Abstract
OBJECTIVE
The objective of this study was to determine the effect of non-occupational exposure to manganese on postural balance.
METHODS
Residents living near a ferromanganese refinery provided hair and blood samples after postural balance testing. The relationship between hair manganese and postural balance was analyzed with logistic regression. Following covariate adjustment, postural balance was compared with control data by analysis of covariance.
RESULTS
Mean hair manganese was 4.4 µg/g. A significantly positive association was found between hair manganese and sway area (EO, p=0.05; EC, p=0.04) and sway length (EO, p=0.05; EC, p=0.04). Postural balance of residents was significantly larger than controls in 5 out of 8 postural balance outcomes.
CONCLUSION
Preliminary findings suggest subclinical impairment in postural balance among residents chronically exposed to ambient Mn. A prospective study with a larger sample size is warranted.
Introduction
Southwestern Ohio is currently home to the only ferromanganese alloy producer in the United States. Located a few miles outside Marietta, Ohio, the refinery has been in operation under different ownerships for over fifty years. Local residents have become increasingly concerned about Manganese (Mn) emissions from this refinery. Their concerns have been supported by Environmental Protection Agency (USEPA) Risk-Screening Environmental Indicators (RSEI) using 2002 toxic release inventory (TRI) data. Combining regional population size, emission volume with chemical characteristics, emissions from this refinery were estimated to pose the greatest health risk from air pollution for Ohio residents.1
Excessive Mn exposure can lead to neuromotor symptoms closely resembling Parkinson’s disease. These parkinsonian symptoms include postural tremors or difficulties with speech, balance and gait. Neurotoxicity from occupational exposures has been demonstrated at ambient Mn concentrations approximating 1 mg/m3.2–4 The health consequences of ambient Mn exposure at lower concentrations in a non-occupational environment remain unclear. A recent study of subjects exposed to lower concentrations by residing near a processing facility in Mexico demonstrated differences with upper extremity and hand coordination.5
Postural balance testing has proven useful in identifying subtle neuromotor abnormalities secondary to exposures to jet fuel6, solvents7, alcohol8 pesticides9 and lead10, 11. Several occupational studies have utilized postural balance testing in the context of Mn exposure.4, 12–16 Only one previous study has evaluated the influence of chronic non-occupational Mn exposure on postural balance.17 The current study investigated the relationship between hair and blood Mn concentrations with measures of postural balance. We then collectively compared postural balance measurements of study residents with measurements from an unexposed control group from a previous study.
Methods
Subjects
This cross-sectional study evaluated postural balance and biological samples of Marietta area residents who met specific inclusion and exclusion criteria. The study was approved by the University of Cincinnati’s Institutional Review Board (IRB). Residents were identified from a community profile survey distributed through the local organization Neighbors for Clean Air. The study’s procedures, risks, and benefits were explained both verbally and in writing to all residents, who then provided written informed consent prior to their participation.
Inclusion criteria required the primary residence of each resident to be within a ten mile radius of the refinery for a minimum of three consecutive years. A distance of ten miles from the refinery was used in an effort to maximize subject participation by including southwest Marietta. The current residential address of each resident was confirmed by a global positioning system (GPS). It was also necessary for each resident to complete a self-reported medical history for screening of neurological disorders.
Exclusion criteria were chosen to minimize covariates that would affect postural balance based on the presence of occupational exposure, neurological issues or resident discomfort. Any resident with a known history of occupational Mn exposure was allowed to undergo testing but ultimately was excluded from the statistical analysis. Any resident with a history of Meniere’s or Parkinson’s disease, multiple sclerosis, blindness, stroke, seizure or upper respiratory or inner ear infection within two weeks was excluded from statistical analysis. Those with difficulty standing unaided for at least three minutes or those having pain and discomfort from standing for a short time were not permitted to undergo testing.
Our general estimates of ambient exposure were based on information published from a recent ATSDR pilot study. Concentrations of Mn within air typically approximate 0.023 µg/m3.18 It was estimated that the mean daily ambient exposure surrounding the refinery range from 0.10 to 2.0 µg/m3 dependent on residential direction and distance from the refinery.19 Modeling from this study supported that ambient exposure was relatively higher for residents living close to the refinery than residents living farther away. More specifically, dispersion maps indicate residences within approximately 1 to 2 miles of the refinery likely had the greatest ambient Mn exposure.19
With exception of three residents which were tested at the University of Cincinnati campus, all residents were tested over a two day period in Marietta, Ohio during October 2006. Thirty two residents completed balance testing with three residents eventually excluded from all statistical analysis. Two male residents were current or recent employees of the refinery. An additional female was excluded as her measures of postural balance under multiple test conditions and hair Mn revealed outlying values. While there was no known diagnosis of diabetic neuropathy in the resident, the potential for this condition to influence postural balance outcomes led to her exclusion from all statistical analysis.
Postural Balance Testing
Postural balance is a complex function controlled by the central and peripheral nervous systems. An upright posture requires an integration of sensory inputs to the brain with motor outputs directing body musculature for proper coordination. Visual, proprioceptive and vestibular pathways provide sensory information relevant for maintaining postural balance.
