Abstract
Myocardial infarction (MI) rapidly depletes the endogenous cardiac progenitor-cell pool, and the inefficient recruitment of exogenously administered progenitor cells limits the effectiveness of cardiac-cell therapy. Recent reports indicate that interactions between the CXC chemokine stromal-cell–derived factor 1 (SDF-1) and its receptor CXC chemokine receptor 4 (CXCR4) critically mediate the ischemia-induced recruitment of bone-marrow—derived circulating stem/progenitor cells, but the expression of CXCR4 in cardiac progenitor cells is very low. Here, we studied the influence of hypoxia on CXCR4 expression in cardiac progenitor cells, on the recruitment of intravenously administered cells to ischemic heart tissue, and on the preservation of heart function in a murine MI model. We found that hypoxic preconditioning increased CXCR4 expression in cardiosphere-derived, Lin−/c-kit+ progenitor (CLK) cells and markedly augmented CLK-cell migration (in vitro) and recruitment (in vivo) to the ischemic myocardium. Four weeks after surgically induced MI, infarct size and heart function were significantly better in mice administered hypoxia-preconditioned CLK cells than in mice treated with cells cultured under normoxic conditions. Furthermore, these effects were largely abolished by the addition of a CXCR4 inhibitor, indicating that the benefits of hypoxic preconditioning are mediated by the SDF-1/CXCR4 axis, and that therapies targeting this axis may enhance cardiac-progenitor-cell—based regenerative therapy.
Keywords: Cardiac progenitor cells, Hypoxia, CXCR4, Cell migration, Myocardial infarction
Introduction
Ischemic heart disease and consequent heart failure remain the leading cause of morbidity and mortality worldwide.1 Traditional therapies, such as angioplasty and thrombolytic agents, can relieve only the cause of infarction; no existing medication or procedure can effectively replace cardiac scarring with functional contractile tissue. However, newer therapies that incorporate recently identified populations of progenitor cells may regenerate cardiac tissue directly by inducing neovasculogenesis and cardiogenesis.2-10 Resident cardiac progenitor cells may be particularly suitable for resurrecting dead myocardium because they are endogenous components of the adult heart and appear to be responsible for the physiologic and pathologic turnover of cardiac myocytes and other cardiac cells.11 Cardiac progenitor cells are self-renewing, clonogenic, and multipotent, giving rise to myocytes, vascular smooth-muscle cells, endothelial cells, and neural-crest cells.2, 6, 12
Although cardiac progenitor cells may seem to be the obvious choice for cell-based cardiac repair, the success of this approach is determined, in part, by the same factors that cause the endogenous cardiac-repair system to fail. Within a day of MI, 40% of resident cardiac progenitor cells are depleted,8 and the barriers imposed by cardiac damage may limit the proliferation and self-renewal of the remaining resident cells, thereby preventing restoration of the progenitor-cell pool. At present, these limitations are often addressed by using ex-vivo expansion protocols to generate a sufficient number of cells for transplantation into the ischemic heart. However, only a very small number of transplanted stem/progenitor cells are retained in the ischemic myocardium,13 and poor cell retention is one of the primary barriers to the effectiveness of cell therapy.14 Thus, techniques that enhance the recruitment and retention of transplanted cardiac progenitor cells are crucial to adequately replenish the resident progenitor-cell pool and to maximize its regenerative potential.
The ischemic heart produces numerous cytokines, chemokines, and growth factors that may influence stem-cell—mediated repair.14, 15 SDF-1 is critically involved in the recruitment and tissue retention of hematopoietic cells,16-20 and the expression of both SDF-121 and its receptor, CXCR4,22 are upregulated in ischemic heart tissue. SDF-1 secretion from the injured heart is stimulated by hypoxia, and both ischemic/hypoxic preconditioning23 and SDF-115-17 enhance the recruitment of bone-marrow (BM)—derived progenitor cells to ischemic myocardium. CXCR4 is normally expressed in BM hematopoietic cells16-20 and is upregulated by hypoxia.18, 24 The high level of CXCR4 expression in hematopoietic cells is thought to result, at least partially, from the relatively low oxygen tension in BM.18, 25 Cancers that evolve from mutations in von Hippel-Lindau, a tumor-suppressor gene, regain cellular hypoxic response and CXCR4 expression, which is directly linked to cancer metastasis and malignancy.26 Clearly, the expression and function of CXCR4 is differentially conserved in different cell types.27 However, it is not known whether hypoxia regulates CXCR4 expression in cardiac progenitor cells or whether hypoxic preconditioning influences cardiac-progenitor—cell recruitment through the SDF-1/CXCR4 axis.
In this study, we provide evidence that hypoxia induces CXCR4 expression in cardiosphere-derived, Lin−c-kit+ progenitor (CLK) cells. Hypoxic preconditioning markedly augments CLK-cell recruitment to the ischemic myocardium in a CXCR4-dependent manner and enhances the benefit of cardiac-cell therapy for treatment of myocardial infarction.
