Abstract
Background
The timing of puberty has well known impact on anthropometric and psychosocial outcomes. Multiple methods have been used to determine pubertal timing, but all with limitations. A uniformly applicable method is needed for different study designs and study populations.
Objectives
The objectives of the study are 1) to propose a new method using statistics modeling to determine relative timing of pubertal maturation; 2) to validate the new method by evaluating its relationship with pubertal growth and timing parameters, including age at menarche, age onset of areolar maturation, age of peak height velocity, age at attainment of adult height, adult height, peak height velocity, BMI and percent body fat; and 3) to contrast the new method with relative timing of menarche on these pubertal parameters.
Methods
Using the National Heart Lung and Blood Institute Growth and Health Study (NGHS) data, an ordinal logistic modeling was used to assess relative timing of pubertal maturation.
Results
The proposed method demonstrated good reliability and strong associations with all pubertal timing parameters, also BMI and percent body fat. Timing was not significantly associated with adult height and peak height velocity.
Conclusion
The proposed method is highly feasible, easy to implement, and valid. The study demonstrated important differences between the relationships of relative timing of secondary sexual characteristics and the timing of menarche on pubertal parameters. The study also demonstrates that individuals with early or late timing at one point of time are likely to maintain the same relative timing throughout puberty.
Keywords: Timing of Puberty, Ordinal Logistic Regression, Statistical Modeling, Validation, Generalize Estimating Equation (GEE)
Introduction
The pubertal maturation process represents a critical biological and psychological period of the life span. The timing of pubertal maturation has potentially profound implications for multiple health outcomes, including anthropometric and psychosocial parameters [1–4]. Accelerated physical growth, appearance of secondary sexual characteristics and memorable events such as initiation of menarche or spermarche mark the critical events throughout pubertal maturation. Thus, age at peak height velocity, age at onset of secondary sexual characteristics, and age at menarche or spermarche are often used for determining timing of pubertal maturation. In order to reliably capture age at peak height velocity or age at onset of secondary sexual characteristics, longitudinal follow-up at least yearly, and preferably every six months is required. Age at menarche or spermarche occurred relatively later, and may not be accurately reported due to recall bias [5, 6]. Because a longitudinal study design is often not feasible due to the costs and logistical difficulties, and that age at menarche or spermarche is available only for adolescents who had been far enough along in their pubertal development to experience initiation of menarche or spermarche, alternative approaches to the determination of timing of puberty are needed.
Perceived relative timing refers to the relative advance or delay of pubertal development for an adolescent with respect to their gender and age-matched peers. It has been reported typically through responses by parents or adolescents to questions in standard formats, such as those in the Pubertal Development Scale (PDS) questionnaire [7]. PDS is a subjective measure, being influenced by psychosocial characteristics of the individual such as their social norm, self-esteem, and body image. A more objective approach has been utilizing statistical modeling for determining the relative timing of puberty [8, 9].
Marshal and Tanner introduced a system of staging pubertal maturation on a 1–5 scale, ranged from “prepubertal” to “mature stage”, based on the appearance of secondary sexual characteristics. Currently, Tanner maturation stage has been widely utilized in research and clinical studies. Tanner maturation stage assessed by a trained clinician is considered “gold” standard [10], and a reliable measure [11, 12]. Recently, new approaches have been proposed, including Garn and Falkner areola staging to better determine breast development independently from adiposity [13], and testicular volume to better determine genital development [14].
By regressing biological age to pubertal maturational stage within a homogeneous population (same gender and race/ethnicity), statistical modeling provides estimation of the expected mean pubertal maturational stage as a function of age. The regression residuals correspond to the deviation of an adolescent’s maturation stage from the expected population mean maturational stage, thus providing relative advance or delay of the adolescent compared to the gender-age-matched peers. Determination of relative timing of pubertal maturation via statistical modeling has many advantages: it is easy to obtain, does not require longitudinal study designs, and is applicable to both gender across all stages of puberty. Currently, linear regression modeling has been used [8, 9], but with limitations. First, the linear regression analysis assumes a linear relationship between the chronological age and maturation stage; while biologically, a non-linear S-shape relationship is expected with the maturation stage plateaus at both younger and older ends of age. Second, it assumes a normal distribution, which may not be reasonable for the ordinal scaled maturation stage. Further, no study has been done to evaluate the validity of the statistical modeling approach for determination of relative timing of puberty.