Postural balance data was collected using a hall-effect type force platform system, Model ACS-110, Advanced Mechanical Technology Inc. (AMTI), Watertown, MA. As the resident stood quietly on the platform, six signals were measured: Fx (force in the horizontal plane perpendicular to the posterior-anterior axis of the resident), Fy (force in the horizontal plane parallel to the posterior-anterior axis of the resident), Fz (force downward, perpendicular to the horizontal plane), Mx (moment about the Fx axis), My (moment around the Fy axis), and Mz (moment around the Fz axis).7, 20 The three orthogonal forces and three moments created by the residents while standing quietly were captured at a frequency of 50 Hz. The recorded forces and moments were amplified and processed by our custom software, (“Kinelysis” Copyright All Rights Reserved, University of Cincinnati) allowing calculation of x-y coordinates corresponding to the resident’s center of pressure (CP). The recorded data was used to calculate the sway length and sway area as outcome measures of postural balance.
The CP of each resident naturally travels as postural balance is maintained during each test condition. The measurements of postural balance which served as dependent variables were total sway length (SL) and total sway area (SA). The SL was the distance traveled in cm by the CP during each test condition. The SA included the area in cm2 enclosed inside the perimeter outlined by the CP movement pattern within the horizontal plane.
All residents underwent a 30 second trial for four different test conditions: (EO): eyes open on the platform, (EC): eyes closed on the platform, (FO): eyes open on a 4 inch thick foam pad placed on the platform and (FC): eyes closed on a 4 inch thick foam pad placed on the platform. These tests were then repeated in reverse order. The replicated measures for each test condition were averaged for a single mean value for SL and SA.
Daily set up for balance testing followed a well established and standardized protocol to maintain identical test conditions for all residents. A standard 18 kg weight was analyzed on the platform at five specific locations prior to daily testing of residents to confirm calibration of equipment. These measurements were required to locate the CP of the standard weight within < 2% error for postural balance testing to proceed.20
Each resident was instructed to stand in a standardized position on the center of the platform without socks or shoes. With heels placed together, feet were separated 30° around a triangular wood block placed between the feet. A tracing of the feet was then made onto a thin sheet of white art paper overlying the platform. This tracing was taped to the platform to serve as a guide for consistent foot placement.
Blood and hair sampling
Following postural balance testing, Mn levels were directly measured for each resident in whole blood and hair. Blood Mn has been identified as a suitable marker for group-based evaluations of inhaled manganese in the form of MnO2.21,22 This has not however been reliably supported at an individual level. Hair as a biological marker is relatively more convenient to collect than blood or urine. Hair analysis has been utilized as a reliable indicator of exposure with concentrations within different segments of hair strands reflecting the time history of exposure.23 The proximal aspect of hair is thought to provide a more accurate measure of body burden. Validation of hair Mn requires comprehensive research including detailed exposure history and nutritional status, particularly iron stores. There is preliminary evidence supporting an association between Mn concentrations in scalp hair and Mn exposure from water ingestion.24, 25 A previous study has found greater scalp hair Mn concentrations in workers exposed to ambient Mn as compared to unexposed workers.26 The relationship between scalp hair as a measure of ambient exposure or internal dose is not fully understood. And while blood Mn has been identified as a suitable marker for group-based evaluations of inhaled Mn, a recent validation study of hair Mn revealed no correlation between hair Mn and blood.27 A lack of correlation between blood and hair Mn could be attributed to unique biological transport and retention process in both media.
A certified phlebotomist collected whole blood samples utilizing single-use syringes for venipuncture and techniques to ensure minimal extraneous lead contamination. A clean alcohol pad was used to wipe the top of all blood tubes. Skin preparation utilized an alcohol pad working outward in a circular fashion from the estimated point of venipuncture. The area was then dried with clean gauze. These steps were repeated prior to specimen collection. A vacutainer system was used to collect approximately 7 ml of whole blood into two 4 ml purple top tubes containing ethylenediamine tetraacetic acid (EDTA). The first tube was used for DNA extraction, if the resident provided consent. The second tube was used for metal analysis due to possible manganese contamination from use of a steel needle. Blood tubes were immediately inverted 7 to 10 times in order to mix whole blood with EDTA and then refrigerated at 4° Celsius until laboratory analysis.
Prior to analysis, all hair samples were washed with nitric acid and the collection table was cleaned with a Sani-Cloth® germicidal disposable wipe. Blades of ceramic scissors were cleaned with an alcohol wipe. Hair collection was taken from the posterior vertex of the head. Gloves were worn throughout the specimen collection protocol. A minimum number of 20 hairs and length of 1 cm was attempted to be cut. Hair length measuring ≤ 12 cm was cut as close to the scalp as possible. For hair length measuring ≥ 13 cm, 12 cm of hair was measured from the scalp, then cut and discarded. The remaining sample was then cut as closely to the scalp as possible. Hair analysis was conducted on a hair sample ≤ 12 cm proximal to the scalp. Hair samples were taped onto a prelabeled index card identifying the scalp end of the sample and resident identification.
All sample analysis was performed by the Hematology and Environmental Laboratory at the University of Cincinnati Department of Environmental Health following previously validated methods.22, 28 For hair analysis, 10 mg from each sample were dissolved in 1 ml 65% nitric acid and kept at 90°C for 60 min. The lysate was then equilibrated for 24 hours at room temperature, diluted with 4 ml of double-distilled water and analyzed by atomic absorption spectroscopy (AAS).22 Blood lead levels were measured using an ESA model 2014 Anodic Stripping Voltammeter with strip chart recording.
