Abstract
Recent theory and empirical research has suggested that fear and anxiety are distinct processes with separable neurobiological substrates. Furthermore, a laboratory procedure has been developed to manipulate fear vs. anxiety independently via administration of predictable or unpredictable electric shock, respectively. Benzodiazepines appear to selectively reduce anxiety but not fear in this procedure. The primary aim of this experiment was to determine if alcohol produced a similar selective reduction in anxiety. Intoxicated (target blood alcohol concentration of 0.08%) and non-intoxicated participants viewed a series of colored squares separated by variable inter-trial intervals (ITI) in three conditions. In the predictable shock condition, shocks were administered contingently during every square. In the unpredictable shock condition, shocks were administered non-contingently during both squares and ITIs. In the no-shock condition, no shocks were administered at any time. Alcohol significantly reduced startle potentiation during cues signaling unpredictable but not predictable shock, consistent with the thesis that alcohol selectively reduces anxiety but not fear. In addition, alcohol’s effect on startle potentiation during unpredictable shock was mediated by vigilance. This anxiolytic effect may clarify the nature of alcohol’s reinforcing effects in social and problem drinkers.
Keywords: Alcohol, startle response, anxiety, fear, stress response dampening
The stress reducing properties of alcohol are well known and occasionally pursued by all drinkers. However, individuals who drink primarily for stress reduction (vs. other motives such as increasing positive emotions or social/celebratory motives) are at increased risk for developing alcohol use disorders (Cooper et al., 1995; Schroder & Perrine, 2007). Alcohol use disorders are also highly co-morbid with certain anxiety disorders (Grant et al., 2004; Kushner et al., 1990). Moreover, stress exposure is a powerful precipitant for relapse to alcohol use among alcohol dependent users (Brown et al., 1995). Thus, identifying the mechanism(s) underlying alcohol’s effect on stress is important in order to better understand both social and problematic alcohol use. Recent basic and applied research with both animals (Sullivan et al., 2004; Walker & Davis, 1997a; Davis, 1989) and humans (Grillon et al., 2006; Curtin et al., 2001; Hogle & Curtin, 2006; Piper & Curtin, 2006) has synthesized precise laboratory manipulations of stress with sensitive measurement procedures to parse the stress response into its constituent components. In particular, this research has suggested that fear and anxiety arise from dissociable neurobiological substrates that can be disentangled by careful manipulation of stimulus contingencies. Of particular importance, recent research with these methods has demonstrated selective effects of anxiolytic drugs (i.e., benzodiazepines) on anxiety but not fear (Grillon et al., 2006). In this paper, we report on the first experiment to use similar methods and measurement procedures to test if alcohol has comparable selective effects on anxiety in humans.
Alcohol Use and Stress
Few would debate the important role that stress plays in the patterns of alcohol use among both social and problem drinkers. “Stress response dampening” is one of the most common expectations that people, social and problem drinkers alike, report regarding the acute effects of alcohol use (Goldman et al., 1987). Even children who have yet to use alcohol themselves expect stress relief from alcohol by observing older drinkers (Christiansen et al., 1982). Not surprisingly, given these robust outcome expectancies associated with alcohol use, drinking to cope with stress is one of the primary motives that individuals report for their use of alcohol as well (Cooper et al., 1995).
Support for an important role of the alcohol use-stress relationship in problem drinking is also well established. For example, drinkers who report emotion regulation broadly as a primary motive for their alcohol use (i.e., either drinking to increase positive emotion or to decrease stress) display increased patterns of alcohol use. However, only drinking to decrease stress has unique connections to alcohol problems (Cooper et al., 1995; Schroder & Perrine, 2007). The high rates of co-morbidity between alcohol use disorders and a subset of anxiety disorders (e.g., Generalized Anxiety Disorder, Post-Traumatic Stress Disorder) also highlight the risk for clinically significant problems associated with stress-related drinking (Grant et al., 2004; Creamer et al., 2001; Kessler et al., 1995). In addition, stressors reliably reinstate alcohol and other drug use among abstinent alcohol or drug-dependent humans and animals (Brown et al., 1995; Lê et al., 1998; Shaham, 2000; Shiffman et al., 1989).
Research on alcohol-emotion interactions has been dominated by the stress response dampening model. This model proposes that alcohol affects emotional response to aversive events broadly, across situations and stimuli (Sher, 1987). Using a variety of eliciting stimuli and measurement techniques, the results of these studies have been inconsistent (Curtin & Lang, 2007; Greeley & Oei, 1999). Over the last 15 years, several more complex models that focus on attention and appraisal mechanisms have been developed (Sayette, 1993; Steele and Josephs, 1990). While these newer models appear promising, more precise tools for eliciting and measuring emotional response are needed to clarify the mechanisms involved.
Fear vs. Anxiety: Methods, Mechanisms, and Measures
The startle reflex (Davis, 1989) provides an attractive, non-invasive methodology for examining the effects of drugs on affective response in animals and humans. The startle response to an abrupt, intense stimulus (e.g., loud noise) increases above baseline when elicited in the presence of a cue that has been paired contingently with an aversive unconditioned stimulus (e.g., electric shock: Curtin et al., 2001; Grillon et al., 1991; Grillon & Davis, 1997). This effect is referred to as fear-potentiated startle and substantial research with animals has confirmed that projections from the central nucleus of the amygdala (CeA) to the primary startle circuit are responsible for this startle potentiation (see Davis, 1992, for a review).
More recently, research has identified other manipulations that also potentiate the startle reflex in animals and humans. For example, corticotropin-releasing factor (CRF; a stress hormone) has been observed to potentiate startle when administered to rats (Swerdlow et al., 1986; Liang et al., 1992). Walker and Davis (1997a) demonstrated that exposure to bright light increases startle above baseline in rats, a nocturnal species. Similarly, in humans, exposure to darkness (Grillon, et al., 1997b; Grillon et al., 2007b) and unpredictable electric shock (Grillon et al., 2004) also increase startle response magnitude.
There are important differences in the nature of the response produced by CRF, non-contingent (unpredictable) shocks, and light/darkness manipulations vs. cue-contingent (predictable) electric shock administration. Specifically, cue contingent administration of electric shock produces phasic fear-potentiated startle only during the punctate cues that predict imminent shock administration. In contrast, CRF, unpredictable shock, and light/darkness manipulations produce more sustained potentiation of the startle reflex. Moreover, Davis and colleagues have demonstrated elegant double dissociations in the neural substrates underlying startle potentiation across these two classes of manipulations in animals (Walker & Davis, 1997b, 2008; Walker et al., 2003). Specifically, lesions of the central nucleus of the amygdala (CeA) abolished fear-potentiated startle to predictable shocks but not potentiation of startle to CRF and bright light exposure. In contrast, lesions of the bed nucleus of the stria terminalis (BNST) abolished startle potentiation to CRF and bright light exposure but not fear-potentiated startle to cues predicting shock. Similar involvement of the BNST has been confirmed during unpredictable shock administration (Walker & Davis, 2008).
Given the nature of the eliciting stimuli and the time course of the response across these two categories of manipulations, researchers have offered these manipulations as laboratory models of fear vs. anxiety (Davis, 2006; Grillon, et al., 2006). Specifically, contingent cue-electric shock pairings involve simple, punctate stimuli that are highly predictive of imminent aversive stimulation (electric shock administration in the next few seconds). The phasic fear-potentiation of startle selectively during these cues in response to this manipulation is proposed to model the fear response. In contrast, non-contingent (unpredictable) shock, light/darkness, and CRF involve more complex, diffuse contextual cues that are more static, or of longer duration, and provide little information about when aversive stimulation will occur. The sustained potentiation of startle response in these manipulations is proposed to model anxiety.
Alcohol and other drug effects on fear vs. anxiety
Recent research has tested for differential effects of various benzodiazepines on fear vs. anxiety in humans using these methods. In a series of studies, Baas et al. (2002) found that diazepam blocked the potentiation of startle induced by darkness but did not affect startle potentiation to a specific threat cue. This work suggests that explicit-cue fear conditioning is insensitive to the effects of diazepam. Grillon and colleagues (2006) manipulated shock predictability to elicit fear vs. anxiety. Specifically, by pairing a distinct cue with shock and instructing participants that shocks could only occur during cue presentation, they were able to evoke fear to that cue. Alternatively, during periods in which shock administration was unpredictable, they generated a sense of anxiety in participants. The authors found that benzodiazepines selectively reduced startle potentiation during unpredictable shock blocks but not during blocks where shock was specifically paired with a distinct threat cue.