Materials and Methods
Isolation of cardiosphere-derived, Lin−c-kit+ progenitor (CLK) cells and bone-marrow mononuclear cells (BMMNCs)
CLK cells were generated from the hearts of 2-month-old, male, C57BL/6 mice (Harlan Bioproducts for Science, Inc., Indianapolis, IN). Hearts were harvested via a protocol approved by the Institutional Animal Care and Use Committee of California Polytechnic State University, then CLK cells were isolated via a 2-step procedure. In step 1, cardiac explants were cultured as described by Messina et al6 for 2-3 weeks, then the small, round, phase-bright cells that had migrated from the adherent explants and proliferated over a fibroblast layer were collected with D-Hanks. In step 2, Lin−c-kit+ cells were isolated from the phase-bright cells through the use of a hematopoietic Lin-depletion cocktail (StemCell Technologies, Vancouver, BC, Canada) followed by magnetic-activated cell sorting (MACS) with CD117 magnetic beads (Miltenyi Biotec Inc., Auburn, CA) as instructed by the manufacturers’ protocols. The selected CLK cells were cultured and maintained in complete media containing DMEM/F12, 10% fetal calf serum, 200 mM L-glutamine, 55 nM ß-mercaptoethanol, 1% MEM nonessential amino acids, and 5 ng/mL basic fibroblast growth factor (Invitrogen Corporation). CLK cells were passaged 10 times at 5-day intervals before use in subsequent experiments. BMMNCs were isolated as described previously.28
Experimental CLK-cell culture
CLK cells were incubated under normoxic conditions or in a BD GasPak™ EZ Pouch (0.1% O2) (BD, Franklin Lakes, NJ) for various lengths of time before use in in vitro analyses; the completeness of oxygen consumption was confirmed with anaerobic indicator strips. For in vivo experiments, CLK cells were incubated under normoxic or hypoxic conditions for 6 hours with or without AMD3100 (5 μg/mL).
CLK-cell transduction
Virus production and CLK-cell infection
Viral vectors encoding CXCR4 shRNA and Nkx2.5-GFP were produced by transfection of 293FT cells with the lentiviral backbone plasmid, an envelope plasmid (pMD2.G), and a packaging plasmid (psPAX2). Two days later, virus-containing supernatants were collected, then the supernatant and 8 μg/mL polybrene were applied to CLK cells; the medium was replaced on the following day. Transduced cells were selected with 10 μg/mL puromycin beginning on the third day after transfection and continuing until 1 week after all mock-transfected cells had died or throughout the generation of stable, clonal, transduced cell lines.
The CXCR4 shRNA backbone plasmids (pLKO.1 puro CXCR4 shRNA) were created from double-stranded oligonucleotides corresponding to exon 2 of mouse CXCR4 (NM_009911) and flanked by sequences compatible with the sticky ends of Age1 and EcoR1 (Online Table II). The oligonucleotides were annealed and inserted into the pLKO.1-TRC cloning vector (Addgene plasmid 13425).
The Nkx2.5-eGFP backbone plasmid (pRRLSIN.cPPT.hNkx2.5-GFP.WPRE) was created by amplifying a 2.3-kb fragment of human Nkx2.5 genomic DNA (Promega Corporation, Madison, WI) via PCR with primers containing EcoRV and AccIII restriction sites on the ends (Online Table II). The hNkx2.5 fragment was inserted into the pRRLSIN.cPPT.PGK-GFP.WPRE plasmid (Addgene plasmid 12252) via EcoRV and AccIII digestion.
Transfection with the retroviral vector pCL-MFG-LacZ (Imgenex Corporation, San Diego, CA) was performed in Retronectin dishes (Takara Bio Inc., Shiga, Japan) as described previously.29
Small-interference RNA (siRNA) transfection
Pre-designed siRNA sequences for mouse hypoxia-inducible factor 1α (HIF-1α) (NM_010431) and a non-targeting siRNA were obtained from Ambion (Austin, TX). The double-stranded mouse HIF-1α siRNA (Online Table II) corresponded to Exon 9 of mouse HIF-1α. For transfection, CLK cells were incubated with siRNA complexes in serum-free medium for 4 hours by using a Silence™ siRNA Transfection II kit (Austin, TX), then the medium was changed and cells were incubated for an additional 48 hours before hypoxic or normoxic treatment.
CLK-cell gene expression
Gene expression in CLK cells was evaluated via flow cytometry,29 immunofluorescent analyses of cultured slides and tissue sections,29 quantitative real-time RT-PCR,30 and Western blotting30 as described previously and as summarized in the Supplemental Methods.
CLK-cell migration
CLK-cell migration was measured with the QCM™ Chemotaxis 96-Well Cell Migration Assay (Millipore, Billerica, MA) as described in the Supplemental Methods.
Myocardial Infarction (MI) and CLK-cell administration
MI was induced in adult male C57BL/6 mice (10-12 weeks of age) via permanent ligation of the middle of the LAD coronary artery as described previously.28 Mice were anesthetized with Isoflurane delivered at 2-4% during surgery. Buprenex (1-2 mg/kg, intraperitoneal injection) was administered following surgery and continued every 12 hours for 3 days to reduce pain. The MI protocol was approved by the Animal Care and Use Committee of Northwestern University and California Polytechnic State University. Mice were randomized and received intravenous injections of CLK cells (1×106 cells/mouse in 100 μL CLK-cell medium) within 1 hour after MI surgery.