Ordinal logistic regression is a less commonly used statistical modeling technique than linear regression. It is a specific modeling technique for an ordinal type of outcome, such as pubertal maturation stage. Just like the commonly used binary logistic regression, ordinal logistic regression models the log-odds of cumulative probabilities of the ordinal outcome as a linear regression function of the predictive variables. Mathematically, if a continuous outcome is classified into multiple ordered categories, ordinal logistic regression modeling could obtain unbiased beta estimates as if fitting a linear regression model to a continuous outcome. The ordinal logistic regression maintains ordinal nature of the outcome, provides estimation of the expected probabilities for each of the ordered categories, and further calculates the mean score of expected outcome, for a given set of predictive variables.
The literature provides ample evidence that timing of puberty is related to body fat [15]. A recent genetic study suggested that the same gene that regulates pubertal growth may also explain body fat attainment [16]. However, it is less clear whether timing of puberty is related to adult height and peak height velocity. While some studies noted the relationship between timing of puberty and adult status [17–19], others reported no association [20, 21]. Such inconsistency may be due to the different measures used for pubertal timing.
Increasingly, the literature suggest that onset of menarche and onset of puberty may represent distinct biological phenomena [2, 22]. Onset of puberty may be driven by a heritable trait such as genetic influence, while the onset of menarche is influenced by multiple factors including genes, nutrition factors and environmental exposure [23]. As suggested by the February 2008 special issue of Pediatrics (Vol.121, Suppl. 3), re-analyzing the same existing study data using different indexes of pubertal time should help shed light on various issues concerning pubertal development.
We propose that ordinal logistic modeling could be used to provide a valid approach to determination of the relative timing of puberty. To illustrate this new approach, we analyzed the National Heart Lung and Blood Institute Growth and Health Study (NGHS), a large multi-site longitudinal study that followed a group of 9 and 10 year old black and white girls for 10 years annually. We choose to analyze the NGHS study because it provides rich growth and pubertal timing related data, including age at menarche, BMI, and percent body fat, and allows for evaluation of age at peak height velocity, age at attainment of full adult height and final adult height. This is also a re-analysis of a published study by Biro et al [2], using the same dataset and variables. Unlike the previous study, which utilized age at menarche as the pubertal timing measure, this study used the relative time of puberty as determined by ordinal logistic regression modeling. Same as the previous study, the analyses were done separately for black and white girls, due to the well know racial differences in pubertal maturation [2, 11].
The specific aims of this study are:
To illustrate that ordinal logistic modeling can be used to determine relative timing of pubertal maturation by conducting secondary data analyses using the NGHS study ;
To validate the newly proposed approach to the determination of relative timing of pubertal maturation by evaluating its relationship with pubertal growth and timing parameters: age at menarche, age at appearance of areolar stage2, age of peak height velocity, age at attainment of adult height, adult height, peak height velocity, BMI and percent body fat;
To contrast the two pubertal timing parameters, relative timing of breast development vs. relative timing of menarche, by comparing the results from this study with the results from the Biro et al. study [2].
Methods
Study Design and Population
This is a secondary data analysis using the National Heart Lung and Blood Institute Growth and Health Study (NGHS). The NGHS was a cohort study that recruited a sample (N = 2378) of 9 and 10 year old black and white girls from three sites (Cincinnati, OH; Richmond, CA; and Washington DC) in 1987–1988, and followed up annually for 10 years. The study was designed to follow the development of obesity and related cardiovascular disease risk factors in girls going through pubertal transition. During the annual visits, physical examinations included height, weight, skin fold, and pubertal maturation. Participants were also interviewed for age and date of menarche. Detailed descriptions of the study design and population have been reported elsewhere [24].
The study was approved by the Institutional Review Boards of the University of Cincinnati and Children’s Hospital Medical Center, Cincinnati, OH; University of California at Berkeley; and Westat/Group Health Association in Rockville, MD. All parents/legal guardians gave informed consent, and all participants over the age of 12 gave their assent.