Melia et al. (1996) provide preliminary data to support the predicted dissociation between alcohol’s effect on fear vs. anxiety in rodents. Specifically, they examined alcohol’s effect in rats on acquisition of phasic fear response (measured by behavioral freezing) to tones contingently paired with electric shock vs. sustained fear response when placed in the training cage context without tone presentation. In this study, alcohol had minimal effect on freezing during tone presentation. In contrast, sizable dose dependent effects of alcohol were observed on freezing when placed in the cage (where shock had been administered) but without explicit tone presentation. These results dovetail nicely with the report of selective effects of benzodiazepines on anxiety in humans reported above.
Curtin and colleagues have used fear-potentiated startle in humans to examine alcohol’s effect on fear during simple, punctate cues that predict electric shock (Curtin et al., 1998, 2001). In both studies, alcohol did not reduce fear-potentiated startle when these simple cues were the focus of attention1. To our knowledge, no research has synthesized these lines of research in order to examine specific dissociations between the effects of alcohol on fear vs. anxiety in humans.
The current study
Based on this brief review, we hypothesized that a moderate dose of alcohol would selectively reduce anxiety but not fear in human participants. To test this thesis, we employed a variant of the methods developed by Grillon et al. (2006) to precisely manipulate fear vs. anxiety (via the administration of predictable vs. unpredictable electric shock, respectively) and measured eyeblink startle response potentiation to index affective response. Predictable shock was expected to produce brief, phasic potentiation of the startle response only during the cues that predicted electric shock administration, consistent with the elicitation of a punctate fear response to these cues that indicated high probability of imminent threat. In contrast, unpredictable shock was expected to produce a sustained potentiation of the startle response across both cues and inter-trial intervals, consistent with the elicitation of a more sustained anxiety response in these blocks where clear information about threat probability and imminence was not provided. We predicted that alcohol would selectively reduce startle potentiation during unpredictable but not predictable shock blocks.
Method
Participants
Sixty-four participants (32 females) were recruited from the community via online advertisements. Preliminary study eligibility was assessed during a phone screening session. Participants were required to be between 21–35 years of age and to report recent experience with the dose of alcohol to be administered in the study. Participants were excluded if they reported a history of alcohol-related problems or a medical condition for which alcohol use was contraindicated. Participants who met these criteria were scheduled for an experimental session and told to abstain from alcohol use for 24 hours, and all food and beverages other than water for four hours, prior to their experimental session. Participants were compensated $10/hour for their time. Descriptive information on participants' age, gender, drinking habits, and alcohol related problems is provided in Table 1.
Table 1.
Means (and Standard Deviations) for Individual Differences in Self-Reported Drinking Variables
| Measure | Placebo | Alcohol |
|---|---|---|
| Age (years) | 23.8 (2.6) | 23.0 (2.2) |
| Gender | ||
| Female | 50% (n=16) | 50% (n=16) |
| Male | 50% (n=16) | 50% (n=16) |
| Current Alcohol Use/Problems | ||
| Frequency (occasions/week) | 2.9 (3.0) | 2.6 (1.7) |
| Quantity (drinks/occasion) | 4.0 (2.9) | 3.8 (2.1) |
| Alcohol Problems | 4.9 (2.0) | 4.5 (2.0) |
NB: Non-demographic data for one male placebo participant are missing.
Alcohol problems are measured with Young Adult Alcohol Problems scale (Hurlbut & Sher, 1992).
General Procedure
Consent and screening
Upon arrival at the lab, participants provided proof of age and signed a consent form approved by the IRB. All participants also completed a medical screening questionnaire to verify their report from the phone screening. Female participants were administered an in-stream urine pregnancy test, with a negative result required for participation. A pre-experiment blood alcohol concentration (BAC) of 0.00% was verified via breathalyzer. Participants were first informed about the electric shock procedure during the consent procedure and were offered an opportunity to ask questions about it at this time. No participants withdrew from the experiment at this or any other point during the study.
Pre-task startle reactivity assessment
Prior to consuming study beverages, participants completed a procedure to assess their overall startle response magnitude. This pre-task startle reactivity was used as a covariate in the main analyses to reduce the impact of individual differences in startle response magnitude (e.g., Miller & Chapman, 2001). In this pre-task assessment, participants viewed a series of eight colored squares presented on a computer monitor. Each square was presented for 6s with a variable inter-trial interval (ITI; range 5s – 12s). Mean overall startle reactivity was based on six startle-eliciting noise probes presented during cues and ITI periods. (see Eyeblink Startle Response Magnitude section below).
Beverage manipulation
Equal numbers of male and female participants were randomly assigned to the alcohol and placebo beverage groups. All participants, regardless of beverage group assignment, were informed that they had been assigned to the alcohol group and would receive a moderately impairing dose of alcohol equivalent to 2–3 drinks in 1 hr for a 160-lb man. Participants assigned to the alcohol group received a beverage consisting of fruit juice mixed with 100 proof vodka (Smirnoff Blue Label) in a 3:1 juice to vodka ratio designed to produce a peak BAC of 0.08% approximately 30 minutes after completion of beverage consumption. Participants assigned to the placebo group received a volume-matched beverage consisting of fruit juice mixed with water poured from a vodka bottle in their presence (see Curtin & Fairchild, 2003 for a description of the dosing formula and placebo-related procedures). The total beverage was evenly divided into two drinks, each consumed in 15 minutes, for a total drinking period of 30 minutes. The experimental session began after a 15 minute post-drinking absorption period. Participants’ BACs were measured at two points during the experiment: just prior to the start of the main procedure, and immediately following the completion of the main procedure.
Shock sensitivity assessment
To control for individual differences in shock sensitivity, the intensity of shocks received during the main procedure was calibrated to each participant’s individual tolerance threshold. This assessment was performed after completion of the drinking period to prevent beverage group differences in shock tolerance that might occur due to alcohol’s possible analgesic effects.
Main procedure
The main procedure was a modified version of a task developed by Grillon et al. (2006) to manipulate fear vs. anxiety in the laboratory. Participants were instructed that they would complete seven blocks of trials. In each block, participants viewed a series of five colored square cues presented for 6 seconds each and separated by a variable ITI (range: 19–23s). These cues were presented in one of three Block Types: Unpredictable shock blocks (U), Predictable shock blocks (P), and No-shock blocks (N). Participants completed two blocks of unpredictable shocks, two blocks of predictable shocks, and three blocks of no shocks in one of two between subject block orders: UNPNPNU or PNUNUNP. A message was presented on the monitor to indicate the onset of each block type. In addition, text indicating the block type remained on the screen in the upper left corner throughout the block and the color of the square cues varied across the three block types. Block duration was 140s per block and the entire procedure required approximately 17 minutes to complete.
In Unpredictable shock blocks, participants were instructed that electric shocks could be administered at any point during the block, both during the cues and in the ITI. A total of five shocks were administered across the two unpredictable shock blocks (two during the cues and three during the ITI). In Predictable shock blocks, participants were instructed that electric shocks would be administered only during the cues and that no shocks would ever be administered during the ITI. Five electric shocks were administered in each predictable shock block (i.e., during every cue; ten total shocks) at 5.5s post cue onset. In No-shock blocks, participants were instructed that no shocks would be administered either during the cues or the ITIs. The No-shock block was included as a non-aversive control condition from which to calculate startle potentiation during cues in Predictable and Unpredictable shock blocks.
Individual difference measures, debriefing, and release
After completing the main procedure, all participants answered two questions to assess the success of our placebo. First, they estimated the content of their total beverage in terms of standard alcoholic drinks and reported their level of intoxication on a five point Likert scale (anchors were “not at all intoxicated” and “extremely intoxicated”). Following these placebo check questions, all participants provided information on their drinking history (current frequency and quantity of alcohol use) and problems (Young Adult Alcohol Problems Screening Test; Hurlbut & Sher, 1992). Following this, participants were debriefed and those in the placebo condition were paid and dismissed. Participants who had received alcohol remained at the study site until their BAC reached .02% or lower, at which point they were paid and dismissed.