Blood samples were collected from 28 of the 29 residents. Hair samples were collected from the 29 residents; however the sample amount was insufficient for accurate laboratory analysis for six residents. An outlying measurement of 366 µg/g and recent use of a hair dye product led to the exclusion of an additional resident from all analysis involving hair measurements.
Several studies have suggested urine Mn to be ineffective as a reflection of ambient Mn exposure.16, 22, 29 Considering the inconsistency of urine Mn in both occupational and environmental studies, analysis of urine Mn was not included in our study. Previous studies have shown elevated blood lead levels to be associated with postural balance abnormalities.10, 11 A recent occupational study using a benchmark approach estimated a new lower threshold for blood lead levels associated with postural sway changes in adults.30 The 95% confidence limits of their new lower threshold estimate were 12.1 to 17.3 µg/dl (mean 14.4). With a mean blood lead level of 1.8 µg/dl (0.6 to 6.2 µg/dl) in our study, blood lead was subsequently excluded from the present analysis. There was no evidence of abnormal lead exposure from surveys in our study or within the study providing control data.
Statistical Analysis
Previous studies have identified covariates beyond the study exclusion criteria possibly affecting either sway length or sway area.7, 9, 20, 31 Covariates included within the analysis were age, gender, height, weight, alcohol intake, caffeine intake, and tobacco usage. Alcohol intake was defined as 12 oz. beer equivalents within 48 hours of testing, caffeine intake as 8 oz. caffeinated drinks consumed prior to testing, and tobacco usage as average number of cigarettes smoked per day. The height and weight of each resident was measured on the day of balance testing and combined into a height/weight ratio (HT/WT) for all statistical analysis.
Statistical Analysis System (SAS) software was used for statistical analysis. Measurements of SL, SA, hair Mn, and blood Mn were natural log transformed (Ln) to approximate normal distributions of these variables in the statistical analysis. PROC MEANS was used to calculate descriptive statistics. PROC UNIVARIATE was utilized to check the normality of dependent and independent variables using the Shapiro-Wilk normality statistic. PROC CORR was used to check test-retest reliability with Spearman and Pearson correlation coefficients.
Hair Mn measurements were utilized with PROC REG to perform a multiple linear regression that evaluated the relationship between hair Mn and postural balance. Initial linear regression models for SA and SL, with b0 to b7 as the regression coefficients, were the following:
Ln of sway dependent variable (SL or SA) = b0 + b1 (Ln of hair Mn) + b2 (Age) + b3 (Gender) + b4 (HT/WT) + b5 (Caffeine) + b6 (Tobacco) + b7 (Alcohol).
The natural log of hair Mn and the covariates Age, Gender and HT/WT were forced to remain in the model regardless of p value. A backward elimination strategy systematically removed Caffeine, Tobacco and Alcohol as potential covariates unless an association with a p value ≤ 0.10 was demonstrated. As SA and SL were expected to increase with increased exposure, a one-tailed alpha of 0.05 was used for statistical inference. Demographics and significant covariates of residents included in the regression evaluating hair Mn and postural balance are provided within Table 1.
Table 1.
Demographics and significant covariate data for regression models evaluating hair Mn and postural balance (n=22)
| Variable | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Age (years) | 51.5 | 9.7 | 31.0 | 68.0 |
| Height (inches) | 64.9 | 3.6 | 58.8 | 70.5 |
| Weight (pounds) | 176.4 | 50.2 | 89.5 | 292.0 |
| HT/WT* | 0.40 | 0.11 | 0.21 | 0.66 |
| Tobacco (n=4)* | 17.5 | 5.0 | 10 | 20 |
| Alcohol (n=1)* | 3.0 | - | 3.0 | 3.0 |
| Blood Lead (µg/dl) | 1.8 | 1.2 | 0.6 | 6.2 |
| Ln Hair Mn* | 1.23 | 0.70 | 0.17 | 2.52 |
| Hair Mn (µg/g) | 4.4 | 3.3 | 1.2 | 12.4 |
| Gender | Female = 16; Male = 6 | |||
Significant in multivariate analysis
Ln denotes natural logarithm transformed
Tobacco – in number of average cigarettes/day
Alcohol – in number of drinks within 48 hrs prior to testing
Using control data collected from a previous study6, the SA and SL of residents were compared to those of a control group for four test conditions. The previous study evaluated the effect of cumulative low-level jet fuel exposure on aircraft maintenance personnel. Controls for that study were considered unexposed in regard to occupational and environmental neurotoxicants and consisted of 25 subjects from the military, the University of Cincinnati and other sources. Available social and occupational histories do not indicate any history of abnormal Mn exposure. To better match the younger age range of the control data, residents over the age of 59 from our study were excluded from this specific analysis (Table 2). This led to the exclusion of 6 residents ranging in age from 60 to 68 years. Survey data listing the number of alcoholic drinks consumed within 48 hours of testing was unavailable for 3 controls. Only residents and controls with survey data documenting no alcohol consumption within 48 hours of testing were included in the analysis. This led to the exclusion of 3 controls and 1 resident from this particular analysis.
Table 2.