Measurements Used in the Analysis
Pubertal maturation stage was assessed by trained female research assistants during the annual physical exam, utilizing Tanner pubic hair criterion [25, 26], and utilizing the system of Garn and Falkner for areolar stages, a method highly correlated to breast stages, and has been shown to be more accurate and less subjective than Tanner breast criterion [13, 27–30]. Of notes, Areolar stage 2 is the same as the Tanner breast stage 2. Anthropometric measures included height, weight, and the sum of skin fold (SSF) thickness at triceps, subscapular, and suprailiac sites. Two measures were repeated for all anthropometric measures; if the two measures differed by more than a preset amount, a third measure was taken. The average of two closest measures was calculated and used in the current analysis. The percent body fat was derived from the triceps and suscapular skin folds using the formulae of Slaughter, et al [31]. Body mass index was calculated using the average height and weight measures (weight/height2).
Age at menarche was established by structured interview of the adolescent during the annual examination. Most of the girls experienced onset of menarche (>99% in 9 year old cohort) during the course of the study, and thus were able to reliably recall their age at menarche [32]. The age of onset of secondary sexual characteristics was defined by age of appearance of areolar stage 2. Height velocity was calculated by the increase in height divided by the time interval between the two consecutive visits. The peak height velocity was identified by the maximum value for each participant, and the corresponding age was recorded as the age of peak height velocity. Adult height was determined by first identifying three consecutive visits (t−1, t, t+1) where changes in height were less than 1.5 cm; the averaged height of those visits was used as the adult height, and the age of first such visit was recorded as the age adult height was attained. Compared to the height recorded at age 18, the averaged height is almost identical (difference of 0.37+/−0.57 cm). In addition, height at the age at menarche was also determined by an interpolation method using data from the two visits when the onset of menarche was reported.
Statistical Methods
Regressing biological ages on the areola stage by race using ordinal logistic regression at each visit, we calculated expected mean score of pubertal maturation, and plotted the mean score against age. The expected mean score is a continuous value ranges from 1 (pre-pubertal) to 4 (full maturation), representing the relative position of the pubertal maturation process. For example, mean score of 2.5 represents a maturational status that is half way between stages 2 and 3.
The relative timing is computed as the deviation score for each individual by subtracting her areolar stage from the sample mean; higher positive values corresponded to later timing of puberty. We further categorize adolescent as early, later, or on time by the upper and lower 20th percentile. The deviation score itself was also used as a continuous measure. Model fit was assessed using a procedure by Hosmer and Lemeshow [33], resulted χ2(9) value of 8.70 (P = 0.53) for white and 6.26 (P=0.29) for black, suggesting good model fit.
Re-analyzing the previous study [2], we considered the same pubertal growth parameters including adult height, peak height velocity, BMI, and percent body fat, the relative timing of puberty was used instead of age onset of menarche as the marker for pubertal timing. In addition, we also considered other timing parameters including age of onset of menarche, age of appearance of areolar stage 2, age of peak height velocity, and age of attaining adult height. Their associations with relative timing of puberty were evaluated using generalized estimating equations (GEE) [34]. GEE is a statistics technique that is designed to taking into account repeated measures from the same individual across different longitudinal visits. In addition, the GEE technique provided robust statistics inferences, even when the correlation matrix is miss-specified. Intra-cluster correlation coefficients (ICC) were estimated for the proposed new measure to examine its reliability. All analyses were conducted stratified by race and age cohort at recruitment, 9 year-old and 10 year-old cohort. Since the results from the two cohorts are parallel in every respect of analyses, only the results from the 9 year-old cohort are reported.
For the purpose of contrasting the relative timing of areolar (breast) development and the age of onset of menarche, the results from the current study were compared with the results from the earlier parallel study [2]. Analyses revealed different results in the outcomes of adult height and peak height velocity, thus we conducted GEE analyses including both timing variables in the corresponding models. For the adult height, GEE analyses also considered height at onset of menarche, and the change in height from onset of menarche to adult height, stratified by race and age cohort.
