsaw, IN) and the size of the particles (mean particle size 2.3 μm with a range of 0.1-68 μm) was similar to debris retrieved from periprosthetic tissues of patients revised for aseptic loosening [16].
The mouse model was established as described previously [15]. Briefly, mice were anesthetized with Xylazine and Ketamine, and a 5-mm medial parapatellar incision made to expose the patellar ligament. The patella was dislocated laterally to expose the tibial plateau, and the cartilage of the tibial plateau was partially removed using a #11 scalpel. The center of the tibial plateau was reamed to form a circular indentation and the proximal 5 mm of the tibial intramedullary canal was reamed with a 0.8 mm dental drill through the center of the indentation. A titanium alloy pin was then press-fit into the canal in a manner that the surface of pin head became flush with the cartilaginous surface of the tibial plateau and did not interfere with the motion of the knee. To mimic prosthetic wear, 10 μl of a titanium alloy particle suspension (4 × 104 particles of Ti-6Al-4V) was pipetted into the tibia canal before insertion of pin implant during surgery, and 40 μl of Ti-particles were intra-articularly injected into the prosthetic joint every 4 weeks following surgery. Mice (n=18) which did not receive titanium particle intra-articular injections throughout the course provided stable pin-implantation controls.
2. AAV-Mediated in vivo gene transfer
The recombinant adeno-associate virus transfer vector coding for OPG (pAAV-CMV-OPG-IRES-EGFP) was generated via multiple sub-cloning steps as detailed previously [17], and packaged in the Gene Core Facility, University of North Carolina (UNC) at Chapel Hill, NC, who prepared the purified rAAV-OPG-IRES-EGFP using type-2 rAAV. The rAAV-LacZ control vectors were also obtained from the Gene Core Facility, UNC.
The mice with titanium pin implants and Ti-particle challenges were divided into 3 groups at 3 weeks post-op: (1) mice in the OPG group were given a peri-articular injection of rAAV-OPG-eGFP at 107 pfu into the implanted joint (n=24); (2) 107 pfu of AAV vectors coding for LacZ was periarticularly injected into the implanted joints of LacZ control mice (n=24); (3) 18 positive control mice received a peri-articular injection of virus-free PBS. The mice with neither Ti-particle challenge nor viral infection (n=18) were kept as group 4 (stable control).
The mice were sacrificed at time points of 2, 4, 12 and 24 weeks after viral transfection. One half of the implanted knee joints (3 or 4 animals at 12 and 24 weeks time points per group) were harvested for histological evaluation, while the other half number of implanted joints, along with the lungs, liver, spleen, kidneys, muscles from contralateral limb, and lymph nodes of subiliac and axillary regions were snap-frozen and stored for molecular analyses and/or transgene detection.
3. Micro-Computerized Tomography (Micro CT) Scans
All mice were scanned immediately following surgery using an eXplore Locus MicroCT system (GE Medical Systems, London, ON, Canada) to confirm the successful position of pin implantation. The parameters for the scans were 45 μm isotropic voxel size, 400 projections, 400 ms exposure time, 80 KW voltages, and 450 μA current. The micro-CT scans were repeated at termination. Following acquisition and reconstruction, the image data were analyzed using the GEHC MicroView® software to generate isosurfaces of the region of interest (ROI) and to calculate the bone mineral densities (BMD) of the tibia bone surrounding the titanium pin.
4. Histological and Immunohistochemical (IHC) Analyses
Formalin-fixed prosthetic joints were decalcified in a solution containing 22% formic acid and 10% sodium citrate before paraffin-embedding. The sections were stained with hematoxylin and eosin to examine new bone formation or bone erosion around the prosthetic pin, and to evaluate debris-associated inflammation, including periprosthetic tissue formation and cellular infiltration. Modified Trichrome staining was performed to examine bone collagen changes [17]. Briefly, the sections were deparaffinized and hydrated before equilibrating in Bouin's solution (70% Picric acid, 5% Glacial acetic acid and 10% formaldehyde) at 56°C for one hour. The sections were then incubated in phosphomolybdic (0.21% w/v)-phosphotungstic acid (0.21% w/v) for 10 minutes, followed by aniline blue solution (2.5% aniline blue in 2% acetic acid) stain for 5 minutes. The sections were then incubated in 1% acetic acid for four minutes before dehydration in graded alcohol. The bone collagen acquired a blue stain, with the stain density proportionate to the collagen content [17]. Immunohistochemical (IHC) stains were carried out to illustrate pro-inflammatory cytokines and mediators of osteoclastogenesis at periprosthetic site. Antibodies against IL-1, TNF, IL-6, CD68, and RANKL were applied according to the instructions of antibody vendors; using methods for IHC reported previously [18]. In negative control sections, the primary antibody was replaced with appropriately diluted irrelevant antisera. To visualize the exogenous gene expression, X-gal stain was employed to trace LacZ gene transduced cells as described previously [17]
Digital images of IHC stained sections and modified Trichrome stained sections were captured. The levels of the positive stains and localizations were evaluated in four different fields per section and expressed as integrated optical density (IOD), using the Image-Pro Plus software package (Media Cybernetics, Silver Spring, MD) [17]. To determine changes of bone collagen content, the integrated optical densities (IOD) of the peri-implant bone tissues from treatment groups were normalized against IODs obtained from bone tissues from the particle-free stable prosthesis group. The data was expressed as the percent loss of bone collagen content.