Eyeblink startle response measurement
Electromyographic activity in the orbicularis oculi muscle was sampled at 2000Hz with a bandpass filter (30–500Hz) from electrodes placed under the right eye according to published guidelines (Van Boxtel et al., 1998). Eyeblink startle response was measured in response to startle-eliciting noise probes (50ms of 102dB white noise with near instantaneous rise time). Noise probes were presented at 5 seconds post onset of square cues and either 13 or 15s post cue offset during the ITIs. A minimum of 13s separated each startle probe from any preceding startle eliciting events (i.e., another startle probe; electric shock). A total of 42 noise probes were presented across Unpredictable (6 probes during cue; 6 during ITI), Predictable (6 during cue; 6 during ITI), and No-shock blocks (9 during cue; 9 during ITI). Offline processing of eyeblink startle magnitude included epoching (−50 to 250ms surrounding noise probe), smoothing (signal rectification followed by 30Hz lowpass filter), and baseline correction. Eyeblink startle magnitude was scored as the peak response between 20–120ms post-probe onset.
Results
Manipulation Checks
Blood alcohol concentration
The mean BAC of participants in the alcohol group was .071% (SD=.016) immediately prior to the start of the main procedure and .076% (SD=.010) immediately post-procedure. Mean peak BAC (i.e., highest BAC across the two measurements) was .079% (SD=.012).
Placebo manipulation2
To evaluate our placebo manipulation, we compared the alcohol and placebo groups on two placebo manipulation check questions that were completed at the conclusion of the experiment. Participants in the alcohol group reported that their beverage contained significantly more alcohol (M=3.5 drinks, SD=1.3, Range=0–5 drinks) than did placebo participants (M=1.9 drinks, SD=1.0, Range=2–6 drinks), t(62)= 5.56, p< .001. However, perceived alcohol content was significantly above 0 in both beverage groups (p’s < .001 for one sample t-test in each beverage group). Participants in the alcohol group also reported that they were significantly more intoxicated (M=3.0, SD=0.6, Range= 2–4), than did placebo participants, (M=1.7, SD=0.6, Range=1–3), t(62)= 8.83, p< .001. Nonetheless, perceived level of intoxication was significantly elevated above 0 in both beverage groups (p’s < .001 for one sample t-test in each beverage group).
Beverage Group effects on startle response: Unpredictable vs. predictable shock
To test for predicted selective effects of alcohol during unpredictable vs. predictable shock cues, we examined startle response in a General Linear Model (GLM) with Beverage Group (Alcohol vs. Placebo) as a categorical between subject factor and Block Type (Unpredictable shock vs. Predictable shock vs. No-shock) and Cue (Cue vs. ITI) as within subject factors. Pre-task startle reactivity (mean-centered) was included as a quantitative covariate. See Table 2 for startle response means (and standard deviations) across Beverage Group, Block Type, and Cue. Multivariate statistics are reported for all effects involving Block Type because the data did not meet the sphericity assumption necessary for univariate tests. Partial eta-squared (ηp 2) indices of effect size are reported for all significant effects. Raw score regression coefficient (B) effect size estimates from the GLM and regression analyses are also reported for significant 1df effects3.
Table 2.
Means (and Standard Deviations) for Startle Response by Beverage Group, Block Type, and Cue
| Block Type | Placebo Group | Alcohol Group |
|---|---|---|
| No Shock Blocks | ||
| Cue | 130.7 (49.1) | 50.5(47.4) |
| ITI | 160.7 (52.3) | 56.4(50.4) |
| Predictable Shock Blocks | ||
| Cue | 196.9 (51.9) | 112.1(70.8) |
| ITI | 168.0 (50.4) | 67.3(63.3) |
| Unpredictable Shock Blocks | ||
| Cue | 202.8 (57.0) | 96.9(55.5) |
| ITI | 201.1 (51.0) | 97.6(54.7) |
NOTE: Startle response means and standard deviations are covariate-adjusted based on pre-task startle response reactivity
A significant main effect of Beverage Group was observed, F(1, 61)= 62.57, p< .001, ηp 2= .51, B=−96.6, indicating that alcohol reduced overall startle response consistent with the well-replicated suppressive effect of alcohol on auditory processing and reflexive responding. A significant effect of the between subject pre-task startle reactivity covariate was also detected, F(1,61)=165.38, p< .001, ηp 2= .73, B=79.1, confirming the expected strong positive relationship between pre-task and main session startle response that justified its inclusion as a covariate in these analyses.
As expected, a significant Block Type × Cue interaction was observed, indicating that the cue effects differed significantly across block types, F(2,60)= 34.05, p< .001, ηp 2= .53. More importantly, the predicted Beverage Group × Block Type × Cue interaction was significant, F(2,60)= 4.60, p= .014, ηp 2= .13, indicating that the pattern of cue effects across blocks was moderated by Beverage Group. This 3-way interaction was decomposed into four separate simple interaction contrast analyses to clarify the response to predictable and unpredictable cues and the moderating effect of Beverage Group on response to these cues. Pre-task startle reactivity was retained as a covariate in all follow-up analyses.
Predictable shock
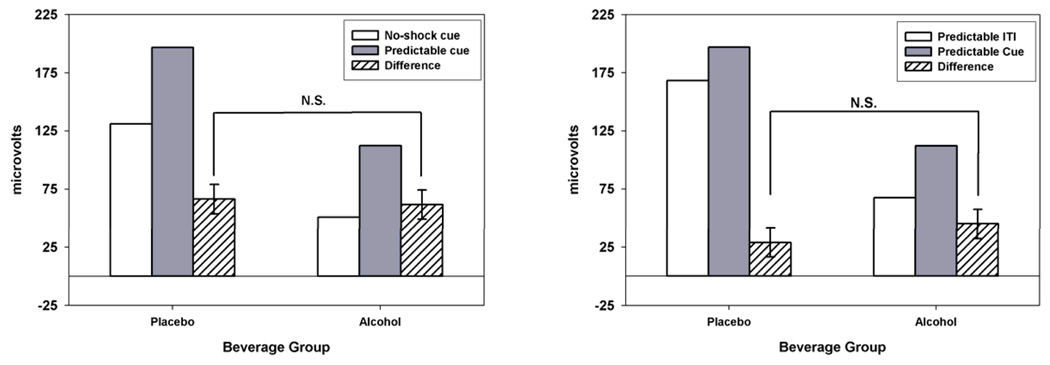
A Beverage Group × Predictable shock cue vs. No-shock cue simple effect analysis was conducted to test for alcohol’s effect on startle potentiation to predictable shock cues. Startle potentiation to predictable shock cues is best referenced to no-shock cues because the no-shock cues provide an important control for the attentional (visual) foreground across the contrast (i.e., both conditions involve visual cues). Figure 1 (left panel) displays startle response during predictable shock cues (gray bars) and no-shock cues (white bars). Predictable shock cue vs. No-shock cue difference scores (i.e., an index startle potentiation to predictable cues) are displayed with hatched bars. The Predictable shock cue vs. No-shock cue contrast was significant, F(1,61)= 102.46, p< .001, ηp 2= .63, B=63.9, confirming that startle response was potentiated during cues that were associated with predictable administration of electric shock. Beverage Group did not significantly moderate this contrast, F(1,61)= 0.13, p= .716, ηp 2= .00, B=−4.6. In other words, alcohol did not reduce startle potentiation during predictable shock cues (vs. no-shock cues).
Figure 1. Startle Response and Startle Potentiation during Predictable Shock Cues.
Left Panel: Raw startle response is displayed for Predictable shock (gray) and No-shock (white) cues by Beverage group. Startle potentiation difference scores (Predictable shock cue – No-shock cue) are displayed (hatched) with standard errors for the Beverage group effect. Predictable shock cues produced significant startle potentiation relative to No-shock cues (difference > 0; p< .001). The non-significant Beverage Group effect on startle potentiation indicates that alcohol does not reduce startle potentiation to predictable shock cues relative to no-shock cues. For all figures, the Beverage group effect on startle potentiation difference scores is statistically equivalent to the Beverage group × Cue type interaction for raw startle response.
Right Panel: Raw startle response is displayed for Predictable shock cues (gray) and Predictable shock ITIs (white) by Beverage group. Startle potentiation difference scores (Predictable cue – Predictable ITI) are displayed (hatched) with standard errors for the Beverage group effect. Predictable shock cues produced significant startle potentiation relative to the Predictable shock ITI period (difference > 0; p< .001). The non-significant Beverage group effect on startle potentiation indicates that alcohol does not reduce startle potentiation to predictable shock cues relative to the ITI period in the same predictable blocks.