CLK-cell recruitment
One day after surgically induced MI and intravenous injection of CLK cells transfected with a vector (pCL-MFG-LacZ) coding for lacZ expression (CLK-LacZ cells), mouse hearts were harvested and CLK-LacZ cell recruitment was quantified by measuring β-galactosidase enzymatic activity in protein extracts from the ischemic heart tissue. Cardiac protein extracts were prepared and β-galactosidase activity was assayed by using the Luminescent beta-galactosidase Reporter System 3 (Clontech) and a Monolight 3010 (Pharmingen) as directed by the manufacturer’s protocol.
CLK-cell differentiation
One month after surgically induced MI and intravenous injection of CLK-LacZ cells, mouse hearts were harvested, embedded in OCT compound, snap-frozen, cut into 5-μm sections, and double-immunostained with FITC-conjugated anti-lacZ antibody (1:500, [Abcam Inc.]) and with anti-cTnI (1:100, [Santa Cruz Biotechnology, Inc.]), anti-vW Factor (1:100,Sigma), or anti-SMA (1:100, Zymed Lab Inc., San Francisco, CA) antibodies. Primary antibodies were resolved via secondary staining with streptavidin Alexa Fluor 555-conjugated, goat anti-rabbit Alexa Fluor 555-conjugated. Nuclei were counterstained with Draq5 (Alexis Biochemicals, San Diego, CA). Sections were examined under a Leica TCS confocal microscope (Leica Microsystems Inc.).
Echocardiography
Echocardiographic measurements were performed 1, 2, and 4 weeks after MI with a Vevo 770TM high-resolution ultrasound biomicroscope (VisualSonics Inc., Toronto, Ontario, Canada) and Vevo analysis software (Vevo 2.2.3, [VisualSonics Inc.]) as described previously31 and as summarized in the Supplemental Methods.
Histological assessments
Twenty-eight days after surgically induced MI and intravenous injection of CLK cells, the vasculature was labeled by injecting 50 μL Lectin I (Vector laboratories) into the tail vein, and mice were euthanized 10 min later. The hearts were harvested, and body weights and heart weights were measured, then infarct area was evaluated via Masson Trichrome elastic tissue staining, infarct wall thickness and infarct size were analyzed using a computerized digital image analysis system (Image Pro, version 4.5; [MediaCybernetics, Inc., Bethesda, MD]), and vascular density was evaluated via immunofluorecent staining with an anti-Lectin I antibody (Vector Laboratories) as described previously28 and as summarized in the Supplemental Methods. All surgical procedures and pathohistological analyses were performed by investigators blinded to treatment assignment.
Statistical analysis
All values are expressed as mean ± SEM. Differences between two groups were analyzed via the Student t-test, and differences between three or more groups were evaluated via one-way ANOVA with bonferroni correction. A p value of less than 0.05 was considered significant.
Results
Generation and phenotypic characterization of cardiosphere-derived, Lin−c-kit+ progenitor (CLK) cells
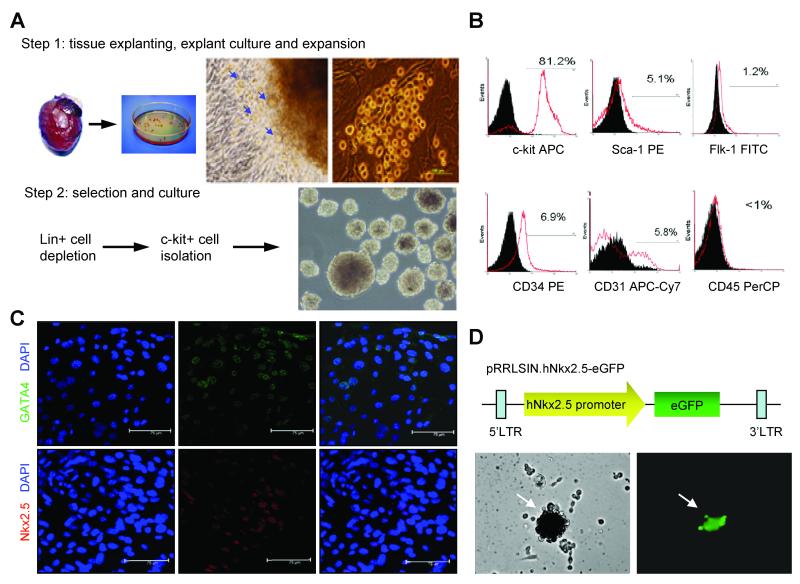
CLK cells were obtained with a two-step procedure: cardiospheres were grown from enzymatically-digested adult hearts6 and expanded, then the CLK cells were isolated by using a hematopoietic lineage-depletion cocktail followed by enrichment for c-kit+ cells via magnetic-activated cell sorting2 (Figure 1A); before depletion, 4% of cardiosphere cells were positive for c-kit expression. After sorting, surface marker expression was profiled by flow cytometry. More than 80% of the sorted cells were positive for c-kit expression (Figure 1B), and the cardiac-specific transcriptional factors GATA4 and Nkx2.5 (Figure 1C) were expressed by 46.9%±22.5% and 31.6%±5.7% of the cells, respectively. Smaller populations of the CLK cells expressed Sca-1 (5.1%), Flk-1 (1.2%), CD34 (6.9%), or CD31 (5.8%), and less than 1% of cells expressed the hematopoietic-lineage marker CD45. The cardiac-progenitor—cell lineage was verified by assessing GFP expression in CLK cells transfected with a lentiviral vector coding for GFP expression regulated by the human Nkx2.5 promoter, a cardiac-specific promoter that is activated during development (Figure 1D).