5. Tartrate-Resistant Acid Phosphatase (TRAP) Stain for Osteoclasts
A commercial Kit (Sigma) was used for histological TRAP (EC3.1.3.2) staining using paraffin-sectioned prosthetic joints after pin removal. Following deparaffinzation and rehydration, the sections were permeated in a microwave oven for 30 seconds before brief fixation in buffered acetone. The sections were then incubated at 37°C for one hour in 0.1 M acetate buffer (pH 5.2), containing 0.5 mM naphthol AS-BI phosphoric acid, 2.2 mM Fast Garnet GBC and 10 mM sodium tartrate. The reaction was stopped by washing in several changes of distilled water. The presence of dark purple staining granules in the cytoplasm was considered as the specific criterion for identifiying TRAP positive cells.
6. Gene Expression Profile and Transgene Product Assessment
Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was performed to assess the influence of gene transfer on osteoclastogenesis. The expression of transgene (OPG) at the local delivery site and the remote organs/tissues were also examined. The hard and soft tissues were minced on dry ice followed by a Polytron (PT-MR2100; Kinematica, Lucerne, Switzerland) homogenization. Total RNA from the homogenates was extracted following the manufacturer's instructions. cDNA was reverse transcribed from 0.5 μg of total RNA in 40 ml of a reaction mixture containing 1× PCR buffer, 500 mM each of nucleotide triphosphates (dNTP), 0.5 U/μl of ribonuclease inhibitor, 2.5 mM of random hexamers, 5.5 mM of MgCl2, and 1.25 U/μl of reverse transcriptase (Perkin Elmer, CT). The reaction mixture was incubated in a DNA Thermal Cycler (Perkin Elmer, CT) at 25°C for 10 minutes, 48°C for 5 minutes followed by 95°C for 5 minutes. The expression of pro-inflammatory cytokines IL-1β, TNFα, IL-6, IL-10, RANKL, CPK, and CTR, as well as the human OPG transgene was determined by real-time PCR using the ABI Prism 7700 sequence detector (PE-Applied Biosystems, Foster City, California) as described previously [19]. With the Ct value of GAPDH as an internal control and mean Ct value of target gene expression from stable control samples (no Ti-particle challenges) as the calibrators (0% expression change), the comparative quantification of gene expressions of the samples from virus-free (particle-induced osteolysis control), LacZ and OPG treatment groups was calculated according to the formula given in the manufacturer's manual [20]. Selected reaction mixtures after RT-PCR were electrophoresed on 1.8% agarose gels to confirm the amplification of the correct target genes.
Enzyme linked immunosorbent assays (ELISA) were performed on periprosthetic tissue homogenates to assess therapeutic transgenes production and cytokine expression. The ELISA utilizes capture and detection monoclonal antibody pairs against different epitopes of the various cytokine molecules, and a standardized protocol was previously described [18]. The antibodies against human OPG distinguish the transgene products from endogenous murine OPG.
7. Statistical Analysis
The data from 4 independent experiments were analyzed for this study. A total of 84 animals were used in the experiments and divided into 4 groups (stable control, virus-free, LacZ-transduced, and OPG-gene modified). Three (3) animals in each group were sacrificed at 2 and 4 weeks, respectively, to evaluate transgene expression and dissemination. At least 6 animals (8 in virus-treated groups) in each group were evaluated at the 12- and 24-week time-points. Statistical analysis between groups was performed by the ANOVA test; with the LCD formula for post hoc multiple comparisons, using the SPSS software package (SPSS v14.0. Chicago, IL). Non-parametric data such as normalized gene expression profiles were analyzed using Chi Square test (SPSS). A p value of less than 0.05 was considered as a significant difference. Data were expressed as mean ± standard error of the mean.
Results
1. Operative Outcome
The mice tolerated the surgical procedure well and ambulated with the implanted limb within 3 days after surgery. The incisions healed with no complications and the sutures were removed 10 days after surgery. Injections of Adeno-associated viral vectors appeared to exert no influence on the daily activities of the animals. There were no infections or tumorogenesis observed during the course of the experimentation. The macroscopic examination of the prosthetic joints at sacrifice revealed metal pins positioned in proximal tibiae without migration, and no scratches nor inflammatory responses on the opposing articulating joint surfaces. There were no obvious macroscopic differences among different treatment groups, except that it was very difficult to dissociate the pin implants from tibiae in the particle-free stable prosthesis group.
2. Transgene Expression and Dissemination
Real-time PCR was conducted to evaluate the transgene expression during the course of the experiments. The OPG mRNA expression was comprehensively elevated in samples extracted from OPG gene transferred prosthetic joint homogenates, while the samples from other groups provided background readings (Figure 1). The transgene expression could be detected at 2 weeks after gene transfer and last till the animal sacrifice at 6 months. ELISA confirmed the OPG protein levels at the gene transduction site in a range of 60-100 ng/mg total protein. DNA extracted from various organs and tissues of the animals with OPG-gene therapy were examined by conventional PCR with human OPG primers for the transgene incorporation. No positive PCR product was detected, except for the periprosthetic tissue where the in vivo gene transfer was executed (Figure 1). All the prosthetic joint samples with LacZ-gene transfer exhibited typical dark blue coloration under X-gal stain as illustrated previously [15].