A Beverage Group × Predicable shock cue vs. Predicable shock ITI analysis was also conducted. The Predictable shock cue vs. Predictable shock ITI contrast provides an alternative method to index startle response potentiation to predictable cues. See right panel of Figure 1 for startle response during predictable shock cue and ITI in predictable shock blocks. The Predictable shock cue vs. ITI contrast difference scores are also displayed. The Predictable shock cue vs. ITI contrast was significant, F(1,61)= 35.36, p< .001, ηp 2= .37, B=36.8. . This confirms that predictable shock administration produced a phasic potentiation of startle response only during the cue (but not ITI) period in predictable shock blocks. As above, Beverage Group did not moderate this contrast, F(1,61)= 1.64, p= .206, ηp 2= .03, B=15.9.
These two analyses confirm that:
Startle response is potentiated during cues that are predictably paired with electric shock relative to both no-shock cues and the ITI period in the predictable shock blocks.
This startle potentiation during predictable shock cues is phasic. In other words, it is observed only during the cue but not the ITI period, as expected.
There was no evidence that alcohol reduced startle potentiation to these predictable shock cues in either analysis. In fact, although not significant, the beverage group effect on startle potentiation was in the opposite direction in the second analysis (i.e., startle response potentiation was approximately 16µV greater in the alcohol group than the placebo group).
Unpredictable shock
As with predictable cues, startle potentiation to unpredictable shock cues is best referenced to no-shock cues to control for visual attention foreground. Therefore, a Beverage Group × Unpredictable shock cue vs. No-shock cue simple effect analysis was conducted to test for alcohol’s effect on startle potentiation to unpredictable shock cues. See left panel of Figure 2 for startle response during unpredictable shock cues and no-shock cues, and the startle potentiation contrast difference scores. The Unpredictable shock cue vs. No-shock cue contrast was significant, F(1,61)= 120.55, p< .001, ηp 2= .66, B=59.2, confirming that startle response was potentiated during cues that were associated with unpredictable administration of electric shock. However, in contrast to earlier analysis of predictable shock cues, Beverage Group did significantly moderate this contrast, F(1,61)= 5.65, p= .021, ηp 2= .09, B=−25.7. In other words, alcohol significantly reduced the potentiation of the startle response observed during unpredictable cues (relative to no-shock cues; see hatched bars on Figure 2 left panel).
Figure 2. Startle Response and Startle Potentiation during Unpredictable Shock Cues and ITI.
Left Panel: Raw startle response is displayed for Unpredictable shock (gray) and No-shock (white) cues by Beverage group. Startle potentiation difference scores (Unpredictable cue – No-shock cue) are displayed (hatched) with standard errors for the Beverage group effect. Unpredictable shock cues produced significant startle potentiation relative to No-shock cues (difference > 0; p< .001). The significant Beverage Group effect (p= .021) on startle potentiation indicates that alcohol selectively reduces startle potentiation to Unpredictable shock cues relative to No-shock cues.
Right Panel: Raw startle response is displayed for the Unpredictable ITI period (gray) and No-shock ITIs period (white) by Beverage group. Startle potentiation difference scores (Unpredictable ITI – No-shock ITI) are displayed (hatched) with standard errors for the Beverage group effect. Unpredictable ITIs produced significant startle potentiation relative to the No-shock ITI period (difference > 0; p< .001). The test non-significant Beverage Group effect on startle potentiation indicates that alcohol does not reduce startle potentiation during the unpredictable ITI period relative to the comparable ITI period in the No-shock blocks.
To determine if the significant alcohol effect on unpredictable shock cues was also observed during the ITI period of the unpredictable shock blocks, we conducted a Beverage Group × Unpredictable shock ITI vs. No-shock ITI analysis. As predicted, the Unpredictable shock ITI vs. No-shock ITI contrast was significant, F(1,61)= 65.84, p< .001, ηp 2= .52, B=40.8, indicating that the startle response potentiation that was observed during unpredictable shock cues was maintained into the ITI period of unpredictable shock blocks. However, Beverage Group did not significantly moderate this contrast, F(1,61)= 0.01, p= .934, ηp 2= .09, B= 0.8. These two analyses confirm that:
Similar to results for predictable shock cues, startle response is also potentiated during cues that are unpredictably paired with electric shock relative to cues during the no-shock blocks.
In contrast to predictable shock cues, startle response potentiation during unpredictable shock cues is sustained throughout the unpredictable shock block (i.e., observed during both cue and ITI periods)4.
Most importantly, startle potentiation during unpredictable cues was significantly reduced by alcohol. This is in contrast to the absence of any effect of alcohol on startle potentiation predictable shock cues. Furthermore, this alcohol effect is confined to the cue period in the unpredictable shock blocks. Alcohol did not reduce startle potentiation during the ITI period in unpredictable shock blocks.
Beverage Group effects on potentiated startle: Unpredictable vs. predictable shock cues
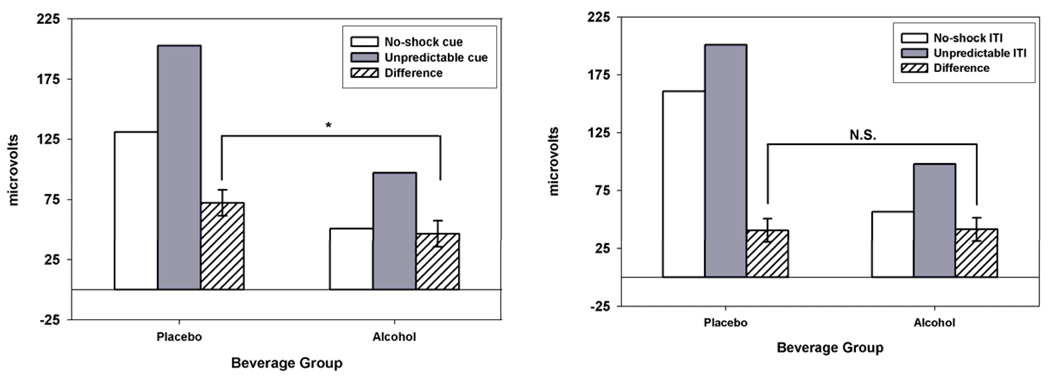
An analysis was conducted on potentiated startle scores for predictable and unpredictable shock cues. Specifically, a Beverage Group (Alcohol vs. Placebo) × Block Type (Unpredictable vs. Predictable) GLM was conducted on startle potentiation scores (i.e., startle response for predictable/unpredictable shock cue vs. no-shock cue). As with all earlier analyses, pre-task startle reactivity (mean-centered) was included as a covariate. Figure 3 displays startle potentiation scores by Beverage Group and Block Type. This analysis addresses two questions. First, it provides a comparison of the magnitude of startle potentiation produced across predictable vs. unpredictable shock cues via the test of the Block Type main effect. Selective deficits are most clearly established when tasks/stimuli are matched with respect to the magnitude of response they generate (Chapman & Chapman, 1973). Second, the analysis provides an explicit test of whether Beverage Group effects are significantly greater for unpredictable vs. predictable shock cues (i.e., a Beverage Group × Block Type interaction).
Figure 3. Startle Potentiation to Predictable and Unpredictable Shock Cues by Beverage Group.
Startle potentiation difference scores are displayed for Predictable (Predictable cue – No-shock cue) and Unpredictable (Unpredictable cue – No-shock cue) shock conditions. These startle potentiation differences scores were displayed separately in the left panels of Figure 1 & Figure 2. These data are presented here together to facilitate direct comparison of startle potentiation magnitude across the two manipulations. Error bars represent standard errors of the Beverage group effects. The significant Beverage Group × Block Type interaction (p= .032) indicates that alcohol has a larger effect on Unpredictable vs. Predictable shock cue startle potentiation. The simple effect of Beverage Group is significant for Unpredictable shock cues (p=.021) but not for Predictable shock cues (p= .716).
The main effect of Block Type was not significant, F(1,61)= 0.95, p= .333, ηp 2= .00, B= 4.7, indicating that unpredictable and predictable cues produced comparable potentiation of the startle response (59.2 vs. 63.9µV, respectively)5. The Beverage Group × Block Type interaction was significant, F(1,61)= 4.82, p= .032, ηp 2= .07, B= 21.1, indicating that the Beverage Group effect on unpredictable cue startle potentiation was significantly greater (reduction of 25.7µV; p=.021) than the Beverage Group effect on predictable cue startle potentiation (reduction of 4.6µV; p= .716).
