Abstract
Background
The association of anal cancer with human papillomavirus (HPV) infection is well established; however, little is known about epidemiology of anal HPV in healthy women. We investigated patterns of duration and clearance of anal HPV infection in a cohort of healthy women in Hawaii.
Methods
Viral and non-viral determinants of anal HPV clearance were examined through a longitudinal cohort study of 431 sexually active women. At baseline and at 4-month intervals, interviews were conducted and cervical and anal cell specimens were obtained for HPV DNA detection.
Results
Fifty percent of the 431 women experienced a total of 414 incident anal HPV infections at one or more clinic visits from baseline through an average 1.2 year follow-up period. Of these infections, 58% cleared during follow-up. The clearance rate for a high-risk (HR) anal infection was 9.2 (95% confidence interval (CI): 6.9–11.9) per 100 woman-months with a median duration of 150 days (95% CI: 132–243 days). The slowest clearing HR-HPV types were HPV-59 (median, 350 days) and HPV-58 (median, 252 days). Median clearance times for HPV-16 and -18, the predominant types associated with anal cancer, were 132 days and 212 days, respectively. Non-viral factors that delayed clearance of anal HPV included douching, long-term tobacco smoking and anal sex.
Conclusions
The majority of anal HPV infections resolve in a relatively short time. Although anal HPV is commonly acquired in healthy women, its rapid clearance suggests limited efficacy of HPV testing as an anal cancer screening tool.
Keywords: human papillomavirus, clearance, duration, cohort study, anal cancer
INTRODUCTION
Anal cancer is an uncommon malignancy and similar to cervical cancer, a causal role for high-risk human papillomavirus (HPV) infection has been postulated. A key feature of the disease is higher incidence among women compared to men. In the U.S., there will be an estimated 5,070 new cases of anal cancer in 2008, with 3,050 cases occurring in women and 2,020 cases in men [1]. The reason for this sex difference is uncertain, although women with a history of cervical dysplasia and cervical cancer [2, 3], a history of anal receptive intercourse [4, 5], and multiple sexual partners [4, 6] are at greater risk for this disease.
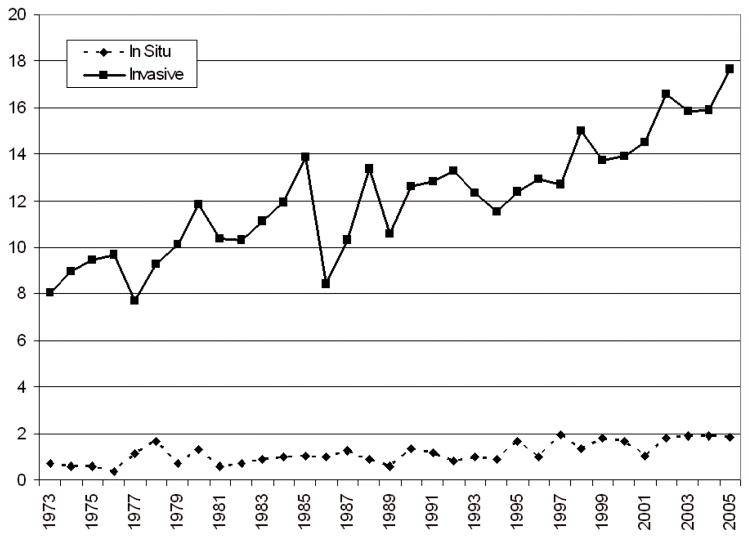
HPV infection of the anal canal has now been established as a risk factor for anal precursor lesions [2, 7, 8] and anal cancer [9]. Although estimates vary, at least 70% of squamous cell carcinomas of the anus are HPV-related, most of these with oncogenic types HPV-16 and -18. Only a few investigators have examined prevalence and incidence of anal HPV infection, and these have been limited to HIV-infected and immunocompromised populations [8, 10, 11]. Little is known regarding the natural history of anal HPV among non-HIV infected women, particularly the correlates of HPV clearance among women who are generally healthy and not at high risk of sexually transmitted infections. Such knowledge is important because anal cancer incidence has increased worldwide during the past several decades [12–14]. Invasive anal squamous cell carcinoma rates increased by 1.7% per year among females in the U.S. between 1973 and 2005 (Figure 1). During the same period, a marked annual increase in the rate of in situ tumors was observed among females (2.9% per year).
Figure 1.
Age-adjusted incidence rates (per 1,000,000) of anal cancer among women: 1973–2005.
Source: Survey Epidemiology and End Results (SEER) program data file, “Incidence SEER 9 Registries Limited-Use, Nov 2007 Sub (1973–2005)”. Trends and incidence rates calculated using SEER*STAT software [15].
Although not generally recognized, the recent U.S. Food and Drug Administration licensure of a prophylactic vaccine against oncogenic HPV types 16 and 18 may have a profound effect on the incidence of anal cancer, potentially reducing the incidence of anal malignancy among women by 2,000 or more cases. An urgent need exists to improve our understanding of anal HPV epidemiology, to facilitate screening and prevention methods to combat this malignancy. In a longitudinal cohort study of Hawaii women, we examined the natural history of anal HPV infection to identify risk factors and patterns of occurrence [16]. The objective of the present report was to determine viral and non-viral factors associated with the duration and clearance patterns of anal HPV.
MATERIALS AND METHODS
Subject Recruitment and Follow-Up
Between 1998 and 2003, a cohort of sexually active women was established [16–19] for a longitudinal study of cervical and anal HPV infection. Cohort participants were recruited among women attending five clinics on Oahu, Hawaii, who were able to read, understand, and sign an informed consent form and medical release form approved by the University of Hawaii Institutional Review Board. Potentially eligible patients included those with appointments for new or annual gynecologic examinations or for family planning services, who were not pregnant or postpartum within the past six months, and had no plans to relocate within a year.
Collection of anal specimens following the gynecologic examination was optional. At the first and subsequent visits, anal cell specimens were obtained among willing women using a Dacron swab moistened with sterile water. The swab was inserted 1.5 to 2.0 cm into the anus and placed in 1.0 mL buffered medium (Digene Corp., Gaithersburg, MD). Women entering the cohort were asked to return every four months for repeat examination and testing.
Upon completion of each clinical examination, the study coordinator administered a study questionnaire to the participant. The baseline interview included demographic and sexual activity data, history of tobacco and alcohol use. A second, more detailed questionnaire, conducted during the second visit after a four-month interval, included gynecological, menstrual, reproductive and sexual histories, hormone use, medical history, history of sexually transmitted infections, and income. Information covered in the baseline questionnaire (sexual activity, tobacco and alcohol use) was also updated. The questionnaire used at subsequent interviews was modified slightly for use during the follow-up period; questions included changes in sexual and reproductive information during the intervening period between clinic visits.