Figure 1.
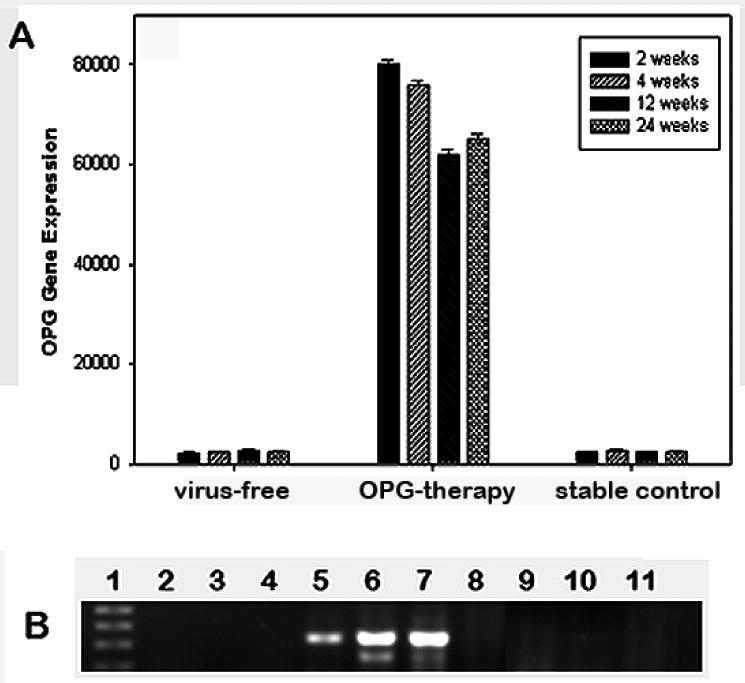
Panel A summarizes successful OPG transgene expression at the periprosthetic site during the course of the animal experiments by real-time RT-PCR. Panel B shows an agarose gel of conventional PCR products against OPG primers on various tissues of OPG-treated animals for transgene dssermination. Lane 1: DNA ladder; Lanes 2-4: LacZ-infected prosthetic tissue; Lanes 5-7: OPG transduced prosthetic tissue homogenates; Lanes 8-11: organs of OPG mouse: 8 – liver, 9 – lungs, 10 – lymph nodes; 11 – muscle.
3. Histological Analyses
Histological evaluation of the pin prosthesis model on H&E sections clearly indicated the proper location of the implant and new bone ingrowth in the particle-free stable pin implantation group (Figure 2A). Consistent with our previous results [15], periprosthetic pseudo-membranes developed at two weeks post-surgery in mice with Ti-particle injections at the bone-implant interface, and the thickness of this periprosthetic membrane increased during the course of the experiment. However, OPG gene modification resulted in a dramatic slowing and reversal of the osteolysis of periprosthetic bone. Figure 2 illustrates the typical histological appearance of tibia sections at 6 months after OPG gene modification. While severe inflammation and bone resorption were present in the particulate debris-containing prosthetic tibiae treated with AAV-LacZ (Figure 2D) or virus-free PBS control (Figure 2B), OPG gene transfer significantly ameliorated the periprosthetic membrane formation and bone resorption (Figure 2C), and appears quite comparable to sections from stable pin-implantation control mice without particle stimulation (Figure 2A).
Figure 2.
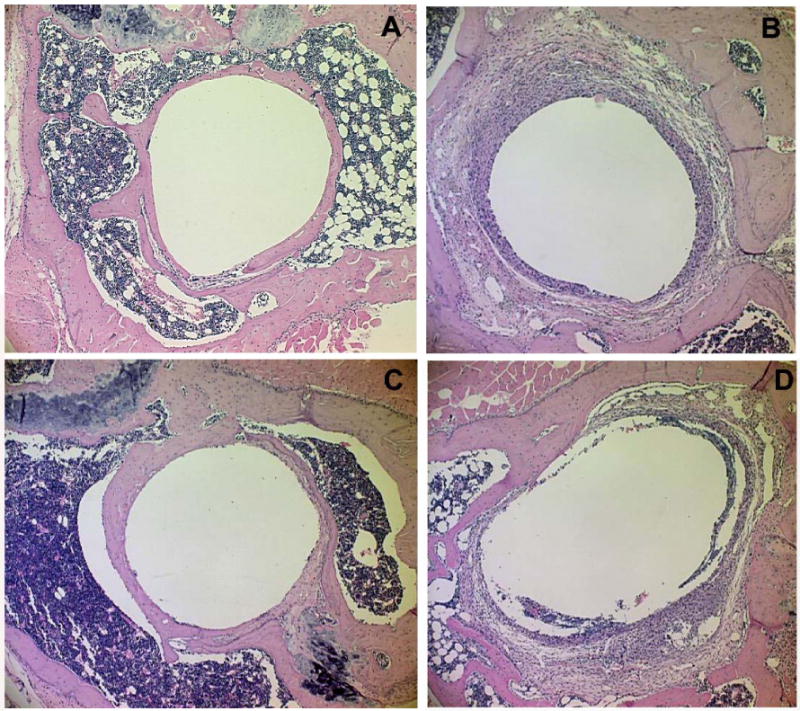
The histological appearances of the pin prosthesis tibiae at 6 months following gene modifications with or without particle challenges: (A) stable pin-implantation without particles stimulation; (B) Ti-particle challenged prosthesis failure control, without viral infection; (C) OPG gene transfer significantly prevented the periprosthetic membrane formation and bone resorption; while (D) Ti particle-containing prosthetic tibiae treated with AAV-LacZ.