Mediation of Beverage Group effect by attention
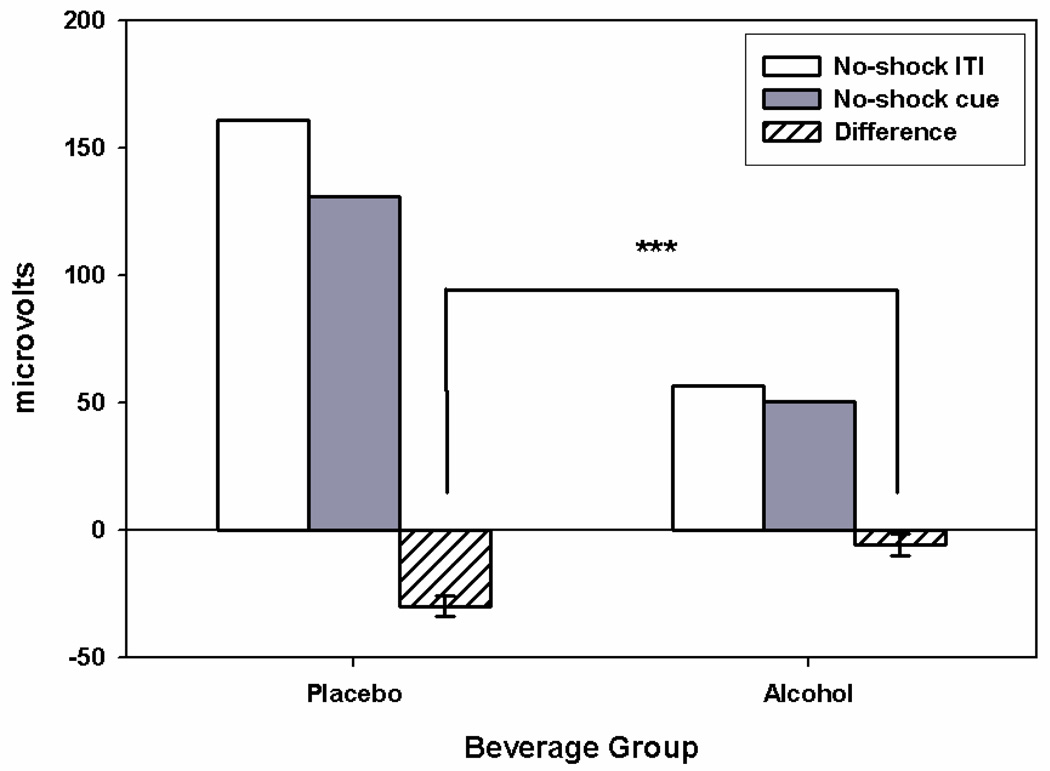
The startle response to acoustic probes is generally inhibited when participants direct attention toward other sensory modalities (e.g., attention to the visual cues reduces startle response to auditory probes; Anthony, 1985). As such, the startle inhibition that was observed during no-shock cues relative to the ITI period in no-shock blocks served as an index of participants’ attention to the visual cues during the experiment. To test for an alcohol effect on attention to the visual cues, we conducted a Beverage Group × Cue (Cue vs. ITI) GLM on startle response in the no-shock blocks (see Fig 4). Pre-task startle reactivity (mean-centered) was included as a quantitative covariate. As expected, a significant main effect of Cue was observed with startle response to acoustic probes significantly reduced during the no-shock cues relative to the ITI period, F(1, 61)= 37.74 p< .001, ηp 2= .38, B=−17.9. More importantly, the Beverage Group × Cue interaction was significant, F(1, 61)= 16.99, p< .001, ηp 2= .22, B= 24.1, indicating that the magnitude of the attentional inhibition of startle was reduced by alcohol. Intoxicated participants directed less attention toward the visual no-shock cues.
Figure 4. Startle Response and Startle Inhibition during No-shock Blocks.
Raw startle response is displayed for No-shock cues (gray) and the No-s hock ITI period (white) by Beverage group. Startle inhibition difference scores (No-shock cue – No-shock ITI) are displayed (hatched) with standard errors for the Beverage group effect. No-shock cues produced significant startle inhibition relative to the No-shock ITI period (difference scores < 0; p< .001). The significant Beverage Group effect (p< .001) on startle inhibition difference scores indicates that alcohol reduced startle inhibition associated with attention to the visual no-shock cues.
Subsequent analyses demonstrated that the magnitude of startle inhibition during visual cues in the no-shock blocks was significantly correlated with startle potentiation during unpredictable shock cues, partial r = −.42, p< .001. Participants who directed more attention toward the no-shock cues also displayed increased startle potentiation during unpredictable shock cues. To a lesser degree, the same relationship was observed between startle inhibition during no-shock cues and startle potentiation during predictable cues, partial r = −.27, p= .032.
These results raised the possibility that the significant Beverage Group effect on startle potentiation during unpredictable shock cues may be mediated by attention. To formally test this, we conducted the three regression analyses described by Baron & Kenny (1986) to establish mediation (see Table 3 for results of regression analyses). Consistent with all previous analyses, we included pre-task startle reactivity as a covariate in these mediation analyses and mean-centered all predictors. First, we regressed startle inhibition during no-shock cues (i.e., the putative attention mediator) on Beverage Group. Significant overall inhibition was observed during no-shock cues (i.e., intercept in this regression), B= −17.9, SE= 2.9, t(61)= 6.14, p< .001. As confirmed above, alcohol significantly reduced startle inhibition to no-shock cues, B= 24.1, SE= 5.9, t(61)= 4.12, p< .001. Second, we regressed startle potentiation during unpredictable shock cues (i.e., our dependent measure of anxiety) on Beverage group. Significant startle potentiation was observed during unpredictable shock cues (i.e., intercept in this regression), B= 59.2, SE= 5.4, t(61)= 10.99, p< .001. As confirmed earlier, alcohol significantly reduced startle potentiation to these unpredictable shock cues, B= −25.7, SE= 10.8, t(61)= 2.38, p< .021. The critical mediation test was provided in the third and final regression analysis, in which startle potentiation to unpredictable shock cues was regressed simultaneously on both Beverage Group and Attentional startle inhibition during no-shock cues. In this analysis, startle inhibition remained as a significant predictor of startle potentiation during unpredictable shock cues, B= −16.7, SE= 6.1, t(60)= 2.74, p= .008. However, the Beverage Group effect was reduced and not significant, B= −10.9, SE= 1.6, t(60)= 0.93, p= .354. These results confirm that the Beverage Group effect on startle potentiation to unpredictable shock cues was mediated by alcohol’s effect on attentional startle inhibition. A similar conclusion is supported by the Sobel test (Sobel, 1982), which directly tests the indirect (mediated) effect. The Sobel test confirms that the mediated pathway is significant, z= 2.28, p= .023.
Table 3.
Mediation of Alcohol Effect on Anxiety via Vigilance
| Mediation Regression Analysis Step 1: Dependent variable is No-shock cue startle inhibition (mediator) | ||||
|---|---|---|---|---|
| Predictor | B | SE | t | p |
| Intercept | −17.9 | 2.9 | 6.14 | .000 |
| Pre-task Baseline | −9.1 | 3.0 | 3.08 | .003 |
| Beverage Group | 24.1 | 5.9 | 4.12 | .000 |
| Mediation Regression Analysis Step 2: Dependent variable is unpredictable shock cue startle potentiation | ||||
| Predictor | B | SE | t | p |
| Intercept | 59.2 | 5.4 | 10.99 | .000 |
| Pre-task Baseline | 24.1 | 5.5 | 4.41 | .000 |
| Beverage Group | −25.7 | 10.8 | −2.39 | .021 |
| Mediation Regression Analysis Step 3: Dependent variable is unpredictable shock cue startle potentiation | ||||
| Predictor | B | SE | t | p |
| Intercept | 59.2 | 5.1 | 11.55 | .000 |
| Pre-task Baseline | 18.5 | 5.6 | 3.31 | .002 |
| Beverage Group | −10.9 | 11.6 | 0.93 | .354 |
| No-shock cue startle inhibition | −16.7 | 6.1 | 2.74 | .008 |
NOTE: Outcomes of critical statistical tests to support mediation are bold and underlined.