Laboratory Analysis
HPV DNA was extracted from exfoliated cervical and anal cell specimens using commercial reagents (Qiagen Inc, Valencia CA). Specimens were analyzed for presence of HPV DNA by PCR using a modified version of the PGMY09/PGMY11 primer system [20], which showed good sensitivity and accuracy of HPV DNA detection in recent validation studies [21,22]. HPV DNA-positive specimens were genotyped using a reverse line blot detection method [23] for 36 different HPV types including high-risk (HR) types 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 70, 73, and 82; low-risk (LR) types 6, 11, 42, 54, 61, 72, 81, and 89; and undetermined-risk types 44, 62, 67, 71, 83, and 84 [24, 25] (Roche Molecular Systems, Pleasanton, CA). We defined the risk (oncogenic potential) associated with various HPV types using the International Agency for Research on Cancer definition [25]. HPV-positive specimens subsequently found to be negative in the genotyping assay were considered unclassified HPV-positive specimens. All specimens were also tested for the human β-globin gene as an internal control for sample sufficiency. 163 (20.3%) specimens testing negative for β-globin were considered to be insufficient and were excluded from analyses.
Statistical Analysis
Analyses were conducted using SAS version 9.1.3 (SAS Institute, Inc) and limited to the 431 women who completed at least two clinic visits and the detailed questionnaire at visit 2. To prevent bias in estimates due to left censoring, we only considered incident infections, i.e. infections first detected at the second or subsequent visit. Duration of infection was the time from the first detection of anal HPV infection until the first negative result for that infection. Kaplan-Meier method was used to calculate median duration of anal and cervical HPV infections grouped by oncogenic risk and phylogenetic species. HPV type-specific clearance rates per 100 person-months were calculated for all detected anal HPV genotypes and grouped by the number of other genotypes present at the clinic visit preceding clearance of the index infection. Poisson exact confidence intervals were constructed for all clearance rates [26]. Clearance rates for five age groups were calculated using the same techniques, and were used to construct age-specific anal HPV clearance curves.
The association of anal HPV clearance with exposures of interest was modeled through Cox regression using days since infection acquisition as the time metric, after adjustment for age at study entry. Infections with unclassified HPV types were excluded from the analysis. Because nearly 10% of study participants did not provide the number of their lifetime sexual partners, this factor was not included as an adjustment variable. Other adjustment factors were considered, but their inclusion in the models did not result in over 10% change in the parameter estimates [27] nor in a significantly better fit according to the likelihood ratio test. The proportional hazards assumption for Cox models was verified by plotting scaled Schoenfeld residuals against time to HPV clearance [28]. Relative risks (RR), as estimated by hazard ratios, and 95% confidence intervals (CI) were used as measures of association. Because each subject was allowed to experience more than one clearance event throughout the course of the study, we used a robust sandwich variance estimate [29], aggregated over subjects, to prevent artificially deflated standard errors and confidence interval estimates.
RESULTS
Among the 431 women included in this analysis, 215 (50%) experienced at least one incident anal HPV infection, and 177 (41%) were HPV negative on all study visits. The remaining 9% had a prevalent infection at baseline but no incident infections. Baseline and follow-up included 1508 visits in which anal specimens were collected (median, 3.5 visits/subject). The cumulative follow-up experience for this cohort was 7004 woman-months (mean, 487 days). The prevalence of anal HPV infection at enrollment was 42% (183 HPV-positive cases). The cohort had a multiethnic composition, with the majority of subjects non-Caucasian women. The median age of cohort participants was 40 years (mean, 39.4 years); 14% of women were current tobacco smokers and 23% were current alcohol drinkers at baseline [16].
The rate of clearance of incident anal HPV infections was 8.57 (CI: 6.89–10.54) per 100 woman-months (Table 1). HR-HPV infections cleared faster (9.16; CI: 6.94–11.87 per 100 woman-months) than LR-HPV infections (7.85; CI: 5.68–10.57 per 100 woman-months). Accordingly, the median duration of LR-HPV infections (224 d) exceeded that of HR-HPV infections (150 d). Two HR-HPV types, HPV-59 and -58, and two types of undetermined oncogenicity, HPV-83 and -71, had the longest clearance times. Among the alpha-papillomavirus species, the fastest clearing were alpha-5 infections (15.23; CI: 6.96–28.91 per 100 woman-months), and the slowest clearing were alpha-10 infections (7.71; CI: 3.33–15.19 per 100 woman-months).
Table 1.
Median duration and clearance rates of anal HPV infection, by genotype and number of coinfections.
| Genotype | No. incident infectionsa |
Women- months of follow-upb |
Median duration (d)c (95% CI)d |
Pct. cleared within 1 yr |
All incident infections |
No coinfectionf |
One or more coinfectionf |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| No. cleared infectionse |
Clearance rate per 100 women- months (95% CI)d |
No. cleared infectionsg |
Clearance rate per 100 women- months (95% CI)d |
