Abstract
The mammalian target of rapamycin (mTOR) has emerged as an important therapeutic target for diffuse large B-cell lymphoma (DLBCL), as recent studies have demonstrated that 30% of relapsed patients respond to mTOR inhibitors. Why some lymphomas are resistant is incompletely understood. In the present study, we demonstrated that rapamycin inhibits mTORC1 in DLBCL lines and primary tumors but is minimally cytotoxic. Subsequent investigations revealed that rapamycin also activated eIF4E and the mTORC2 target Akt, suggesting a potential mechanism of rapamycin resistance. Furthermore, knockdown of the mTORC2 component rictor, but not the mTORC1 component raptor, inhibited rapamycin-induced Akt phosphorylation in lymphoma cells. Addition of the histone deacetylase inhibitor (HDI) LBH589 (LBH) overcame rapamycin resistance by blocking mTOR, thus preventing Akt activation. Further studies support the involvement of the protein phosphatase PP1 in LBH-mediated Akt dephosphorylation, which could be mimicked by knockdown of HDAC3. This is the first demonstration that a HDI such as LBH can overcome rapamycin resistance through a phosphatase that antagonizes mTORC2 activation. These results provide a mechanistic rationale for a clinical trial of a combination of HDI and mTOR inhibitors for DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL), an aggressive form of non-Hodgkin lymphoma (NHL), is the most common type of lymphoma in the United States. With rituximab-based chemoimmunotherapy such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, approximately 60% of DLBCL patients are cured.1,2 Salvage chemotherapy followed by stem cell transplantation is able to produce durable remissions in a minority of relapsed patients, and improved therapy is required for those who relapse after second-line treatment.
Because deregulation of the PI3 kinase (PI3K)/mTOR pathway occurs in many human diseases,3,4 targeting the mTOR pathway with small molecule inhibitors has become an intense area of research. Key components of this pathway, including Akt and mTOR, regulate cell growth and survival.5 The mTOR kinase exists as 2 complexes. The rapamycin-sensitive mTOR complex 1 (mTORC1 or raptor/mTOR), consists of mTOR, raptor, and mLST8. mTORC1 regulates translation initiation through 2 distinct pathways: ribosomal p70 S6 kinase (p70S6K) and eukaryotic translation initiation factor 4E (eIF4E) binding proteins (4E-BPs). In one pathway, mTORC1 phosphorylates and activates the ribosomal protein S6. In the second pathway, mTORC1 directly phosphorylates 4E-BP1 causing its dissociation from the translation initiation factor eIF4E. This allows eIF4E to stimulate cap-dependent RNA translation. In the absence of mTORC1 activation, 4EBP1 binds tightly to eIF4E, preventing it from binding to 5′-capped mRNA.6 The mTOR complex 2 (mTORC2 or rictor/mTOR), which contains mTOR, rictor, and mLST8, is rapamycin insensitive and functions to regulate the survival kinase Akt by phosphorylation of serine 473.5
Recent clinical trials of the mTORC1 inhibitors temsirolimus and everolimus, both analogues of the parent compound rapamycin, have demonstrated overall response rates (ORRs) of approximately 30% for relapsed DLBCL.7 This single-agent activity of mTOR inhibitors in heavily pretreated DLBCL patients highlights the importance of the PI3K/mTOR pathway in these cells. To exploit the sensitivity of lymphomas to mTOR inhibitors through effective therapies, it is important to understand the mechanistic basis for resistance of DLBCL to mTOR inhibition.
Histone deacetylase inhibitors (HDIs) have emerged as a potentially promising new class of anticancer drugs. The inhibition of histone deacetylases (HDACs) by HDIs results in increased gene-specific histone acetylation, which can lead to reactivation of silenced genes, morphologic reversion of transformed cells, differentiation, inhibition of cell growth, induction of apoptosis, and inhibition of angiogenesis in cancer cell lines.8,9 Several structurally diverse classes of synthetic compounds have been identified as HDIs.10,11 HDACs are involved in the pathogenesis of some lymphomas, notably cutaneous T-cell lymphoma.12 Vorinostat, a potent oral HDI belonging to the class of hydroxamic acid–containing hybrid polar molecules, is now FDA approved for relapsed cutaneous T-cell lymphoma.13 The potential role of HDACs in other lymphoma is not well understood.
LBH589 (LBH) is a cinnamic acid hydroxamate HDI currently being tested in clinical trials for various malignancies. LBH inhibits cell proliferation and induces apoptosis in preclinical models. Moreover, LBH exhibits antileukemic effects in phase 1 studies.14 The goals of the current studies were to investigate the mechanisms of resistance of DLBCL to mTOR inhibition and to determine whether a HDI such as LBH can overcome this resistance. Our results demonstrate that important and unanticipated effects of LBH on mTORC1/mTORC2 signaling in DLBCL cells lead to synergistic inhibition of lymphoma cell survival when rapamycin and LBH are combined.
Methods
Reagents
LBH589 (panobinostat) was provided by Novartis-Pharmaceutical. Annexin V–fluorescein isothiocyanate was obtained from BD Biosciences. Rapamycin and antibodies (total and phospho) specific for p85, PDK1, Akt (Ser 473), S6 (Ser 235/236), mTOR (Ser 2448), 4E-BP1(Thr 37/46), eIF4E (Ser 219), rictor, raptor, H3, H4, and all the HDACs were obtained from Cell Signaling Technology. Antibodies for protein phosphatase 1 (PP1) and PP1 alpha (PP1a) were from Santa Cruz Biotechnology. Calyculin A and ZVAD-fmk were purchased from Calbiochem and R&D Systems, respectively. PI3K inhibitors LY294002 and wortmannin were purchased from Calbiochem. Akt-specific inhibitor A443654 was a kind gift from S.H.K.
Lymphoma samples
Tumor samples from DLBCL were obtained through the University of Iowa/Mayo Lymphoma SPORE Biospecimens Core. All samples were surgical waste, and patients signed informed consent to provide excess tissue for research on a protocol approved by the Mayo Clinic institutional review board, in accordance with the Declaration of Helsinki. Mononuclear cells were isolated from lymph node biopsies by mechanical disruption over a wire mesh screen followed by Ficoll-Hypaque centrifugation. A total of 20 primary samples of DLBCL were used for this study. SUDHL-6 (DHL-6), OCI-Ly7 (Ly7), and OCI-Ly3 (Ly3) DLBCL cell lines were a gift from Dr Margaret Shipp (Dana-Farber Cancer Center). Ly7 and Ly3 cell lines were cultured in IMDM medium supplemented with 10% fetal calf bovine serum. DHL-6 was maintained in RPMI with 10% fetal calf bovine serum.