Discussion
In this experiment, we examined the effect of alcohol on affective response in two distinct conditions involving either predictable or unpredictable administration of electric shock. Previous research with both animals and humans has suggested that predictable vs. unpredictable threat exposure can serve as valid laboratory models for fear vs. anxiety response, respectively (Walker et al., 2003; Walker & Davis, 2008; Grillon, 2008). In this experiment, predictable shock cues indicated high probability of imminent threat and produced robust phasic potentiation of the startle response only during these cues. In contrast, both threat probability and imminence was lower during unpredictable shock cues. Despite this, these unpredictable shock cues also produced robust startle potentiation, which was sustained throughout the Unpredictable shock block. In fact, these two manipulations produced comparable magnitude of startle response potentiation during cue presentation (see Figure 3 and related analyses). The comparable strength of these manipulations provided an opportunity to examine predicted differential effects of alcohol on startle response potentiation during unpredictable vs. predictable shock cues with confidence that such effects would not be the spurious result of unmatched manipulations of anxiety vs. fear (Chapman & Chapman, 1973).
In this experiment, alcohol selectively reduced startle potentiation associated with anxiety response during unpredictable shock cues but not fear response during predictable shock cues. To our knowledge, this is the first experiment to demonstrate such a dissociation for alcohol in humans. In addition, we demonstrated that alcohol reduced vigilance, as measured by attention to the threat irrelevant no-shock cues in no-shock blocks. Moreover, this putative vigilance effect mediated alcohol’s anxiolytic effect during unpredictable shock cues (see also Sher et al., 2007). In the sections that follow, we elaborate on the theoretical and clinical implications of these results, as well as limitations and important future directions for research.
Previous research that specifically examined alcohol’s effect on fear response has failed to observe direct robust effects of alcohol on fear when threat cues were presented in the focus of attention (Curtin et al., 1998, 2001). The results associated with predictable shock cues in this experiment are consistent with this previous research. Alcohol did not reduce startle potentiation during simple visual cues that unambiguously predicted imminent administration of electric shock. In contrast, recent research has demonstrated selective effects of anxiolytic drugs on anxiety but not fear response (Baas et al., 2002; Grillon et al., 2006). In this experiment, we confirmed that a moderate dose of alcohol appears to have a similar selective effect on anxiety using comparable laboratory procedures (i.e., unpredictable shock administration).
The selective anxiolytic effect of alcohol observed in this experiment only during unpredictable shock cues may provide an explanation for the heterogeneous stress response dampening effects of alcohol observed in earlier research. As reviewed earlier, it is clear that drinkers believe that alcohol reduces stress, broadly defined, but consistent confirmation of this effect in the laboratory has been elusive (Curtin & Lang, 2007; Greeley & Oei, 1999). However, laboratory methods used to elicit stress response have been quite varied in this literature. The implicit assumption that alcohol should have comparable effects regardless of the affect eliciting stimuli and nature of the associated negative affective response may be untenable. Evidence is rapidly accruing from multiple sources that fear and anxiety are distinct processes with dissociable neural substrates (Walker et al., 2003; Walker & Davis, 2008; Grillon, 2008). Thus, it should not be surprising that alcohol has different effects on fear vs. anxiety as suggested by the results of this experiment. Unfortunately, researchers often aggregate alcohol challenge results across experiments with varied procedures to attempt to reach broad conclusions about stress response dampening effects. This may have slowed efforts to understand when and how alcohol affects negative emotions.
Consideration of this distinction between fear and anxiety may also clarify the mechanism proposed by important cognitive models of the alcohol-emotion nexus (Steele & Josephs, 1990; Sayette, 1993). Steele and Josephs (1990) proposed that alcohol’s effect on emotional response is mediated via impairment in attention when intoxicated, such that intoxicated individuals display reduced response to threats that are presented in the periphery of salient distracters (see Curtin et al., 1998, 2001 for empirical support using fear potentiated startle). Similarly, Sayette (1993) suggested that alcohol reduces negative emotional response in situations where stressors would not be adequately appraised due to the nature and timing of the threats. The observed dissociation between alcohol’s effect on fear vs. anxiety in the context of predictable and unpredictable threats may offer a novel perspective on the mechanism(s) proposed by these cognitive models. Salient distracters and other manipulations that degrade threat cue appraisal may make aversive events less predictable, producing an ongoing anxious state in these environments. These environments also place higher demands on vigilance processes because of the absence of clear predictive information about threat onset, imminence and/or probability. Alcohol appears to reduce anxiety in these environments. In contrast, when explicit threats are presented in the focus of attention, the onset and nature of the threat are more predictable, and phasic fear response is more tightly coupled to threat onset. Attentional demands are reduced in these environments because the threat is salient, well-defined, and easily appraised. Alcohol appears to be ineffective at reducing fear response in these environments.
Of course, further research clarifying the critical parameters that distinguish fear from anxiety is necessary. Davis (2006) has proposed that fear and anxiety result from “two phenomenologically and anatomically dissociable response systems” (p. 750). As reviewed earlier, the CeA appears to be critically involved in a rapid response system that mediates brief, phasic “fear” response to explicit, simple cues that predict imminent, highly probable threat. Fear is linked to action tendency and immediate defensive behavior. In contrast, Davis (2006) argues that the BNST mediates a slower onset but more sustained anxiety response that occurs in complex multimodal environments where threats are more ambiguous, abstract, or otherwise ill-defined and temporal precision regarding threat onset and/or probability is reduced. Activity in this anxiety response system may persist even after termination of a specific threat. Generally, anxiety is future oriented, involves more complex cognitive processing of symbolic representations of danger, and is not associated with well-coordinated, organized behavioral response. Moreover, vigilance is heightened, but attention is less focused on specific stimuli during anxiety than fear (Cornwell et al., 2008; Hasler et al., 2007).
It is clear that the neurobiological substrates of fear and anxiety, including both the CeA and the BNST, play an important role in attention as well as affective response (Davis & Whalen, 2001). However, the attentional changes that covary with fear vs. anxiety may be different in nature. Cornwell et al. (2008) demonstrated that fear-relevant, high probability, imminent threat of electric shock was associated with narrowly focused attention on stimuli in the threat-related sensory modality (in this case, tactile). Others have proposed that when potential threats exist but are not imminent, vigilance is increased and the individual will be broadly attentive to stimuli, across modalities, that may be at all threat relevant (Blanchard & Blanchard, 1989, Fanselow, 1994).
Startle inhibition during no-shock cues in this experiment appeared to index processes associated with this more diffuse vigilance state. Inhibition of acoustic startle response indicated participants’ tendency to attend to the visual cues that were not themselves threat-relevant nor in the same modality as the threatening electric shocks. However, these no-shock cues resembled stimuli that did predict threat at other times (i.e. predictable shock visual cues). Inhibition of startle during ambiguous no-shock cues was more strongly correlated with unpredictable shock cue startle potentiation. Alcohol reduced both this vigilance-relevant startle inhibition and anxiety-relevant unpredictable shock cue startle potentiation. In fact, alcohol’s effect on anxiety was mediated via its effects on vigilance. This pattern of results provides additional support for the expected coupling of cognitive and affective changes when the neurobiological substrates associated with anxiety-relevant unpredictable threat are activated. Moreover, the results provide preliminary evidence that these processes are particularly sensitive to alcohol administration.
The contrast of alcohol’s effect on startle potentiation during unpredictable shock cues vs. the unpredictable shock ITI period provides further support that attentional processes associated with vigilance and threat cue processing may be involved in the anxiolytic effects of alcohol. Alcohol administration significantly reduced startle potentiation during unpredictable shock cue presentation. However, alcohol was ineffective at reducing startle potentiation that was sustained into the ITI period after unpredictable shock cue termination. In combination with mediation analyses, this pattern of results suggests that intoxicated participants may have failed to attend and respond to unpredictable shock cues because the threat associated with these cues was not clear given the lower probability of shock administration during these cues.