Demographic and significant covariate data for ANCOVA
| Variable | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| Residents (n = 22) Female = 13; Male = 9 | ||||
| Age (years)* | 47.6 | 10.3 | 20.0 | 59.0 |
| Height (inches) | 65.7 | 4.2 | 58.8 | 73.5 |
| Weight (pounds) | 193.6 | 65.3 | 89.5 | 360.0 |
| HT/WT* | 0.37 | 0.12 | 0.20 | 0.66 |
| Controls (n = 22) Female = 10; Male = 12 | ||||
| Age (years)* | 35.0 | 8.1 | 24.0 | 57.0 |
| Height (inches) | 69.8 | 13.8 | 52.4 | 106.6 |
| Weight (pounds) | 168.9 | 8.8 | 152.0 | 185.4 |
| HT/WT* | 0.41 | 0.07 | 0.32 | 0.63 |
Significant in multivariate analysis
The comparison between residents and controls utilized PROC GLM to determine the least squares mean (LS mean) of SA and SL in an analysis of covariance (ANCOVA). Potential covariates of age, gender, height, weight, tobacco, and caffeine were also included in the ANCOVA. The initial regression models comparing residents and controls, with c0 to c5 as the regression coefficients, were the following:
Ln of sway dependent variable (SA or SL) = c0 + c1 (Age) + c2 (Gender) + c3 (HT/WT) + c4 (Tobacco) + c5 (Caffeine).
Age, Gender and HT/WT were forced into the model regardless of p value. A backward elimination strategy was again followed to systematically remove Tobacco and Caffeine as covariates unless a p value ≤ 0.10 was demonstrated. Alcohol was not included as a potential covariate within the regression as only residents and controls documenting no alcohol consumption were included. Demographic and significant covariate data for residents and controls included in the ANCOVA are provided within Table 2.
Results
For the 29 residents used in the statistical analysis, ages ranged from 19 to 68 years (mean 50). The radial distances from the residents’ home address to the refinery ranged from 0.3 to 9.7 miles (mean 5.8 miles). Blood and hair sampling demonstrated a mean blood Mn of 9.4 µg/L (4.2 to 21.7) and a mean hair Mn 4.4 µg/g (1.2 to 12.4). There was a significant association between hair Mn and blood Mn with a Pearson correlation coefficient of 0.59 (p<0.004). While not statistically significant, hair Mn was greater in the residents living within 6 radial miles of the refinery (n=11, mean 5.1 µg/g) than residents living farther away (n=11, mean 3.7 µg/g).
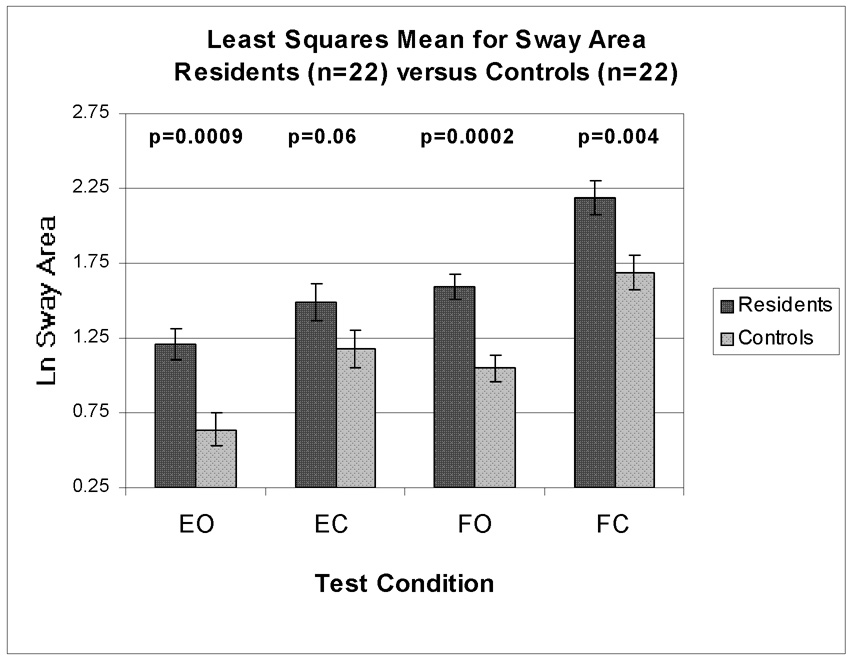
The test-retest Spearman correlation coefficients between the first and second postural balance trials were 0.93 for SL and 0.78 for SA. This is consistent with previous studies and supports acceptable reproducibility for sway variables.6, 7 Pearson correlation coefficients between measures of postural balance and hair Mn were all positive and reached statistical significance for SL under EO and EC test conditions (Table 3). Following covariate adjustment within the regression analysis, hair Mn reached statistical significance with SA and SL under EO and EC test conditions (Table 4). The covariate of HT/WT was significant with SA and SL under EC and FO test conditions respectively. Within these conditions, the negative relationship suggests that heavier and/or shorter residents had larger measurements of postural balance. Covariates representing alcohol and tobacco usage were inversely related with SA under the EO test condition. While this was not expected, this relationship may have been due to chance and the small number of residents within our study using these products (Table 1). Age, gender, and caffeine were not significant within the regression analysis. When blood lead was included within regression models, a significant association remained between hair Mn and SA under EO and EC test conditions (results not shown). In the ANCOVA, the LS mean for SA was significantly greater for residents in comparison to controls under EO (89%), FO (52%), and FC (30%) test conditions (Figure 1). The LS mean for SL was also significantly larger for residents in comparison to controls under FO (4%) and FC (5%) test conditions (Figure 2).