Modified Trichrome staining was performed to investigate changes in the bone collagen content [17]. The periprosthetic bone tissues challenged with titanium particles and treated with LacZ gene exhibited tremendous focal and general reduction in staining compared to sections from the stable implantation group and the OPG gene therapy group. Comparison of mean Trichrome staining by computerized image analysis system suggested that OPG gene transfer significantly preserved the bone collagen loss during the course of Ti-particle challenge (p<0.01, Figure 3).
Figure 3.
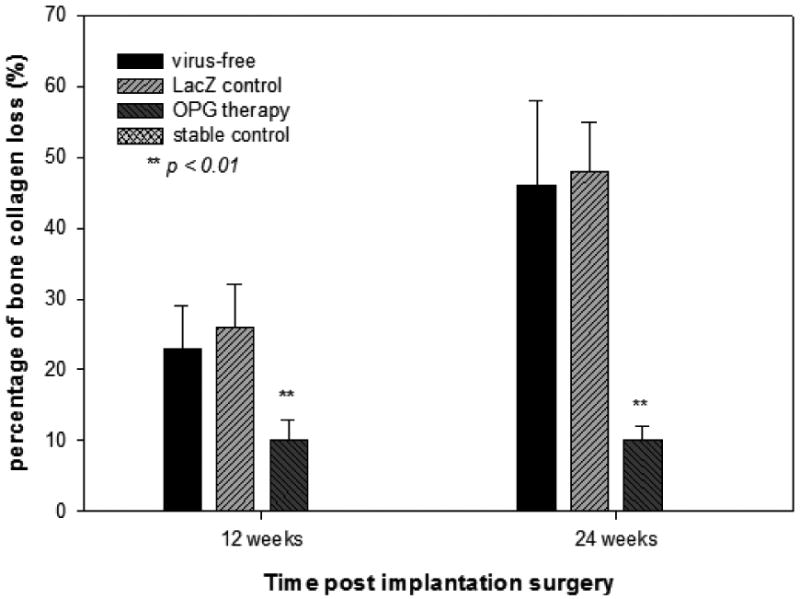
Summary of the bone collagen degradation quantified by a computerized image analysis system using image-Pro® on the modified Trichrome staining sections. The integrated optical density readings from 4 areas per tissue sections and at least 3 or 4 animals per time point per group were averaged and compared. The data is expressed as the percentage of bone collagen loss against the readings from the stable control group (**p< 0.01).
4. MicroCT Evaluation
MicroCT scans indicated that the implants were well fixed with no obvious migration up to 6 months in the surgery cases without particle challenge. However, titanium particle injections resulted in a significant reduction in periprosthetic bone mineral density (BMD). In contrast, particle-challenged pin implantation mice treated with local AAV-OPG viral vector infections clearly showed the preservation of BMD at the end of the experiment (Figure 4).
Figure 4.
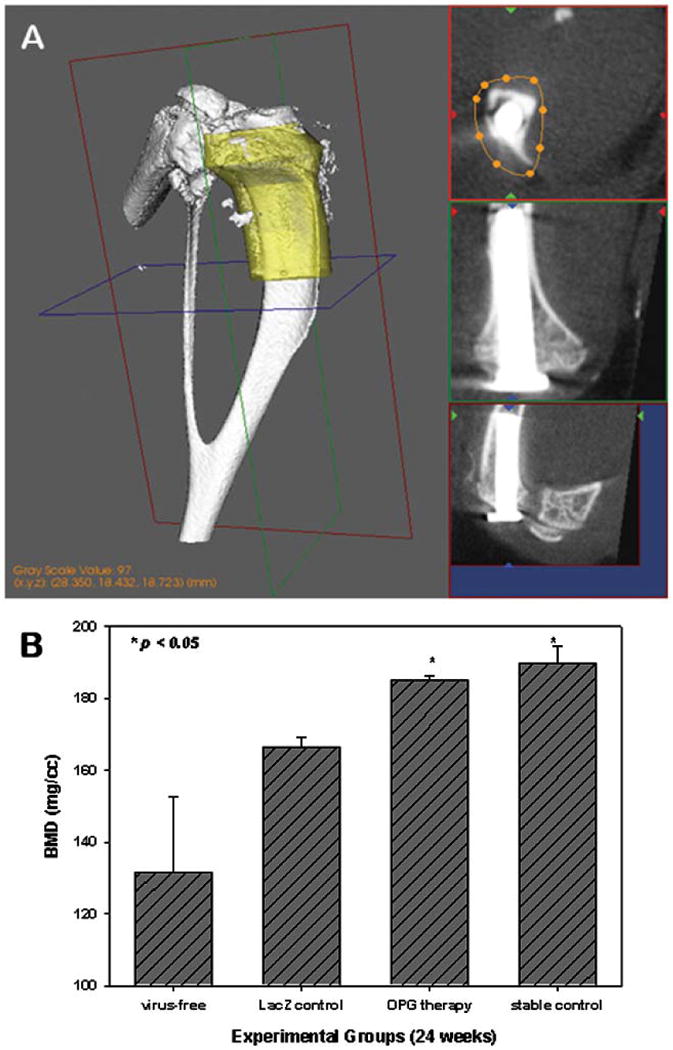
A GE microCT system was used to quantify periprosthetic bone mineral density (BMD) in the yellow normalized volume of Interest (VOI, panel A). OPG gene modification markedly preserved the periprosthetic BMD in comparison with the LacZ gene-transferred and the virus-free control groups (Panel B, n ≥ 6, p<0.05).