No. cleared infectionsh | Clearance rate per 100 women-months (95% CI)§ | |||||
| Any HPVi | 119 | 780 | 238(159–280) | 70 | 58 | 7.44(5.65–9.62) | 36 | 4.62(3.23–6.39) | 22 | 2.82(1.77–4.27) |
| HR HPVj | 107 | 622 | 150.0(132–243) | 74 | 57 | 9.16(6.94–11.87) | 24 | 3.86(2.47–5.74) | 33 | 5.30(3.65–7.45) |
| 16 | 17 | 102 | 132.0(105–348) | 82 | 12 | 11.77(6.08–20.56) | 2 | 1.96(0.24–7.09) | 10 | 9.81(4.70–18.04) |
| 18 | 13 | 55 | 211.5(140–365) | 83 | 6 | 10.83(3.97–23.57) | 0 | (−6.66) | 6 | 10.83(3.97–23.57) |
| 31 | 10 | 41 | 160.0(125–250) | 100 | 7 | 16.95(6.81–34.92) | 0 | (−8.93) | 7 | 16.95(6.81–34.92) |
| 33 | 2 | 4 | 119.0(N/Ak) | 100 | 1 | 25.21(0.64–140.46) | 0 | (−93.00) | 1 | 25.21(0.64–140.46) |
| 35 | 3 | 15 | 161.0(146–176) | 100 | 2 | 13.19(1.60–47.64) | 0 | (−24.32) | 2 | 13.19(1.60–47.64) |
| 39 | 6 | 20 | 119.0(106–135) | 100 | 3 | 14.93(3.08–43.62) | 0 | (−18.35) | 3 | 14.93(3.08–43.62) |
| 45 | 6 | 61 | 210.0(103–763) | 60 | 5 | 8.15(2.65–19.02) | 3 | 4.89(1.01–14.29) | 2 | 3.26(0.39–11.78) |
| 51 | 14 | 46 | 131.0(124–147) | 100 | 7 | 15.14(6.09–31.20) | 3 | 6.49(1.34–18.96) | 4 | 8.65(2.36–22.15) |
| 52 | 18 | 114 | 135.0(131–161) | 83 | 11 | 9.64(4.81–17.25) | 2 | 1.75(0.21–6.33) | 9 | 7.89(3.61–14.97) |
| 53 | 25 | 116 | 174.0(132–245) | 100 | 11 | 9.46(4.72–16.92) | 4 | 3.44(0.94–8.80) | 7 | 6.02(2.42–12.40) |
| 56 | 15 | 100 | 160.0(138–644) | 71 | 8 | 8.02(3.46–15.79) | 2 | 2.00(0.24–7.24) | 6 | 6.01(2.21–13.09) |
| 58 | 9 | 25 | 252.0(117–272) | 100 | 3 | 12.18(2.51–35.59) | 0 | (−14.98) | 3 | 12.18(2.51–35.59) |
| 59 | 8 | 53 | 350.5(146–434) | 50 | 4 | 7.59(2.07–19.42) | 0 | (−7.00) | 4 | 7.59(2.07–19.42) |
| 66 | 7 | 42 | 131.0(82–392) | 80 | 5 | 11.77(3.82–27.48) | 2 | 4.71(0.57–17.01) | 3 | 7.06(1.46–20.65) |
| 68 | 8 | 35 | 136.0(124–146) | 83 | 6 | 17.39(6.38–37.85) | 0 | (−10.69) | 6 | 17.39(6.38–37.85) |
| 70 | 10 | 82 | 134.0(127–182) | 87 | 8 | 9.81(4.24–19.33) | 4 | 4.91(1.34–12.56) | 4 | 4.91(1.34–12.56) |
| 73 | 11 | 46 | 125.0(120–134) | 100 | 9 | 19.64(8.98–37.28) | 4 | 8.73(2.38–22.35) | 5 | 10.91(3.54–25.46) |
| 82 | 2 | 13 | 133.5(132–135) | 100 | 2 | 15.54(1.88–56.15) | 1 | 7.77(0.20–43.30) | 1 | 7.77(0.20–43.30) |
| LR HPVl | 77 | 548 | 224.0(163–267) | 76 | 43 | 7.85(5.68–10.57) | 18 | 3.28(1.95–5.19) | 25 | 4.56(2.95–6.73) |
| Low risk | ||||||||||
| 6 | 7 | 68 | 180.0(118−) | 81 | 5 | 7.40(2.40–17.27) | 0 | (−5.46) | 5 | 7.40(2.40–17.27) |
| 11 | 1 | 0 | N/Ak | 0 | N/Ak | N/Ak | N/Ak | |||
| 42 | 7 | 29 | 159.0(98−) | 100 | 4 | 13.97(3.81–35.77) | 1 | 3.49(0.09–19.46) | 3 | 10.48(2.16–30.62) |
| 54 | 10 | 49 | 170.0(85–359) | 100 | 7 | 14.40(5.79–29.68) | 1 | 2.06(0.05–11.46) | 6 | 12.35(4.53–26.87) |
| 61 | 17 | 97 | 186.0(126–274) | 78 | 10 | 10.33(4.95–19.00) | 5 | 5.17(1.68–12.05) | 5 | 5.17(1.68–12.05) |
| 81 | 10 | 89 | 156.5(125–286) | 87 | 8 | 8.96(3.87–17.65) | 3 | 3.36(0.69–9.81) | 5 | 5.60(1.82–13.06) |
| 89 | 9 | 50 | 187.0(135–225) | 100 | 5 | 10.08(3.27–23.52) | 0 | (−7.44) | 5 | 10.08(3.27–23.52) |
| Undetermined risk | ||||||||||
| 44 | 6 | 60 | 141.0(112–514) | 60 | 4 | 6.67(1.82–17.07) | 2 | 3.33(0.40–12.04) | 2 | 3.33(0.40–12.04) |
| 62 | 13 | 108 | 170.0(128–365) | 80 | 9 | 8.34(3.81–15.83) | 0 | (−3.42) | 9 | 8.34(3.81–15.83) |
| 67 | 2 | 4 | 123.0(N/Ak) | 100 | 1 | 24.39(0.62–135.89) | 0 | (−89.97) | 1 | 24.39(0.62–135.89) |
| 71 | 6 | 36 | 238.0(163−) | 67 | 2 | 5.55(0.67–20.03) | 1 | 2.77(0.07–15.45) | 1 | 2.77(0.07–15.45) |
| 83 | 11 | 67 | 239.0(137−) | 70 | 5 | 7.42(2.41–17.32) | 4 | 5.94(1.62–15.20) | 1 | 1.48(0.04–8.27) |
| 84 | 19 | 97 | 160.0(108–280) | 89 | 9 | 9.25(4.23–17.55) | 2 | 2.05(0.25–7.42) | 7 | 7.19(2.89–14.82) |
| Speciesm | ||||||||||
| 1 | 16 | 74 | 176.0(129–225) | 100 | 8 | 10.76(4.64–21.20) | 1 | 1.34(0.03–7.49) | 7 | 9.41(3.78–19.39) |
| 3 | 70 | 383 | 181.0(135–240) | 82 | 31 | 8.09(5.50–11.49) | 14 | 3.66(2.00–6.13) | 17 | 4.44(2.59–7.11) |
| 5 | 16 | 59 | 132.0(126–146) | 100 | 9 | 15.23(6.96–28.91) | 4 | 6.77(1.84–17.33) | 5 | 8.46(2.75–19.74) |
| 6 | 47 | 240 | 160.0(132–245) | 85 | 21 | 8.75(5.42–13.38) | 8 | 3.34(1.44–6.57) | 13 | 5.42(2.89–9.27) |
| 7 | 51 | 267 | 145.5(128–210) | 78 | 24 | 8.99(5.76–13.38) | 6 | 2.25(0.83–4.89) | 18 | 6.75(4.00–10.66) |
| 9 | 61 | 278 | 145.0(131–161) | 91 | 29 | 10.42(6.98–14.97) | 4 | 1.44(0.39–3.68) | 25 | 8.98(5.81–13.26) |
| 10 | 14 | 104 | 160.5(118–514) | 70 | 8 | 7.71(3.33–15.19) | 2 | 1.93(0.23–6.96) | 6 | 5.78(2.12–12.58) |
Overall number of genotype-specific incident infections: 414, including HR-HPV: 184; LR-HPV: 118; unclassified types: 112.
Months of follow-up for subjects at risk of clearing an infection. Calculated by summing the durations across women for a specific HPV type or group.
Estimated by product-limit (Kaplan-Meier) method.
CI, confidence interval.
Number of genotype-specific cleared infections: 179, including HR-HPV: 110; LR-HPV: 69.
Other HPV genotypes detected at the clinic visit preceding clearance of the index infection.
Number of genotype-specific cleared infections with no coinfection: 46, including HR-HPV: 27; LR-HPV: 19.
Number of genotype-specific cleared infections with 1+ coinfection: 133, including HR-HPV: 83; LR-HPV: 50.
From acquisition of the first HPV type until clearance of the last HPV type. An HPV-group specific infection was considered to have occurred at the visit (after visit 1) of the first detection of an anal HPV infection in the group of interest. The infection was considered resolved at the first subsequent visit where the anal specimen was negative for HPV infection in that group.