Figure 1. Isolation, expansion, and phenotypic characterization of cardiosphere-derived, Lin−c-kit+ progenitor (CLK) cells.
(A) Isolation and expansion of CLK cells. Step 1: cardiac explants were cultured for 2-3 weeks (panels 1 and 2); phase-contrast microscopy revealed cells migrating from the primary culture of mouse ventricular explants (panel 3, blue arrow); the migrated cells (round, phase-bright) aggregated and proliferated over the fibroblast coating (panel 4). Step 2: CLK cells were isolated from the phase-bright cells via lineage-depletion and c-kit+ enrichment (panels 1 and 2), then expanded in culture (panel 3). (B) Flow cytometric analyses of CLK cells for expression of the cell-surface markers c-kit, Sca-1, Flk-1, CD34, CD31, and CD45. (C) Immunofluorescent staining of CLK cells for expression of the cardiac transcription factors GATA4 (green) and Nkx2.5 (red); cell nuclei were counterstained with DAPI (blue). (D) GFP expression in CLK cells transfected with a lentiviral vector coding for GFP expression regulated by the human Nkx2.5 promoter.
CXCR4 and paracrine-factor expression in CLK cells
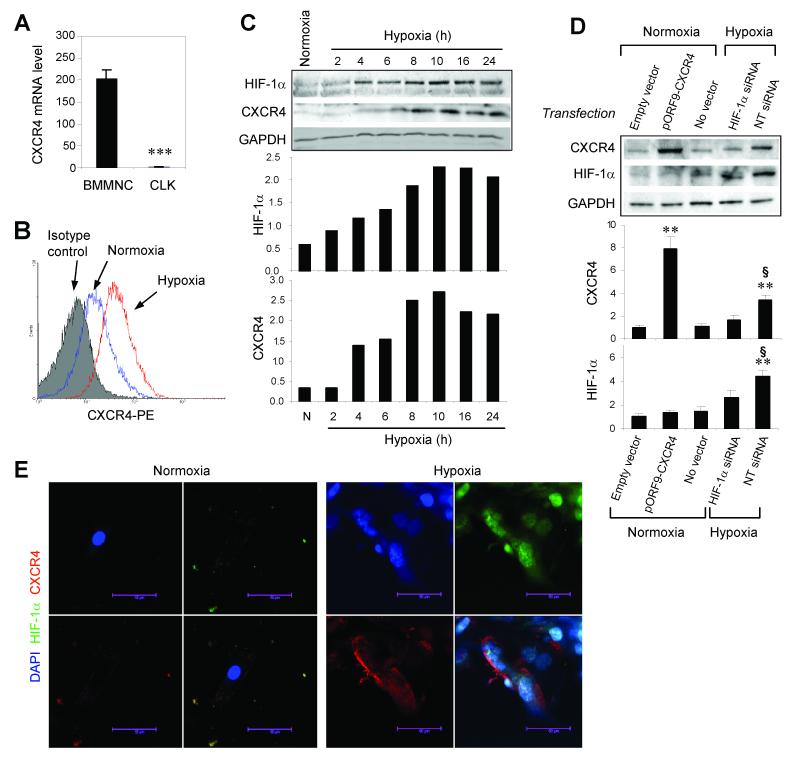
Because CXCR4 plays a critical role during SDF-1-mediated recruitment of circulating stem/progenitor cells to ischemic myocardium,16, 17 we evaluated the expression of CXCR4 in CLK cells. When cultured under normoxic conditions, CXCR4 mRNA expression was much lower in CLK cells than in freshly isolated BMMNCs (Figure 2A); however, both the number of cells expressing CXCR4 (Figure 2B) and the amount of CXCR4 protein expressed (Figure 2C) increased after just 4 hours in hypoxic culture; CXCR4 protein expression peaked at 8-10 hours. The elevation in CXCR4 expression was preceded by an increase in the expression of HIF-1α, and transfection of CLK cells with HIF-1α siRNA impaired hypoxia-induced CXCR4 expression (Figure 2D). Immunofluorescent staining revealed that the hypoxia-induced increase in CXCR4 expression occurred both at the cell membrane and in the cytoplasm, whereas the concurrent increase in HIF-1α expression was observed in the cytoplasm and the nucleus (Figure 2E).
Figure 2. CXCR4 expression in CLK cells.