4. Immunohistochemistry Examination and TRAP staining
Immunohistochemical assessment using anti-mouse cytokine antibodies revealed a profound accumulation of TNF and IL-1 expressing cells at the interface between the pin and surrounding bone in the virus-free sections. CD68+ macrophages were also aggregated in the particle-stimulated periprosthetic membranes (Figure 5). However, there was a lack of periprosthetic inflammatory membranes formed in the OPG gene-modification group, and only sporadic CD68+ cells were present at the bone-implant interface (Figure 5). TRAP staining was performed to identify mature osteoclasts at the bone-implant interface. There was a marked reduction of TRAP+ cells in the sections from OPG-gene modified animals in comparison with LacZ-treated and virus-free groups (Figure 5).
Figure 5.
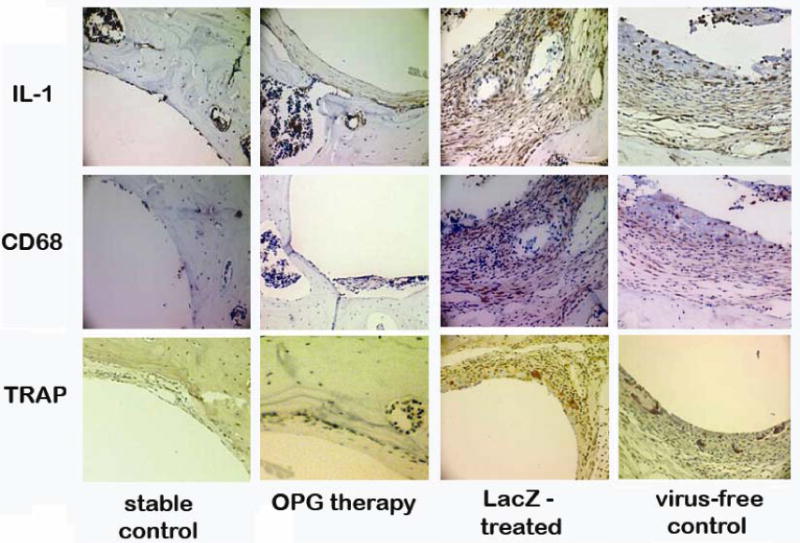
Typical IHC stained periprosthetic tissue sections against mouse IL-1 (upper panels), and mouse CD68 (middle panels) to reveal IL-1 expressing and CD68+ cells. TRAP staining to identify mature osteoclast at the bone-implant interface (lower panels). Four experimental groups are illustrated in columns.
5. Molecular and Immunologic Analyses
Pro-inflammatory cytokines and osteoclastogenesis gene expression profiles in the samples were evaluated by real-time PCR and summarized in Figure 6. While virus-free and LacZ controls promoted strong TNF, IL-1, CPK and RANKL expression, specimens receiving OPG gene modification resulted in significantly lower expressions of these genes (Figure 6). There was no meaningful difference among groups in the gene expression of IL-6, IL-10, and CTR in the current study.
Figure 6.
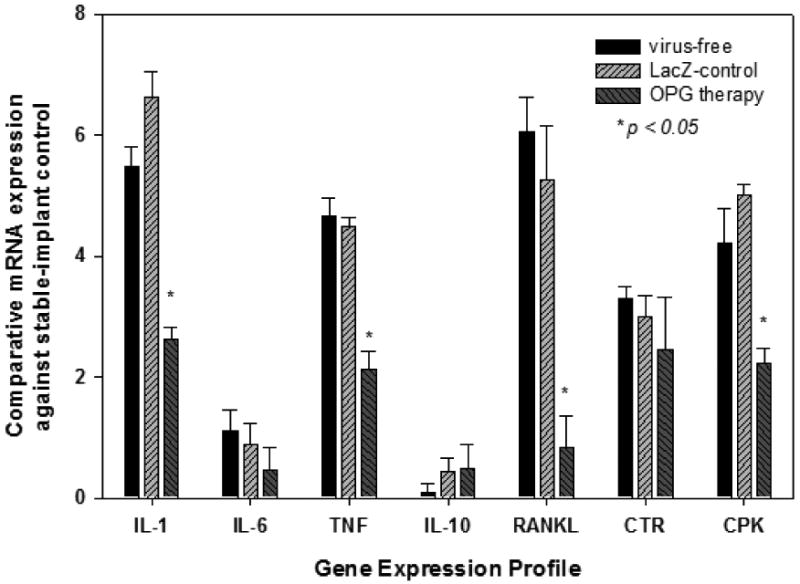
Gene Expression Profiles responding to the in vivo gene modifications during the course of the experimentation. The data are expressed as comparative gene copies against readings from the stable-implant control (see Materials & Methods, *p<0.05).