Immunohistochemistry
Sections (4 μm thick) were cut from formalin-fixed paraffin-embedded tissue blocks. After drying at 60°C for 60 minutes, the slides were deparaffinized. Endogenous peroxidase activity was quenched by incubation of sections in a 1:1 mixture of 3% hydrogen peroxide and absolute methanol. The slides were stained using DAKO autostainer under standard conditions, rinsed well, counterstained with hematoxylin for 30 seconds, and mounted with aqueous mounting media. All slides were observed with light microscopy (Olympus AX70, 400×/0.75 numeric aperature; Olympus America). All images were captured with SPOT RT camera and software (Diagnostic Instruments).
Cell survival by annexin V/PI
Malignant cells from DLBCL patients (0.5 × 106 cells/mL) or from DLBCL lines were incubated in 48-well plates (0.5 mL/well). Cells were cultured in the presence of various concentrations of LBH or rapamycin at 37°C, stained using 1 μg/mL annexin V–FITC for 30 minutes at 4°C, washed once in annexin V binding buffer, stained with 0.5 μg/mL propidium iodide (PI), and analyzed by flow cytometry (FACSCalibur; Becton Dickinson). Data analysis was performed with FlowJo software (TreeStar).
Proliferation assay
Cells from DLBCL lines were cultured for 48 hours at 37°C in 96-well round-bottom microtiter plates (Costar) at a density of 5 × 104 cells/well in the presence of various concentrations of LBH. Before harvesting, cells were pulsed with 1 μCi (0.037 MBq) tritiated thymidine (3H-TdR; Amersham) for 18 hours. 3H-TdR incorporation levels were determined using a Beckman scintillation counter (GMI).
Western blot analysis
Cells (5 × 106) were incubated with various concentrations of LBH or rapamycin at 37°C for various lengths of time, as indicated. Cells were washed with cold PBS and lysed with radioimmunoprecipitation assay lysis buffer supplemented with 1 mM phenylmethylsulfonyl fluoride and protease inhibitors (Roche Diagnostic). After clarification of the lysates by centrifugation, proteins were resolved on 10% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were blocked and probed with antibodies as indicated and subsequently incubated with peroxidase-conjugated secondary antibody. Bound antibodies were visualized using Pierce Super Signal (Amersham Biosciences). After incubation at room temperature for 15 minutes in stripping buffer (Pierce Biotechnology), blots were reprobed with additional antibodies.
Coimmunoprecipitation assay
To the cleared lysates, 5 μg specific antibody was added and complexes were allowed to form by incubating with rotation for 60 minutes at 4°C. A 50% slurry (25 μL) of protein A–Sepharose was then added and the incubation continued for 1 hour. Immunoprecipitates captured with protein A–Sepharose were washed 4 times with radioimmunoprecipitation assay buffer and analyzed by immunoblotting.
siRNA transfection
DHL-6 and Ly7 cells were transfected with human B cell Nucleofector kit (Amaxa Biosystems). Briefly, 10 × 106 cells were transiently transfected with 250 nM or 500 nM rictor or raptor (Dharmacon) or HDAC3 or HDAC4 siRNA (Santa Cruz Biotechnology) using U-15 program. For c-Myc inhibition, c-Myc siRNA kit (Cell Signaling Technologies) was used according to the supplier's instructions.
Results
Activation of the mTOR signaling pathway in lymphoma samples from DLBCL patients
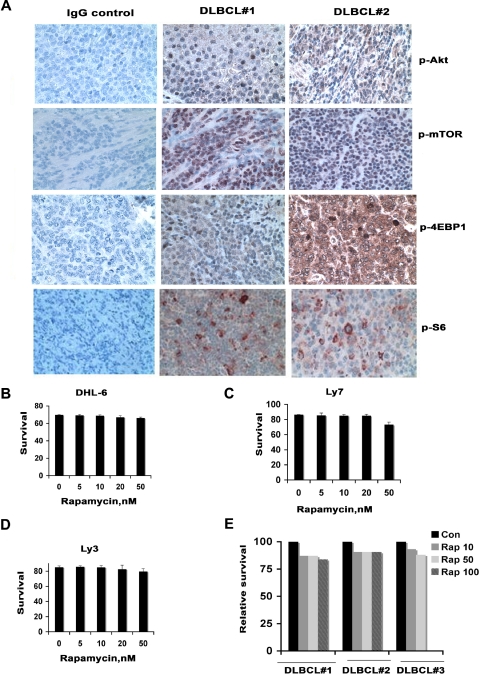
To examine activation of the mTOR pathway in fresh human lymphoma tissues, immunohistochemistry was performed on primary tumor samples from 10 DLBCL patients using antibodies against mTOR and its downstream targets the ribosomal protein S6 (S6rp) and 4EBP1. Phosphorylation of mTOR, S6, 4EBP1, and Akt was detected in all 10 lymphomas (Figure 1A).
Figure 1.
Activation of mTOR signaling in DLBCL. (A) Constitutive activation of the mTOR signaling pathway in patient samples of DLBCL. Activation of Akt, mTOR, S6rp, and 4EBP1 was assessed in DLBCL tissue by immunohistochemistry with phospho-specific antibodies for Akt mTOR or S6rp or 4EBP1. (B-E) Effect of rapamycin on DLBCL survival. After DHL-6, Ly7, and Ly3 cell lines (B-D) or primary cells from 3 DLBCL patients (E) were treated with the indicated concentrations of rapamycin for 48 hours, survival was assessed using annexin and propidium iodide followed by flow cytometry. Data from 1 representative experiment are shown for each cell line (n = 3). Bar graph shows mean ± standard deviation (SD) from 3 determinations.