Results
Table 1 provides descriptive statistics for the study participants by race (N=615, 53% White and N = 541, 47% Black). At the baseline, greater than 99% of the girls were premenarcheal. The mean age at menarche was 12.7+/−1.15 years in white, and 12.0+/−1.14 years in black participants (P<.0001). More than 80% (83.4%) of the white girls and 63.7% of black girls were areolar stage 1 at the baseline (P<.0001).
Table 1.
Descriptive Statistics of Participant Characteristics by Race
| White (N=615) |
Black (N=541) |
|||||
|---|---|---|---|---|---|---|
| Variables | N | Mean | SD | N | Mean | SD |
| Baseline Participant Age (yr) | 615 | 9.54 | 0.29 | 541 | 9.54 | 0.28 |
| Baseline Height (cm) | 612 | 137.02 | 5.99 | 533 | 139.28 | 6.92 |
| Baseline Weight (kg) | 612 | 33.14 | 7.32 | 534 | 36.20 | 9.34 |
| Baseline BMI (kg/m2) | 612 | 17.54 | 3.10 | 533 | 18.50 | 3.74 |
| Baseline SSF Percent fat (%) | 608 | 20.22 | 7.26 | 535 | 20.71 | 9.11 |
| Age of Menarche (yr) | 576 | 12.66 | 1.15 | 518 | 12.00 | 1.14 |
| Peak height velocity (cm/yr) | 463 | 8.04 | 1.19 | 463 | 8.08 | 1.53 |
| Age reach PHV (yr) | 463 | 11.94 | 0.98 | 463 | 11.47 | 0.87 |
| Computed adult height (cm) | 519 | 164.69 | 6.06 | 490 | 163.19 | 6.48 |
| Age attain full adult height (yr) | 366 | 16.89 | 1.03 | 380 | 16.37 | 1.18 |
BMI: Body Mass Index; SSF: Sum Skin Fold thickness; PHV: Peak Height Velocity; SD: Standard Deviation.
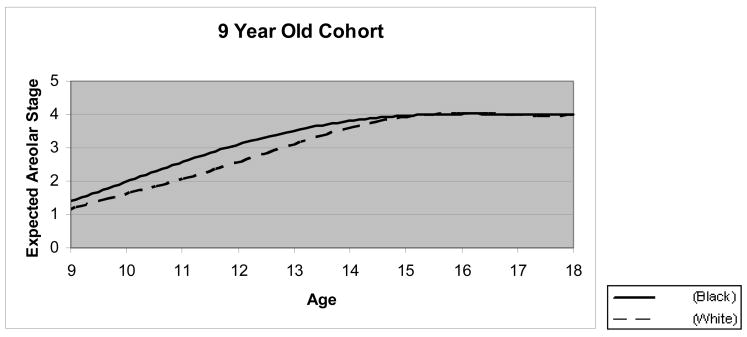
Figure 1 presents the expected areolar stage at a given age by race. This non-linear curve started at the expected mean areolar stage of 1.17+/−0.05 for the white and 1.41+/−0.12 for the black at age 9, then steadily increased and approached full maturation (stage 4) by age 15. Black girls entered into puberty (areolar stage 2) approximately one year earlier than the white girls, and remained more advanced until age 15. GEE analyses revealed significant racial differences in pubertal maturation stage up to 15 years of age (P < .0001). Survival analysis for interval censored age of the appearance of areolar stage 2 estimated the median age at10.40+/−0.02 in white and 9.65+/−0.02 in black girls. Intra-cluster correlation coefficients (ICC) of the relative timing were estimated at 0.73 in white and 0.74 in black.
Figure 1.
Expected Areolar Stage at a Given Age by Age Cohort and by Race from Ordinal Logistic Modeling
Solid line presents black girls, and dashed line presents white girls
Table 2 presents GEE analyses, stratified by race. When one considers three timing groups, i.e. early, on-time and late relative timing of puberty, the ages of menarche in years were estimated at 12.08, 12.68 and 13.23 in the white girls; and 11.54, 12.00 and 12.52 in the black girls. Age at menarche was significantly different in three timing groups (P < .0001).
Table 2.