Discussion
This study examined the therapeutic effects of OPG gene transfer in blocking the titanium particle-associated periprosthetic bone resorption, using a newly characterized murine model of weight-bearing knee pin implant. Loosening of joint arthroplasty components due to osteolysis represents the major long-term complication following total joint replacement. Although the precise pathogenesis of aseptic loosening remains to be established, the cumulative evidence indicates that particulate biomaterial debris generated from the mechanical wear of prosthetic components play a critical role [21, 22]. Studies have suggested that wear debris particles may promote periprosthetic inflammation and subsequent bone resorption, and lead to the prosthesis failure [15, 18]. Attenuation of the debris associated inflammation and reversion of implant surrounding bone resorption would thus appear to hold considerable promise as a therapeutic strategy for this complication.
As first noted in 1990, the in vitro maturation of osteoclasts requires the presence of marrow stromal cells or their osteoblast progeny [23]. It is now clear that these accessory cells express two molecules that are essential and sufficient to promote osteoclastogenesis. One of these 2 molecules is recognized as the osteoclast differentiation factor (RANKL). It binds to its physiologic signaling receptor, RANK, on membranes of macrophages and osteoclast precursors, thereby providing signals required for their survival, maturation, and activation [11]. Osteoprotegerin (OPG) has been identified as a potent negative regulator of the osteoclast differentiation factor, which is also naturally produced in stromal cells and several other cell types. It is the balance between the expression of the stimulator of osteoclastogenesis, RANKL, and the inhibitor, OPG that dictates the quantity of bone resorption and remodeling [24]. Thus, OPG may be an effective therapeutic candidate to retard the process of wear debris-associated inflammatory osteolysis and aseptic loosening of the prosthetic joint. Major problems in the approach to treating a localized chronic inflammatory/osteolytic disorder such as debris-associated aseptic loosening include the lack of adequate suppressive agents and effective specific therapeutic delivery systems. While conventional systemic administration of biological drugs relies on vascular perfusion to the local sites of loosening (requiring relatively high doses and repeated administration) viral vector–mediated gene transfer provides a novel means to deliver therapeutic genes to the site of disease to express therapeutic proteins in a persistent and localized manner [25]. Using retroviral vectors encoding IL-1 receptor antagonist, we and others have successfully attenuated orthopaedic particle–induced inflammation and experimental animal arthritis [18, 26, 27]. However, retroviral vectors have the disadvantage of only infecting actively dividing cells. Adeno-associated viral vectors (AAV) have shown some distinct advantages over other viral vectors; they can infect both dividing and non-dividing host cells and provoke a limited host immune response, unlike the adenoviral vectors [25, 28]. AAV vectors also have better long-term transgene expression. Using the air pouch osteolysis model and the mouse calvaria model, we and others have successfully incorporated OPG gene mediated by AAV and demonstrated that OPG gene transfer in the models effectively protected against particle-induced bone resorption provoked by a range of orthopaedic biomaterials including ultra high molecular weight polyethylene and titanium-alloy [17, 29]. Due to the short-term nature of theses models, the therapeutic effects and long term safety issues of OPG gene modification in progressive osteolytic conditions during the weight-bearing implant loosening process have not been evaluated.
We have recently established a new long term mouse model of knee prosthesis failure to mimic the clinical wear debris-associated periprosthetic osteolysis process [15]. Due to the fast metabolic rate per body mass and short life span (2-3 years) [30, 31], up to 6 months of interactions between the weight-bearing implant, particulate debris, and surrounding bone in mouse may represent a subclinical long-term prosthesis failing model, equivalent to about 10 years of human life length of the joint prosthesis. The pin-implantation model reveals an inflammatory pseudo-membrane at the interface between the pin-implant and surrounding bone as early as 2 weeks after periprosthetic titanium particle challenges. Periprosthetic bone resorption was also noticed on MicroCT images at 3 weeks [15]. It might be argued that the wear debris challenge should be introduced after implant osseointegration has been achieved to more fully reflect the clinical situation, but unpublished data from our lab has confirmed that delayed and repeated Ti-particle challenges (started three weeks after implant surgery and repeated once a month) results in periprosthetic osteolysis with a similar pattern to the current report; and implanted pins that only received an initial Ti-particle injection without subsequent challenges did regain stability. It appears that only constant particle stimulation promotes periprosthetic bone resorption in this model, therefore it appears that this progressing osteolytic implant-loosening model is a relevant platform to evaluate the therapeutic influence of in vivo gene therapy. AAV-OPG therapy was locally administered to the prosthetic joints at 3 weeks post–operation and Ti-particle challenge and compared with irrelevant viral transfection (AAV-LacZ) and non-treatment controls. Data from stable implants without particle stimulations were used as positive controls. The exogenous OPG gene actively inhibited Ti particle–induced osteoclastogenesis with diminished TRAP positive stains and a reduction of the gene expression levels of RANK ligand and the osteoclast makers (CPK and CTR). Consequently, periprosthetic bone resorption and bone collagen degradation were dramatically ameliorated. The therapeutic effectiveness of the gene modification was sustained for 6 months during constant particle challenge. Interestingly, the detection levels of pro-inflammatory cytokines such as IL-1 and TNF were also decreased at the prosthetic joints in OPG-modification group compared to controls. While further studies are warranted to confirm if blockage of RANK-RANKL pathway influences the interactions of cytokine expressions, the data obtained here is associated with the restoration of osseointegration of pin implant and elimination of particle infiltration at the pin-implant interface, since there was a marked reduction of periprosthetic inflammatory tissue present in OPG-treated animals at 6 months.