DLBCL cells are resistant to rapamycin cytotoxicity
The finding that the mTOR signaling pathway is constitutively activated in primary DLBCL tissue suggested that mTOR inhibition might inhibit growth of these cells. Three representative DLBCL cell lines, DHL-6, Ly7, and Ly3, were treated with increasing doses of rapamycin (5 nM-100 nM) for 48 hours and assessed for the effects on proliferation and survival. These concentrations of rapamycin were chosen based on achievable concentrations of rapamycin in humans. Rapamycin produced less than a 10% inhibition of cell survival in all cell lines without evidence of a dose-dependent effect (Figure 1B-D). Because DLBCL lines differ from fresh primary DLBCL samples in terms of growth rates, altered microenvironment, and the absence of interacting stroma, we treated malignant cells from 3 different DLBCL patients with rapamycin in vitro and assessed lymphoma cell survival at 48 hours. As observed in the cell lines, the survival of the primary DLBCL cells was minimally reduced in response to rapamycin (Figure 1E).
Although rapamycin does not affect lymphoma cell survival, we did demonstrate partial inhibition of proliferation in all of the DLBCL lines. This was not, however, dose dependent (supplemental Figure 1A-B, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results suggest that lymphoma cells may have a potential mechanism to bypass growth inhibition caused by rapamycin.
Inhibition of mTOR by rapamycin increases the target of mTORC2 while decreasing mTORC1 targets
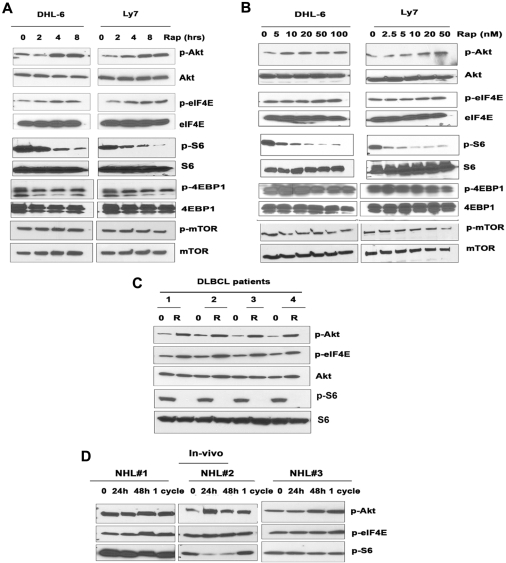
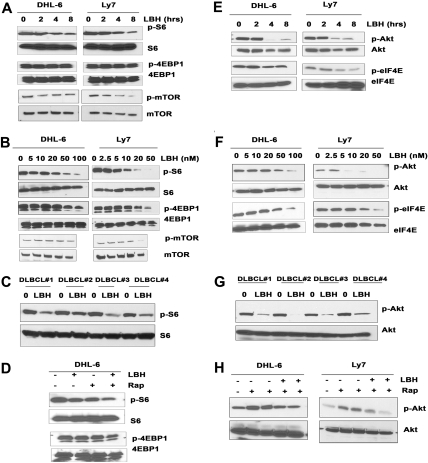
The results in Figure 1 prompted us to ask whether rapamycin was actually blocking mTOR and how rapamycin differentially affected mTORC1 versus mTORC2 in DLBCL cells. The effects of rapamycin on mTORC2 signaling in DLBCL were investigated by examining the mTORC2-mediated phosphorylation of Akt on Ser 473. Unexpectedly, both cell lines exposed to rapamycin exhibited a dose- and time-dependent increase in Akt phosphorylation (Figure 2A-B). In addition, the phosphorylation of eIF4E, a molecule downstream of mTORC1 that is involved in cell growth (Figure 2A-B), was also increased. mTOR inhibition by rapamycin would be expected to inhibit eIF4E phosphorylation (via dephosphorylation of 4E-BP1) rather than stimulate eIF4E phosphorylation. How mTOR inhibition activates eIF4E phosphorylation remains unclear. It has been demonstrated that the MEK/ERK and p38 MAPK signaling pathways activate eIF4E through Mnk1-mediated phosphorylation of eIF4E.15 Further studies are ongoing to investigate the role of Mnk1 in rapamycin-induced eIF4E phosphorylation. To correlate the dynamic changes in phospho-Akt and phospho-eIF4E with the inhibition of mTOR in response to rapamycin, we examined the effects of rapamycin dose and exposure time. Phosphorylation of Akt and eIF4E increased soon after a 4-hour exposure to rapamycin and was sustained for up to 24 hours (Figure 2A). Primary DLBCL samples exposed to rapamycin in vitro also contained elevated levels of p-Akt and p-eIF4E (Figure 2C).
Figure 2.
Effect of rapamycin on mTORC1/mTORC2 signaling. DHL-6 and Ly7 cells were treated with 50 nM rapamycin for 2, 4, and 8 hours (A) or for 24 hours (B) with the indicated rapamycin concentration. Phosphorylation of Akt, eIF4E, S6, 4EBP1, and mTOR was assessed by Western blot analysis. (C) Malignant cells from DLBCL patients (n = 4) were treated with 50 nM rapamycin for 6 hours before Akt, eIF4E, and S6 phosphorylation was analyzed by Western blot. (D) Levels of PAkt and p-eIF4E were evaluated in peripheral blood samples from 3 patients (n = 3) with aggressive lymphoma treated in the clinic with single-agent temsirolimus.
After treatment with rapamycin, the phosphorylation of S6rp, 4EBP1, and mTOR was reduced in a time- and dose-dependent manner (Figure 2A-B), confirming the ability of rapamycin to inhibit mTORC1 signaling. However rapamycin reduced pS6rp more effectively than p4EBP1. Likewise, rapamycin diminished the phosphorylation of S6 in all DLBCL samples examined (n = 4; Figure 2C). Thus, despite a lack of cytotoxicity, mTORC1 is indeed being inhibited by rapamycin in DLBCL cells. Taken together, our results suggest that rapamycin triggers a negative feedback mechanism by activating survival pathways involving Akt and eIF4E that may attenuate the antiproliferative effects mediated through mTORC1.
mTOR inhibition also causes increased Akt signaling in malignant lymphoma cells in vivo
To further explore rapamycin-induced Akt phosphorylation, we examined peripheral blood samples from patients with relapsed aggressive B-cell NHL who were treated with single-agent temsirolimus on a clinical trial.16,17 Phosphorylation of Akt and eIF4E was detectable at baseline and was increased in each of the 3 patient samples at 24 hours, at 48 hours, and after 4 weekly doses (1 cycle) of temsirolimus (Figure 2D). Due to the unavailability of additional temsirolimus-treated patient samples, instead of probing with total Akt or eIF4E, we probed the same blot for p-S6 to determine the status of mTORC1 in the same samples. These results provide the first evidence that rapamycin derivatives such as temsirolimus are capable of increasing Akt signaling in patients treated with these inhibitors.
