Abstract
Higher levels of procoagulant factors and factor XII deficiency may be risk factors for first venous thromboembolism (VTE). We studied associations of coagulation factors IX through XIII with risk of future VTE in 2 general population samples. Using a nested case-control study combining the 21 860 participants of the Atherosclerosis Risk in Communities study and the Cardiovascular Health Study, we determined antigenic levels of these coagulation factors in primarily pre-event blood samples from 462 participants who subsequently developed VTE and 1047 participants who remained free of VTE. Only elevated levels of factors IX and XI were associated with increased risk of VTE after adjustment for age, sex, race, and study. For factor IX, the odds ratio (OR) was 1.4 (95% confidence interval [CI], 1.0-2.0) comparing the top to bottom quintile. The OR for factor XI was higher: 2.0 (95% CI, 1.4-2.9). With further adjustment for body mass index and diabetes, only elevated factor XI remained associated with VTE risk: OR 1.8 (95% CI, 1.3-2.7). Associations were similar by study and whether the thrombosis was idiopathic or secondary. Factor XII deficiency was not related to VTE risk. Among these procoagulant factors, only elevated factor XI was a risk factor for VTE.
Introduction
Venous thromboembolism (VTE) is a common vascular disease tied etiologically to the balance between the endogenous procoagulant and anticoagulant systems. Several studies have confirmed that higher levels of factors II, V, and VIII are associated with VTE risk.1 Associations of factor VII and fibrinogen with VTE are less consistent.1 Most information on levels of other coagulation factors and risk of first VTE has come from the Leiden Thrombophilia Study (LETS) and the Multiple Environmental and Genetic Assessment of risk factors for venous thrombosis (MEGA) studies, which are both case-control studies. LETS reported higher VTE risk in persons in the upper parts of the population distributions of factors IX,2 X,3 and XI,4 but not factor XII.5 Other reports suggest that factor XII deficiency is related to risk of first or recurrent thrombosis6–9 although findings have been inconsistent.5 An inverse association of factor XIII (activity and the Val 34 Leu polymorphism) with VTE has been reported, but results for the factor XIII gene variant have been mixed.10,11 In a study of postoperative asymptomatic deep vein thrombosis (DVT), higher factor VIII, but not factors VII or IX, was associated with DVT risk.12
Confirmation of these results in other populations, preferably in prospective studies, is important for several reasons. (1) Most published data on procoagulant factors and risk of DVT are from 2 Dutch studies in which blood samples were obtained after the VTE. (2) Most results have not included adjustment for obesity, a major VTE risk factor. (3) There are few data concerning pulmonary embolus (PE). (4) Many clinicians measure some of these factors in patients with thrombosis.13 It is unclear that such measurements are clinically useful. Here, we report results on associations of levels of coagulation factors IX through XIII with risk of future VTE in the Longitudinal Investigation of Thromboembolism Etiology (LITE). We hypothesized that low levels of factor XII (Hageman factor) and high levels of factors IX, X, XI, and XIII would be risk factors for VTE and that these would add to the risk associated with other VTE risk factors. We previously reported associations of higher FVIII and von Willebrand factor with greater VTE risk in this prospective study.14,15
Methods
Subjects
The LITE consists of participants of the Cardiovascular Health Study (CHS) and the Atherosclerosis Risks in Communities (ARIC) studies,16 population-based observational studies examining risk factors for cardiovascular diseases in 6 U.S. communities.17,18 In 1987 to 1989, 15 792 men and women 45 to 64 years of age were enrolled in ARIC; 27% were black. In 1989 to 1990, 5201 men and women 65 to 100 years of age were enrolled in the CHS. An additional 687 black participants were enrolled in the CHS in 1992 to 1993. All participants provided written informed consent with methods approved by institutional review committees at all participating institutions, in accordance with the Declaration of Helsinki.
Baseline risk factor data included medical history and measured height and weight. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Race was categorized as black or white based on participant self-identification. Diabetes was defined as fasting glucose more than or equal to 126 mg/dL, and nonfasting glucose was defined as more than or equal to 200 mg/dL or use of diabetes medication. The ARIC participants were followed by clinic visits every 3 years, annual telephone calls, and surveillance of community hospitals. The CHS participants were followed with alternating clinic visits and telephone calls every 6 months. Clinic visits ended in 1998 for ARIC and 1999 for CHS, and thereafter telephone calls and searches of the Centers for Medicare and Medicaid Services database (CHS only) continued to identify all hospitalizations.