From acquisition of the first HR-HPV type until clearance of the last HR-HPV type.
The indicated statistic could not be estimated from the available data.
From acquisition of the first LR-HPV type until clearance of the last LR-HPV type. Includes genotypes of undetermined oncogenic risk.
α-Papillomavirus species; species 1 comprises types 42 and 89; species 3 comprises types 61, 62, 81, 83, and 84; species 5 comprises types 51 and 82; species 6 comprises types 53, 56, and 66; species 7 comprises types 18, 39, 45, 59, 68, and 70; species 9 comprises types 16, 31, 33, 35, 52, 58, and 67; and species 10 comprises types 6, 11, and 44.
The presence of another HPV genotype increased the clearance rate of both HR-and LR-HPV infections, but the difference was not statistically significant. The rate of clearance of anal HR-HPV infections was 3.86 (95% CI: 2.47–5.74) per 100 woman-months among women without a coinfection at the visit prior to clearance, and 5.30 (95% CI: 3.65–7.45) per 100 woman-months among women with one or more HPV coinfections. This pattern was observed across most HPV groups. Compared to infections with a single HPV genotype, the clearance rate among women with one or more coinfections was over 4 times higher for HPV-16, -52, -54, and species alpha-1 and -9; nearly 3 times higher for HPV-56, -42, -84, and species alpha-7 and -10, although the confidence intervals overlapped.
Based on Cox regression, the risk of clearance of LR-HPV infection was higher in the presence of HPV-61 (RR: 3.95; CI: 1.83–8.55) or any two other genotypes (RR: 2.54; CI: 1.18–5.46) (Table 2). Coinfections with other HPV types tended to increase the chances of clearance of LR-HPV infection, although the differences were not significant. The presence of other genotypes did not substantially affect the clearance of HR-HPV. A higher number of co-existing genotypes did not change anal HPV clearance rates.
Table 2.
Clearance of anal HPV infection by presence of coinfecting genotypes.
| High-risk HPV |
Low-risk HPVf |
Any HPV |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Subjectsb | Eventsc | Hazard Ratiod | Subjectsb | Eventsc | Hazard Ratiod | Subjectsb | Eventsc | Hazard Ratiod | |
| Coinfectiona | N | N | (95% CI)e | N | N | (95% CI)e | N | N | (95% CI)e |
| HR HPV: not present | 21 | 19 | |||||||
| present | 29 | 39 | 1.41(0.83–2.39) | ||||||
| LR HPV: not present | 26 | 27 | |||||||
| present | 24 | 44 | 1.06(0.70–1.61) | ||||||
| alpha-9: not present | 21 | 23 | 26 | 19 | 44 | 42 | |||
| present | 15 | 24 | 1.16(0.69–1.97) | 14 | 17 | 1.36(0.76–2.44) | 20 | 41 | 1.29(0.88–1.88) |
| alpha-7: not present | 21 | 20 | 22 | 19 | 41 | 39 | |||
| present | 10 | 22 | 1.18(0.71–1.97) | 13 | 18 | 1.00(0.52–1.91) | 18 | 40 | 1.02(0.69–1.49) |
| alpha-6: not present | 18 | 19 | 28 | 19 | 44 | 38 | |||
| present | 10 | 16 | 1.18(0.64–2.16) | 10 | 14 | 1.41(0.72–2.77) | 13 | 30 | 1.37(0.91–2.08) |
| alpha-3: not present | 26 | 27 | 10 | 5 | 34 | 32 | |||
| present | 20 | 47 | 1.11(0.72–1.71) | 9 | 8 | 2.49(0.89–6.97) | 24 | 55 | 1.28(0.86–1.90) |
| HPV-16: not present | 25 | 25 | 28 | 19 | 50 | 44 | |||
| present | 7 | 10 | 1.42(0.81–2.49) | 5 | 6 | 1.34(0.43–4.24) | 9 | 16 | 1.51(0.88–2.58) |
| HPV-51: not present | 24 | 24 | 27 | 19 | 47 | 43 | |||
| present | 3 | 8 | 1.25(0.66–2.36) | 2 | 1 | 1.24(0.14–11.44) | 5 | 9 | 1.39(0.74–2.61) |
| HPV-52: not present | 23 | 25 | 27 | 19 | 47 | 44 | |||
| present | 7 | 11 | 1.35(0.68–2.70) | 5 | 5 | 1.79(0.60–5.31) | 8 | 16 | 1.70(0.98–2.94) |
| HPV-53: not present | 23 | 23 | 28 | 19 | 49 | 42 | |||
| present | 6 | 8 | 1.01(0.50–2.07) | 5 | 6 | 2.01(0.82–4.96) | 8 | 14 | 1.37(0.79–2.40) |
| HPV-61: not present | 27 | 27 | 23 | 14 | 48 | 41 | |||
| present | 4 | 17 | 1.57(0.83–2.98) | 6 | 8 | 3.95(1.83–8.55)* | 6 | 25 | 2.04(1.24–3.37) |
| HPV-62: not present | 27 | 27 | 25 | 19 | 50 | 46 | |||
| present | 7 | 17 | 1.05(0.58–1.87) | 6 | 5 | 0.67(0.12–3.60) | 8 | 22 | 0.97(0.55–1.71) |
| HPV-84: not present | 27 | 27 | 24 | 17 | 47 | 44 | |||
| present | 7 | 12 | 0.78(0.46–1.33) | 4 | 4 | 0.84(0.29–2.42) | 8 | 16 | 0.85(0.54–1.36) |
| No. of coinfections | |||||||||
| none | 25 | 27 | 20 | 19 | 43 | 46 | |||
| 1 | 24 | 36 | 1.12(0.73–1.72) | 24 | 30 | 1.59(0.93–2.73) | 30 | 66 | 1.29(0.92–1.79) |
| 2 | 8 | 11 | 1.30(0.65–2.59) | 8 | 9 | 2.54(1.18–5.46) | 9 | 20 | 1.76(1.05–2.95) |
| 3+ | 7 | 36 | 1.08(0.71–1.66) | 6 | 11 | 0.94(0.45–1.95) | 7 | 47 | 1.13(0.79–1.62) |
| P for trend | 0.74 | 0.76 | 0.47 | ||||||
Coinfection with the specified HPV type at the clinic visit preceding clearance of the index infection.
The number of subjects who completed the questionnaire and at least 2 clinical visits.
The number of cleared incident HPV infections during the study period.
Estimated using Cox regression, adjusted for age of participants at study entry.
CI, confidence interval. Estimates indicated with a ‘*’ are statistically significant after Bonferroni correction for multiple comparisons.
Includes undetermined-risk HPV types.