(A) CXCR4 mRNA expression in CLK cells and in freshly-isolated BMMNCs was evaluated with quantitative real-time RT-PCR. Values are presented as mean±SEM (n=3 per group, ***p<0.001). (B) Flow cytometry analyses of CXCR4 protein expression in CLK cells cultured under hypoxic or normoxic conditions for 4 hours. (C) Representative Western blots of HIF-1α, CXCR4, and GADPH protein expression in CLK cells cultured under normoxic or hypoxic conditions for 2, 4, 6, 8, 10, 16, and 24 hours (upper panel); quantification of HIF-1α and CXCR4 protein levels (normalized to GADPH protein levels) (lower panels). Representative data from three independent experiments are shown. (D) Representative Western blots of CXCR4, HIF-1α, and GADPH expression in CLK cells transfected with a CXCR4-expressing plasmid (pORF9-CXCR4), HIF-1α siRNA, non-targeting (NT) siRNA, or the control vehicle and cultured under hypoxic conditions for 8 hours (upper panel); quantification of CXCR4 and HIF-1α protein levels (normalized to GADPH protein levels) expressed as a ratio relative to the level observed in normoxia-cultured cells transfected with the empty vector (lower panels) (n=3 independent experiments; **, p<0.01 versus normoxia+empty vector group; §, p<0.05 versus hypoxia+HIF-1α siRNA group). (E) Immunofluorescent staining for HIF-1α (green) and CXCR4 (red) protein expression in CLK cells cultured under hypoxic or normoxic conditions for 8 hours; nuclei were counterstained with DAPI (blue).
To determine whether hypoxic preconditioning may influence the potential paracrine mechanisms induced by CLK cells, we performed protein array experiments32 to compare the levels of more than 300 mouse cytokines released from hypoxia- and normoxia-treated CLK cells into the culture medium. Hypoxic treatment was associated with higher levels of numerous factors, including chemokines (e.g., TCA-3, SDF-1, 6Ckine), vascular growth factors (e.g., VEGF, osteopontin, bFGF, erythropoietin, stem-cell factor), and factors involved in cardiac differentiation (e.g., Activin A, TGF-β, and Dickkopf homolog-1) (Online Figure I and Online Table I). Because SDF-1 contributes to stem/progenitor-cell recruitment, we performed ELISA to confirm that hypoxia upregulated SDF-1α expression; hypoxic treatment of CLK cells for 6 hours dramatically increased the production of SDF-1 (hypoxia: 736.5±81.87 pg/mL, normoxia: 254.5±29.51 pg/mL; p=0.0015) (Online Figure II). Thus, both CXCR4 expression and SDF-1 production are induced by hypoxia.
CLK-cell migration, recruitment, and differentiation
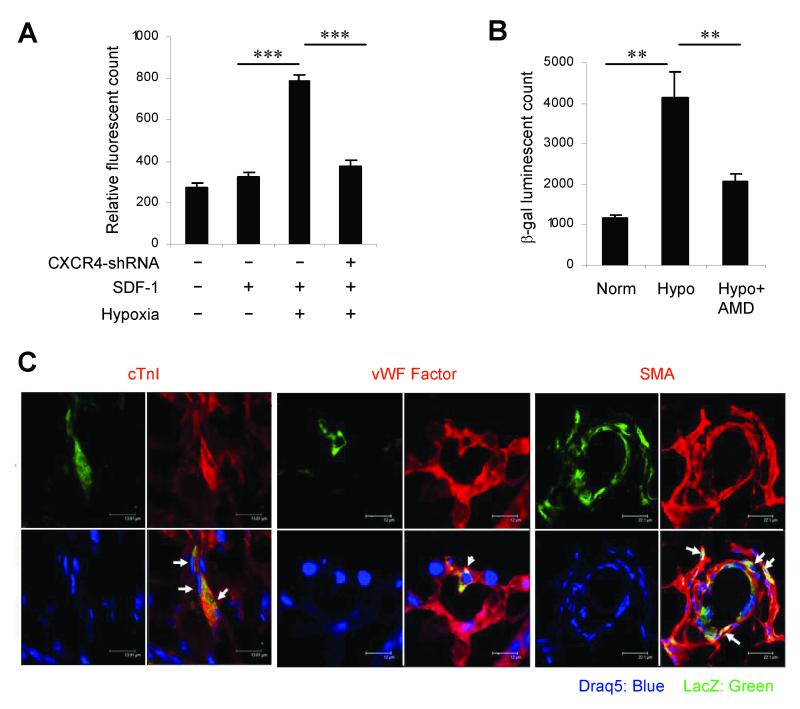
To determine whether hypoxia-induced CXCR4 expression influenced CLK-cell migration toward SDF-1, we cultured CLK cells under normoxic or hypoxic conditions for 4 hours, then performed an in-vitro cell-migration assay. Migration toward SDF-1 was significantly greater among hypoxia-preconditioned CLK cells than normoxia-cultured CLK cells (Figure 3A); however, when CXCR4 expression was knocked down by infecting CLK cells with a lentiviral vector encoding CXCR4 shRNA, the hypoxia-induced enhancement of CLK-cell migration was abolished. Collectively, these observations indicate that the migratory activity of CLK cells is increased by hypoxia, and that the mechanism of enhancement is dependent on a HIF-1α-mediated increase in CXCR4 expression.
Figure 3. CLK-cell migration, recruitment, and differentiation.