Table 3.
Bivariate Correlations between Dependent and Independent Variables
| Independent Variables |
||||
|---|---|---|---|---|
| Test Condition |
Dependent Variables |
Ln Hair Mn (n=22) |
Ln Blood Mn (n=28) |
|
| EO | SA | r | 0.24 | 0.16 |
| (p) | 0.28 | 0.42 | ||
| SL | r | 0.42 | 0.11 | |
| (p) | 0.05* | 0.57 | ||
| EC | SA | r | 0.29 | 0.11 |
| (p) | 0.18 | 0.58 | ||
| SL | r | 0.45 | 0.05 | |
| (p) | 0.03* | 0.79 | ||
| FO | SA | r | 0.32 | 0.11 |
| (p) | 0.15 | 0.59 | ||
| SL | r | 0.21 | −0.02 | |
| (p) | 0.35 | 0.93 | ||
| FC | SA | r | 0.24 | −0.06 |
| (p) | 0.28 | 0.76 | ||
| SL | r | 0.22 | −0.06 | |
| (p) | 0.33 | 0.76 | ||
Denotes statistical significance reaching p ≤ 0.05
Ln denotes natural logarithm transformed
Table 4.
Linear regression models relating postural balance measurements with Ln Hair Mn (n=22)
| Test | Dependent Variable | Independent Variable | Parameter Estimate | Standard Error | P Value (one tailed) | Model R2 |
|---|---|---|---|---|---|---|
| EO | SA | Intercept | 1.28 | 0.65 | 0.035 | 0.52 |
| Ln Hair Mn | 0.21 | 0.12 | 0.05 | |||
| Age | −0.0047 | 0.01 | 0.31 | |||
| Gender | 0.16 | 0.19 | 0.21 | |||
| HT/WT | −0.48 | 0.99 | 0.31 | |||
| Alcohol | −0.31 | 0.14 | 0.02 | |||
| Tobacco | −0.021 | 0.014 | 0.09 | |||
| SL | Intercept | 3.66 | 0.30 | 0.0001 | 0.31 | |
| Ln Hair Mn | 0.10 | 0.061 | 0.05 | |||
| Age | −0.002 | 0.005 | 0.27 | |||
| Gender | 0.12 | 0.09 | 0.10 | |||
| HT/WT | 0.49 | 0.40 | 0.12 | |||
| EC | SA | Intercept | 2.19 | 0.79 | 0.0129 | 0.34 |
| Ln Hair Mn | 0.30 | 0.16 | 0.04 | |||
| Age | −0.006 | 0.012 | 0.30 | |||
| Gender | 0.21 | 0.25 | 0.21 | |||
| HT/WT | −2.45 | 1.06 | 0.02 | |||
| SL | Intercept | 3.70 | 0.41 | 0.0001 | 0.32 | |
| Ln Hair Mn | 0.16 | 0.085 | 0.04 | |||
| Age | 0.0004 | 0.006 | 0.47 | |||
| Gender | 0.21 | 0.13 | 0.06 | |||
| HT/WT | 0.25 | 0.55 | 0.33 | |||
| FO | SA | Intercept | 1.44 | 0.50 | 0.01 | 0.17 |
| Ln Hair Mn | 0.13 | 0.10 | 0.12 | |||
| Age | −0.002 | 0.008 | 0.39 | |||
| Gender | 0.19 | 0.16 | 0.13 | |||
| HT/WT | 0.10 | 0.67 | 0.44 | |||
| SL | Intercept | 3.67 | 0.38 | 0.0001 | 0.23 | |
| Ln Hair Mn | 0.04 | 0.08 | 0.33 | |||
| Age | 0.0007 | 0.0060 | 0.456 | |||
| Gender | 0.14 | 0.12 | 0.14 | |||
| HT/WT | 0.95 | 0.51 | 0.04 | |||
| FC | SA | Intercept | 2.17 | 0.83 | 0.009 | 0.11 |
| Ln Hair Mn | 0.18 | 0.17 | 0.16 | |||
| Age | −0.010 | 0.01 | 0.22 | |||
| Gender | 0.02 | 0.26 | 0.47 | |||
| HT/WT | 0.55 | 1.10 | 0.31 | |||
| SL | Intercept | 4.14 | 0.60 | 0.0001 | 0.14 | |
| Ln Hair Mn | 0.08 | 0.12 | 0.25 | |||
| Age | −0.003 | 0.009 | 0.38 | |||
| Gender | 0.11 | 0.19 | 0.28 | |||
| HT/WT | 1.00 | 0.80 | 0.12 | |||
Ln denotes natural logarithm transformed
Fig 1.
All test conditions adjusted for Age, Gender, Height/Weight Y error bars represent standard error
Fig. 2.