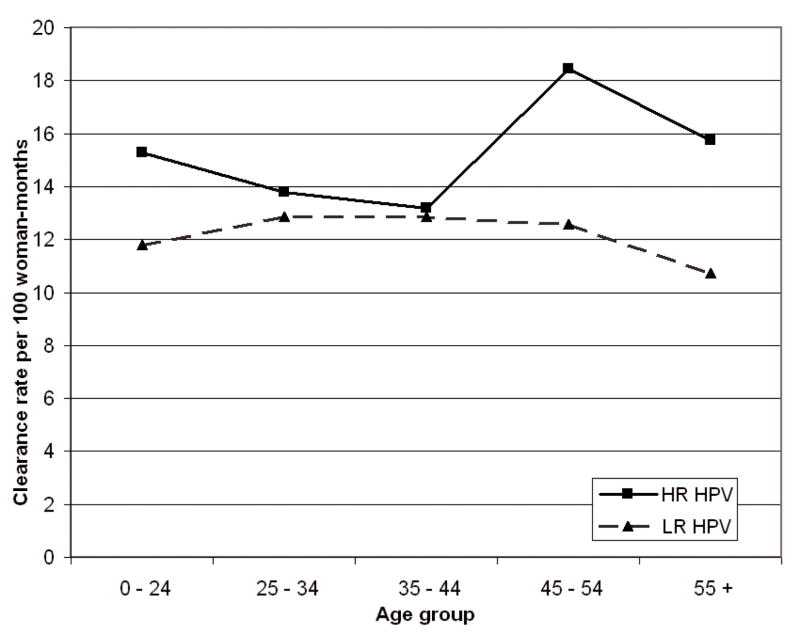
No significant association was observed between the age of participants at baseline and anal HPV clearance (Table 3). Clearance rates of HR-HPV were slightly elevated for those 45 years and older, while clearance rates of LR-HPV were nearly constant across age groups and consistently lower than those of HR-HPV (Figure 2). Older age at first sexual intercourse was associated with higher clearance rates for any HPV, while greater number of lifetime sexual partners reduced clearance rates, but the associations were not significant. Women who took oral contraceptives for 2–4 years exhibited higher anal HPV clearance rates, but no particular trend was observed.
Table 3.
Baseline risk factors for clearance of anal HPV infection.
| High-risk HPV |
Low-risk HPVe |
Any HPV |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjectsa N (=64) |
Eventsb N (=110) |
Hazard Ratioc (95% CI)d |
P for trend |
Subjectsa N (=58) |
Eventsb N (=69) |
Hazard Ratioc (95% CI)d |
P for trend |
Subjectsa N (=89) |
Eventsb N (=179) |
Hazard Ratioc (95% CI)d |
P for trend |
|
| Age (years) | ||||||||||||
| < 25 | 16 | 22 | 15 | 19 | 22 | 41 | ||||||
| 25 – 34 | 17 | 27 | 1.12(0.71–1.74) | 13 | 13 | 1.23(0.63–2.40) | 22 | 40 | 1.18(0.81–1.71) | |||
| 35 – 44 | 16 | 28 | 1.07(0.70–1.64) | 9 | 11 | 0.94(0.46–1.93) | 20 | 39 | 1.06(0.73–1.55) | |||
| 45 – 54 | 7 | 14 | 1.05(0.52–2.12) | 13 | 14 | 0.93(0.54–1.62) | 15 | 28 | 0.93(0.61–1.43) | |||
| >= 55 | 8 | 19 | 1.04(0.64–1.67) | 0.98 | 8 | 12 | 0.86(0.45–1.63) | 0.49 | 10 | 31 | 0.97(0.66–1.42) | 0.57 |
| Ethnicity | ||||||||||||
| Japanese | 4 | 5 | 4 | 7 | 6 | 12 | ||||||
| Caucasian | 33 | 62 | 1.65(0.99–2.74) | 34 | 42 | 0.90(0.47–1.71) | 48 | 104 | 1.24(0.82–1.86) | |||
| Hawaiian | 8 | 21 | 1.81(0.97–3.39) | 4 | 5 | 0.55(0.24–1.29) | 9 | 26 | 1.22(0.73–2.03) | |||
| Filipino | 4 | 4 | 1.60(0.62–4.14) | 3 | 4 | 0.76(0.24–2.49) | 6 | 8 | 1.04(0.49–2.22) | |||
| other | 15 | 18 | 1.42(0.77–2.62) | 13 | 11 | 0.86(0.43–1.74) | 20 | 29 | 1.10(0.70–1.75) | |||
| Education | ||||||||||||
| <= high school | 12 | 25 | 14 | 14 | 19 | 39 | ||||||
| some college | 17 | 25 | 1.14(0.68–1.91) | 14 | 17 | 3.37(1.70–6.70) | 23 | 42 | 1.85(1.23–2.78) | |||
| college grad | 24 | 35 | 1.26(0.76–2.09) | 23 | 27 | 2.74(1.44–5.25) | 36 | 62 | 1.82(1.23–2.70) | |||
| graduate degree | 11 | 25 | 0.99(0.62–1.58) | 0.89 | 7 | 11 | 1.46(0.70–3.03) | 0.04 | 11 | 36 | 1.24(0.85–1.82) | 0.11 |
| Income | ||||||||||||
| < 7,500 | 17 | 27 | 15 | 16 | 23 | 43 | ||||||
| 7,500 – 19,999 | 11 | 22 | 0.72(0.40–1.27) | 14 | 19 | 0.98(0.53–1.84) | 18 | 41 | 0.85(0.56–1.28) | |||
| 20,000 – 49,999 | 23 | 41 | 0.86(0.57–1.30) | 19 | 20 | 1.30(0.70–2.44) | 31 | 61 | 1.04(0.74–1.48) | |||
| >= 50,000 | 10 | 16 | 1.25(0.67–2.31) | 0.70 | 9 | 13 | 1.62(0.86–3.06) | 0.11 | 14 | 29 | 1.38(0.89–2.12) | 0.16 |
| Number of pregnancies | ||||||||||||
| none | 28 | 48 | 26 | 29 | 38 | 77 | ||||||
| 1 | 8 | 13 | 1.26(0.73–2.20) | 12 | 11 | 1.01(0.53–1.91) | 15 | 24 | 1.09(0.73–1.62) | |||
| 2 | 10 | 14 | 0.86(0.55–1.34) | 5 | 5 | 1.19(0.62–2.30) | 12 | 19 | 0.93(0.65–1.34) | |||
| >= 3 | 18 | 35 | 0.83(0.54–1.26) | 0.36 | 15 | 24 | 0.85(0.49–1.46) | 0.57 | 24 | 59 | 0.83(0.60–1.16) | 0.3 |
| Age at first sexual intercourse | ||||||||||||
| < 16 | 13 | 29 | 14 | 17 | 19 | 46 | ||||||
| 16 – 17 | 23 | 33 | 0.94(0.65–1.36) | 18 | 21 | 1.39(0.79–2.44) | 31 | 54 | 1.07(0.79–1.46) | |||
| 18 – 19 | 15 | 31 | 0.95(0.61–1.48) | 15 | 18 | 1.51(0.80–2.86) | 21 | 49 | 1.14(0.79–1.63) | |||
| >= 20 | 12 | 15 | 1.46(0.85–2.53) | 0.39 | 10 | 11 | 1.77(0.79–3.93) | 0.12 | 17 | 26 | 1.54(0.97–2.43) | 0.09 |
| Number of lifetime sexual partners | ||||||||||||
| < 2 | 1 | 9 | 1 | 2 | 1 | 11 | ||||||