Davis, Walker, and their colleagues (Walker & Davis, 2008; see also Grillon, 2008) have indicated that threat probability, threat imminence, and the time-course of the response (phasic vs. sustained) are all important dimensions along which fear and anxiety response, and their neurobiological substrates, can be dissociated. However, the initial tasks that have used predictable vs. unpredictable shock have confounded these three dimensions, making it difficult to determine which, if any, are key causes rather than correlates. For .example, we have recently demonstrated that selective manipulation of threat probability, while holding threat imminence constant, can affect the time course of startle potentiation (Curtin, 2008; unpublished data). In this experiment, shocks are always presented during cue presentation only (similar to predictable shock blocks). However, the percentage of cues that are paired with shock varied from 20% to 100%. High probability (100%) shock cues produced phasic startle potentiation only during cue presentation. However, cues that are infrequently paired with shock elicited startle potentiation that is sustained into the ITI period even though shocks are never administered during the ITIs. This suggests that threat probability may be the critical moderator that determines if threat exposure elicits phasic fear vs. more sustained anxiety response. Clearly, further careful experimental research is needed to clarify the role these various factors play in the elicitation and characterization of fear and anxiety.
Clinical implications
These results also may have important implications for understanding drug use motivational processes in addiction itself. Many classic and contemporary models of addiction identify adaptations in stress systems as a critical mechanism in the development of addiction across many classes of drugs (Solomon & Corbit, 1974; Koob & LeMoal, 2001; Baker et al., 2004). In short, repeated homeostatic adjustments in stress systems during periods of acute intoxication eventually lead to chronic compensatory adaptations in the structures involved in emotional response and its regulation. These adaptations persist beyond periods of acute use and result in dysregulated negative affect (e.g., increased anxiety) on cessation of drug use. Reliable report of increased negative affect during withdrawal from most common “addictive” drugs (e.g., alcohol, nicotine, opiates; see Baker et al., 2004 for review) supports this assertion. We propose that systematic laboratory acute drug challenge (e.g., alcohol administration) and drug withdrawal studies in humans using varied addictive drugs can provide an important method to identify these stress-related adaptations that support addictive drug use. Specifically, paradigms that reveal antagonistic/compensatory effects during drug challenge vs. drug withdrawal are ideal candidates to advance this effort. Similar acute anxiolytic effects have been demonstrated for benzodiazepines in this paradigm (Baas et al., 2002; Grillon et al., 2006). Conversely, withdrawal from nicotine produces a compensatory increase in anxiety in this paradigm (Hogle et al., in press; Grillon et al., 2007; see also Hogle & Curtin, 2006). Pronounced activation of the BNST has been observed during withdrawal from opiates (Aston-Jones et al., 1999; Delfs et al., 2000). We believe this body of research provides preliminary support that adaptations in anxiety and its neurobiological substrates may represent one cross-drug mechanism that contributes to the motivation to use drugs among addicts.
Limitations and Additional Future Directions
This experiment used a well-studied laboratory task for independently manipulating fear and anxiety. However, confidence in this selective alcohol effect will be increased by replication with other methods used to manipulate anxiety in the laboratory (e.g., darkness, Grillon et al., 2007b; CO2 challenge, Zvolensky et al., 1999). Moreover, future research must clarify the eliciting conditions and cognitive and behavioral consequences that distinguish anxiety from other negative emotions (e.g., fear, depression). As noted earlier, threat predictability appears to be important to distinguish between fear and anxiety. However, predictability itself may be a function of the complexity of the cueing stimuli, the probability of threat occurrence, and the duration of the threat, among other parameters (see also Zvolensky, Lejuez, & Eifert, 2000). These parameters are an active topic of study in basic affective science (e.g., Mol et al., 2007). More precise measurement of vigilance using event related brain potentials (e.g., Curtin et al., 2001) can extend the preliminary findings associated with startle inhibition to no-shock cues and help to explicate differences in the cognitive consequences of fear vs. anxiety manipulations.
This experiment only administered one dose of alcohol designed to produce a moderate blood alcohol level (0.08%). A next important step will be to investigate possible dose response effects on anxiety vs. fear. Other recent research has examined alcohol dose response effects using emotionally valent images to elicit affective response (Donohue et al., 2007). In this research, alcohol selectively reduced startle potentiation to unpleasant images without any concurrent effect on positive emotional response to pleasant images. However, this selective effect on negative emotional response was only observed at higher blood alcohol levels. Unfortunately, slide viewing tasks do not provide the necessary precision to parse fear vs. anxiety. Therefore, future research with the current methods can confirm if the alcohol dose-response effect observed by Donohue et al. is limited to anxious negative affect or occurs more broadly (i.e., includes fear and other negative affective response; see also Sher & Walitzer, 1986). Future research could also use both placebo and true no-alcohol comparison groups to more precisely tease apart pharmacological and expectancy (and compensatory) effects.
In this paper, we reviewed recent evidence that fear and anxiety can be distinguished both phenomenologically and anatomically. Basic research in affective science has validated laboratory procedures that selectively manipulate fear vs. anxiety, using potentiated startle to measure affective response. On this theoretical and methodological foundation, we demonstrated that a moderate acute dose of alcohol selectively reduced anxiety but not fear response. This selective effect may help to explain inconsistency in the literature on the stress response dampening effects of alcohol. Moreover, these results provide clear direction for future research on the cognitive and neural mechanisms of alcohol’s actions. Clinically, acknowledgement of a selective effect of alcohol on anxiety may provide insight into patterns of alcohol use disorder co-morbidity with anxiety disorders, and possibly, the nature of addiction itself. More broadly, this program of research highlights the potential to advance our understanding of the motivation to use drugs and the etiology of human addiction by attending to basic research in affective neuroscience.
Acknowledgements
This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism to J. Curtin (R01AA15384) and a National Institute of Mental Health Training Program in Emotion Research Fellowship to C. Moberg (5T32MH018931-18). The authors would like to thank Jason Jaber for the important role he played in data collection.
Footnotes
These studies did provide support for a reduction in fear potentiated startle in a divided attention condition that focused attention away from the threat cues. These observations are consistent with predictions from the Attention Allocation model. However, it may be that divided attention/distraction may alter the nature of the emotional response from fear to something more like anxiety. We will return to this idea in the discussion.
The decision to use a placebo, as opposed to a true no-alcohol, comparison group was a reasoned one. First, as noted by Greeley and Oei (1999) in their review of the preceding decade of alcohol and stress response research, placebo effects are rarely observed in this area. They further concluded that the majority of stress response dampening effects, when present, appear to have a clearly pharmacological basis. Moreover, correlational analyses in the current study failed to detect any significant relationship between our placebo manipulation check questions and either overall startle response or startle potentiation associated with either predictable or unpredictable electric shock.
Even though placebo effects are very unlikely for the reasons provided above, we believe that attempting to control for alcohol consumption expectancy as best possible (via use of a placebo comparison group) represents the most rigorous method to establish a pharmacological effect of alcohol. It is clear that participants in the alcohol group believed that they had consumed alcohol. Therefore, to unambiguously conclude that differences between beverage groups result from pharmacological effects of alcohol, it is important that the comparison group also believe that they consumed alcohol. Thus, possible subtle effects resulting from this belief (i.e., a reduction in stress based on the belief that alcohol should reduce stress or conversely any compensatory response associated with attempt to combat the expected effects of alcohol) are held constant across our beverage groups, to the best of our ability. As indicated by manipulation check analyses, we were successful in establishing an expectation of alcohol consumption and related intoxication among participants in both the alcohol and placebo groups. However, as is typical with these manipulations, we were not entirely successful in matching level of expectancy across the beverage groups.
All effects were mean-centered such that the intercept, when reported, indicates overall unweighted dependent variable mean. Dichotomous contrasts were unit-weighted such that B can be interpreted as the mean difference between the two levels of the contrast (i.e., level 1 mean – level 2 mean). Quantitative effects were standardized such that Bs can be interpreted as the change in the dependent variable associated with a 1 standard deviation increase on the quantitative effect.
In addition to being significantly potentiated relative to no-shock ITI, focused contrasts confirm that startle response during unpredictable ITI is significantly higher than during predictable ITI, F(1,61)= 42.77, p< .001. ηp 2= .41, B= 31.7. Moreover, startle response is comparable during unpredictable ITI and unpredictable cues, F(1,61)= 0.02, p= .904, ηp 2= .00, B=−0.5
A more conservative test of the Block Type effect conducted in the control/placebo group also failed to detect any significant difference between startle potentiation produced by predictable vs. unpredictable cues, F(1,30)= 1.22, p= .279, B= 6.4, ηp 2= .04.
