Abstract
Proteins critical for vesicle formation by the Coat Protein I (COPI) complex are being identified, but less known has been the role of specific lipids. Brefeldin-A ADP-Ribosylated Substrate (BARS) acts in the fission step of COPI vesicle formation. Here, we show that BARS induces membrane curvature in cooperation with phosphatidic acid (PA). This revelation has allowed us to further delineate COPI vesicle fission into two sub-stages: i) an earlier stage of bud neck constriction, in which BARS and other COPI components are needed, and ii) a later stage of bud neck scission, in which PA generated by phospholipase D2 (PLD2) is needed additionally. Moreover, in contrast to the disruption of the Golgi seen upon perturbing the core COPI components (such as coatomer), inhibition of PLD2 results in milder disruptions, predicting that such COPI components have additional role(s) in maintaining Golgi structure other than through COPI vesicle formation.
Vesicles formed by the COPI complex act in transport among Golgi cisternae, and also retrograde transport from the Golgi to the endoplasmic reticulum (ER) 1, 2. ADP-Ribosylation Factor 1 (ARF1) regulates COPI in these events 3, 4. Like all small GTPases, ARF1 is activated by a guanine nucleotide exchange factor (GEF), and deactivated by a GTPase-activating protein (GAP). Consistent with ARF1 being a key regulator of COPI vesicle formation, inhibition of the GEF activity that activates ARF1, such as pharmacologically through brefeldin-A 5, 6, inhibits COPI vesicle formation 7. Notably however, such inhibition also disrupts Golgi integrity 8. Perturbation of coatomer (the core components of the COPI complex 9) also compromises Golgi integrity 10. These correlative effects have led to a current view that COPI transport is critical to maintaining Golgi structure.
Even though ARF1 and coatomer have been proposed originally to be the only cytosolic proteins needed for COPI vesicle formation 11, additional proteins are being identified in recent years. Besides having a traditional role as a negative regulator of the ARF GTPase cycle, ARFGAP1 acts also as an ARF1 effector, behaving as a key component of the COPI complex 12, 13. This finding has led to the subsequent identification of BARS as a key protein that acts at the fission step of COPI vesicle formation 14, 15. Moreover, even though the ability of BARS to induce membrane fission has been attributed previously to an acyltransferase activity 16, this activity has been found to be dispensable for COPI vesicle fission 14. Providing an explanation, a recent study has found that the previously characterized acyltransferase activity is not intrinsic to BARS 17. However, this finding also raises a new key question: how does BARS induce membrane fission?
RESULTS
Deformation of liposomes by BARS requires PA
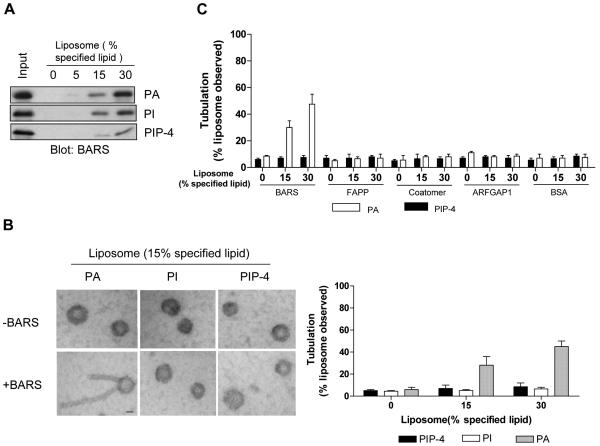
As the better characterized fission factors possess an intrinsic ability to generate membrane curvature 18-21, we initially sought to determine whether BARS had a similar ability. When different lipids were spotted onto a filter and then incubated with purified BARS, we initially found that BARS bound directly to phosphatidylinositol (PI), PI(4)-phosphate [PIP-4], and phosphatidic acid (PA) (Fig S1A). Moreover, when liposomes were generated with a lipid composition that mimicked Golgi membrane (Table S1), increasing levels of particular phospholipids found above to interact with BARS led to increased binding by BARS (Fig 1A). An even more notable finding was discerned when these liposomes were examined by electron microscopy (EM). Whereas PA allowed BARS to induce liposome tubulation, neither PI nor PIP-4 allowed this effect (Fig 1B). In contrast, other proteins, including COPI components and FAPP (four-phosphate-adaptor protein), which has been shown to bind PIP-4 22 and we further confirmed by its binding to PIP-4-containing liposomes (Fig S1B), could not induce liposome deformation (Fig 1C).