| 2 – 5 | 20 | 23 | 0.89(0.48–1.64) | 20 | 22 | 0.84(0.20–3.55) | 32 | 45 | 0.79(0.47–1.33) | |||
| >= 6 | 40 | 72 | 0.79(0.49–1.28) | 0.31 | 35 | 41 | 0.72(0.17–3.04) | 0.47 | 53 | 113 | 0.71(0.44–1.15) | 0.18 |
| Oral contraceptive use at baseline | ||||||||||||
| never | 10 | 19 | 11 | 13 | 14 | 32 | ||||||
| ever | 54 | 91 | 0.99(0.65–1.50) | 47 | 56 | 1.09(0.68–1.77) | 75 | 147 | 1.08(0.79–1.49) | |||
| past user | 34 | 64 | 1.07(0.69–1.66) | 32 | 37 | 1.09(0.65–1.84) | 50 | 101 | 1.14(0.81–1.60) | |||
| current user | 20 | 27 | 0.82(0.49–1.36) | 15 | 19 | 1.10(0.58–2.08) | 25 | 46 | 0.95(0.64–1.42) | |||
| Years of regular oral contraceptive pill use | ||||||||||||
| none | 19 | 33 | 17 | 21 | 24 | 54 | ||||||
| < 2 years | 7 | 19 | 1.03(0.66–1.62) | 4 | 6 | 0.99(0.37–2.66) | 9 | 25 | 1.15(0.77–1.72) | |||
| 2 – 4 | 15 | 19 | 1.52(0.93–2.49) | 15 | 18 | 2.08(1.19–3.62) | 21 | 37 | 1.72(1.21–2.46) | |||
| 5 – 9 | 12 | 11 | 0.79(0.46–1.37) | 9 | 10 | 0.88(0.46–1.68) | 17 | 21 | 0.82(0.54–1.24) | |||
| >= 10 | 11 | 28 | 0.84(0.54–1.32) | 0.39 | 13 | 14 | 0.75(0.41–1.38) | 0.40 | 18 | 42 | 0.83(0.57–1.20) | 0.22 |
| Non-contraceptive hormone use | ||||||||||||
| never | 57 | 94 | 50 | 60 | 78 | 154 | ||||||
| past user | 5 | 11 | 1.19(0.59–2.38) | 6 | 6 | 0.83(0.45–1.53) | 9 | 17 | 0.99(0.62–1.58) | |||
| current user | 2 | 5 | 0.84(0.53–1.34) | 2 | 3 | 0.62(0.26–1.49) | 2 | 8 | 0.72(0.46–1.14) | |||
| Tobacco smoking history | ||||||||||||
| never | 38 | 61 | 31 | 37 | 52 | 98 | ||||||
| ever | 26 | 49 | 0.73(0.52–1.01) | 27 | 32 | 0.86(0.55–1.36) | 37 | 81 | 0.78(0.60–1.02) | |||
| past | 13 | 27 | 0.75(0.50–1.15) | 13 | 16 | 1.03(0.60–1.77) | 16 | 43 | 0.84(0.60–1.17) | |||
| current | 13 | 22 | 0.70(0.47–1.04) | 14 | 16 | 0.74(0.43–1.29) | 21 | 38 | 0.73(0.53–1.01) | |||
| Pack-years of tobacco use | ||||||||||||
| never | 38 | 61 | 31 | 37 | 52 | 98 | ||||||
| < 2 | 9 | 18 | 0.95(0.59–1.51) | 9 | 9 | 0.93(0.47–1.84) | 13 | 27 | 0.92(0.63–1.35) | |||
| 2 – 10 | 11 | 22 | 0.65(0.44–0.96) | 9 | 12 | 0.88(0.44–1.76) | 13 | 34 | 0.76(0.54–1.07) | |||
| >= 10 | 6 | 9 | 0.60(0.31–1.18) | 0.03 | 9 | 11 | 0.81(0.46–1.42) | 0.45 | 11 | 20 | 0.67(0.43–1.03) | 0.03 |
| Alcohol history | ||||||||||||
| never | 30 | 50 | 24 | 28 | 39 | 78 | ||||||
| ever | 34 | 60 | 1.07(0.78–1.46) | 34 | 41 | 0.85(0.55–1.32) | 50 | 101 | 0.97(0.76–1.25) | |||
| past | 18 | 34 | 1.25(0.85–1.85) | 21 | 22 | 0.84(0.51–1.40) | 27 | 56 | 1.03(0.76–1.40) | |||
| current | 16 | 26 | 0.90(0.62–1.30) | 13 | 19 | 0.86(0.49–1.49) | 23 | 45 | 0.90(0.66–1.23) | |||
| Lifetime ethanol intake (drinks) | ||||||||||||
| none | 30 | 50 | 24 | 28 | 39 | 78 | ||||||
| < 250 | 16 | 23 | 1.17(0.76–1.82) | 12 | 15 | 0.89(0.47–1.69) | 21 | 38 | 1.02(0.71–1.48) | |||
| 250 – 1099 | 6 | 7 | 1.74(0.97–3.12) | 12 | 15 | 1.31(0.72–2.36) | 13 | 22 | 1.37(0.87–2.14) | |||
| >= 1100 | 12 | 30 | 0.92(0.61–1.40) | 0.96 | 10 | 11 | 0.52(0.28–0.98) | 0.21 | 16 | 41 | 0.80(0.57–1.13) | 0.46 |
| Condom use at baseline | ||||||||||||
| no | 43 | 77 | 44 | 56 | 62 | 133 | ||||||
| yes | 21 | 33 | 1.37(0.94–2.00) | 14 | 13 | 0.67(0.35–1.28) | 27 | 46 | 1.03(0.74–1.44) | |||
| Use of douching at baseline | ||||||||||||
| no | 53 | 96 | 48 | 59 | 74 | 155 | ||||||
| yes | 11 | 14 | 0.48(0.33–0.71) | 10 | 10 | 0.62(0.36–1.07) | 15 | 24 | 0.54(0.39–0.74) | |||
| History of anal sex | ||||||||||||
| never | 47 | 77 | 41 | 55 | 62 | 132 | ||||||
| ever | 17 | 33 | 1.05(0.69–1.59) | 17 | 14 | 0.44(0.24–0.79) | 27 | 47 | 0.73(0.53–1.03) | |||
| past | 11 | 23 | 1.42(0.91–2.24) | 9 | 7 | 0.44(0.21–0.92) | 17 | 30 | 0.89(0.60–1.34) | |||
| current | 6 | 10 | 0.66(0.39–1.12) | 8 | 7 | 0.43(0.19–0.99) | 10 | 17 | 0.54(0.35–0.86) | |||
The number of subjects who completed at least 2 clinical visits and had an incident anal HPV infection.