The HDAC inhibitor LBH inhibits the survival and proliferation of lymphoma cells by inhibiting HDAC3 and HDAC4 expression
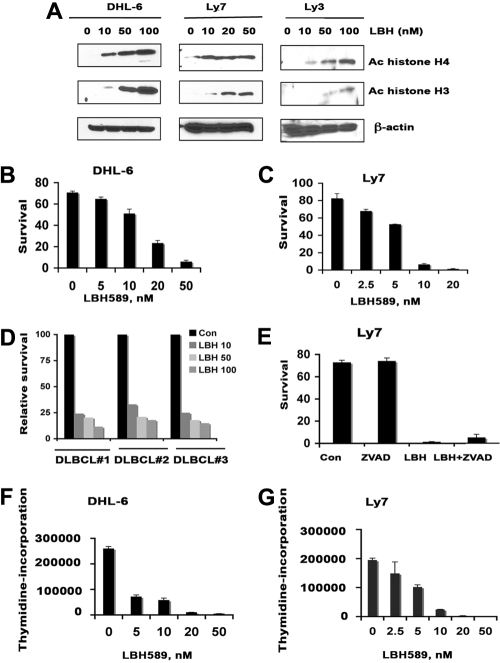
In a parallel set of experiments, we investigated whether the HDI LBH would inhibit proliferation and survival of DLBCL cells. To verify that LBH inhibits its targets in these cells, the DHL-6, Ly7, and Ly3 lines were treated with increasing LBH concentrations and assayed by immunoblotting for changes in histone acetylation. As expected, there was a progressive increase in acetylated histones H4 and H3 (Figure 3A). Further analysis demonstrated that increasing doses of LBH (5-100 nM) also suppressed the survival of each cell line in a dose-dependent manner at 48 hours (Figure 3B-C). IC50 values for these cell lines were 20 nM (DHL-6) and 10 nM (Ly7). Subsequent testing showed that all DLBCL clinical specimens (n = 3) were also sensitive to LBH, even at the lowest dose (10 nM) of the drug (Figure 3D). Pretreatment of LY7 cells with a caspase inhibitor Z-VAD-FMK did not prevent LBH-induced cell death (Figure 4E). These results suggest that LBH-induced apoptosis of DLBCL cells involves a caspase-independent mechanism. LBH also inhibited 3H-thymidine incorporation in a concentration-dependent manner (Figure 3F-G).
Figure 3.
Effect of LBH on survival, proliferation, and histone acetylation in diffuse large B-cell lymphoma cells. (A) DHL-6, Ly7, and Ly3 cells were treated with indicated concentrations of LBH589 for 24 hours. Western blot analysis of acetylated histones H3 and H4 were performed using specific antibodies. (B-C) DHL-6 and Ly7 diffuse large B-cell lymphoma (DLBCL) cells were treated with indicated concentrations of LBH589 for 48 hours and survival was assessed by annexin V/PI staining by flow cytometry. Data from 1 representative experiment are shown for each cell line (n = 3). (D) Primary cells from DLBCL patients were treated with various doses of LBH589 and survival was assessed by annexin V/PI staining. (E) LY7 cells were pretreated with 50 μM Z-VAD-fmk alone or with LBH589 (50 nM) for 48 hours, and survival was assessed by annexin V/PI staining. (F-G) DHL-6 and Ly7 cells were treated with indicated doses of LBH589 for 48 hours and cell proliferation was assessed by 3H-thymidine incorporation. Error bars indicate mean ± SD from 3 determinations.
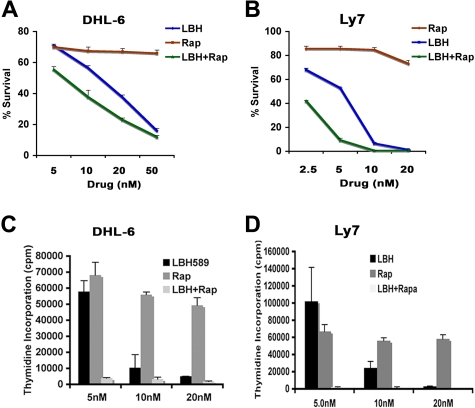
Figure 4.
Cotreatment with LBH and rapamycin exerts synergistic cytotoxic effects against DLBCL cells. (A-B) DHL-6 (A) and Ly7 (B) cells were treated with the indicated concentrations of LBH589 and/or rapamycin for 48 hours and survival was assessed as described before. (C-D) Effect of LBH + Rap on DLBCL cell proliferation. DHL-6 and Ly7 cells were treated with the indicated doses of LBH and/or rapamycin for 48 hours and cell proliferation was assessed by 3H-thymidine incorporation. One representative experiment is shown for each line (n = 3). Error bars indicate mean ± SD from 3 determinations.
Subsequent experiments investigated whether the mechanism of LBH cytotoxicity on DLBCL cells was HDAC dependent and, if so, which specific HDAC enzymes were inhibited. We have found that Ly7 and DHL-6 cells express HDAC1-6 but neither cell line expresses HDAC7 (supplemental Figure 2). When cells were treated with LBH, HDAC3 and HDAC4 expression was specifically inhibited in Ly7 and DHL-6 cells, with little effect on other HDACs (supplemental Figure 2). These data suggest that HDAC3 and HDAC4 may be important to the growth of DLBCL.
Rapamycin and LBH synergistically inhibit survival and proliferation of DLBCL cells
Combined treatment of DHL-6 cells with 5, 10, or 20 nM rapamycin with the same concentrations of LBH for 48 hours significantly reduced the survival of DHL-6 cells (Figure 4A). To determine whether this shift in IC50 for the combinations reflected drug synergy, we calculated the combination index (CI) using the Chou-Talalay equation.18 Formal mathematic analysis indicated that rapamycin and LBH were synergistic, as indicated by CI values of less than 1.0 (ie, CI was 0.5 at 5 nM, 0.8 at 10 nM, and 0.9 at 20 nM). Similarly, cotreatment of Ly7 cells with 2.5, 5.0, or 10 nM rapamycin with the same doses of LBH for 48 hours also significantly decreased the survival of Ly7 cells (Figure 4B). The CI values for Ly7 cells were more synergistic than for DHL-6 cells (ie, CI was 0.1 at 2.5 nM, 0.3 at 5 nM, and 0.4 at 10 nM). The LBH/rapamycin combination also reduced proliferation of DHL-6 and Ly7 cells more effectively than either agent alone (Figure 4C-D).