Figure 1. Liposome tubulation by BARS requires PA.
(A) Binding by BARS to liposomes with increasing level of additional specified lipid. (B) EM examination of liposomes bound by BARS; bar, 200 nm. The fraction that exhibited tubulation was quantified, with mean and standard error from three experiments shown. (C) Quantitation for the ability of other proteins to induce liposome tubulation, as analyzed by EM.
Recently, diacylglycerol (DAG) had been suggested to act in COPI vesicle fission 23. However, we found that BARS could not bind DAG, either directly to DAG when spotted on a filter (Fig S2A) or when DAG was added to liposomes (Fig S2B). Moreover, BARS could not induce deformation of such liposomes (Fig S2C). We also ruled out that the selective ability of BARS to induce liposome deformation in the presence of PA could be attributed somehow to an acyltransferase activity that tightly associated with recombinant BARS when generated by bacterial expression 17, as BARS prepared from bacteria lacking this activity behaved similarly (Fig S3).
COPI vesicle formation requires PA generated by PLD2
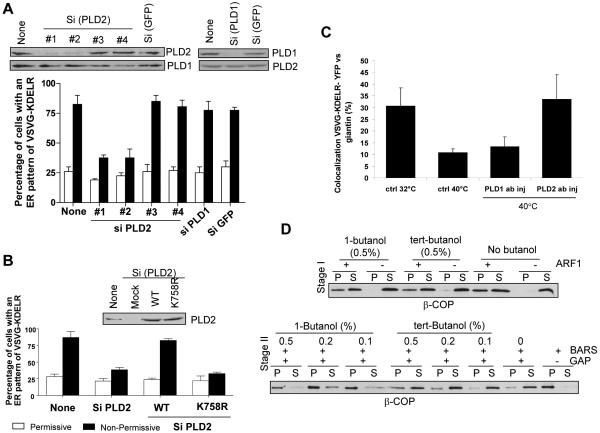
As these initial results suggested that PA played an integral role with BARS to generate membrane curvature, we further pursued this intriguing possibility in one of the better characterized contexts of BARS function, COPI vesicle fission 14, 15. Initially, because phospholipase D (PLD) activity had been well characterized to generate PA and two isoforms of PLD had been identified 24, 25, we tested whether either PLD form had any role in COPI transport. Using a previously established in vivo assay for COPI-dependent transport, which tracked the redistribution of a chimeric KDEL receptor (KDELR) from the Golgi to the ER 14, 15, we found that PLD2 depletion by small interfering ribonucleic acid (siRNA) treatment of cells inhibited COPI transport, but PLD1 depletion did not have a similar effect (Fig 2A). Moreover, expression of a siRNA-resistant form of wild-type PLD2 reversed the effect of the inhibitory oligonucleotides (Fig 2B). In this context, a siRNA-resistant form of a catalytically dead PLD2 mutant could not rescue similarly (Fig 2B), implicating the catalytic activity of PLD2 being critical for COPI transport. We also found that the microinjection of an anti-PLD2 antibody selectively inhibited the COPI transport assay (Fig 2C).
Figure 2. Perturbation of PA generated by PLD2 affects COPI transport.
(A) Gel shows knockdown of proteins (upper panels) with loading controls (lower panels). Graph shows quantitation of retrograde transport of the chimeric KDELR, with the mean from three experiments and standard error shown. (B) Gel shows knockdown of proteins. Graph again shows quantitation of retrograde transport of the chimeric KDELR. (C) Quantitation of retrograde transport of the chimeric KDELR upon microinjection of different antibodies in conditions, with the mean from three experiments and standard error shown. (D) First stage shows ARF1-dependent recruitment of coatomer to Golgi membrane (above). Second stage shows COPI vesicle formation, as reflected by the release of coatomer from Golgi membrane (below).
To gain further mechanistic insight into how PLD2 acted in COPI transport, we next turned to the COPI vesicle reconstitution system, as previously established 12-15. Moreover, as PA formation by PLD activity could be blocked by the addition of a primary alcohol, such as 1-butanol 25, we initially used this pharmacologic agent to screen for potential effects in the first and/or the second stage of the vesicle reconstitution system. 1-butanol selectively affected the second stage, and as a control, tert-butanol did not have a similar effect (Fig 2D). We also noted that PA could be generated from diacylglycerol (DAG) by the activity of a DAG kinase (DGK). Thus, as multiple forms of DGK exist 26, we screened for their potential involvement by examining the effect of two pharmacologic inhibitors 27, 28. Neither one alone, nor in combination, had significant effects on COPI vesicle formation (Fig S4). Thus, we subsequently focused on how PA generated by PLD activity would act in COPI vesicle formation.