The number of cleared incident HPV infections during the study period.
Estimated using Cox regression, adjusted for age of participants at study entry.
CI, confidence interval.
Includes undetermined-risk HPV types.
Figure 2.
Clearance rates of anal HPV infection, by age group and oncogenic risk.a
aClearance rates among the 215 participants who experienced an incident anal HPV infection during the study period. HR-HPV: any high-risk HPV genotype; LR-HPV: any low-risk HPV genotype.
History of tobacco smoking was associated with non-significantly reduced risk of anal HPV clearance, with a tendency toward increased HR-HPV persistence by pack-years of tobacco smoking (Table 3). A lower risk of HR-HPV clearance was also observed in women who douched. Current practice of anal sex significantly reduced the chances of anal HPV clearance overall, and among women with LR-HPV infections. Including these factors in a multivariate Cox model yielded similar risk estimates (data not shown).
DISCUSSION
In this study of anal HPV duration and clearance among healthy adult women, we found that anal HPV infections were moderately common [16], and had relatively short duration. Most anal HPV infections in our study were transient, with 87% of cleared infections clearing within 1 year. The median duration of anal HR-HPV infections was 5 months, shorter than the median duration of 8 months for cervical HR-HPV in the same group of women [19]. The latter figure is in agreement with the 8–20 month median duration of cervical HR-HPV reported by most other studies [30]. It is unclear whether the much lower incidence of anal cancer compared to cervical cancer is due to faster clearance of anal HPV or to different biology. Faster clearance of anal HR-HPV, as compared to cervical HR-HPV, may result from a number of factors. A higher concentration of keratinized cells in the epithelial tissues of the anus may hinder HPV persistence and facilitate more rapid clearance. Whether non-specific immunity of the gastrointestinal tract could contribute to reduced duration of anal HR-HPV is unclear and warrants further investigation. Association between cervical and anal HPV in our cohort will be the subject of a separate report.
The effect of multiple genotypes on clearance of anal HPV is largely unexplored. Hernandez et al. [17] speculated that women with multiple-type anal HPV infections are more susceptible to such infections due to impaired immune function or other factors. In the present study, clearance of both HR-and LR-HPV anal infections was enhanced in the presence of one or more other HPV genotypes. This could possibly be explained by competition among the established HPV infections, generally not found in the cervix [31, 32]. An effect of multiple infections has also been found in our cohort for anal HPV acquisition. Goodman et al. [16] reported that the risk of acquisition of a new anal HPV infection was increased in the presence of another HPV genotype, and that certain genotypes, such as HPV-16, had greater effect on the acquisition of new HPV infections.
Overall, anal infections with LR-HPV types took longer to resolve in our cohort than did HR-HPV types. This is in contrast to findings from most cervical HPV natural history studies in which LR-HPV types generally clear faster than HR-HPV types [30, 33]. HPV-16, the most common oncogenic genotype found in association with cervical malignancy, tends to persist longer in cervical tissue than other types [34]. In contrast, anal HPV-16 infections generally cleared within ~4 months in our study, much faster than many other genotypes. The ability to rapidly clear HPV-16 may protect anal cells from clonal progression of the persistently infected epithelium to anal pre-cancer and invasion. Our observation supports the hypothesis that certain HPV types have different tropism to the anus than to the cervix [17]. Frisch et al. [6] found LR-HPV DNA in only 4% of anal cancers; therefore, longer persistence of LR-HPV types is unlikely to influence their oncogenic potential. Nonetheless, the presence of LR-HPV genotypes may delay clearance of concurrent HR-HPV infections or, through weakening the host’s immune system, increase susceptibility to subsequent acquisition of high-risk anal HPV.
Non-viral factors that delayed clearance of anal HPV in our study included long-term tobacco smoking. Tobacco smoking has been established as a risk factor for anal cancer [4, 35], but it is not associated with increased risk of anal HPV acquisition in our cohort [16] and other studies [10]. The results of the present analysis suggest that the effect of smoking in the etiology of anal cancer is in part due to a longer clearance time, but not higher acquisition rate of anal HPV. Behavioral factors, such as current practice of anal sex, also reduced the risk of anal HPV clearance in our cohort. One possible explanation is that increased exposure through anal intercourse contributes to continued reinfection. Since we are unable to distinguish HPV infections that were resolved and reacquired between clinic visits from those that persisted from one visit to the next, this reinfection with the same HPV genotype may help explain the slower clearance we have found in this study. An association of douching with reduced clearance time may also be a result of inadvertent reinfection of the anus with HPV.
If anal cancer rates continue increasing in the U.S., more consideration should be given to the implementation of anal cancer screening programs. However, it is unclear whether anal HPV testing would be an effective cancer screening technique. The potential benefits of cervical HR-HPV testing as a cancer screening tool have recently been addressed in the literature. In a study of 10,154 women, Mayrand et al. [36] reported sensitivity of HPV testing for cervical intraepithelial neoplasia to be 94.6%, as compared to 55.4% sensitivity for the Pap smear. Given the highly transient nature of anal HPV infections and a relatively low incidence of anal cancer, a test for anal HPV is more likely to pick up short-term infections that resolve spontaneously, rather than persistent infections with the potential to progress to precancerous lesions. Therefore, as a cancer screening tool, anal HPV testing would likely have lower sensitivity and positive predictive value than cervical HPV testing and thus may not be as cost-effective.
Further, because we do not completely understand how specific HPV types affect anal cancer development, it is unclear which HPV genotypes should be targeted in a population-wide anal HPV testing program. Further research is necessary to address these issues. The risk factors identified in this report that hinder clearance of anal HPV may be helpful in designing a screening program based on risk stratification, whereby individuals with higher perceived risk for anal cancer would be given priority screening [37].
The advantages of this study include a long mean follow-up period of ~16 months and a relatively short interval between visits. The unique ethnic composition of Hawaii population enabled us to compare anal HPV clearance rates in women from various racial and ethnic groups. Limitations of this study included its recruitment scheme. Study subjects were recruited among college students and patients of health maintenance organizations, so our results may not be generalizable to the entire population. In addition, collection of anal specimens was optional; women who chose to provide them comprised 66% of cervical study participants and differed from those who did not with respect to age and ethnicity [17]. Another limitation of this and other longitudinal studies of HPV infection is the assumption that the same genotype detected at consecutive visits represents the same persistent infection, rather than reinfection after clearance. We defined clearance as a single negative visit after a positive, whereas some investigators have argued in favor of a two-negative-visit definition. In our study, the same genotype reappeared after a negative in 39 subjects, indicating that these genotypes could have been missed during PCR hybridization-based genotyping.
In summary, the differences in duration between anal and cervical HPV infections found in this cohort suggest a different disease natural history at these two sites. Shorter overall duration of anal HPV infection could reduce sensitivity of anal HPV testing, rendering it less cost-effective than cervical HPV testing as a cancer screening tool. The potential of anal HPV testing for anal cancer prevention should be re-evaluated when more affordable, cost-effective HPV testing is developed and when the longer-term effects of HPV vaccination on the prevalence of HPV-16 are better understood.