LBH effectively targets mTORC1 signaling
In experiments designed to determine the effect of LBH on the mTORC1 targets S6 and 4EBP1, we demonstrated that single-agent LBH was able to inhibit the phosphorylation of S6 and 4EBP1 along with mTOR in a time- and dose-dependent manner (Figure 5A-B). LBH also inhibited the phosphorylation of S6 in all 4 DLBCL patient samples examined (Figure 5C). Next, we determined the effects of cotreatment with LBH and rapamycin on the mTORC1 signaling proteins. Doses of LBH and rapamycin that were noninhibitory as single agents were in combination able to produce more inhibition of the phosphorylation of S6rp and 4EBP1 in DHL-6 cells than treatment with either agent alone (Figure 5D).
Figure 5.
Effect of LBH on mTORC1 and mTORC2. (A-B) DHL-6 and Ly7 DLBCL lines were treated with 100 nM LBH for the indicated times (A) or with the indicated concentrations (B) of LBH for 24 hours and then subjected to Western blot analysis using p-S6 or p-4EBP1 or p-mTOR antibody. (C) Malignant cells from DLBCL patients (n = 4) were treated with 100 nM LBH for 6 hours and analyzed by Western blot. (D) DHL-6 cells were cotreated with 10 nM LBH and 10 nM rapamycin for 24 hours before S6 and 4EBP1 phosphorylation was analyzed. Effect of LBH on mTORC2 targets. (E-F) DHL-6 and Ly7 DLBCL lines were treated with 100 nM LBH for the indicated times (E) or with the indicated concentrations (F) of LBH for 24 hours and then analyzed for Akt or eIF4E phosphorylation. (G) Malignant cells from DLBCL patients (n = 4) were treated with 100 nM LBH for 6 hours and were analyzed for Akt or eIF4E phosphorylation. (H) DHL-6 and Ly7 cells were treated with 100 nM LBH and 20 nM rapamycin for 24 hours before analysis of Akt phosphorylation.
LBH inhibits constitutive as well as rapamycin-induced Akt (mTORC2) phosphorylation
Because our previous experiments demonstrated that one compensatory effect of rapamycin treatment in DLBCL cells was phosphorylation of Akt, we investigated the effect of LBH on Akt. LBH caused a dose-dependent decrease in the Ser 473 phosphorylation of Akt in DHL-6 and Ly7 cells without changing the level of total Akt (Figure 5E). In addition to the effects on Akt, LBH also inhibited the phosphorylation of eIF4E in a time- and dose-dependent manner (Figure 5E-F). Similar effects of LBH on the phosphorylation of Akt and eIF4E were observed in samples from DLBCL patients (n = 4; Figure 5G). Rapamycin treatment alone increased Akt and eIF4E phosphorylation levels in both DLBCL lines. This effect on p-Akt could be effectively blocked if LBH was added with the rapamycin (Figure 5H).
Mechanism of LBH-mediated Akt dephosphorylation
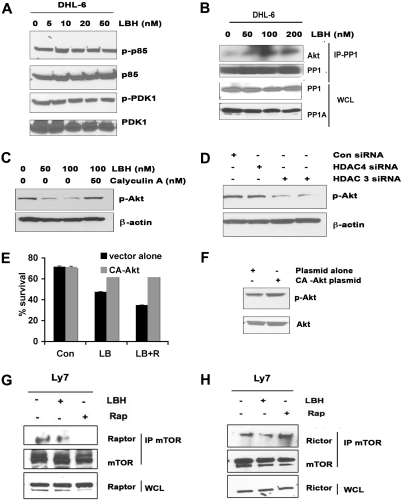
Potential mechanisms of LBH-mediated Akt dephosphorylation are through the deactivation of upstream proteins or the activation of an Akt-specific phosphatase. We treated DHL-6 cells with LBH and assessed the expression levels of a series of signaling proteins related to the regulation of Akt signaling, including the p85 regulatory subunit of PI3K and phosphoinositide-dependent protein kinase 1 (PDK-1). As shown in Figure 6A, LBH exposure did not alter the expression or phosphorylation of either of these signaling proteins. The reported ability of HDAC inhibitors to disrupt HDAC-PP1 complex formation19,20 led us to investigate whether LBH-induced alterations in PP1 could contribute to the observed effects on Akt phosphorylation. We first examined the possibility that LBH might be increasing phosphatase expression, but observed that LBH did not increase the total levels of PP1 and PP1A (Figure 6B). Instead, immunoprecipitation experiments revealed that LBH treatment resulted in increased binding of PP1 to Akt (Figure 6B). To determine whether this increased binding of PP1 to Akt might be contributing to increased Akt phosphorylation, cells were treated with LBH in the absence or presence of the PP1 inhibitor calyculin A. As indicated in Figure 6C, calyculin A diminished the effect of LBH on Akt phosphorylation. To validate the role of HDAC3 and HDAC4 in Akt deactivation, we used siRNAs to selectively knock down the expression of HDAC3 and HDAC4 and a control siRNA as a negative control (supplemental Figure 3A). We showed that repression of HDAC3 but not HDAC4 led to decreased Akt phosphorylation, whereas control siRNA transfection had no effect on phospho-Akt levels (Figure 6D). The effects of LBH on Akt are clear, but to better understand whether this blockage of Akt contributes to LBH-induced apoptosis, DHL-6 cells were transiently transfected with constitutively active Akt (CA-Akt). Significant protection from cell death was observed in CA-Akt–expressing cells after treatment with LBH alone or LBH plus rapamycin (Figure 6E). The function of the CA-Akt construct was monitored by enhancement of p-Akt (Figure 6F).
Figure 6.