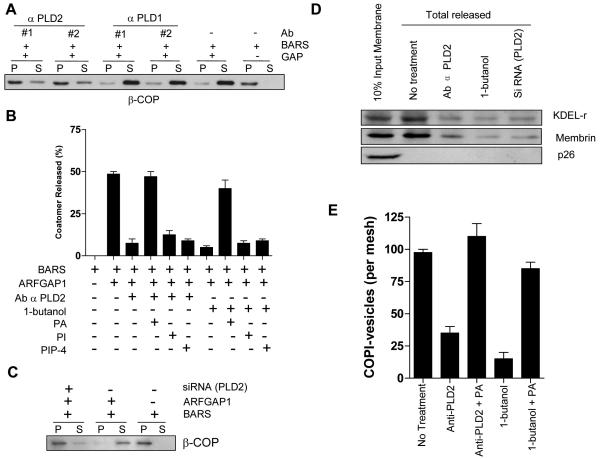
Initially, using antibodies directed against either PLD1 or PLD2, we found that only antibodies against PLD2 inhibited the second stage of the COPI vesicle reconstitution system (Fig 3A). This inhibition was reversed by the addition of liposomes that contained PA, but not those that contained PI or PIP-4 (Fig 3B). The antibody that we had used to target PLD2 likely acted with specificity in inhibiting COPI vesicle formation. First, it was used previously to detect a Golgi pool of PLD2 29. Second, the protein detected by this antibody in western blotting was reduced upon siRNA against PLD2 (as seen above in Fig 2A). Third, the antibody detected one predominant protein band on Golgi membrane used for the COPI vesicle reconstitution system (Fig S5C). Confirming the effect of the blocking antibody, we also prepared Golgi membrane from cells treated siRNA against PLD2 (Fig S5A), and found that COPI vesicle reconstitution was similarly inhibited (Fig 3C). In this setting, the level of BARS associated with Golgi membrane was not appreciably altered (Fig S5B).
Figure 3. COPI vesicle formation requires PA generated by PLD2.
(A) Vesicle formation as reflected by the release of β–COP from Golgi membrane at the second stage. (B) Quantitation of vesicle formation as reflected by the release of β–COP from Golgi membrane at the second stage. The mean from three experiments with standard error is shown. (C) Vesicle formation as reflected by the release of β–COP from Golgi membrane at the second stage. (D) Cargo sorting into COPI vesicles, as reflected by the release of cargo proteins from Golgi membrane at the second stage. (E) Vesicle formation assessed by immunogold EM. The mean from three experiments with standard error is shown.
To examine cargo sorting, we focused on the KDELR and membrin as representative COPI cargoes 14. First verifying that only KDELR and membrin were selectively released from Golgi membrane during the second-stage incubation, we then found that this release was inhibited by the different ways of perturbing PLD2 (Fig 3D). In contrast, p26 as control remained with Golgi membrane in either condition. Moreover, by quantitative immunogold electron microscopy (EM), the level of COPI-positive vesicles generated after the second-stage incubation was reduced by PLD inhibition, which was reversed by the addition of PA-containing liposomes (Fig 3E).
Effects of PLD2 depletion on COPI vesicle fission and Golgi structure
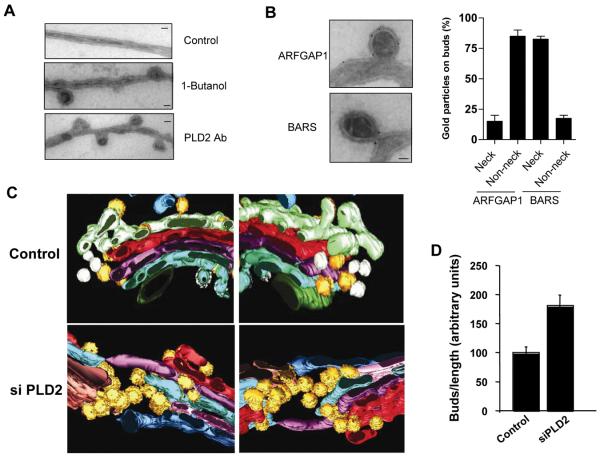
To examine in more detail how PA generated by PLD2 activity acted in COPI vesicle formation, we initially inspected Golgi membrane in the COPI vesicle reconstitution system by EM. Strikingly, inhibition of PLD2 induced the accumulation of buds with constricted necks (Fig 4A). Immunogold labeling showed that whereas ARFGAP1 showed a diffuse distribution on such buds, BARS showed a more restricted distribution at the neck of these buds (Fig 4B). These results were consistent with ARFGAP1 having been found to act in conjunction with coatomer as a coat component 12, 13, and BARS having a more restricted role in COPI vesicle fission 14, 15.