Although virus-mediated gene therapy appears to be an attractive tool for treatment of many genetic and non-genetic disorders, the risks of permanent gene therapy have become a concern after the unfortunate episode of clinical trials in children with X-linked severe combined immunodeficiency (X-SCID) [32]. In addition, a recently published study suggested a very long term existence (6 years) of rAAV particles in the target organ after successful in vivo gene transfer [33] and a separate study reported that injection of a recombinant AAV serotype 2 evoked strong immune responses against transgene products [34]. In our current long-term model of the prosthetic failure, the in vivo transgene products were assessed for up to 6 month. The transgene was expressed at the local transfection site through the course of the experiment. However, there was no transgene dissemination detected in remote organs and tissues, and no tumorigenesis was noticed post viral infections. Overall, the experiment suggested that this therapeutic approach was safe and effective.
Conclusions
This study suggests that the long-term mouse model of knee prosthesis failure is a useful tool to evaluate therapeutic approaches to debris-associated osteolysis, and that rAAV-mediated OPG gene transfer appears to be a feasible and effective mechanism to treat the wear debris–associated osteolysis and aseptic loosening. Further studies are warranted to fully understand the molecular mechanisms of the therapeutic influences and the safety concerns, and to seek different therapeutic protocols to prevent, slow down or reverse the periprosthetic osteolysis based on the clinical stages of this long-term complication.
Acknowledgments
This work was supported by a NIH grant 5R03AR054929 to S-Y.Y. The authors wish to thank Dr. Bin Wu and Dr Ling Bai for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keener JD, Callaghan JJ, Goetz DD, Pederson DR, Sullivan PM, Johnston RC. Twenty-five-year results after Charnley total hip arthroplasty in patients less than fifty years old: a concise follow-up of a previous report. J Bone Joint Surg Am. 2003;85-A(6):1066–1072. doi: 10.2106/00004623-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Kawamoto K, Hasegawa Y, Iwase T, Iwasada S, Kanamono T, Iwata H. Failed cementless total hip arthroplasty for osteoarthrosis due to hip dysplasia. A minimum five-year follow-up study. Bull Hosp Jt Dis. 1998;57(3):130–135. [PubMed] [Google Scholar]
- 3.Kim YH, Kim VE. Cementless porous-coated anatomic medullary locking total hip prostheses. J Arthroplasty. 1994;9(3):243–252. doi: 10.1016/0883-5403(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 4.Toumbis CA, Kronick JL, Wooley PH, Nasser S. Total joint arthroplasty and the immune response. Semin Arthritis Rheum. 1997;27(1):44–47. doi: 10.1016/s0049-0172(97)80036-4. [DOI] [PubMed] [Google Scholar]
- 5.Buly RL, Huo MH, Salvati E, Brien W, Bansal M. Titanium wear debris in failed cemented total hip arthroplasty. An analysis of 71 cases. J Arthroplasty. 1992;7(3):315–323. doi: 10.1016/0883-5403(92)90056-v. [DOI] [PubMed] [Google Scholar]
- 6.Goodman SB, Fornasier VL, Kei J. Quantitative comparison of the histological effects of particulate polymethylmethacrylate versus polyethylene in the rabbit tibia. Arch Orthop Trauma Surg. 1991;110(3):123–126. doi: 10.1007/BF00395792. [DOI] [PubMed] [Google Scholar]
- 7.Shanbhag AS, Hasselman CT, Rubash HE. The John Charnley Award. Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop Relat Res. 1997;344:33–43. [PubMed] [Google Scholar]
- 8.Goldring SR, Schiller AL, Roelke M, Rourke CM, O'Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am. 1983;65(5):575–584. [PubMed] [Google Scholar]
- 9.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408(6812):600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 10.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97(4):1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim N, Odgren PR, Kim DK, Marks SC, Jr, Choi Y. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci U S A. 2000;97(20):10905–10910. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 14.Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg Am. 1995;77(7):1103–1114. doi: 10.2106/00004623-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Yang SY, Yu H, Gong W, Wu B, Mayton L, Costello R, et al. Murine model of prosthesis failure for the long-term study of aseptic loosening. J Orthop Res. 2007;25(5):603–611. doi: 10.1002/jor.20342. [DOI] [PubMed] [Google Scholar]