Generalized Estimating Equation Results of Associations of Relative Timing of Puberty with other Timing Parameters
| Early (<20%-tile) | On-time (20–80 %-tile) | Late (>80%-tile) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Onset Age (yr) | Race | Beta | SD | LSM | 95% CL | LSM | 95%CL | LSM | 95% CL | |||
| Age@Menarche | White | 0.66*** | 0.05 | 12.08 | 11.94 | 12.22 | 12.68 | 12.59 | 12.77 | 13.23 | 13.08 | 13.37 |
| Black | 0.51*** | 0.05 | 11.54 | 11.40 | 11.68 | 12.00 | 11.89 | 12.10 | 12.52 | 12.36 | 12.67 | |
| Age@Onset1§ | White | 0.97*** | 0.03 | 9.59 | 9.47 | 9.71 | 10.44 | 10.34 | 10.54 | 11.19 | 11.07 | 11.31 |
| Black | 1.03*** | 0.04 | 8.77 | 8.62 | 8.92 | 9.61 | 9.48 | 9.74 | 10.60 | 10.46 | 10.74 | |
| Age@PHV1 | White | 0.52*** | 0.05 | 11.44 | 11.32 | 11.55 | 11.95 | 11.86 | 12.04 | 12.43 | 12.28 | 12.57 |
| Black | 0.38*** | 0.05 | 11.09 | 10.99 | 11.20 | 11.45 | 11.38 | 11.52 | 11.84 | 11.69 | 12.00 | |
| Age@FAH1 | White | 0.32*** | 0.06 | 16.55 | 16.36 | 16.73 | 16.89 | 16.79 | 16.99 | 17.15 | 17.01 | 17.29 |
| Black | 0.29*** | 0.05 | 16.11 | 15.93 | 16.29 | 16.33 | 16.21 | 16.46 | 16.64 | 16.47 | 16.81 | |
1 Age@onset = Age at the onset of areolar stage 2; Age@PHV: Age at the Peak Height Velocity; Age@FAH: Age at the final adult height;
LSM = Least Square Mean; 95% CL = 95% Confidence Limit;
P value <.0001
Considering interval censored age onset of AR2, estimated median and the corresponding 95% CL are reported instead of LSM and its 95% CL.
When relative timing was modeled as a continuous variable, GEE results noted that one stage advance in relative timing of puberty was associated with 0.66+/−0.05 and 0.51+/−0.05 years of delay in age at menarche in white and black girls correspondingly. Similar to the age at menarche, girls from the early or late areolar timing group had significantly (P <0.0001) early or late age appearance of areolar stage 2, age of peak height velocity, and age at attainment of adult height than the mid (20 ~ 80%-tile) group correspondingly, regardless of their race.
Table 3 presents results of the GEE analyses that examined the association between relative timing and growth parameters. The findings suggest a consistent graded pattern for BMI and percent body fat. Early timing girls had significantly (P <0.0001) higher BMI and percent body fat, and late timing girls had lower BMI and percent body fat, than on-time girls.
Table 3.
Generalized Estimating Equation Results of Associations of Relative Timing of Puberty with Growth Parameters
| Early (<20%-tile) | On-time (20–80 %-tile) | Late (>80%-tile) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race | Beta | SD | LSM | 95% CL | LSM | 95%CL | LSM | 95% CL | ||||
| Body Mass | White | −1.72*** | 0.15 | 21.15 | 20.69 | 21.60 | 19.48 | 19.17 | 19.78 | 18.29 | 17.88 | 18.69 |
| Index (kg/m2) | Black | −1.82*** | 0.20 | 22.87 | 22.24 | 23.50 | 21.42 | 21.00 | 21.84 | 19.70 | 19.11 | 20.28 |
| %Body Fat | White | −3.02*** | 0.32 | 25.51 | 24.58 | 26.45 | 22.48 | 21.84 | 23.12 | 20.21 | 19.36 | 21.06 |
| (SSF) | Black | −3.34*** | 0.42 | 27.16 | 25.80 | 28.52 | 24.31 | 23.41 | 25.21 | 21.34 | 20.23 | 22.44 |
| Final Adult | White | 0.33NS | 0.34 | 164.34 | 163.39 | 165.29 | 164.76 | 164.21 | 165.31 | 164.47 | 163.59 | 165.36 |
| Height (cm) | Black | −0.16 NS | 0.31 | 163.20 | 162.21 | 164.18 | 163.15 | 162.57 | 163.74 | 162.98 | 162.01 | 163.95 |
| Peak Height | White | −0.10 NS | 0.07 | 8.09 | 7.90 | 8.29 | 8.06 | 7.94 | 8.17 | 7.98 | 7.83 | 8.12 |
| Velocity (cm/yr) | Black | 0.05 NS | 0.08 | 7.99 | 7.74 | 8.24 | 8.17 | 8.04 | 8.31 | 8.01 | 7.82 | 8.20 |
LSM = Least Square Mean; 95% CL = 95% Confidence Limit;
P value <.0001;
P value <.05;
P value > 0.05.