Potential cases of VTE were identified from baseline to December 31, 2002, with median follow-up of 12.7 years in ARIC and 9.8 years in CHS. Cases were ascertained in 2 phases, first from enrollment to the end of 1998, then from 1998 through 2002. Hospital records were obtained and events validated by 2 physicians.16 VTE events required a positive duplex or Doppler ultrasound or venogram, high probability ventilation-perfusion scan, or positive chest computed tomography. Cases were classified as incident (no self-reported VTE history before baseline) or recurrent (self-reported VTE history before baseline), and as secondary (associated with active cancer, or within 90 days of major trauma, surgery, or marked immobility, such as coma, paralysis, or orthopedic-induced limitation) or idiopathic (not associated with the prior risk factors).16
A nested case-control study design was used to evaluate associations of coagulation factor levels and VTE. We randomly selected 2 controls, frequency-matched to VTE cases by 5-year age group, gender, race, follow-up time, and study (ARIC, CHS). Controls had not had VTE as of the selected follow-up date. Baseline blood samples were retrieved from storage freezers for cases and controls. When sample availability was limited, samples were retrieved from a later blood collection, almost always before events occurred. Of 548 cases and 1097 controls, 1520 of 1645 had sufficient blood samples for analysis and 18 using warfarin at baseline were excluded. Of the remaining 1502 participants, 7 who were selected as controls later developed VTE and were included in the analysis as both a control and a case. The analysis included 462 VTE cases and 1047 controls.
Laboratory methods
Blood was drawn in the morning after an overnight fast at baseline and 3 years later in both studies, and 7 years later in the ARIC. Samples were centrifuged at 4°C for 30 000g-minutes and stored in −70°C or colder freezers. Baseline factor VIII coagulant activity (VIIIc) was measured by one-stage clotting assays during enrollment in all participants except the additional CHS black cohort.19,20 In the nested case-control sample, factor IX, X, XI, XII, and XIIIA subunit antigenic levels were measured in citrate plasma by sandwich enzyme-linked immunoassays using reagents with affinity-purified polyclonal antibodies from Enzyme Research Laboratories. Assays were rarely done using post-VTE event blood samples. Coefficients of variation for control samples with 100% levels were 5.4%, 4.9%, 6.8%, 5.0%, and 6.9%, respectively. D-dimer was measured by immunoturbidometric analysis on the STA-R instrument (Liatest D-dimer; Diagnostica Stago). Factor V Leiden and prothrombin 20210A genotypes were determined by standard methods.
Statistical analysis
Coagulation factor levels were the independent variables, and their associations with each other and covariates of interest were examined using Spearman correlation coefficients or Wilcoxon rank-sum tests. Coagulation factor levels were then categorized into quintiles and were also dichotomized (at 60% for factor XII deficiency [∼ 2.5th percentile] and at the top decile for the other factors). Factor VIIIc and D-dimer were categorized as abnormal based on prior studies as levels in the top quartile and top tertile, respectively.14,21 Unconditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Robust variance estimates were used to account for data from the 7 controls who later became cases. Models examined each coagulation factor separately, first adjusting for age, sex, race, and study, and then also adjusting for baseline BMI and diabetes status, which were risk factors for VTE in LITE. Separate models were fit adding factor VIII level. Analyses were repeated in subsets (idiopathic vs secondary VTE and ARIC vs CHS). Additive interactions among VTE risk factors were assessed by estimating ORs after cross-classifying participants by each pair of risk factors, then calculating the relative excess risk due to interaction percentage, which is the percentage of disease attributable to the joint exposure.22,23
Results
Baseline coagulation factor levels and characteristics of the 462 participants who developed VTE and 1047 who did not are shown in Table 1. Those who developed VTE had a higher mean BMI than those who did not and were more likely to have diabetes. Cases had higher mean levels of factors IX and XI than noncases. Among the 462 VTE cases, 196 were idiopathic, 314 were DVT, and 148 were PE with or without DVT.
Table 1.
Baseline characteristics of participants who developed venous thromboembolism and those who did not: LITE
| Baseline characteristic | N* | No VTE (n = 1047) | VTE (n = 462) | P |
|---|---|---|---|---|
| Age, y† | 1509 | 62.3 (10.0) | 62.9 (10.0) | .34 |
| Male sex, %† | 1509 | 43% | 44% | .88 |
| Black race, %† | 1509 | 26% | 27% | .92 |
| Study, % ARIC† | 1509 | 63% | 59% | .22 |
| BMI, kg/m2 | 1503 | 27.5 (5.0) | 29.1 (5.6) | < .001 |
| Diabetes | 1498 | 11% | 17% | .003 |
| Factor VIII, % | 1415 | 128 (38) | 143 (48) | < .001 |
| D-dimer, μg/mL | 1494 | 0.59 (0.67) | 0.73 (0.79) | < .001 |
| Factor V Leiden heterozygote/homozygote, % | 1413 | 3.0% | 10.3% | < .001 |
| Prothrombin 20210A heterozygote/homozygote, % | 1417 | 2.1% | 3.5% | .14 |
| Factor IX, % | 1498 | 144 (31) | 147 (33) | .04 |
| Factor X, % | 1495 | 139 (35) | 139 (36) | .88 |
| Factor XI, % | 1497 | 132 (36) | 139 (37) | < .001 |
| Factor XII, % | 1494 | 141 (46) | 143 (45) | .53 |
| Factor XIII, % | 1499 | 141 (39) | 142 (42) | .65 |
Values are mean (SD) or percent prevalence. P values are from the χ2 or the t test.