References
- Anthony BJ. In the blink of an eye: Implications of the reflex modification for information processing. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in Psychophysiology. Vol. 1. Greenwich, CT: JAI Press; 1985. pp. 167–218. [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: A target site for noradrenergic actions in opiate withdrawal. Annals of the New York Academy of Sciences. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Baas JM, Grillon C, Bocker KB, Brack AA, Morgan CA, III, Kenemans JL, Verbaten MN. Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology. 2002;161:233–247. doi: 10.1007/s00213-002-1011-8. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Progress in Neuropsychopharmacology and Biological Psychiatry. 1989;13:S3–S14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability, and adult alcohol relapse. Journal of Studies on Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Chapman L, Chapman J. Problems in the measurement of cognitve deficit. Psychological Bulletin. 1973;79:380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Christiansen BA, Goldman MS, Inn A. Development of alcohol-related expectancies in adolescents: separating pharmacological from social-learning influences. Journal of Consulting and Clinical Psychology. 1982;50(3):336–344. doi: 10.1037//0022-006x.50.3.336. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. Journal of Personality and Social Psychology. 1995;69(5):990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Echiverri AM, Covington MF, Grillon C. Modality-specific attention under imminent but not remote threat of shock. Psychological Science. 2008;19(6):615–622. doi: 10.1111/j.1467-9280.2008.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer M, Burgess P, McFarlane AC. Post-traumatic stress disorder: findings from the Australian National Survey of Mental Health and Well-being. Psychological Medicine. 2001;31:1237–1247. doi: 10.1017/s0033291701004287. [DOI] [PubMed] [Google Scholar]
- Curtin JJ. Alcohol reduces startle potentiation to low but not high probable threat. 2008 Manuscript in preparation. [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: Implications for regulation of behavior during response conflict. Journal of Abnormal Psychology. 2003;112(3):424–436. doi: 10.1037/0021-843x.112.3.424. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Lang AR. Alcohol and emotion: Insights and directives from affective science. In: Rottenberg J, Johnson S, editors. Emotion and Psychopathology: Bridging Affective and Clinical Science. Vol. 8. Washington, DC: American Psychological Association; 2007. pp. 191–213. [Google Scholar]
- Curtin JJ, Lang AR, Patrick CJ, Stritzke WGK. Alcohol and fear-potentiated startle: The role of competing cognitive demands in the stress-reducing effects of intoxication. Journal of Abnormal Psychology. 1998;107(4):547–565. doi: 10.1037//0021-843x.107.4.547. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Lang AR, Patrick CJ, Cacioppo JT, Birbaumer N. Alcohol affects emotion through cognition. Psychological Science. 2001;12(6):527–531. doi: 10.1111/1467-9280.00397. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear-potentiated startle. In: Davis M, Jacobs BL, Schoenfeld RI, editors. Annals of the New York Academy of Sciences. Vol. 563. 1989. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61(8):741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Donohue KF, Curtin JJ, Patrick CJ, Lang AR. Intoxication Level and Emotional Response. Emotion. 2007;7:103–112. doi: 10.1037/1528-3542.7.1.103. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Goldman MS, Brown SA, Christiansen BA. Expectancy theory: Thinking about drinking. In: Blane HT, Leonard KE, editors. Psychological theories of drinking and alcoholism. New York: Guilford Press; 1987. pp. 181–226. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou P, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Greeley J, Oei T. Alcohol and tension reduction. In: Leonard KE, Blane HT, editors. Psychological theories of drinking and alcoholism. 2nd ed. New York: Guilford Press; 1999. pp. 14–53. [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology. 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Avenevoli S, Daurignac E, Merikangas KR. Fear-potentiated startle to threat, and prepulse inhibition among young adult nonsmokers, abstinent smokers, and nonabstinent smokers. Biological Psychiatry. 2007a;62:1155–1161. doi: 10.1016/j.biopsych.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Duncko R, Covington MF, Kopperman L, Kling MA. Acute stress potentiates anxiety in humans. Biological Psychiatry. 2007b;62:1183–1186. doi: 10.1016/j.biopsych.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118(5):916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pellowski M, Merikangas KR, Davis M. Darkness facilitates the acoustic startle in humans. Biological Psychiatry. 1997b;42:453–460. doi: 10.1016/S0006-3223(96)00466-0. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. Journal of Neuroscience. 2007;27(23):6313–6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ, Kaye JT. Negative affect during nicotine withdrawal: insights from explicit-cue and contextual fear conditioning models. Nicotine and Tobacco Research. 10 (in press). [Google Scholar]
- Hurlbut SC, Sher KJ. Assessing alcohol problems in college students. Journal of American College Health. 1992;41(2):49–58. doi: 10.1080/07448481.1992.10392818. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Beitman BD. The relation between alcohol problems and the anxiety disorders. American Journal of Psychiatry. 1990;147(6):685–695. doi: 10.1176/ajp.147.6.685. [DOI] [PubMed] [Google Scholar]
- Lê AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. Journal of Neuroscience. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M. Behavioral Genetic Models of Alcoholism and Drinking. In: Leonard KE, Blane HT, editors. Psychological theories of drinking and alcoholism. 2nd ed. New York: Guilford Press; pp. 372–421. [Google Scholar]
- Melia KR, Ryabinin AE, Corodimas KP, Wilson M, LeDoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–322. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mol N, Baas JMP, Grillon C, van Ooijen L, Kenemans JL. Startle potentiation in rapidly alternating conditions of high and low predictability of threat. Biological Psychiatry. 2007;76:43–51. doi: 10.1016/j.biopsycho.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Piper ME, Curtin JJ. Tobacco withdrawal and negative affect: An analysis of initial emotional response intensity and voluntary emotion regulation. Journal of Abnormal Psychology. 2006;115(1):96–102. doi: 10.1037/0021-843X.115.1.96. [DOI] [PubMed] [Google Scholar]
- Sayette MA. An appraisal-disruption model of alcohol's effects on stress responses in social drinkers. Psychological Bulletin. 1993;114:459–476. doi: 10.1037/0033-2909.114.3.459. [DOI] [PubMed] [Google Scholar]
- Schroder KE, Perrine MW. Covariations of emotional states and alcohol consumption: evidence from 2 years of daily data collection. Social Science & Medicine. 2007;65(12):2588–2602. doi: 10.1016/j.socscimed.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Research Reviews. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Peuser K, Erickson DJ, Wood MD. Stress-Response-Dampening Effects of Alcohol: Attention as a Mediator and Moderator. Journal of Abnormal Psychology. 2007;116:362–377. doi: 10.1037/0021-843X.116.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ. Stress response dampening. In: Blane HT, Leonard KE, editors. Psychological Theories of Drinking and Alcoholism. New York: Guilford Press; 1987. pp. 227–271. [Google Scholar]
- Sher KJ, Walitzer KS. Individual differences in the stress-response dampening effect of alcohol: A dose-response study. Journal of Abnormal Psychology. 1986;95:159–167. doi: 10.1037//0021-843x.95.2.159. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Smoking relapse crises: The Stay Quit Line project. In: Loberg T, Miller WR, Nathan PE, Marlatt GA, editors. Addictive behaviors: Prevention and early intervention. Netherlands: Swets & Zeitlinger Publishers; 1989. pp. 253–273. [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equations models. In: Leinhart S, editor. Sociological methodology. Vol. 1982. San Francisco: Jossey-Bass; 1982. pp. 290–312. [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: temporal dynamics of affect. Psychological Review. 1974;81(2):119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Steele C, Josephs R. Alcohol myopia: Its prized and dangerous effects. American Psychologist. 1990;45:921–933. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, LeDoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing response elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: Blockade by chlordiazepoxide. Psychopharmacology. 1986;88(2):147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Van Boxtel A, Boelhouwer AJW, Bos AR. Optimal EMG signal bandwidth and interelectrode distance for the recording of acoustic, electrocutaneous, and photic blink reflexes. Psychophysiology. 1998;35:690–697. [PubMed] [Google Scholar]
- Walker DL, Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biological Psychiatry. 1997a;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. Journal of Neuroscience. 1997b;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Structure & Function. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Eifert GH, Lejuez CW, McNeil DW. The effects of offset control over 20% carbon-dioxide-enriched air on anxious responding. Journal of Abnormal Psychology. 1999;108(4):624–632. doi: 10.1037//0021-843x.108.4.624. [DOI] [PubMed] [Google Scholar]
- Zvolensky M, Lejuez C, Eifert G. Prediction and control: operational definitions for the experimental analysis of anxiety. Behaviour Research and Therapy. 2000;38:653–663. doi: 10.1016/s0005-7967(99)00090-x. [DOI] [PubMed] [Google Scholar]