GEE analyses were also conducted to examine the effect of age at menarche on adult height, as well as peak height velocity. The adult height attained by the early menarche girls was 2.4 cm shorter than mid menarche girls, and 3.6 cm shorter than late menarche girls among the white girls (162.5 cm, 164.9 cm and 166.1 cm respectively, P <.0001 ). Such results were consistently presented for both races. In a similar fashion, peak height velocity was greatest in early menarche girls and lowest in later menarche girls among the white girls (8.28 cm/yr, 8.17 cm/yr and 7.51 cm/yr respectively, P <.0001). After adjusting for age at menarche, relative timing of areolar stage remained non-significant in predicting adult height and peak height velocity. However, the adjusted timing of areolar stage had a significant effect on height attained at the onset of menarche (beta = −1.48 and −1.72 in white and black girls correspondingly, P < .0001), and on the change in height from the onset of menarche to adult height (beta = 0.98 and 1.14 in white and black girls correspondingly, P < .0001).
Discussion
This study proposed a new approach to the determination of relative timing of puberty using ordinal logistic regression modeling. Re-analyzing NGHS study by Biro et al, this study demonstrated the general utility of the proposed method. By examine the relationship between the relative timing of puberty with growth and pubertal parameters, the study results suggested this simple method provide a meaningful and valid new approach to the assessment of relative timing of puberty.
The proposed approach was shown to have significant associations with other age specific pubertal parameters, including age at menarche, age of appearance of areolar stage 2, age of peak height velocity, and age at attainment of adult height. Consistent with current literatures [2, 16, 19, 22], the study has shown that the relative timing correlates well with BMI and percent body fat during puberty. Early maturing girls have significantly higher BMI and percent body fat than on-time girls, and much greater than later-maturing.
The intra-cluster coefficients of 0.73 and 0.74 demonstrate a pattern of good reliability within race groups. This suggests that the relative timing of puberty measured at different ages during puberty is consistent throughout the pubertal transition period. That is, an individual with early or late timing at one point of time is likely to be early or later throughout the pubertal transition period. Similar finding was reported in Smolak et al [35].
This study demonstrated that adult height was the same regardless of relative timing of areolar development. However, both this and Biro et al. study [2], demonstrated girls with earlier menarche had shorter adult height. Contemporary studies have noted a secular downward trend in onset of puberty in developed countries [36–38], yet adult height is not decreasing. Consistent with previous studies [22, 38], this finding further suggests that menarche and the onset of puberty represent increasingly different biologic phenomenon.
This study has several limitations. Since the relative timing of puberty in this study is based on the maturation stage, it may be impeded by the potential misclassification bias related to maturation stage [39]. Utilizing areolar rather than breast maturation stage in this study maybe a limitation, however the two rating systems are highly correlated (r =0.94) [13]. The NGHS enrolled only girls, in addition, 27% white and 36% of black girls were already advanced to areolar stage 2 or higher at the initiation of the study, thus their age appearance of areolar stage 2 were left censored, in other words, only the upper bound but not the lower bound of the age appearance of areolar stage 2 is observed. This is why we have analyzed the age onset of areolar stage 2 as an interval censored data using survival regression. Additionally the NGHS, although represented by broad socioeconomic diversity, included only black and white participants recruited from three sites, who were born in the late 1970’s.
Our proposed approach could be adapted to more recently generate nationally representative datasets, such as NHANES III. This should allow estimation of expected population statistics for maturation stage corresponding to a range of biological age across pubertal maturational process, within gender and racial groups. Such statistics could be used as reference norms for researchers or clinicians to determine the relative timing of puberty for an adolescent, during the pubertal transition process.