(A) CLK cells were seeded into the upper chamber (4×104 cells/well) of the ChemoTx cell-migration system, and the lower chamber was filled with 125 ng/mL recombinant human SDF-1, then the chambers were incubated at 37°C for 4 hours under hypoxic or normoxic conditions (n=8 per group); a subset of CLK cells were transfected with lentiviral CXCR4 shRNA before culture. The number of cells that had migrated to the lower chamber were quantified with a nucleic acid-binding fluorescent dye (***p < 0.001). (B-C) CLK cells were transfected with a vector coding for lacZ expression, cultured under hypoxic (Hypo) or normoxic (Norm) conditions for 6 hours, then intravenously injected into mice within 1 hour after surgically induced MI. (B) One day later, hearts were harvested, and recruitment was quantified by measuring β-galactosidase enzymatic activity in protein extracts from the ischemic heart tissue (**p<0.01). (C) One month after surgical MI and CLK-cell injection, hearts were harvested and sectioned, then sections were double-immunostained for co-expression of lacZ (green) and the lineage-specific proteins (red) cardiac troponin I (cTnI), von Willebrand factor (vWF), or smooth muscle actin (SMA); nuclei were counterstained with Draq5 (blue). The double-positive cells (yellow, identified with white arrows) in panels 1, 2, and 3 identified CLK cells that had differentiated into cardiomyocytes, endothelial cells, and smooth-muscle cells, respectively. Representative sections from 3 infarcted hearts in animals administered hypoxia-preconditioned CLK cells are displayed.
To determine whether hypoxia-induced CLK-cell migration was accompanied by enhanced CLK-cell recruitment to ischemic myocardium, and to assess the differentiation of the recruited CLK cells, CLK cells were transduced with retroviral vector pCL-MFG-LacZ, cultured for 6 hours under normoxic or hypoxic conditions, then injected intravenously into mice within 1 hour after surgical induction of MI. Recruitment was assessed 24 hours after CLK-cell injection; heart tissue was harvested, and the recruited CLK cells were quantified by measuring β-galactosidase enzymatic activity in protein extracts from the ischemic heart tissue (Figure 3B). Hypoxic preconditioning was associated with an ~2.5-fold increase in CLK-cell recruitment to ischemic myocardium. CLK-cell differentiation was assessed 4 weeks after CLK-cell injection; mouse hearts were harvested, serially sectioned, and co-immunostained for expression of lacZ and other cell-type—specific markers. The lacZ+ cells in the ischemic myocardium co-expressed cardiac troponin I, von Willebrand factor, and smooth-muscle actin (Figure 3C), indicating that the transplanted CLK cells had differentiated into cardiomyocytes, endothelial cells, and vascular smooth-muscle cells, respectively.
Therapeutic potential of hypoxia-preconditioned CLK cells
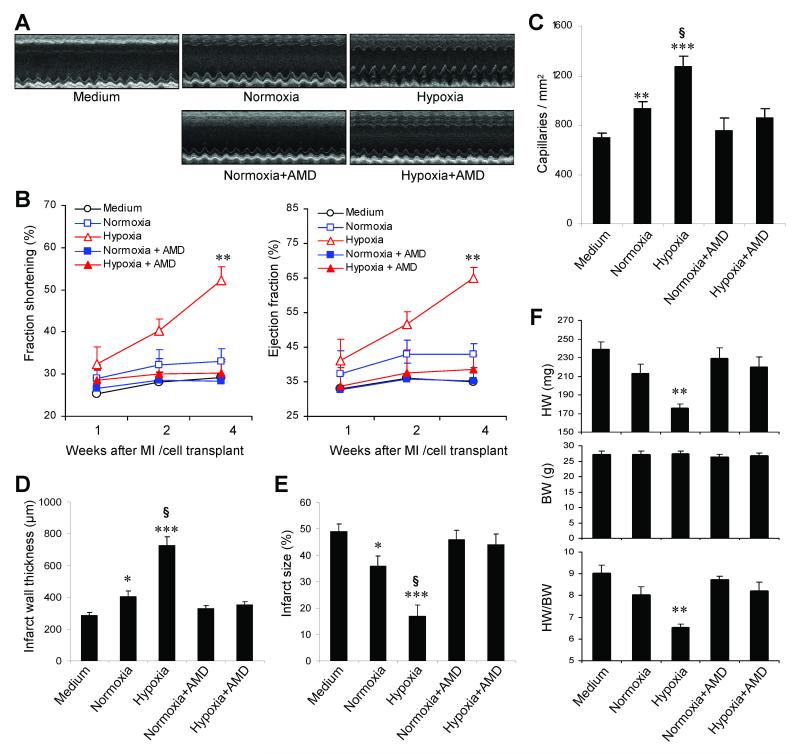
After observing the dramatic hypoxia-induced increase in CLK-cell recruitment to ischemic myocardium, we investigated whether hypoxic preconditioning enhanced the therapeutic benefit of CLK cells. CLK cells were cultured under normoxic or hypoxic conditions for 6 hours with or without the CXCR4 antagonist AMD3100, then intravenously injected into mice after surgical MI; echocardiographic measurements of cardiac function were performed 1, 2, and 4 weeks later (Figure 4A), then hearts were harvested for histological analyses. At week 4, functional analyses indicated that both left-ventricular fractional shortening and ejection fraction were better preserved in mice administered hypoxic-preconditioned CLK cells than in mice treated with normoxia-cultured CLK cells, but the enhancement was blocked when hypoxic-preconditioned CLK cells were incubated with AMD3100 (Figure 4B). Cardiac functional parameters were higher in mice treated with normoxia-cultured CLK cells than in mice administered the culture medium, but the difference did not reach statistical significance. Histological analyses indicated that peri-infarct capillary density (Figure 4C) and infarct wall thickness (Figure 4D) in the left ventricle were significantly greater, and infarct size (Figure 4E) was significantly smaller, in hearts from mice administered hypoxic-preconditioned cells than from mice treated with normoxia-cultured cells, and hypoxic-preconditioned cells were also associated with lower heart weights and a lower heart-weight:body-weight ratio (Figure 4F); again, the apparent benefit of hypoxic preconditioning was abolished when hypoxic-preconditioned CLK cells were incubated with AMD3100. Collectively, these results suggest that the therapeutic benefits of CLK-cell administration after MI is enhanced by hypoxic preconditioning via a CXCR4-dependent mechanism.