Seven participants were counted as both a case and a control because of the sampling method.
Frequency matching variables.
Table 2 shows the interrelations of coagulation factors and VTE risk factors at baseline among participants who remained free of VTE. The coagulation factors were modestly correlated with each other, in particular the vitamin K–dependent factors, factors IX and X, which were also each modestly associated with factor XI. Factor XII was most closely associated with factor X, and factor XIII was moderately correlated with factors IX, X, and XI. Factor VIIIc was most strongly correlated with factor IX, but this association was weak to moderate (Spearman correlation coefficient, 0.19). D-dimer was correlated only with factor IX, though weakly. BMI was higher only with higher levels of factor IX, and to a lesser extent, factor X. All of the coagulation factors were higher in women than men, with the largest gender difference for factor XII. Older age was associated with higher levels of factors IX and XIII and lower levels of factors X, XI, and XII. Coagulation factor levels did not differ by factor V Leiden or prothrombin 20210 genotype.
Table 2.
Interrelations of coagulation factors and venous thromboembolism risk factors among participants without venous thromboembolism: LITE
| Coagulation factor, % |
|||||
|---|---|---|---|---|---|
| Factor IX | Factor X | Factor XI | Factor XII | Factor XIII | |
| Continuous covariates (Spearman correlation coefficient) | |||||
| FIX, % | 1 | ||||
| FX, % | 0.45‡ | 1 | |||
| FXI, % | 0.31‡ | 0.38‡ | 1 | ||
| FXII, % | 0.13‡ | 0.34‡ | 0.17‡ | 1 | |
| FXIII, % | 0.22‡ | 0.24‡ | 0.22‡ | 0.13‡ | 1 |
| Age, y | 0.17‡ | −0.09* | −0.14‡ | −0.08* | 0.09† |
| FVIII, % | 0.19‡ | 0.03 | 0.12† | 0.06 | 0.02 |
| BMI, kg/m2 | 0.28‡ | 0.12‡ | 0.07* | −0.02 | −0.04 |
| D-dimer, μg/mL | 0.14‡ | 0.003 | 0.03 | −0.04 | 0.03 |
| Binary covariates (coagulation factor means compared by Wilcoxon rank-sum test) | |||||
| Sex, F/M | 147/139‡ | 144/132‡ | 138/123‡ | 151/129‡ | 143/137* |
| Race, white/black | 142/148* | 138/140 | 131/134 | 147/125‡ | 139/145* |
| Study, ARIC/CHS | 140/150‡ | 139/138 | 135/127‡ | 142/139 | 137/148‡ |
| Diabetes, no/yes | 142/157‡ | 139/139 | 130/144† | 142/132* | 140/142 |
| Factor V Leiden, GG/AG or AA | 144/143 | 138/140 | 132/138 | 142/139 | 141/138 |
| Prothrombin 20210A, GG/AG | 144/138 | 139/136 | 132/129 | 141/147 | 141/138 |
P ≤ .05.
P ≤ .005.
P < .001.
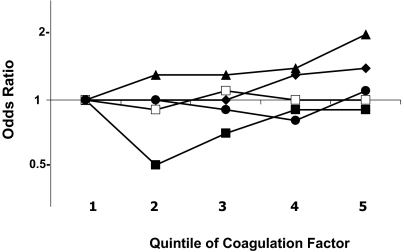
Figure 1 shows the ORs of VTE by quintiles of each coagulation factor, adjusted for age, sex, race, and study. VTE risk was higher with increasing levels of factors IX and XI, but not for the other factors. For factor IX, the ORs (95% CIs) for the fourth and fifth quintile were similar at 1.3 (0.9-1.9) and 1.4 (1.0-2.0), relative to the first quintile. Results were similar comparing the top decile to all lower values: 1.4 (1.0-1.9). Factor XI had more of a graded increase in risk with each increasing quintile, with ORs in the third, fourth, and fifth quintiles of 1.3 (0.9-1.9), 1.4 (1.0-2.1), and 2.0 (1.4-2.9), respectively. The OR for the top decile of factor XI compared with all lower values was 1.3 (0.9-1.8). Similar ORs were found for the top decile of factors X and XIII compared with all lower values, at 1.3 (0.9-1.8) for each. Results for factors X, XII, and XIII are shown in detail in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Results for all coagulation factors were nearly identical after exclusion of 37 cases classified as recurrent VTE, or of 51 cases and 30 controls who self-reported a history of VTE at baseline (data not shown).