This study has proposed a new method to determine relative timing of puberty, utilizing the ordinal logistic regression approach. It provides a valid alternative to determine relative timing of puberty. The proposed approach is highly feasible and is easy to implement in different study designs and study populations. The study concluded good intra-cluster correlation coefficients, suggesting that relative timing of puberty measured at different ages during puberty is fairly consistent throughout the pubertal transition period. Furthermore, this study demonstrated important differences when contrasting outcomes of biologic phenomena, using relative timing of areolar development from timing of menarche.
Acknowledgments
1RO1DA01965-01A1, NIDA, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bin Huang, Center for Epidemiology and Biostatistics, Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati.
Frank M. Biro, Division of Adolescent Medicine, Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati.
Lorah Dorn, Division of Adolescent Medicine, Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati.
References
- 1.Ito M, Yamada M, Hayashi K, et al. Relation of early menarche to high bone mineral density. Calcified Tissue International. 1995;57(1):11–14. doi: 10.1007/BF00298989. [DOI] [PubMed] [Google Scholar]
- 2.Biro F, McMahon RP, Striegel-Moore R, et al. Impact of timing of pubertal maturation on growth in black and white female adolescents: The National Heart, Lung, and Blood Institute Growth and Health Study. The Journal of Pediatrics. 2001;138(5):636–643. doi: 10.1067/mpd.2001.114476. [DOI] [PubMed] [Google Scholar]
- 3.Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28(1):51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- 4.Kaltiala-Heino R, Marttunen M, Rantanen P, et al. Early puberty is associated with mental health problems in middle adolescence. Social Science & Medicine. 2003;57(6):1055–64. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- 5.Coleman L, Coleman J. The Measurement of Puberty: A Review. Journal of Adolescence. 2002;25:535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- 6.Cooper GS, Bell M, Hardy R, et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health. 2006;60:993–997. doi: 10.1136/jech.2005.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen AC, Crockett L, Richards M, et al. A self-report of pubertal status: Reliability, validity, and initial norms. Journal of Youth & Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 8.Dorn L, Ponirakis A, Susman E. Pubertal timing and adolescent adjustment and behavior: Conclusions vary by rater. Journal of Youth and Adolescence. 2003;32:157–167. [Google Scholar]
- 9.Ellis BJ, Garber J. Psychosocial antecedents of variation in girls' pubertal timing: maternal depression, stepfather presence, and marital and family stress. Child Development. 2000;71(2):485–501. doi: 10.1111/1467-8624.00159. [DOI] [PubMed] [Google Scholar]
- 10.Dorn LD, Dahl RE, Woodward HR, et al. Defining the boundaries of early adolescence: a user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10(1):30–56. [Google Scholar]
- 11.Herman-Giddens ME, Slora EJ, Wasserman RC, et al. Secondary sex characteristics and menses in young girls seen in office practice: A study from the pediatric research in office settings network. Pediatrics. 1997;99(4):505–512. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 12.Dorn LD, Susman EJ, Nottelmann ED, et al. Perceptions of puberty: Adolescent, parent, and health care personnel. Developmental Psychology. 1990;26(2):322–329. [Google Scholar]
- 13.Biro FM, Falkner F, Khoury P, et al. Areolar and breast staging in adolescent girls. Adolescent and Pediatric Gynecology. 1992;5:271–272. [Google Scholar]
- 14.Largo RH, Prader A. Pubertal development in Swiss girls. Helvetica Paediatrica Acta. 1983;38:229–243. [PubMed] [Google Scholar]
- 15.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121:S208–S217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 16.Silventoinen K, Haukka J, Dunkel L, et al. Genetics of pubertal timing and its associations with relative weight in childhood and adult height: The Swedish young male twins study. Pediatrics. 2008;121(4):E885–E891. doi: 10.1542/peds.2007-1615. [DOI] [PubMed] [Google Scholar]