Effect of LBH on upstream proteins involved in Akt activation. (A) After DHL-6 cells were treated with the indicated doses of LBH for 24 hours, cell lysates were immunoblotted for p-p85 and p-PDK1. (B) Immunoprecipitation of PP1 in lysates of LBH-treated cells, followed by immunoblotting with Akt. Simultaneously LBH-treated whole-cell lysates (WCLs) were immunoblotted with PP1 and PP1A. (C) Effect of protein phosphatase inhibitor calyculin A on LBH-induced Akt dephosphorylation. DHL-6 cells were exposed to LBH and calyculin A (50 nM) for 24 hours and cell lysates were immunoblotted. (D) Repressed expression of HDAC3, but not HDAC4, led to Akt dephosphorylation in a manner similar to that of LBH treatment. The cell lysates were immunoblotted with antibodies against phospho-Akt. (E) DHL-6 cells were transiently transfected with CA-Akt and empty vector, and subsequently treated with the combination for 48 hours, and survival was assessed. Bar graph shows mean ± SD. (F) CA-Akt–transfected cells were analyzed for Akt activation using p-Akt antibody. (G-H) Effect of LBH on rictor/raptor complex. Ly7 cells were treated with rapamycin (10 nM) or LBH (50 nM) or the combination for 24 hours. WCLs and mTOR immunoprecipitates prepared from the lysates were analyzed by Western blot for the levels of mTOR, rictor, and raptor.
LBH alters the level of intact mTORC2
To determine the effect of LBH on mTORC2, we treated Ly7 cells with LBH and/or rapamycin for 24 hours and compared the amounts of raptor and rictor bound to mTOR. Rapamycin had little effect on the expression levels of mTOR, raptor, or rictor, but as expected, strongly reduced the amounts of raptor recovered with mTOR (Figure 6G) and increased the amount of rictor bound to mTOR (Figure 6H). LBH, on the other hand, diminished the amount of rictor or raptor bound to mTOR (Figure 6G-H).
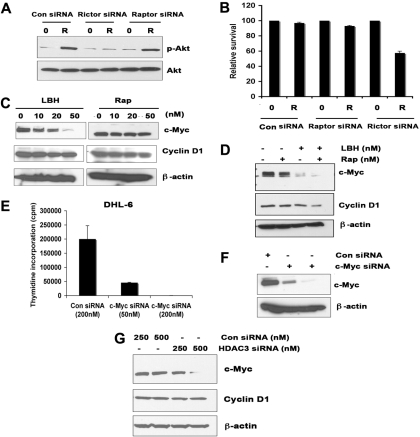
To confirm the importance of mTORC2 but not mTORC1 in the rapamycin-induced Akt phosphorylation, we used small interfering RNA (siRNA) to specifically knock down rictor or raptor (supplemental Figure 3B). As indicated in Figure 7A, knockdown of rictor but not raptor prevented rapamycin-induced phosphorylation of Akt on Ser 473. These results demonstrate that mTORC2 complexes are responsible, at least in part, for the Akt Ser 473 phosphorylation observed upon treatment with the rapamycin. Next, we asked whether inhibition of rictor-mediated signaling could increase the survival of rapamycin-treated DLBCL cells. Rictor or raptor knockdown cells were treated with rapamycin for 48 hours and stained with annexin V/PI to assess survival. As indicated in Figure 7B, down-regulation of rictor but not raptor partially increased the rapamycin-induced sensitivity in DHL-6 cells.
Figure 7.
LBH589 alters the level of intact mTORC2 and c-Myc expression. (A-B) Effect of siRNA knockdown of raptor or rictor. (A) Rictor siRNA blocks phosphorylation of Akt in the presence of rapamycin. Ly7 cells were transiently transfected with raptor or rictor siRNA and then treated with rapamycin (R) or left untreated for 24 hours and subjected to Western blot analysis using p-Akt antibody. (B) After raptor or rictor knockdown, DHL-6 cells were treated with rapamycin (R) or left untreated (0) for 24 hours. Survival was analyzed by staining with annexin V and PI. (C-D) Effect of LBH and rapamycin on cyclin D1 and c-Myc expression. (C) Ly7 cells were treated with the indicated concentrations of LBH589 or rapamycin for 24 hours and then subjected to Western blot analysis using c-Myc and cyclin D1 antibodies. (D) Ly cells were cotreated with 50 nM LBH and rapamycin for 24 hours, and c-Myc and cyclin D1 expression was analyzed. (E-F) DHL-6 cells were transiently transfected with control siRNA or c-Myc siRNA, and proliferation was done as described earlier. Western blot analysis showed successful knockdown of c-Myc (50 nM or 200 nM). (G) HDAC3-transfected DHL-6 cells were analyzed for c-Myc and cyclin D1 expression. Error bars indicate mean ± SD from 3 determinations.
To investigate whether the PI3K (upstream of Akt) was playing any role in rapamycin-induced Akt activation, we blocked the PI3K activity with a PI3K-specific inhibitor (LY294002). We found that LY294002 had minimal effect on rapamycin-induced Akt phosphorylation at AktSer473 with no effect at the AktThr308 site (supplemental Figure 4A). Furthermore, treatment of Ly7 DLBCL cells with a specific Akt inhibitor (A443654) inhibited survival in a dose-dependent manner similar to LBH. Combining A443654 with rapamycin showed significant growth-inhibitory effects (supplemental Figure 4B-C). This again confirms that LBH exerts its cytotoxic effects in part through Akt.
Combined treatment of LBH589 and rapamycin effectively targets c-Myc in DLBCL cells
The mTOR pathway is considered to control the translation of specific mRNA transcripts, some of which are involved in cell- cycle control (eg, cyclin D1 and c-Myc). LBH alone resulted in a dose-dependent decrease in the expression of c-Myc in the Ly7 line, whereas cyclin D1 was not inhibited in either line (Figure 7C). In contrast, rapamycin treatment inhibited cyclin D1 expression, but only after 24 hours at high concentrations (50 nM, 100 nM). Rapamycin failed to inhibit the level of c-Myc in Ly7 cells at any dose (Figure 7C). Importantly, the combination of both rapamycin and LBH attenuated both cyclin D1 and c-Myc levels in Ly7 cells (Figure 7D). To better understand whether the inhibitory effects of LBH on proliferation are mediated through c-Myc down-regulation, we used c-Myc siRNA to inhibit c-Myc expression in DHL-6 cells and demonstrated growth inhibition (Figure 7E-F). We further showed that inhibition of HDAC3 inhibited the c-Myc expression in DHL-6 cells, whereas it has little to no effect on cyclin D1 expression (Figure 7G).