Figure 1.
Odds ratios of venous thromboembolism by quintiles of coagulation factors. Analyses were adjusted for age, sex, race, and study. ♦ represents factor IX; ■, factor X; ▴, factor XI; □, factor XII; and ●, factor XIII. Fifth quintile cutoffs are 167% for factor IX, 161% for factor X, 157% for factor XI, 179% for factor XII, and 171% for factor XIII.
Table 3 shows the impact of added adjustment for BMI and diabetes on the ORs of VTE associated with factors IX and XI. In these models, the positive association of factor IX level with VTE was attenuated to the null (primarily by adjustment for BMI), but the association of higher factor XI with VTE remained strong. For factor IX, adjusted ORs were similar for idiopathic and secondary VTE, and in each cohort. ORs for factor XI in the top quintile relative to the first were similar for idiopathic and secondary VTE and were slightly higher in the older CHS cohort (2.2; 1.2-4.0) than in the ARIC (1.7; 1.0-2.7). The adjusted ORs for factor XI in the top quintile were higher in relation to risk of PE with or without DVT (2.4; 1.3-4.4) than DVT alone (1.7; 1.1-2.5). ORs also did not differ considering only participants with a first-time VTE. For both factors, results were null comparing values above versus below the 90th percentile (factor IX > 184%, factor XI > 176%). Exclusion of those with obesity or diabetes from analysis did not alter interpretation of the findings for either factor. For factors IX and XI, results were similar for cases occurring within 1 year compared with beyond 1 year after factors were measured.
Table 3.
Odds ratios (95% confidence intervals) of venous thromboembolism in relation to quintiles of factor IX and XI: LITE
| Odds ratio (95% CI) of VTE by quintile |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Factor IX, % | < 118 | 118-133 | 133-147 | 147-167 | ≥ 167 |
| Overall VTE | |||||
| Model 1 | 1 (reference) | 1.0 (0.7-1.4) | 1.0 (0.7-1.4) | 1.3 (0.9-1.9) | 1.4 (1.0-2.0) |
| Model 2 | 1 (reference) | 0.9 (0.6-1.3) | 0.9 (0.6-1.3) | 1.1 (0.8-1.6) | 1.1 (0.7-1.6) |
| Idiopathic VTE | |||||
| Model 1 | 1 (reference) | 0.8 (0.5-1.4) | 0.9 (0.5-1.5) | 1.3 (0.8-2.1) | 1.3 (0.8-2.1) |
| Model 2 | 1 (reference) | 0.8 (0.5-1.4) | 0.8 (0.5-1.4) | 1.1 (0.7-1.8) | 1.0 (0.6-1.7) |
| Secondary VTE | |||||
| Model 1 | 1 (reference) | 1.1 (0.7-1.8) | 1.1 (0.7-1.7) | 1.4 (0.9-2.1) | 1.5 (1.0-2.4) |
| Model 2 | 1 (reference) | 1.0 (0.6-1.6) | 1.0 (0.6-1.5) | 1.2 (0.7-1.8) | 1.1 (0.7-1.8) |
| ARIC | |||||
| Model 1 | 1 (reference) | 1.0 (0.7-1.6) | 0.8 (0.5-1.2) | 1.6 (1.0-2.4) | 1.5 (1.0-2.4) |
| Model 2 | 1 (reference) | 0.9 (0.6-1.4) | 0.7 (0.4-1.2) | 1.3 (0.9-2.1) | 1.2 (0.7-1.9) |
| CHS | |||||
| Model 1 | 1 (reference) | 0.9 (0.5-1.8) | 1.4 (0.7-2.6) | 1.1 (0.6-2.0) | 1.3 (0.7-2.4) |
| Model 2 | 1 (reference) | 1.0 (0.5-1.9) | 1.3 (0.7-2.4) | 0.9 (0.5-1.7) | 1.0 (0.5-1.8) |
| Factor XI, % | < 104 | 104-120 | 120-134 | 134-157 | ≥ 157 |
| Overall VTE | |||||
| Model 1 | 1 (reference) | 1.3 (0.9-1.9) | 1.3 (0.9-1.9) | 1.4 (1.0-2.1) | 2.0 (1.4-2.9) |
| Model 2 | 1 (reference) | 1.3 (0.9-1.9) | 1.2 (0.8-1.8) | 1.4 (1.0-2.0) | 1.8 (1.3-2.7) |
| Idiopathic VTE | |||||
| Model 1 | 1 (reference) | 1.2 (0.7-2.1) | 1.3 (0.8-2.2) | 1.5 (0.9-2.5) | 2.2 (1.3-3.6) |
| Model 2 | 1 (reference) | 1.2 (0.7-2.1) | 1.3 (0.8-2.2) | 1.4 (0.8-2.5) | 2.0 (1.2-3.4) |
| Secondary VTE | |||||
| Model 1 | 1 (reference) | 1.4 (0.9-2.2) | 1.3 (0.8-2.0) | 1.4 (0.9-2.2) | 2.0 (1.2-3.1) |
| Model 2 | 1 (reference) | 1.3 (0.8-2.1) | 1.2 (0.8-1.9) | 1.4 (0.9-2.2) | 1.7 (1.1-2.7) |
| ARIC | |||||
| Model 1 | 1 (reference) | 1.5 (0.9-2.4) | 1.1 (0.7-1.9) | 1.4 (0.8-2.2) | 1.9 (1.2-3.0) |
| Model 2 | 1 (reference) | 1.4 (0.8-2.3) | 1.1 (0.6-1.8) | 1.3 (0.8-2.1) | 1.7 (1.0-2.7) |
| CHS | |||||
| Model 1 | 1 (reference) | 1.2 (0.7-2.0) | 1.5 (0.9-2.7) | 1.6 (0.9-2.8) | 2.3 (1.3-4.2) |
| Model 2 | 1 (reference) | 1.2 (0.7-2.1) | 1.5 (0.9-2.7) | 1.6 (0.8-2.8) | 2.2 (1.2-4.0) |
Model 1 adjusted for age, sex, race, and study. Model 2 adjusted additionally for BMI and diabetes.