Figure 4. PLD2 is required for at a late stage of COPI vesicle fission.
(A) EM examination of Golgi membrane after the second stage; bar, 50 nm. As comparison, Golgi membrane prior to the incubation is shown as control. (B) The distributions of proteins on Golgi membrane after the second stage as assessed by immunogold EM; bar, 50 nm. Quantitation with the mean from three experiments and standard error is also shown. (C) Three-dimensional reconstruction of a representative Golgi from either control or PLD2-depleted cells is shown in two opposite views. Yellow highlights buds. (D) Quantitation of buds accumulation. The mean and standard deviation, derived from analyzing ten randomly selected Golgi profiles in each condition, are shown.
We next examined the effect of depleting PLD2 in cells. In particular, because EM tomography could distinguish buds from vesicles with better certainty, we used this approach and found that the Golgi had more buds (Fig 4C). Stereological quantitation revealed about a two-fold increase in bud accumulation (Fig 4D). We also detected bud accumulation at the intermediate compartment, also known as the ERGIC (Endoplasmic Reticulum-Golgi-Intermediate Compartment), which is the other organellar compartment that forms COPI vesicles. In this case, unusual morphology of bud accumulation was observed, with some buds appearing in close juxtaposition to, or extending from, others (data not shown).
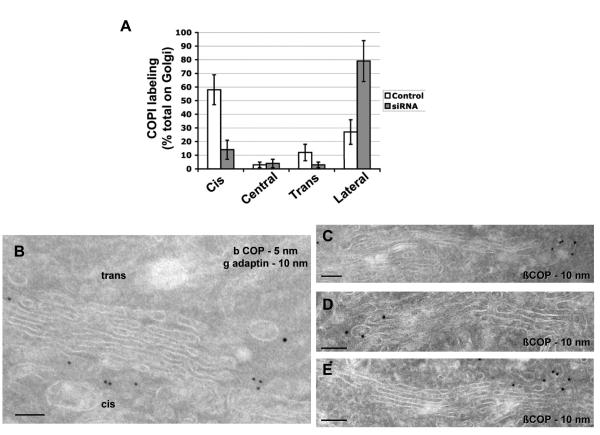
We also assessed Golgi structure and the distribution of COPI using immunogold EM. In cells treated with siRNA against PLD2, we observed increased COPI labeling at the lateral rims of Golgi cisternae (Fig 5A and Fig 5C/D/E). Unexpectedly however, we also observed decreased COPI labeling at the cis-side of the Golgi (Figs 5A and Fig 5C/D/E). Scrutinizing this latter result for a reason, we found that the cis-most cisterna was markedly reduced (compare Figs 5B and 5C/D/E), predicting impaired level of COPI budding at this region. Thus, a reason became apparent regarding why the total level of Golgi buds did not increase more dramatically upon the depletion of PLD2 (seen in Fig 4D). Increased COPI buds at the lateral rims of Golgi stacks was being offset to some degree by reduced COPI budding at the cis-side of the Golgi. EM examination also revealed a relatively intact Golgi structure, except for some swelling and loss of the cis-most cisterna (compare Fig 5A with Fig 5C/D/E). Moreover, immunofluorescence microscopy revealed the distribution of Golgi markers to be relatively unperturbed (Fig S6). These morphologic findings were surprising, as a prevailing view had been that COPI transport supported the integrity of the entire Golgi complex.
Figure 5. Golgi morphology and COPI distribution upon PLD2 depletion.
(A) Relative distributions of COPI labeling at the Golgi. The mean from ten randomly selected Golgi profiles with standard deviation is shown. (B) Golgi in control cells. (C-E) Golgi in PLD2-depleted cells; bar, 200 nm.