All test conditions adjusted for Age, Gender, Height/Weight Y error bars represent standard error
Discussion
Manganese is found naturally within a wide range of foods including green leafy vegetables, nuts, soybeans and oats. And while the most significant and typical route of Mn exposure is dietary intake, healthy adults generally maintain proper Mn homeostasis without difficulty. Almost 98% of ingested Mn is rapidly cleared by the liver and excreted as bile.32 Ingestion as a source of excessive body burden is typically isolated to cases of hepatobiliary failure or chronic consumption of extremely high Mn levels within drinking water.24, 33 There were no known cases of liver disease in our resident population and a study sampling area residential cisterns did not suggest water to be a significant source of Mn exposure.34 Furthermore, practically every resident in this study limited water usage to the regulated city supply.
Unlike ingestion, inhalation of Mn particles bypasses hepatobiliary elimination allowing for a relatively greater dose to reach the CNS (central nervous system). Animal models support that penetration of Mn into the CNS is three orders greater with inhalation than by ingestion.35 The potential of direct olfactory transport is also unique to ambient exposure. Animal models have demonstrated direct transport of ambient Mn into the CNS by the olfactory system.36, 37 The true toxicological significance of olfactory transmission however remains unclear as evidence of neurological damage from olfactory transport has been inconsistent.38–40
Previous non-occupational studies have reported ambient Mn concentrations ranging from 0.003 to 5.86 µg/m3 (mean of 0.42) in Mexico 5 and 0.009 to 0.035 µg/m3 (mean of 0.022) in Southwest Quebec17. Blood Mn measurements for these studies ranged from 5.0 to 31.0 µg/l (mean of 10.16) and 2.5 to 15.9 µg/l (mean of 7.5) respectively. The estimated ambient exposure to our residents was relatively higher than the Southwest Quebec study and within the lower range seen in the Mexico study. The relationship between blood Mn measurements and ambient Mn estimates in our study is generally consistent with those found in other non-occupational studies.
Within our study, hair Mn had a significant relationship with increased response of postural balance outcome measures under the four test conditions. In contrast, blood Mn did not demonstrate a consistent association with postural balance. Our preliminary findings lend support for hair Mn being a more sensitive biologic measure of the influence of Mn on postural balance. As a measure of more recent exposure, blood Mn may be less reliable in the evaluation of changes resulting from prolonged Mn exposure. If postural balance changes resulted from chronic low level Mn exposure, the biological marker representing a longer exposure may reflect these changes more accurately. This can not however be inferred from our study without more specific estimates of resident exposure.
As postural balance testing progresses through test conditions, visual and proprioceptive inputs selectively challenge or negate afferent sensory pathways placing greater dependence on other pathways. During the EO condition, the body utilizes all three sensory afferents: visual, proprioceptive and vestibular pathways. When testing in the EC condition, the visual pathway is removed subsequently increasing dependence on proprioceptive and vestibular pathways. The FO condition incorporates standing on foam to challenge proprioceptive input and increasing dependence on visual and vestibular inputs. The FC condition reduces both visual and proprioceptive inputs, forcing maximum reliance on vestibular function.
While there were large demographic differences between residents and controls, statistical adjustments were made to account for potential confounders of age, gender, height and weight. Following covariate adjustment, the greatest difference in our study between residents and controls was seen in SA under the EO test condition. Resident SA and SL were also significantly greater under FO and FC test conditions. Based on Yasuda et al, these findings support a functional subclinical impairment associated with vestibular and proprioceptive sensory afferents among the residents in our study. While our findings are subclinical, they are consistent with the pathology of Mn neurotoxicity. The primary sites of excessive Mn deposition within the CNS are the globus pallidus and putamen within the basal ganglia. And an important function of the basal ganglia is the integration of sensory feedback from the visual, proprioceptive and vestibular systems.41
This study population provided the unique opportunity to evaluate the influence of low level chronic ambient Mn exposure on the postural balance of a residential population. While Mn exposure within this population was much lower than concentrations found in occupational studies, it is supported that exposure within this resident population is greater than the typical environment. To be included in our study, residents must have lived within a ten mile radius of the refinery over the three previous consecutive years. This focused our evaluation on residents with a reasonable amount of chronic exposure, but can not account for variability between residents. And chronic exposure does not necessarily represent cumulative exposure. The majority of residents had lived in close proximity to the refinery their entire lives, albeit at different locations. Furthermore, the daily migratory patterns of residents can vary widely, potentially increasing or decreasing their relative exposure frequency and duration. Without more specific estimates of ambient exposure over an extended period of time, a valid estimation of cumulative exposure would be difficult.
Any study with voluntary non-random participant selection has potential for selection bias. All interested residents meeting inclusion and exclusion criteria were given an opportunity to take part in our study within the allotted testing period. With residents identified through a community survey, it is likely residents interested in personal or community health would also be interested in participating in our study. It is noteworthy that the only information regarding balance testing given to residents was for the purpose of consent and the verbal directions necessary to complete the protocol. No specific information regarding how postural balance was being measured or different test conditions was discussed with residents.
A cross-sectional study by design can not accurately infer causation. The results of this study are preliminary and should be confirmed by a prospective study with a larger sample size. Our findings do however stimulate questions regarding the consequences of chronic ambient Mn exposure and unknown implications of subclinical abnormalities in postural balance. Plans are underway for future research within this community to include the potential influence of Mn exposure on children.