Figure 4. Therapeutic potential of systemically infused CLK cells.
CLK cells were cultured under normoxic or hypoxic conditions for 6 hours with or without the CXCR4 antagonist AMD3100 (5 μg/mL), then intravenously injected into mice after surgical MI; control mice were administered CLK-cell medium. Echocardiographic measurements were performed 1, 2, and 4 weeks after CLK-cell administration, and mice were sacrificed for histological examinations at week 4. (A) Representative echocardiographic images at week 4. (B) Cardiac function during the 4-week period after surgical MI and CLK-cell administration. FS indicates left-ventricular fractional shortening; EF, left-ventricular ejection fraction. (**, p<0.005 versus any other treatment group). (C) Capillary density in peri-infarct myocardium 4 weeks after MI (**, p<0.01 versus medium group; ***, p<0.001 versus medium group; §, p<0.001 versus hypoxia+AMD3100 group). (D-E) Infarct wall thickness (D) and infarct size (E) 4 weeks after surgical MI and CLK-cell administration. (*, p<0.05 versus medium group; ***, p<0.001 versus medium group; §, p<0.001 versus hypoxia+AMD3100 group). (F) Heart weight (HW), body weight (BW), and heart-weight:body-weight ratio (HW/BW) 4 weeks after surgical MI and CLK-cell administration. (**, p<0.005 versus any other treatment group).
Discussion
Our data indicate that CLK cells from adult mice are a population of cardiac progenitor cells that retain their capacity for self-renewal and clonogenic expansion in vitro and can differentiate into cardiomyocytes, vascular endothelial cells, and smooth-muscle cells after transplantation into ischemic heart tissue. Moreover, we found that hypoxic preconditioning increases CLK-cell migration and induces CXCR4 expression in CLK cells via a mechanism that is dependent, at least in part, on HIF-1α. When administered systemically, the hypoxia-preconditioned CLK cells homed to the infarcted myocardium more efficiently than normoxia-cultured cells, which led to significantly improved cardiac function and reduced infarct size. Furthermore, these effects were largely abolished by CXCR4 inhibition, indicating that the benefits of hypoxic preconditioning are mediated by the SDF-1/CXCR4 axis.
The ischemic myocardium is known to produce an abundance of chemo-active, -attractive, or - repulsive, agents,33, 34 and these chemotactic signals, along with specific cell-matrix and cell-cell interactions, modulate the regenerative capacity of progenitor cells.14 It is now evident that progenitor cells of different origins respond uniquely to each chemokine17, 18, 35 and are governed by different adhesion interactions during homing.27, 28, 36 The SDF-1/CXCR4 axis plays a key role in progenitor-cell homing17 and embryonic cardiogenesis37, 38; our findings suggest that this regenerative pathway is conserved in ex-vivo—cultured adult cardiac progenitor cells, and that the homing of systemically administered cells is enhanced by hypoxic preconditioning. Systemic delivery is less invasive and, consequently, preferable to intramyocardial administration, particularly because the results from clinical trials with BM stem/progenitor cells39 strongly suggest that cell therapy may need to be administered multiple times over an extended period to sustain improvements in cardiac function. Systemically administered cells may also bypass some of the barriers that limit the regenerative capacity of endogenous cardiac progenitor cells.
Recent studies have shown that SDF-1 is cardioprotective, and that pretreatment of BM-derived mesenchymal stem cells (MSCs) with SDF-1 enhances cell survival, proliferation, and engraftment, and improves cardiac function after myocardial infarction in animals.22, 40, 41 Our results indicate that SDF-1 is one of the chemokines and growth factors secreted by hypoxia-preconditioned CLK cells, which suggests that the apparent benefit associated with systemically administered hypoxia-preconditioned CLK cells evolves, at least in part, through paracrine mechanisms. Furthermore, the coincident upregulation of SDF-1 and CXCR4 implies (indirectly) that there may be positive feedback between SDF-1 and CXCR4, which could amplify SDF-1/CXCR4 signaling and, consequently, progenitor-cell homing. If so, pre-treatment of CLK cells with SDF-1 may induce responses similar to those observed with hypoxia preconditioning.