Discussion
The PI3K/Akt/mTOR pathway has become an important focus for cancer therapeutics.21,22 The mTOR inhibitory agents that have been most thoroughly investigated to date as single agents are sirolimus (rapamycin), temsirolimus, and everolimus. Temsirolimus and everolimus are now approved by the FDA for relapsed renal cell carcinoma.23 mTOR inhibitors have also demonstrated impressive single-agent activity in relapsed lymphoma. Weekly intravenous temsirolimus produced an ORR of 38% and 41% in 2 studies of patients with relapsed mantle cell NHL.17,24 Additional trials of temsirolimus have produced similar results in relapsed follicular NHL and DLBCL.25 Preliminary results using oral everolimus have demonstrated that approximately 30% of patients with relapsed DLBCL respond7; the ORR is higher, at 40%, for MCL, Waldenström macroglobulinemia,26 and Hodgkin disease.27,28 These single-agent trials have provided proof-of-concept that mTOR inhibition can produce antitumor responses in relapsed lymphoma.
Analyses of human DLBCL tumor specimens provide support that mTOR signaling pathways are frequently activated in DLBCL tumors, and others have shown that the expression of p-Akt is an adverse prognostic feature.29 Although rapamycin at low concentrations (even at 5 nM) can suppress the phosphorylation of S6rp (indicating that mTOR signaling has indeed been blocked), this results in only a modest antiproliferative effect, with minimal cytotoxicity in DLBCL lines and primary cells from DLBCL patients. This rapamycin resistance is consistent with the previous reports in DLBCL cell lines and other cancers.30,31 In the studies reported herein, we sought to understand the mechanism(s) of mTOR resistance in lymphoma and to use this information in the design of new treatment strategies that could overcome mTOR inhibitor resistance in lymphoma. We found that the mTORC2 target p-Akt was increased in DLBCL cell lines and primary samples after rapamycin exposure. Moreover, we detected significantly increased levels of p-Akt in lymphoma samples from patients treated with intravenous temsirolimus, consistent with the findings of Wang et al32 in lung cancer cell lines. It has been suggested that an equilibrium may exist between mTORC1 and mTORC2 complexes33; therefore, it is possible that inhibition of mTORC1 by an mTOR inhibitor shifts the equilibrium to favor activation of mTORC2, leading to an increase in Akt phosphorylation.34 Sarbassov et al34 reported that prolonged rapamycin treatment in cell types (other than lymphoma) could inhibit the assembly of mTORC2. However in our studies, prolonged treatment of lymphoma cells with high doses of rapamycin only slightly inhibited the Akt activation and did not completely abolish the p-Akt activation (data not shown). In addition, the PI3K inhibitors LY294002 and wortmannin were unable to abrogate rapamycin-induced Akt activation or to inhibit the survival of lymphoma cells. This suggests that PI3K activity is not required for Akt activation by rapamycin. Our data further show that the mTOR-rictor (TORC2) complex can directly phosphorylate Akt at Ser 473. This was demonstrated by our finding that disruption of mTORC2 by knocking down rictor blocked the rapamycin-induced Akt activation. In addition, mTORC1 knockdown with raptor siRNA did not block rapamycin-induced Akt phosphorylation in DLBCL cells. This finding is in contrast with the studies in lung cancer cells where rapamycin increased Akt phosphorylation independent of mTORC232 and consistent with the results of others in different model systems.35 This suggests that rapamycin-induced Akt activation might be tissue and dose specific.
We showed that increased Akt phosphorylation in response to rapamycin most likely contributes to the attenuated antitumor activity of rapamycin. This resistance mechanism can be overcome in vitro in DLBCL cells with the pan-DAC inhibitor LBH589. LBH treatment also inhibits the constitutive activation of Akt in DLBCL. Other studies with another HDI (SAHA) found inhibition of Akt activity in prostate cancer cells36 and mantle cell lymphoma.37 These observations suggest that the Akt signaling pathway might be a common target of HDI in cancer cells. In our experiments, the LBH-induced dephosphorylation of Akt was not mediated through regulators upstream of Akt (PI3K kinase) but rather by activation of the protein phosphatase PP1 that subsequently inactivates Akt by dephosphorylation. These results are consistent with the results of Chen et al.36 Key differences between their results and ours were the use of a different HDI (trichostatin A vs LBH), different tumors (glioma and prostate cell lines vs lymphoma cell lines and primary samples), and different HDACs (HDAC1 and HDAC6 vs HDAC3) in our report.
Other key findings of our studies are the constitutive expression of activated eIF4E in DLBCL, the further increase in eIF4E with rapamycin treatment, and the ability of LBH to prevent this eIF4E activation in vitro. eIF4E expression in primary lymphoma samples (including DLBCL) has been demonstrated by others,38 and the increase in eIF4E with rapamycin has also been described in lung cancer cell lines.30 Because Akt and eIF4E are often associated with cell survival and resistance to cancer therapy,39 our findings imply that the activation of Akt and eIF4E through mTOR inhibition may counteract the anticancer efficacy of mTOR inhibitors. Our coimmunoprecipitation data suggest that LBH alone was able to alter the level of intact mTORC2/mTORC1 by reducing the amounts of rictor or raptor bound to mTOR, which is further decreased when combined with rapamycin. We have also seen that LBH alone was able to inhibit the activation of mTORC1 targets S6rp and 4E-BP1, whereas combined treatment with rapamycin inhibited the phosphorylation of S6rp and 4EBP1 to a greater extent than either agent alone. In support of this model, rapamycin combined with LBH exhibited enhanced synergistic inhibitory effects on survival and proliferation of DLBCL cells in a dose-dependent manner along with induction of histone H3 and H4 acetylation. It is important to note that only nanomolar doses of each agent were needed for a potent synergistic cytotoxic effect on DLBCL cells. These doses should be achievable in humans.
An important function of mTOR is to phosphorylate S6rp and 4E-BP-1, resulting in the translation of mRNAs coding for proteins involved in cell-cycle regulation such as cyclin D1 and c-Myc.40 Deregulation of c-Myc proto-oncogene is implicated in the development of numerous human tumors including lymphoma.40 When overexpressed, c-Myc can trigger proliferation by driving quiescent cells into S-phase in the absence of other mitogenic stimuli. We have shown that all the DLBCL lines overexpress c-Myc protein. Surprisingly, LBH alone and in combination with rapamycin at very low doses was able to completely inhibit c-Myc expression and suppress cellular proliferation.