Acknowledgements
This study was completed in partial fulfillment of the requirements for a degree of Master of Science in the Department of Environmental Health at the University of Cincinnati for the primary author. We would like to gratefully acknowledge Washington State Community College for use of their campus, Dr. Richard Wittberg for assistance with GPS data collection, Stephanie Wessel for assistance with study coordination, and Sandy Roda for assistance with laboratory analysis. This research study was partially supported by the National Institute of Occupational Safety and Health and the University of Cincinnati Education and Research Center Grant #T42/OH008432-03. Additional funding was provided by the National Institute of Environmental Health Sciences: 1R21ES013524-02R21, 5T32ES10957, and P30-ES06096.
References
- 1.Political Economy Research Institute. [Accessed April 27, 2008];Ohio's 'Toxic Twelve' Identifies Top Air Polluters in State. Available at: http://peri2.enomalylabs.com/Ohio-Toxics.343.0.html. [Google Scholar]
- 2.Roels H, Lauwerys R, Buchet JP, et al. Epidemiological survey among workers exposed to manganese: Effects on lung, central nervous system, and some biological indices. Am J Ind Med. 1987;11:307–327. doi: 10.1002/ajim.4700110308. [DOI] [PubMed] [Google Scholar]
- 3.Roels HA, Ghyselen P, Buchet JP, Ceulemans E, Lauwerys RR. Assessment of the permissible exposure level to manganese in workers exposed to manganese dioxide dust. Br J Ind Med. 1992;49:25–34. doi: 10.1136/oem.49.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chia SE, Goh J, Lee G, et al. Use of a computerized postural sway measurement system for assessing workers exposed to manganese. Clin Exp Pharmacol Physiol. 1993;20:549–553. doi: 10.1111/j.1440-1681.1993.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Agudelo Y, Riojas-Rodriguez H, Rios C, et al. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci Total Environ. 2006;368:542–556. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Smith LB, Bhattacharya A, Lemasters G, et al. Effect of chronic low-level exposure to jet fuel on postural balance of US air force personnel. J Occup Environ Med. 1997;39:623–632. doi: 10.1097/00043764-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kuo W, Bhattacharya A, Succop P, Linz D. Postural stability assessment in sewer workers. J Occup Environ Med. 1996;38:27–34. doi: 10.1097/00043764-199601000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya A. Quantitative posturagraphy as an alternative noninvasive tool for alcohol/drug/chemical testing--preliminary thoughts. Drug Chem Toxicol. 1999;22:201–212. doi: 10.3109/01480549909029732. [DOI] [PubMed] [Google Scholar]
- 9.Sack D, Linz D, Shukla R, Rice C, Bhattacharya A, Suskind R. Health status of pesticide applicators: Postural stability assessments. J Occup Med. 1993;35:1196–1202. [PubMed] [Google Scholar]
- 10.Bhattacharya A, Shukla R, Bornschein RL, Dietrich KN, Keith R. Lead effects on postural balance of children. Environ Health Perspect. 1990;89:35–42. doi: 10.1289/ehp.908935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya A, Shukla R, Dietrich KN, Bornschein RL. Effect of early lead exposure on the maturation of children's postural balance: A longitudinal study. Neurotoxicol Teratol. 2006;28:376–385. doi: 10.1016/j.ntt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Chia SE, Gan SL, Chua LH, Foo SC, Jeyaratnam J. Postural stability among manganese exposed workers. Neurotoxicology. 1995;16:519–526. [PubMed] [Google Scholar]
- 13.Kaji H, Ohsaki Y, Rokujo C, Higashi T, Fujino A, Kamada T. Determination of blood and urine manganese (mn) concentrations and the application of static sensography as the indices of mn-exposure among mn-refinery workers. J UOEH. 1993;15:287–296. doi: 10.7888/juoeh.15.287. [DOI] [PubMed] [Google Scholar]
- 14.Kim EA, Cheong HK, Choi DS, et al. Effect of occupational manganese exposure on the central nervous system of welders: 1H magnetic resonance spectroscopy and MRI findings. Neurotoxicology. 2007;28:276–283. doi: 10.1016/j.neuro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Young T, Myers JE, Thompson ML. The nervous system effects of occupational exposure to manganese--measured as respirable dust--in a South African manganese smelter. Neurotoxicology. 2005;26:993–1000. doi: 10.1016/j.neuro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Bowler RM, Nakagawa S, Drezgic M, et al. Sequelae of fume exposure in confined space welding: A neurological and neuropsychological case series. Neurotoxicology. 2007;28:298–311. doi: 10.1016/j.neuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Hudnell HK. Effects from environmental mn exposures: A review of the evidence from non-occupational exposure studies. Neurotoxicology. 1999;20:379–397. [PubMed] [Google Scholar]
- 18.Agency for Toxic Substances and Disease Registry. Atlanta, GA: US Department of Health and Human Services; Toxicological profile for manganese. 2000 September;
- 19.Agency for Toxic Substances and Disease Registry. [Accessed September 10, 2007];Atlanta, GA: US Department of Health and Human Services; Health Consultation: Washington County Air Quality Marietta Ohio. 2007 June 18; Available at: http://www.atsdr.cdc.gov/HAC/pha/marietta3/ATSDRHealthConsultation2007.pdf.