For factor X, adjustment for BMI and diabetes attenuated the ORs for values above the 90th percentile (data not shown). ORs were similarly null for idiopathic and secondary VTE and by study. For factor XIII above the 90th percentile, there was no impact of adjustment for BMI and diabetes, and results for idiopathic or secondary VTE and by study were similar to the overall analysis (data not shown).
There was no evidence of higher risk of VTE with factor XII deficiency. Participants with levels less than 60% compared with higher levels had an adjusted OR of VTE of 0.9 (0.5-1.7). This was 0.7 (0.3-1.9) for idiopathic VTE and 1.1 (0.5-2.3) for secondary VTE.
Factors IX and XI were modestly directly correlated with factor VIIIc (Table 2). In models adjusting for age, sex, race, diabetes, BMI, and factor VIIIc level, the ORs associated with factors IX and XI in the top versus bottom quintile were 1.0 (0.6-1.4) and 1.6 (1.1-2.4), respectively. The adjusted OR per one SD higher factor VIII and factor IX were 1.4 (1.2-1.6) and 1.2 (1.0-1.3), respectively, while the OR for factor VIII in the top quintile was 2.1 (1.4-3.0).
The OR of VTE for D-dimer in the top tertile compared with the bottom tertile, adjusted for age, race, sex, study, BMI, and diabetes was 2.0 (1.5-2.7). The OR for the top versus bottom quintile of factor XI adjusting for these factors plus D-dimer was 1.8 (1.2-2.6). In Table 4, elevated factor XI did not interact on more than an additive scale with other VTE risk factors.
Table 4.
Factor XI and other risk factors in relation to risk of VTE: LITE
| Factor XI > 157% (top quintile) | Additional risk factor* | No. of cases | No. of controls | OR (95% CI)† | RERI % (95% CI) |
|---|---|---|---|---|---|
| D-dimer > 0.56 ng/mL | |||||
| No | No | 195 | 594 | 1 (reference) | −7% (−94% to +80%) |
| No | Yes | 134 | 230 | 1.8 (1.3 to 2.4) | |
| Yes | No | 74 | 132 | 1.5 (1.1 to 2.2) | |
| Yes | Yes | 53 | 73 | 2.2 (1.5 to 3.4) | |
| Factor VIII > 153% | |||||
| No | No | 221 | 627 | 1 (reference) | +27% (−44% to +99%) |
| No | Yes | 87 | 151 | 1.6 (1.1 to 2.1) | |
| Yes | No | 69 | 140 | 1.3 (0.9 to 1.9) | |
| Yes | Yes | 50 | 59 | 2.2 (1.4 to 3.4) | |
| Factor V Leiden† | |||||
| No | No | 278 | 760 | 1 (reference) | −31% (−204% to +142%) |
| No | Yes | 30 | 20 | 4.1 (2.2 to 7.4) | |
| Yes | No | 105 | 185 | 1.5 (1.1 to 2.0) | |
| Yes | Yes | 13 | 10 | 3.7 (1.6 to 8.6) | |
| Prothrombin 20210A† | |||||
| No | No | 299 | 763 | 1 (reference) | +54% (−62% to +170%) |
| No | Yes | 11 | 17 | 1.6 (0.7 to 3.4) | |
| Yes | No | 114 | 194 | 1.4 (1.1 to 1.9) | |
| Yes | Yes | 4 | 3 | 3.2 (0.7 to 14.5) |
Defined as top tertile of D-dimer, top quartile of factor VIII, and heterozygotes plus homozygotes for each mutation.