Our findings highlight the value of pathway-targeted combination therapy to achieve maximal blockade of signaling pathways for cancer treatment. These studies demonstrate for the first time to the best of our knowledge that pan-HDAC inhibitors such as LBH can actually interfere with the mTOR pathway both at the mTORC1 and mTORC2 level and overcome mTOR inhibitor resistance in DLBCL. Our data indicate that LBH overcomes rapamycin resistance by inhibiting Akt activation through 2 mechanisms, (1) through activation of protein phosphatase PP1 and (2) by inhibition of mTORC2 (mTOR-rictor), and not by inhibition of PI3 kinase. Based on these mechanistic preclinical studies, we propose that combination therapy with pan-HDAC and mTOR inhibitors targeting both mTORC1 and mTORC2 may be applicable to a broad spectrum of lymphoma patients, particularly those with aggressive disease.
Supplementary Material
Acknowledgments
This work was supported in part by Iowa/Mayo SPORE CA97274; R01CA127433; R21CA112904 (T.E.W.); and Iowa/Mayo SPORE (CA097274-07DRP6; M.G.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.G. designed and performed research, analyzed and interpreted data, and wrote the paper; S.M.A. and S.K. provided patient specimens and participated in editing the paper; A.J.N. assisted in interpretation of data and editing of the paper; S.H.K. designed research, interpreted data, and wrote the paper; and T.E.W. designed research, provided patient specimens, interpreted data, and wrote and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas E. Witzig, Mayo Clinic College of Medicine, Division of Hematology, 200 SW 1st St, Rochester, MN 55905; e-mail: witzig.thomas@mayo.edu.
References
- 1.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 2.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 3.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 4.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11(8):353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Gingras AC, Gygi SP, Raught B, et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13(11):1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeder CB, Gornet MK, Habermann TM, et al. A phase II trial of the oral mTOR inhibitor everolimus (RAD001) in relapsed aggressive non-Hodgkin lymphoma (NHL) [abstract]. Blood (ASH Annual Meeting Abstracts) 2007;110(11):121. [Google Scholar]
- 8.Schreiber SL, Bernstein BE. Signaling network model of chromatin. Cell. 2002;111(6):771–778. doi: 10.1016/s0092-8674(02)01196-0. [DOI] [PubMed] [Google Scholar]
- 9.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 10.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6(1):38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 11.Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkuhler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 2007;17(3):195–211. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 12.Duvic M, Vu J. Vorinostat in cutaneous T-cell lymphoma. Drugs Today (Barc) 2007;43(9):585–599. doi: 10.1358/dot.2007.43.9.1112980. [DOI] [PubMed] [Google Scholar]
- 13.Mann BS, Johnson JR, He K, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13(8):2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 14.Giles F, Fischer T, Cortes J, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12(15):4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 15.Mahalingam M, Cooper JA. Phosphorylation of mammalian eIF4E by Mnk1 and Mnk2: tantalizing prospects for a role in translation. Prog Mol Subcell Biol. 2001;27:132–142. [PubMed] [Google Scholar]
- 16.Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113(3):508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23(23):5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 18.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 19.Canettieri G, Morantte I, Guzman E, et al. Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat Struct Biol. 2003;10(3):175–181. doi: 10.1038/nsb895. [DOI] [PubMed] [Google Scholar]
- 20.Brush MH, Guardiola A, Connor JH, Yao TP, Shenolikar S. Deactylase inhibitors disrupt cellular complexes containing protein phosphatases and deacetylases. J Biol Chem. 2004;279(9):7685–7691. doi: 10.1074/jbc.M310997200. [DOI] [PubMed] [Google Scholar]
- 21.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11(1–2):32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 23.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 24.Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113(3):508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SM, Pro B, Cisneros A, et al. Activity of single agent temsirolimus (CCI-779) in non-mantle cell non-Hodgkin lymphoma subtypes [abstract]. J Clin Oncol. 2008;26(suppl) Abstract 8514. [Google Scholar]
- 26.Ghobrial IM, Chuma S, Sam A, et al. Phase II trial of the mTOR inhibitor RAD001 in relapsed and/or refractory waldenstrom macroglobulinemia: the Dana Farber Cancer Institute Experience [abstract]. Blood (ASH Annual Meeting Abstracts) 2008;112(11):1011. [Google Scholar]
- 27.Johnston PB, Ansell SM, Colgan JP, et al. Phase II trial of the oral mTOR inhibitor everolimus (RAD001) for patients with relapsed or refractory lymphoma [abstract]. J Clin Oncol. 2007;25(18S) suppl Abstract 8055. [Google Scholar]
- 28.Johnston PB, Ansell SM, Colgan JP, et al. mTOR inhibition for relapsed or refractory Hodgkin lymphoma: promising single agent activity with everolimus (RAD001) [abstract]. Blood (ASH Annual Meeting Abstracts) 2007;110(11):2555. [Google Scholar]
- 29.Uddin S, Hussain AR, Siraj AK, et al. Role of phosphatidylinositol 3′-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108(13):4178–4186. doi: 10.1182/blood-2006-04-016907. [DOI] [PubMed] [Google Scholar]
- 30.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65(16):7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 31.Wanner K, Hipp S, Oelsner M, et al. Mammalian target of rapamycin inhibition induces cell cycle arrest in diffuse large B cell lymphoma (DLBCL) cells and sensitises DLBCL cells to rituximab. Br J Haematol. 2006;134(5):475–484. doi: 10.1111/j.1365-2141.2006.06210.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68(18):7409–7418. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8(3):179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 36.Chen CS, Weng SC, Tseng PH, Lin HP, Chen CS. Histone acetylation-independent effect of histone deacetylase inhibitors on Akt through the reshuffling of protein phosphatase 1 complexes. J Biol Chem. 2005;280(46):38879–38887. doi: 10.1074/jbc.M505733200. [DOI] [PubMed] [Google Scholar]
- 37.Kawamata N, Chen J, Koeffler HP. Suberoylanilide hydroxamic acid (SAHA; vorinostat) suppresses translation of cyclin D1 in mantle cell lymphoma cells. Blood. 2007;110(7):2667–2673. doi: 10.1182/blood-2005-11-026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Rosenwald IB, Hutzler MJ, et al. Expression of the eukaryotic translation initiation factors 4E and 2alpha in non-Hodgkin's lymphomas. Am J Pathol. 1999;155(1):247–255. doi: 10.1016/s0002-9440(10)65118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 40.Bjornsti MA, Houghton PJ. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell. 2004;5(6):519–523. doi: 10.1016/j.ccr.2004.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.