- 17.Hediger ML, Scholl TO, Schall JI, et al. One year changes in weight and fatness in girls during late adolescence. Pediatrics. 1995;96:253–258. [PubMed] [Google Scholar]
- 18.St George IM, William S, Silva PA. Body Size and the menarche: the Duedin study. Journal of Adolescent Health. 1994;15:573–576. doi: 10.1016/1054-139x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- 19.Zacharias L, Rand W, Wurtman R. A prospective study of sexual development and growth in American girls: The statistics of menarche. Obstet Gynecol Survey. 1976;31:325–37. doi: 10.1097/00006254-197604000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Stanhope R, Preece MA, Grand DB, et al. New concepts of growth spurt of puberty. Acta Paediatrica Scand Suppl. 1988;347:30–37. [PubMed] [Google Scholar]
- 21.Tanner J, Davies P. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107:317–329. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- 22.de Ridder CM, Thijssen JHH, Bruning PF, et al. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab. 1992;75:442–446. doi: 10.1210/jcem.75.2.1639945. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee I, Clayton P. The genetic basis for the timing of human puberty. J Neuroendocrinol. 2007;19(11):831–8. doi: 10.1111/j.1365-2826.2007.01598.x. [DOI] [PubMed] [Google Scholar]
- 24.The National Heart Lung and Blood Institute Growth and Health Study Research Group. Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. American Journal of Public Health. 1992;82:1613–20. doi: 10.2105/ajph.82.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall W, Tanner J. Variations in the pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanner J. Growth at adolescence. 2. Oxford, England: Blackwell Scientific Publications; 1962. [Google Scholar]
- 27.Aygun AD, Akarsu S, Guvenc H, et al. Nipple and areola diameter in Turkish pubertal girls. Journal of Adolescent Health. 1998;23:55–57. doi: 10.1016/s1054-139x(97)00272-3. [DOI] [PubMed] [Google Scholar]
- 28.Rohn RD. Nipple (papilla) development in puberty: Longitudinal observations in girls. Pediatrics. 1987;79:745–747. [PubMed] [Google Scholar]
- 29.Morrison JA, Barton BA, Biro FM, et al. Sexual maturation and obesity in 9- and 10-year-old Black and White girls: The National Heart, Lung, and Blood Institute Growth and Health Study. The Journal of Pediatrics. 1994;124(6):889–895. doi: 10.1016/s0022-3476(05)83176-2. [DOI] [PubMed] [Google Scholar]
- 30.Daniel WA, Paulshock BZ. A physician's guide to sexual maturaty rating. Patient Care. 1979;13:122. [Google Scholar]
- 31.Slaughter M, Lohman T, Boileau R, et al. Skinfold equations for estimation of body fatness in children and youth. Human Biol. 1988;60:709–23. [PubMed] [Google Scholar]
- 32.Koo M, Rohan T. Accuracy of short-term recall of age at menarche. Annals of Human Biology. 1997;24:61–64. doi: 10.1080/03014469700004782. [DOI] [PubMed] [Google Scholar]
- 33.Hosmer DW, Lemeshow S. Applied Logistic Regression. In: Barnett V, et al., editors. Probability and Mathematical Statistics. John Wiley & Sons; New York: 1989. pp. 135–173. [Google Scholar]
- 34.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;72:353–358. [Google Scholar]
- 35.Smolak L, Krieg DB, Hayward C, et al. The Reliability of Self-Reported Menarcheal Timing. The Journal of Early Adolescence. 2007;27:386. [Google Scholar]
- 36.Parent AS, Teilmann G, Juul A, et al. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocrine Reviews. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 37.Herman-Giddens ME, Kaplowitz PB, Wasserman R. Navigating the recent articles on girls' puberty in Pediatrics: what do we know and where do we go from here? Pediatrics. 2004;113:911–917. doi: 10.1542/peds.113.4.911. [DOI] [PubMed] [Google Scholar]
- 38.Biro FM, Huang B, Crawford PB, et al. Pubertal correlates in black and white girls. J Pediatr. 2006;148(2):234–40. doi: 10.1016/j.jpeds.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Albert PS, Hunsberger SA, Biro FM. Modeling repeated measures with monotonic ordinal responses and misclassification, with applications to studying maturation. Journal of the American Statistical Association. 1997;92(440):1304–1311. [Google Scholar]