Adjusted for age, race, sex, study, BMI, and diabetes.
Discussion
The main finding of this prospective epidemiologic study is that, among several procoagulant factors (IX-XIII), only elevated factor XI was independently associated with increased risk of future VTE. This association was not confounded by levels of factor VIIIc or D-dimer, which were strong VTE risk factors in this study.14,21 The association was slightly weaker than for factor VIIIc. This association was similar for idiopathic and secondary VTE and among younger and older participants but was stronger for PE than DVT. Elevated factor XI added to the risk associated with some other risk factors, but interactions beyond additivity were not significant. Factor XII deficiency was not associated with increased VTE risk.
Other than factor XII, there are few studies assessing these procoagulant factors in relation to VTE risk. Our finding for elevated factor XI agrees with the LETS and MEGA findings, which suggested a 2.3- and 1.9-fold increased risk of VTE, respectively, after adjustment for age, sex, and oral contraceptive use (the latter assessed in LETS only). Gene discovery studies have confirmed that variation in the factor XI gene was associated with VTE risk,24,25 and the polymorphisms in both studies were in strong linkage disequilibrium. In the LETS and MEGA studies, this gene variation was associated with factor XI levels and adjustment for factor XI weakened but did not eliminate the excess risk associated with gene variation. Taken together with our findings in this prospective study, there is strong evidence for a causal relationship of factor XI variation with VTE risk. Presumably, the reason for this relationship is increased thrombin formation and perhaps fibrin stability26 related to higher levels of factor XI, hypotheses that deserved further study.
There are 2 other studies of elevated factor IX and VTE risk. The LETS reported an OR of 3.0, whereas a study of postmenopausal women in the United Kingdom reported an OR of 2.3 for higher versus lower levels. In the current report, a weak unadjusted association of elevated factor IX with VTE was seen, but this association disappeared after adjustment for BMI, a correlate of higher factor IX and risk factor for VTE, which was not considered in the previous studies. In terms of causation, factor IX polymorphisms have been inconsistently associated with VTE risk,24,25,27 and factor IX gene variation has not been associated with factor IX levels in the context of VTE.27 Overall the findings suggest that, unless factor IX alterations are in the causal pathway between obesity and VTE, it is not a clinically relevant factor.
The LETS, the only study available, reported an OR of 1.8 (1.2-2.7) for factor X antigen above versus below the 90th percentile, adjusting for age, sex, and use of oral contraceptives.3 This differs considerably from our findings, and in LETS the association was confounded by levels of vitamin K-dependent factors. It is possible that changes in factor X level after a VTE led to a spurious association in the case-control study compared with our prospective study, but other factors, such as a large number of contraceptive-related VTE in LETS, high within-person variability of factor X contributing to falsely null findings here, or assay differences could be considered. Finally, the LETS analysis did not find associations of factor X gene variants with factor X levels,3 further supporting no etiologic link of factor X with thrombosis.
The first reported case of factor XII deficiency was in John Hagemen, who died of a PE after a fracture,28 leading to a hypothesis that factor XII deficiency causes VTE. The reasoning is that factor XII deficiency leads to impaired fibrinolysis.29 In agreement with our findings, the first analysis of this question in a large study was by the LETS group, where there was no association of factor XII deficiency with thrombosis risk (OR 0.8; 95% CI 0.6-2.4).5 Results from LETS and other studies included analyses of a polymorphism of the factor XII gene, 46 C → T, which is associated with substantially lower factor XII levels. In 2 studies comprising 250 and 300 cases, and a study involving 81 Japanese cases, homozygosity for this polymorphism was approximately 3- to 3.5-fold more common among VTE cases than controls.7,8,30 In one of these studies, factor XII deficiency was not significantly more common in cases than controls.7 The LETS group31 also did not provide confirmation. More recently, a study evaluating a small number of pregnancy-associated thrombosis cases suggested a 6-fold higher risk of VTE with this polymorphism.9 Gene discovery studies of VTE etiology to date, including one assessing haplotypes of factor XII,24 have not corroborated these associations.25 Given the mixed results from different populations, more work on factor XII and VTE risk is needed.