We also noted that PLD2 depletion resulted in buds with constricted necks. Thus, a prediction was that COPI buds would be essentially committed to becoming vesicles when PLD2 acted. Indeed, we did not observe enhanced Golgi tubulation in cells treated with siRNA against PLD2, either by immunofluorescence microscopy (Fig S6) or by EM (Fig 5). Depletion of PLD2 also did not affect the ability of brefeldin-A (BFA) to induce Golgi tubulation (Fig S7A). Moreover, the kinetics by which BFA induced Golgi redistribution to the ER was not significantly altered (Fig S7B).
We further noted that the induction of liposome tubulation had been used previously to identify transport factors involved in membrane deformation, including fission factors, such as endophilin A for clathrin vesicles 30 and Sar1p for COPII vesicles 31. Further studies using more physiologic membrane subsequently provided better context for how such factors acted in vesicle fission. Thus, as BARS was originally discovered to act in membrane fission by the finding that Golgi tubules were transformed into vesicles 16, we next re-visited this finding. As this fission ability of BARS had been shown to require the addition of cytosol 16, we examined for predicted additional factor(s) in the cytosol that would act in conjunction with BARS for Golgi fission. We first confirmed that the transformation of Golgi tubules into vesicles was arrested at the stage of serial constrictions when PLD2 was inhibited (see figure associated with Table 1). Subsequently, the combination of ARF1, ARFGAP1, and coatomer was found to be sufficient in replacing cytosol to achieve serial constrictions in the presence of PLD2 inhibition (Table 1). Thus, we concluded that COPI-related factors cooperated with BARS to initiate membrane constrictions, while PA generated by PLD2 was needed additionally for the later step of converting these constrictions into vesicles.
Table 1.
Components needed to induce membrane constriction
| Additional factor(s) | Constriction (% Golgi membrane observed) | ||
|---|---|---|---|
| 1-Butanol | Ab α PLD2 | siRNA(PLD2) | |
| Coatomer | 0 | 0 | 0 |
| ARF1 | 0 | 0 | 0 |
| ARFGAP1 | 0 | 0 | 0 |
| Coatomer + ARF1 | 0 | 0 | 0 |
| Coatomer + ARFGAP1 | 0 | 0 | 0 |
| ARF1 + ARFGAP1 | 0 | 0 | 0 |
| Coatomer + ARF1 + ARFGAP1 | 62 ± 5 | 55 ± 8 | 50 ± 8 |

Different protein components as indicated were added to Golgi membrane along with BARS in the presence of PLD2 inhibition. Membrane constriction versus no effect were assessed by EM, with examples of such images shown.
Defining a domain in BARS responsible for membrane deformation
Finally, we performed structure-function analysis on BARS to further elucidate how it induced membrane curvature. As different domains of BARS had been generated previously 14, we initially examine their behavior using the same series of assays that had examined the wild-type form; that is, binding to purified lipids, to defined liposomes, and also by EM examination to assess liposome tubulation. Three forms, previously termed C-Terminal Domain (CTD), Substrate-Binding Domain (SBD), and C-Terminal Portion (CTP) 14, bound directly to specific lipids similar to that seen for the full-length form (Table 2 and Fig S8A) and to defined liposomes (Table 2 and Fig S8B). However, only CTD and SBD induced liposome tubulation (Table 2 and Fig S8C). We also noted that CTP interacted with lipids similar to those seen for the wild-type BARS (Table 2, Figs S8A and S8B). Yet, it could not induce liposome tubulation (Table 2 and Fig S8C), despite containing CTD that was concluded above to be the minimal domain responsible for inducing such tubulation. Thus, these findings explained why CTP was found previously to block COPI vesicle fission in a dominant manner 14, 15.
DISCUSSION
ARF1 and coatomer have been shown to be sufficient to produce vesicular-like structures in the context of liposomes 32-34 However, the use of Golgi membrane has subsequently identified additional critical factors, including ARFGAP1 12, 13, BARS 14/endophilin B 15, and now PA generated by PLD2. As the core machinery of the COPI coat 32, 34, coatomer is expected to act across all stages of vesicle formation. BARS, in contrast, is predicted to act in later stages of COPI vesicle formation, being required when the neck of COPI buds begins to constrict. The role of PA generated by PLD2 is predicted to be even more restricted, as we have found that it is required at a late stage of vesicle fission, when the neck of COPI buds undergoes final scission to generate COPI vesicles. ARFGAP1 has probably the most complex role. Besides behaving as a general coat component 12, 13, its interaction with BARS suggests that it would also play a key role in COPI vesicle fission 14. Along this line, diacylglycerol (DAG) has been found recently to act in COPI vesicle fission by recruiting ARFGAP1 to the Golgi 23. Thus, as DAG is a key lipid that underlies membrane fission in other transport pathways from the Golgi 35, one possibility is that PA may have a dual role. On the one hand, PA likely plays a direct role in allowing BARS to bend membrane during COPI vesicle fission. On the other hand, PA may serve as a source of DAG (such as through a lipid phosphate phosphatase 35), and thereby being indirectly involved in the role of ARFGAP1 in COPI vesicle fission.