Acknowledgments
FUNDING
This study was supported by US Public Health Service grant R01-CA-077318 from the National Cancer Institute, NIH, Department of Health and Human Services.
We extend our gratitude to the staff of the University of Hawaii, Cancer Research Center of Hawaii, and the University of Hawaii University Health Services whose clinical staff conducted specimen collection for the study. Reagents for the HPV PGMY-LB assay were kindly supplied by Roche Molecular Systems.
Abbreviations
- CI
confidence interval
- HPV
human papillomavirus
- HR
high-risk
- LR
low-risk
- PCR
polymerase chain reaction
- RR
relative risk
Footnotes
CONFLICT OF INTEREST STATEMENT
Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Ning L, Killeen J, Goodman MT: No conflicts. Hernandez BY: pending research funding from Merck. Kamemoto L: research funding from GlaxoSmithKline, speakers’ bureau for Merck.
References
- 1.American Cancer Society. Cancer facts and figures. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 2.Moscicki AB, Hills NK, Shiboski S, et al. Risk factors for abnormal anal cytology in young heterosexual women. Cancer Epidemiol Biomarkers Prev. 1999;8:173–8. [PubMed] [Google Scholar]
- 3.Edgren G, Sparen P. Risk of anogenital cancer after diagnosis of cervical intraepithelial neoplasia: a prospective population-based study. Lancet Oncol. 2007;8:311–6. doi: 10.1016/S1470-2045(07)70043-8. [DOI] [PubMed] [Google Scholar]
- 4.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–80. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 5.Holly EA, Ralston ML, Darragh TM, Greenblatt RM, Jay N, Palefsky JM. Prevalence and risk factors for anal squamous intraepithelial lesions in women. J Natl Cancer Inst. 2001;93:843–9. doi: 10.1093/jnci/93.11.843. [DOI] [PubMed] [Google Scholar]
- 6.Frisch M, Glimelius B, van den Brule AJ, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337:1350–8. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 7.Palefsky JM. Human papillomavirus infection and anogenital neoplasia in human immunodeficiency virus-positive men and women. J Natl Cancer Inst Monogr. 1998:15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024166. [DOI] [PubMed] [Google Scholar]
- 8.Williams AB, Darragh TM, Vranizan K, Ochia C, Moss AR, Palefsky JM. Anal and cervical human papillomavirus infection and risk of anal and cervical epithelial abnormalities in human immunodeficiency virus-infected women. Obstet Gynecol. 1994;83:205–11. [PubMed] [Google Scholar]
- 9.Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 10.Palefsky JM, Holly EA, Ralston ML, Da CM, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001;183:383–91. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 11.Piketty C, Darragh TM, Da CM, et al. High prevalence of anal human papillomavirus infection and anal cancer precursors among HIV-infected persons in the absence of anal intercourse. Ann Intern Med. 2003;138:453–9. doi: 10.7326/0003-4819-138-6-200303180-00008. [DOI] [PubMed] [Google Scholar]
- 12.Frisch M, Melbye M, Moller H. Trends in incidence of anal cancer in Denmark. BMJ. 1993;306:419–22. doi: 10.1136/bmj.306.6875.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewster DH, Bhatti LA. Increasing incidence of squamous cell carcinoma of the anus in Scotland, 1975–2002. Br J Cancer. 2006;95:87–90. doi: 10.1038/sj.bjc.6603175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiao EY, Krown SE, Stier EA, Schrag D. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr. 2005;40:451–5. doi: 10.1097/01.qai.0000159669.80207.12. [DOI] [PubMed] [Google Scholar]
- 15.Surveillance Research Program, National Cancer Institute. SEER*Stat software. 2008 ( www.seer.cancer.gov/seerstat) version 6.4.4 [computer program]
- 16.Goodman MT, Shvetsov YB, McDuffie K, et al. Acquisition of anal human papillomavirus infection among women: the Hawaii HPV cohort study. J Infect Dis. 2008;197:957–66. doi: 10.1086/529207. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez BY, McDuffie K, Zhu X, et al. Anal human papillomavirus infection in women and its relationship with cervical infection. Cancer Epidemiol Biomarkers Prev. 2005;14:2550–6. doi: 10.1158/1055-9965.EPI-05-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman MT, Shvetsov YB, McDuffie K, et al. Hawaii cohort study of serum micronutrient concentrations and clearance of incident oncogenic human papillomavirus infection of the cervix. Cancer Res. 2007;67:5987–96. doi: 10.1158/0008-5472.CAN-07-0313. [DOI] [PubMed] [Google Scholar]
- 19.Goodman MT, Shvetsov YB, McDuffie K, et al. Prevalence, acquisition, and clearance of cervical human papillomavirus infection among women with normal cytology: Hawaii HPV cohort study. Cancer Research. 2008:68. doi: 10.1158/0008-5472.CAN-08-1380. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gravitt PE, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gravitt PE, Schiffman M, Solomon D, Wheeler CM, Castle PE. A comparison of linear array and hybrid capture 2 for detection of carcinogenic human papillomavirus and cervical precancer in ASCUS-LSIL triage study. Cancer Epidemiol Biomarkers Prev. 2008;17:1248–54. doi: 10.1158/1055-9965.EPI-07-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castle PE, Gravitt PE, Solomon D, Wheeler CM, Schiffman M. Comparison of linear array and line blot assay for detection of human papillomavirus and diagnosis of cervical precancer and cancer in the atypical squamous cell of undetermined significance and low-grade squamous intraepithelial lesion triage study. J Clin Microbiol. 2008;46:109–17. doi: 10.1128/JCM.01667-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur HH. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24S3:S1–S10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 26.Dobson AJ, Kuulasmaa K, Eberle E, Scherer J. Confidence intervals for weighted sums of Poisson parameters. Stat Med. 1991;10:457–62. doi: 10.1002/sim.4780100317. [DOI] [PubMed] [Google Scholar]
- 27.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 28.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 29.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. Journal of the American Statistical Association. 1989;84:1074–8. [Google Scholar]
- 30.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24 (Suppl 1):S1–15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 31.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 32.Rousseau MC, Pereira JS, Prado JC, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508–17. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 33.Koshiol JE, Schroeder JC, Jamieson DJ, et al. Time to clearance of human papillomavirus infection by type and human immunodeficiency virus serostatus. Int J Cancer. 2006;119:1623–9. doi: 10.1002/ijc.22015. [DOI] [PubMed] [Google Scholar]
- 34.Molano M, Van den BA, Plummer M, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003;158:486–94. doi: 10.1093/aje/kwg171. [DOI] [PubMed] [Google Scholar]
- 35.Daling JR, Sherman KJ, Hislop TG, et al. Cigarette smoking and the risk of anogenital cancer. Am J Epidemiol. 1992;135:180–9. doi: 10.1093/oxfordjournals.aje.a116270. [DOI] [PubMed] [Google Scholar]
- 36.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–88. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 37.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]