Only the LETS has investigated factor XIII A-subunit level and VTE risk,32 where results were similar to those reported here. In a meta-analysis of retrospective studies involving 3165 cases, the factor XIII Val34Leu polymorphism, which is associated weakly with higher factor XIII activity but not with subunit levels, was inversely associated with VTE risk, but this was not confirmed in the subsequently published LITE study, which had the largest sample size of studies to date.11 A mutation in the factor XIII B-subunit, the carrier for the factor XIII A-subunit, leads to increased dissociation of factor XIII in plasma and has also been related to modestly increased thrombosis risk.33
Strengths of our study include the prospective design, with coagulation factors almost always measured before occurrence of events. This feature rules out reverse causality as an explanatory factor. Associations of factor XI with VTE risk were similar for near-term and longer-term time periods, strengthening our conclusions. Participants were followed frequently, and careful ascertainment was used to validate VTE events. The study included 2 general population samples, although some participants, such as those in nursing homes and with advanced medical conditions, were excluded at baseline. Finally, there were a large number of cases, allowing ample power and some subgroup analyses. Limitations should also be considered. We know little about the within-person variability of these coagulation factors, so that levels measured years before a VTE may not reflect levels closer to the time of the event. This could lead to underestimation of associations and an interpretation that associations of factor XI with VTE risk might be larger than we observed. We included participants who self-reported prior VTE in our analysis; however, exclusion of these participants did not alter the findings. Finally, findings related to differential associations for DVT versus PE need to be interpreted cautiously, as case classification in this study might not be reliable.
In conclusion, among these procoagulant factors, only elevated factor XI was a risk factor for VTE in this study. More work on the epidemiology and genetics of factor XI in relation to VTE is needed, including clarification of any role of clinical testing of factor XI level in people at risk for VTE. Testing of other factors studied here in clinical practice does not seem indicated.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC and CHS studies for their important contributions.
This work was supported by the National Heart, Lung, and Blood Institute (grant R01 HL59367, LITE; contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022, ARIC; and contracts N01-HC-85079 through N01-HC-8586, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, U01 HL080295, with additional contribution from the National Institute of Neurological Disorders and Stroke, CHS). The sponsor had no role in analysis or interpretation of this study. A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Footnotes
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.C., A.R.F., and S.R.H. designed and performed research, collected data, interpreted data, and wrote the manuscript; and E.S.O. analyzed and interpreted data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary Cushman, Department of Medicine and Pathology, University of Vermont, 208 South Park Dr, Colchester, VT 05446; e-mail: mary.cushman@uvm.edu.
References
- 1.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62–69. doi: 10.1053/j.seminhematol.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Hylckama Vlieg A, ven der Linden I, Bertina R, Rosendaal F. High levels of factor IX increase the risk of venous thrombosis. Blood. 2000;95(12):3678–3682. [PubMed] [Google Scholar]
- 3.de Visser MC, Poort SR, Vos HL, Rosendaal FR, Bertina RM. Factor X levels, polymorphisms in the promoter region of factor X, and the risk of venous thrombosis. Thromb Haemost. 2001;85(6):1011–1017. [PubMed] [Google Scholar]
- 4.Meijers JC, Tekelenberg WL, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. N Engl J Med. 2000;342(10):696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 5.Koster T, Rosendaal FR, Briet E, Vandenbroucke JP. John Hageman's factor and deep-vein thrombosis: Leiden thrombophilia Study. Br J Haematol. 1994;87(2):422–424. doi: 10.1111/j.1365-2141.1994.tb04937.x. [DOI] [PubMed] [Google Scholar]
- 6.Halbmayer WM, Mannhalter C, Feichtinger C, Rubi K, Fischer M. The prevalence of factor XII deficiency in 103 orally anticoagulated outpatients suffering from recurrent venous and/or arterial thromboembolism. Thromb Haemost. 1992;68(3):285–290. [PubMed] [Google Scholar]
- 7.Tirado I, Soria JM, Mateo J, et al. Association after linkage analysis indicates that homozygosity for the 46C → T polymorphism in the F12 gene is a genetic risk factor for venous thrombosis. Thromb Haemost. 2004;91(5):899–904. doi: 10.1160/TH03-10-0620. [DOI] [PubMed] [Google Scholar]