We also note that PLD activity has been suggested previously to mediate the recruitment of coatomer by ARF1 activation 36, but a later study has concluded that this role cannot be generalized for all cell types 37. Our current findings support the latter conclusion. Moreover, we have found that PLD activity is critical for a late stage of COPI vesicle fission step. This role has been revealed through the use of GTP that allows the GAP activity of ARFGAP1 to be manifested. In contrast, we note that the traditional approach of forming COPI vesicles blocks this activity 38, which would have obscured the ability to examine the late stages of COPI vesicle formation 13. Along this line, our finding that PA generated by PLD2 is critical for COPI vesicle fission also contributes a general understanding of membrane fission. In many cases, it has been difficult to distinguish whether the role of a specific lipid in this process relates simply to the localization of protein fission factors to a particular membrane domain, or a more fundamental role by cooperating with such proteins in generating membrane curvature 18-21. Addressing this issue, we have found that BARS has an intrinsic ability to induce membrane curvature and this ability requires the cooperation of PA.
Finally, EM examination has led us to discern some notable effects on the Golgi when PLD2 is depleted. As expected, we find that COPI buds accumulate at the lateral rims of Golgi cisternae. However, we also find that the cis-most cisterna of the Golgi is markedly compromised upon PLD2 depletion, and thus explaining impaired COPI budding in this region. Notably, this finding indicates that intra-Golgi COPI transport is critical in maintaining the cis-most region of the Golgi. In this respect, previous studies that perturb COPI transport through either ARF1 8 or coatomer 10 have observed more dramatic disruption of the Golgi, which has led to the current notion that COPI transport is critical in maintaining the entire Golgi complex. In considering a reconciling explanation, we note another previous study that suggests coatomer likely to have additional role(s) at the Golgi other than in COPI transport 39. Our current findings further support this possibility, predicting additional role(s) of coatomer in maintaining Golgi structure.
METHODS
Chemicals
GTP, bovine serum albumin (BSA), 1-butanol, tert-butanol, and DGK inhibitors (R59022 and R59949) were obtained (Sigma-Aldrich, St Louis, MO). Different di-oleoyl (DO)-lipids, including DOPA, DOPC, DOPE, DOPS, DOPI, DOPIP-3, DOPIP-4, DOPIP2-3,4, DOPIP2-3,5, DOPIP2-4,5, and DOPIP3-3,4,5, along with cholesterol, sphingomyelin, and 1-palmitoyl-2-oleoyl-DAG were also obtained (Avanti Polar Lipids, Alabaster, AL).
Proteins
Coatomer, myristoylated ARF1, ARFGAP1, and BARS along with its different truncation domains were prepared as described previously 12, 14. BARS was expressed as a GST fusion protein in bacteria deficient in acyltransferase activity (JC201) as previously described 17, and then purified by first releasing from GST using thrombin followed by incubation with p-aminobenzamidine agarose beads (Sigma, St. Louis, MO) to sequester soluble thrombin. Acyltransferase activity of purified BARS was performed as previously described 14. The PH domain of FAPP that bound specifically PIP-4 was generated as previously described 22.
Membranes
Golgi membranes were isolated and washed as previously described 14, 15. Unilamellar liposomes were generated essentially as previously described 30, 32, 34. Briefly, lipids were dissolved in chloroform and then mixed in various molar ratios that mimicked Golgi composition 32, 34; see Supplementary Table 1 for details. After drying, lipid films were hydrated in 50 mM Hepes-KOH, pH7.2, and 100mM KCl, followed by dispersion and then extruded through 400nm polycarbonate membrane (Whatman, Clifton, NJ). Extruded liposomes were then centrifuged at 12,000 × g for 5 min, and the resulting supernatant was stored at 4°C.