- 16.Margevicius KJ, Bauer TW, McMahon JT, Brown SA, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg Am. 1994;76(11):1664–1675. doi: 10.2106/00004623-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Yang SY, Mayton L, Wu B, Goater JJ, Schwarz EM, Wooley PH. Adeno-associated virus-mediated osteoprotegerin gene transfer protects against particulate polyethylene-induced osteolysis in a murine model. Arthritis Rheum. 2002;46(9):2514–2523. doi: 10.1002/art.10527. [DOI] [PubMed] [Google Scholar]
- 18.Yang SY, Wu B, Mayton L, Mukherjee P, Robbins PD, Evans CH, et al. Protective effects of IL-1Ra or vIL-10 gene transfer on a murine model of wear debris-induced osteolysis. Gene Ther. 2004;11(5):483–491. doi: 10.1038/sj.gt.3302192. [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Wu B, Mayton L, Evans CH, Robbins PD, Wooley PH. IL-1Ra and vIL-10 gene transfer using retroviral vectors ameliorates particle-associated inflammation in the murine air pouch model. Inflamm Res. 2002;51(7):342–350. doi: 10.1007/pl00000313. [DOI] [PubMed] [Google Scholar]
- 20.PE Applied Biosystems. TagMan Cytokine Gene Expression Plate I; Protocol. P/N 4304671 ed. 1998:37–53. [Google Scholar]
- 21.Wooley PH, Schwarz EM. Aseptic loosening. Gene Ther. 2004;11(4):402–407. doi: 10.1038/sj.gt.3302202. [DOI] [PubMed] [Google Scholar]
- 22.Holt G, Murnaghan C, Reilly J, Meek RM. The biology of aseptic osteolysis. Clin Orthop Relat Res. 2007;460:240–252. doi: 10.1097/BLO.0b013e31804b4147. [DOI] [PubMed] [Google Scholar]
- 23.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci U S A. 1990;87(18):7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofbauer LC. Osteoprotegerin ligand and osteoprotegerin: novel implications for osteoclast biology and bone metabolism. Eur J Endocrinol. 1999;141(3):195–210. doi: 10.1530/eje.0.1410195. [DOI] [PubMed] [Google Scholar]
- 25.Evans CH, Ghivizzani SC, Kang R, Muzzonigro T, Wasko MC, Herndon JH, et al. Gene therapy for rheumatic diseases. Arthritis Rheum. 1999;42(1):1–16. doi: 10.1002/1529-0131(199901)42:1<1::AID-ANR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Sud S, Yang SY, Evans CH, Robbins PD, Wooley PH. Effects of cytokine gene therapy on particulate-induced inflammation in the murine air pouch. Inflammation. 2001;25(6):361–372. doi: 10.1023/a:1012898513512. [DOI] [PubMed] [Google Scholar]
- 27.Makarov SS, Olsen JC, Johnston WN, Anderle SK, Brown RR, Baldwin AS, Jr, et al. Suppression of experimental arthritis by gene transfer of interleukin 1 receptor antagonist cDNA. Proc Natl Acad Sci U S A. 1996;93(1):402–406. doi: 10.1073/pnas.93.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goater J, Muller R, Kollias G, Firestein GS, Sanz I, O'Keefe RJ, et al. Empirical advantages of adeno associated viral vectors in vivo gene therapy for arthritis. J Rheumatol. 2000;27(4):983–989. [PubMed] [Google Scholar]
- 29.Ulrich-Vinther M, Carmody EE, Goater JJ, K Sb, O'Keefe RJ, Schwarz EM. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Joint Surg Am. 2002;84-A(8):1405–1412. doi: 10.2106/00004623-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 30.de Magalhaes JP, Budovsky A, Lehmann G, Costa J, Li Y, Fraifeld V, et al. The Human Ageing Genomic Resources: online databases and tools for biogerontologists. Aging Cell. 2009;8(1):65–72. doi: 10.1111/j.1474-9726.2008.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen TS, Currier GJ, Wabner CL. Intestinal transport during the life span of the mouse. J Gerontol. 1990;45(4):B129–133. doi: 10.1093/geronj/45.4.b129. [DOI] [PubMed] [Google Scholar]
- 32.Staal FJ, Pike-Overzet K, Ng YY, van Dongen JJ. Sola dosis facit venenum. Leukemia in gene therapy trials: a question of vectors, inserts and dosage? Leukemia. 2008;22(10):1849–1852. doi: 10.1038/leu.2008.219. [DOI] [PubMed] [Google Scholar]
- 33.Stieger K, Schroeder J, Provost N, Mendes-Madeira A, Belbellaa B, Le Meur G, et al. Detection of intact rAAV particles up to 6 years after successful gene transfer in the retina of dogs and primates. Mol Ther. 2009;17(3):516–523. doi: 10.1038/mt.2008.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14(17):1249–1260. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]