- 8.Gallimore MJ, Harris SL, Jones DW, Winter M. Plasma levels of factor XII, prekallikrein and high molecular weight kininogen in normal blood donors and patients having suffered venous thrombosis. Thromb Res. 2004;114(2):91–96. doi: 10.1016/j.thromres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Cochery-Nouvellon E, Mercier E, Lissalde-Lavigne G, et al. Homozygosity for the C46T polymorphism of the F12 gene is a risk factor for venous thrombosis during the first pregnancy. J Thromb Haemost. 2007;5(4):700–707. doi: 10.1111/j.1538-7836.2007.02423.x. [DOI] [PubMed] [Google Scholar]
- 10.Wells PS, Anderson JL, Scarvelis DK, Doucette SP, Gagnon F. Factor XIII Val34Leu variant is protective against venous thromboembolism: a HuGE review and meta-analysis. Am J Epidemiol. 2006;164(2):101–109. doi: 10.1093/aje/kwj179. [DOI] [PubMed] [Google Scholar]
- 11.Cushman M, Cornell A, Folsom AR, et al. Associations of the beta-fibrinogen Hae III and factor XIII Val34Leu gene variants with venous thrombosis. Thromb Res. 2007;121(3):339–345. doi: 10.1016/j.thromres.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowe GD, Haverkate F, Thompson SG, et al. Prediction of deep vein thrombosis after elective hip replacement surgery by preoperative clinical and haemostatic variables: the ECAT DVT Study. European Concerted Action on Thrombosis. Thromb Haemost. 1999;81(6):879–886. [PubMed] [Google Scholar]
- 13.Heit JA. Thrombophilia: common questions on laboratory assessment and management. Hematology Am Soc Hematol Educ Program. 2007:127–135. doi: 10.1182/asheducation-2007.1.127. [DOI] [PubMed] [Google Scholar]
- 14.Tsai AW, Cushman M, Rosamond WD, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). Am J Med. 2002;113(8):636–642. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 15.Ohira T, Cushman M, Tsai MY, et al. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE). J Thromb Haemost. 2007;5(7):1455–1461. doi: 10.1111/j.1538-7836.2007.02579.x. [DOI] [PubMed] [Google Scholar]
- 16.Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the Longitudinal Investigation of Thromboembolism Etiology. Am J Med. 2004;117(1):19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 18.Atherosclerosis Risk in Communities. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 19.Chambless L, McMahon R, Finch A, et al. ARIC Hemostasis Study III: quality control. Thromb Haemost. 1993;70(4):588–594. [PubMed] [Google Scholar]
- 20.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;42(2):264–270. [PubMed] [Google Scholar]
- 21.Cushman M, Folsom AR, Wang L, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101(4):1243–1248. doi: 10.1182/blood-2002-05-1416. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 23.Assmann SF, Hosmer DW, Lemeshow S, Mundt KA. Confidence intervals for measures of interaction. Epidemiology. 1996;7(3):286–290. doi: 10.1097/00001648-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Smith NL, Hindorff LA, Heckbert SR, et al. Association of genetic variations with nonfatal venous thrombosis in postmenopausal women. JAMA. 2007;297(5):489–498. doi: 10.1001/jama.297.5.489. [DOI] [PubMed] [Google Scholar]
- 25.Bezemer ID, Bare LA, Doggen CJ, et al. Gene variants associated with deep vein thrombosis. JAMA. 2008;299(11):1306–1314. doi: 10.1001/jama.299.11.1306. [DOI] [PubMed] [Google Scholar]
- 26.von dem Borne PA, Cox LM, Bouma BN. Factor XI enhances fibrin generation and inhibits fibrinolysis in a coagulation model initiated by surface-coated tissue factor. Blood Coagul Fibrinolysis. 2006;17(4):251–257. doi: 10.1097/01.mbc.0000224843.33216.5f. [DOI] [PubMed] [Google Scholar]
- 27.van Minkelen R, de Visser MC, van Hylckama Vlieg A, Vos HL, Bertina RM. Sequence variants and haplotypes of the factor IX gene and the risk of venous thrombosis. J Thromb Haemost. 2008;6(9):1610–1613. doi: 10.1111/j.1538-7836.2008.03066.x. [DOI] [PubMed] [Google Scholar]
- 28.Ratnoff OD, Busse RJ, Shean RP. The demise of John Hageman. N Engl J Med. 1968;279:760–761. [Google Scholar]
- 29.Mandle RJ, Jr, Kaplan AP. Hageman-factor-dependent fibrinolysis: generation of fibrinolytic activity by the interaction of human activated factor XI and plasminogen. Blood. 1979;54(4):850–862. [PubMed] [Google Scholar]
- 30.Kanaji T, Watanabe K, Hattori S, et al. Factor XII gene (F12)-4C/C polymorphism in combination with low protein S activity is associated with deep vein thrombosis. Thromb Haemost. 2006;96(6):854–855. [PubMed] [Google Scholar]
- 31.Bertina RM, Poort SR, Vos HL, Rosendaal FR. The 46C → T polymorphism in the factor XII gene (F12) and the risk of venous thrombosis. J Thromb Haemost. 2005;3(3):597–599. doi: 10.1111/j.1538-7836.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 32.van Hylckama Vlieg A, Komanasin N, Ariens RA, et al. Factor XIII Val34Leu polymorphism, factor XIII antigen levels and activity and the risk of deep venous thrombosis. Br J Haematol. 2002;119(1):169–175. doi: 10.1046/j.1365-2141.2002.03797.x. [DOI] [PubMed] [Google Scholar]
- 33.Komanasin N, Catto AJ, Futers TS, et al. A novel polymorphism in the factor XIII B-subunit (His95Arg): relationship to subunit dissociation and venous thrombosis. J Thromb Haemost. 2005;3(11):2487–2496. doi: 10.1111/j.1538-7836.2005.01624.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.