Antibodies
Primary antibodies have been described previously 12, 14, 15, 40, including: mouse anti-β-COP (M3A5) and anti-VSVG (BW8G65) antibodies; rabbit anti-β-COP, anti-ε-COP, anti-ζ-COP, anti-γ adaptin of AP1, anti-BARS, anti-KDELR, anti-membrin, and anti-p26 antibodies. Secondary antibodies include Cy2 or Cy3 conjugated donkey antibodies against either mouse IgG or rabbit IgG, horseradish peroxidase-conjugated donkey antibodies against mouse IgG or rabbit IgG, and 10 nm gold-conjugated goat antibodies against rabbit IgG. Affinity-purified rabbit anti-PLD1 and PLD2 antibodies have also been described previously 29, 41. The predominant one used to target PLD2 in the current study has been named antibody #27 previously 29.
Plasmids, cells, and transfections
Mouse PLD2 constructs, wild type and point mutant (K758R), were generated in the mammalian expression vector pCGN by inserting into XbaI and SmaI sites. VSVG (ts045)-KDELR has been described previously 14, 15. VSVG (ts045)-KDELR-YFP was generated in the pEYFP-N1 vector (BD Biosciences, San Jose, CA) by assembly PCR of the VSVG (ts045) luminal domain with the KDELR, and ligation into the HindIII and SalI sites of the expression vector. Culturing of HeLa and COS cells and their transfection with FuGene6 (Roche, Indianapolis, IN) or Lipofectamine 2000 (Invitrogen, Carlsbad, CA) have been described 14, 15.
Lipid dot blot to assess binding by BARS
Purified lipids were spotted onto filter membrane, and then incubated with recombinant BARS. Filters were washed three times, and then incubated with a rabbit antibody against BARS followed by an anti-rabbit secondary antibody conjugated with horseradish peroxidase.
Liposome binding and tubulation assays
Liposomes were incubated with BARS (0.2 μM) in buffer (50 mM Hepes-KOH, pH7.2, 100mM KCl), and the resulting incubation was subjected to centrifugation at 15,000 × g for 20 minutes, followed by immunoblotting for BARS in the resulting pellet (liposome-bound) fraction. Examination of liposomes for tubulation was performed using the technique of whole-mount on Formvar grids as described previously 14.
Knockdown by siRNA
The siRNA sequences against human PLD2 are: #1: 5′- ggacaaccaagaagaaata-3′, #2: 5′- ggaccggcctttcgaagat-3′, #3: 5′-gacctgcactaccgactga-3′, and #4: 5′-cagcatggcggga ctatat-3′ (from Dharmacon, Lafayette, CO). For siRNA against human PLD1, a mixture of sequences was used (SMARTpool, Dharmacon, Lafayette, CO). Transfections were performed using Oligofectamine (Invitrogen, Carlsbad, CA). For siRNA rescue experiments, mouse PLD2 was used to construct siRNA resistant wild-type or catalytic dead mutant (K758R), whereby the sequence targeted by oligo #1 against PLD2 was changed in mouse PLD2 to: 5′-ggactacgaaaaagaagtt-3′.
In vivo COPI transport assay
Retrograde transport of VSVG(ts045)-KDELR and the use of siRNA and rescue in this assay have been described previously 14, 15. Micro-injection of antibodies was performed as previously described 42, with subsequent assessment of colocalization between VSVG-KDELR-YFP and giantin (as Golgi marker) performed using LSM software (Zeiss Imaging, Thornwood, NY).
DGK activity assay
Transfection of myc-tagged DGK–α followed by its isolation from cells and then assay for kinase activity were performed as previously described 43.
COPI vesicle reconstitution system
The two-stage incubation system was performed as described previously 14, 15. For antibody inhibition studies, 100 μg of washed Golgi membrane was preincubated with 5 μl of specific antibody as indicated, and then used for the two-stage incubation system. For rescue experiments that involved adding purified PA to overcome the effect of PLD inhibitors, PA-containing liposomes were generated as previously described 44.
Golgi fission assay
Purified BARS was incubated with washed Golgi membrane along with cytosol, essentially as previously described 15, 16. For experiments that replace cytosol with purified COPI-related components, GTP is added whenever ARF1 is included as a component.