- 20.Bhattacharya A, Morgan R, Shukla R, Ramakrishanan HK, Wang L. Non-invasive estimation of afferent inputs for postural stability under low levels of alcohol. Ann Biomed Eng. 1987;15:533–550. doi: 10.1007/BF02364247. [DOI] [PubMed] [Google Scholar]
- 21.Apostoli P, Lucchini R, Alessio L. Are current biomarkers suitable for the assessment of manganese exposure in individual workers? Am J Ind Med. 2000;37:283–290. doi: 10.1002/(sici)1097-0274(200003)37:3<283::aid-ajim6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Bader M, Dietz MC, Ihrig A, Triebig G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int Arch Occup Environ Health. 1999;72:521–527. doi: 10.1007/s004200050410. [DOI] [PubMed] [Google Scholar]
- 23.Foo SC, Khoo NY, Heng A, et al. Metals in hair as biological indices for exposure. Int Arch Occup Environ Health. 1993;65:S83–S86. doi: 10.1007/BF00381312. [DOI] [PubMed] [Google Scholar]
- 24.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: Pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boojar MM, Goodarzi F, Basedaghat MA. Long-term follow-up of workplace and well water manganese effects on iron status indexes in manganese miners. Arch Environ Health. 2002;57:519–528. doi: 10.1080/00039890209602083. [DOI] [PubMed] [Google Scholar]
- 26.Sukumar A, Subramanian R. Elements in the hair of workers at a workshop, foundry, and match factory. Biol Trace Elem Res. 2000;77:139–147. doi: 10.1385/BTER:77:2:139. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues JL, Batista BL, Nunes JA, Passos CJ, Barbosa F., Jr Evaluation of the use of human hair for biomonitoring the deficiency of essential and exposure to toxic elements. Sci Total Environ. 2008 doi: 10.1016/j.scitotenv.2008.06.002. Epublished ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Vitayavirasuk B, Junhom S, Tantisaeranee P. Exposure to lead, cadmium and chromium among spray painters in automobile body repair shops. J Occup Health. 2005;47:518–522. doi: 10.1539/joh.47.518. [DOI] [PubMed] [Google Scholar]
- 29.Smith D, Gwiazda R, Bowler R, et al. Biomarkers of mn exposure in humans. Am J Ind Med. 2007;50:801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- 30.Iwata T, Yano E, Karita K, Dakeishi M, Murata K. Critical dose of lead affecting postural balance in workers. Am J Ind Med. 2005;48:319–325. doi: 10.1002/ajim.20220. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya A, Shukla R, Bornschein R, Dietrich K, Kopke JE. Postural disequilibrium quantification in children with chronic lead exposure: A pilot study. Neurotoxicology. 1988;9:327–340. [PubMed] [Google Scholar]
- 32.Levy BS, Nassetta WJ. Neurologic effects of manganese in humans: A review. Int J Occup Environ Health. 2003;9:153–163. doi: 10.1179/oeh.2003.9.2.153. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal A, Vaidya S, Shah S, Singh J, Desai S, Bhatt M. Reversible parkinsonism and T1W pallidal hyperintensities in acute liver failure. Mov Disord. 2006;21:1986–1990. doi: 10.1002/mds.21096. [DOI] [PubMed] [Google Scholar]
- 34.Agency for Toxic Substances and Disease Registry. [Accessed September 14, 2006];Atlanta, GA: US Department of Health and Human Services; Health Consultation: Warren Township Cistern Sampling Investigation. 2005 June 18; Available at: http://www.atsdr.cdc.gov/hac/PHA/WarrenTwnshipCistern/WarrennTownshipCisternHC.pdf.
- 35.Andersen ME, Gearhart JM, Clewell HJ., 3rd Pharmacokinetic data needs to support risk assessments for inhaled and ingested manganese. Neurotoxicology. 1999;20:161–171. [PubMed] [Google Scholar]
- 36.Dorman DC, Brenneman KA, McElveen AM, Lynch SE, Roberts KC, Wong BA. Olfactory transport: A direct route of delivery of inhaled manganese phosphate to the rat brain. J Toxicol Environ Health A. 2002;65:1493–1511. doi: 10.1080/00984100290071630. [DOI] [PubMed] [Google Scholar]
- 37.Elder A, Gelein R, Silva V, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorman DC, Struve MF, Clewell HJ, 3rd, Andersen ME. Application of pharmacokinetic data to the risk assessment of inhaled manganese. Neurotoxicology. 2006;27:752–764. doi: 10.1016/j.neuro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Henriksson J, Tjalve H. Manganese taken up into the CNS via the olfactory pathway in rats affects astrocytes. Toxicol Sci. 2000;55:392–398. doi: 10.1093/toxsci/55.2.392. [DOI] [PubMed] [Google Scholar]
- 40.Dorman DC, McManus BE, Parkinson CU, Manuel CA, McElveen AM, Everitt JI. Nasal toxicity of manganese sulfate and manganese phosphate in young male rats following subchronic (13-week) inhalation exposure. Inhal Toxicol. 2004;16:481–488. doi: 10.1080/08958370490439687. [DOI] [PubMed] [Google Scholar]
- 41.Visser JE, Bloem BR. Role of the basal ganglia in balance control. Neural Plast. 2005;12:161–174. doi: 10.1155/NP.2005.161. discussion 263-72. [DOI] [PMC free article] [PubMed] [Google Scholar]