During ischemia, hypoxia stimulates serial adaptive cellular responses which favor the survival of cardiomyocytes,15, 42 and transient episodes of ischemia have been shown to protect the ischemic heart.43 Thus, patients experiencing a cardiovascular event could benefit, at least theoretically, from ischemic preconditioning, but this cardioprotective strategy has yet to be effectively employed clinically.42 One of the primary barriers limiting the effectiveness of ischemic preconditioning is the need to apply this strategy before the ischemic event occurs, rather than after the unanticipated onset of myocardial ischemia experienced by patients who present with acute MI or who survive cardiac arrest. Hypoxic preconditioning may be a more viable therapeutic approach, because the cellular components could be obtained and hypoxia preconditioned in advance for administration after a subsequent ischemic event.
In summary, our data suggest that inducing expression of CXCR4 via hypoxic preconditioning may be a novel approach for enhancing the therapeutic benefit of cardiac progenitor-cell therapy.
Supplementary Material
Acknowledgements
We thank W. Kevin Meisner, PhD, for editorial assistance.
Sources of Funding This work was supported by American Heart Association beginning Grant in Aid 0765094Y and National Institutes of health Grants HL086555-01A1 (to Y.T.), National Institutes of Health Grants HL 77602, MERIT HL27339 (to M.I.P.), American Heart Association Grant 0430135N (to G.Q.).
Footnotes
Disclosures None.
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 3.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 6.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 7.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–1092. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 9.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Wilson RM, Kubo H, Berretta RM, Harris DM, Zhang X, Jaleel N, MacDonnell SM, Bearzi C, Tillmanns J, Trofimova I, Hosoda T, Mosna F, Cribbs L, Leri A, Kajstura J, Anversa P, Houser SR. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res. 2007;100:536–544. doi: 10.1161/01.RES.0000259560.39234.99. [DOI] [PubMed] [Google Scholar]
- 11.Dawn B, Bolli R. Cardiac progenitor cells: the revolution continues. Circ Res. 2005;97:1080–1082. doi: 10.1161/01.RES.0000195610.71671.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, Guo Y, Bolli R, Rokosh G. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 17.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 18.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 19.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Haider H, Ahmad N, Zhang D, Ashraf M. Evidence for ischemia induced host-derived bone marrow cell mobilization into cardiac allografts. J Mol Cell Cardiol. 2006;41:478–487. doi: 10.1016/j.yjmcc.2006.06.074. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 23.Ii M, Nishimura H, Kusano KF, Qin G, Yoon YS, Wecker A, Asahara T, Losordo DW. Neuronal nitric oxide synthase mediates statin-induced restoration of vasa nervorum and reversal of diabetic neuropathy. Circulation. 2005;112:93–102. doi: 10.1161/CIRCULATIONAHA.104.511964. [DOI] [PubMed] [Google Scholar]
- 24.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- 26.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 27.Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, Gavin M, Goukassian DA, Yoon YS, Papayannopoulou T, Asahara T, Kearney M, Thorne T, Curry C, Eaton L, Heyd L, Dinesh D, Kishore R, Zhu Y, Losordo DW. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203:153–163. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang YL, Shen L, Qian K, Phillips MI. A novel two-step procedure to expand cardiac Sca-1+ cells clonally. Biochem Biophys Res Commun. 2007;359:877–883. doi: 10.1016/j.bbrc.2007.05.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin G, Kishore R, Dolan CM, Silver M, Wecker A, Luedemann CN, Thorne T, Hanley A, Curry C, Heyd L, Dinesh D, Kearney M, Martelli F, Murayama T, Goukassian DA, Zhu Y, Losordo DW. Cell cycle regulator E2F1 modulates angiogenesis via p53-dependent transcriptional control of VEGF. Proc Natl Acad Sci U S A. 2006;103:11015–11020. doi: 10.1073/pnas.0509533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu W, Shou W, Payne RM, Caldwell R, Field LJ. A mouse model for juvenile doxorubicin-induced cardiac dysfunction. Pediatr Res. 2008;64:488–494. doi: 10.1203/PDR.0b013e318184d732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, Takatsu Y, Melamed J, di Fagagna F d’Adda, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 33.Vandervelde S, van Luyn MJ, Tio RA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol. 2005;39:363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XF, Wang JF, Matczak E, Proper JA, Groopman JE. Janus kinase 2 is involved in stromal cell-derived factor-1alpha-induced tyrosine phosphorylation of focal adhesion proteins and migration of hematopoietic progenitor cells. Blood. 2001;97:3342–3348. doi: 10.1182/blood.v97.11.3342. [DOI] [PubMed] [Google Scholar]
- 35.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 36.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek K, Zeiher AM, Chavakis T, Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 38.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 39.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 40.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 41.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: united at reperfusion. Pharmacol Ther. 2007;116:173–191. doi: 10.1016/j.pharmthera.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.