Electron microcopy
Examination of reconstituted COPI vesicles and Golgi membrane, using the whole mount technique, has been described previously 14, 15. Examination of serial thin sections after epon-embedding 14 or immunogold labeled cryo-sections of cells 40 has also been described previously. EM tomography was performed as previously described 45. For quantitation of Golgi buds using EM stereology, sections were photographed randomly. Stereological test grid with the standard (500 nm) length was then placed over the images. The intersections of the grid with the contours of the Golgi cisterna (Ic), round profiles (Ir), elongated profiles (Ie) and COPI-coated buds (Ib) were counted and the relative surface area of the buds (ASb) was estimated as Ib/(Ic+Ir+Ie).
Supplementary Material
Acknowledgement
We thank Jian Li and Ming Bai for advice and discussions, and Richard Premont for technical assistance. This work is funded by grants from the NIH to VWH (GM058615), MAF (GM071520), and GD (GM071475), from Telethon (Italy), AIRC (Italy) and MIUR (Italy) to AL and AM, and from the Canadian Institutes of Health Research to SB. HG was supported by a Marie Curie Fellowship.
REFERENCES
- 1.Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. doi: 10.1146/annurev.cellbio.20.010403.105307. [DOI] [PubMed] [Google Scholar]
- 2.Rabouille C, Klumperman J. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol. 2005;6:812–7. doi: 10.1038/nrm1735. [DOI] [PubMed] [Google Scholar]
- 3.Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol. 2003;15:396–404. doi: 10.1016/s0955-0674(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–2. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- 6.Helms JB, Rothman JE. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–4. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- 7.Orci L, et al. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991;64:1183–95. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- 8.Lippincott-Schwartz J, et al. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–36. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 9.Waters MG, Serafini T, Rothman JE. ‘Coatomer’: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991;349:248–51. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- 10.Guo Q, Vasile E, Krieger M. Disruptions in Golgi structure and membrane traffic in a conditional lethal mammalian cell mutant are corrected by epsilon-COP. Journal of Cell Biology. 1994;125:1213–24. doi: 10.1083/jcb.125.6.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orci L, Palmer DJ, Amherdt M, Rothman JE. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993;364:732–4. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- 12.Yang JS, et al. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159:69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SY, Yang JS, Hong W, Premont RT, Hsu VW. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J Cell Biol. 2005;168:281–290. doi: 10.1083/jcb.200404008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JS, et al. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. Embo J. 2005;24:4133–43. doi: 10.1038/sj.emboj.7600873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JS, et al. Key components of the fission machinery are interchangeable. Nat Cell Biol. 2006;8:1376–82. doi: 10.1038/ncb1503. [DOI] [PubMed] [Google Scholar]
- 16.Weigert R, et al. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–33. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- 17.Gallop JL, Butler PJ, McMahon HT. Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature. 2005;438:675–8. doi: 10.1038/nature04136. [DOI] [PubMed] [Google Scholar]
- 18.Farsad K, De Camilli P. Mechanisms of membrane deformation. Curr Opin Cell Biol. 2003;15:372–81. doi: 10.1016/s0955-0674(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 19.McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–6. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- 20.Haucke V, Di Paolo G. Lipids and lipid modifications in the regulation of membrane traffic. Curr Opin Cell Biol. 2007;19:426–35. doi: 10.1016/j.ceb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 22.Godi A, et al. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Ulibarri I, et al. Diacylglycerol is required for the formation of COPI vesicles in the Golgi-to-ER transport pathway. Mol Biol Cell. 2007;18:3250–63. doi: 10.1091/mbc.E07-04-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci. 2005;62:2305–16. doi: 10.1007/s00018-005-5195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer WD, Brown HA, Sternweis PC. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- 26.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 27.de Chaffoy de Courcelles DC, Roevens P, Van Belle H. R 59 022, a diacylglycerol kinase inhibitor. Its effect on diacylglycerol and thrombin-induced C kinase activation in the intact platelet. J Biol Chem. 1985;260:15762–70. [PubMed] [Google Scholar]
- 28.Himpens B, De Smedt H, Bollen M. Modulation of nucleocytosolic [Ca2+] gradient in smooth muscle by protein phosphorylation. Faseb J. 1994;8:879–83. doi: 10.1096/fasebj.8.11.8070638. [DOI] [PubMed] [Google Scholar]
- 29.Freyberg Z, Bourgoin S, Shields D. Phospholipase D2 is localized to the rims of the Golgi apparatus in mammalian cells. Mol Biol Cell. 2002;13:3930–42. doi: 10.1091/mbc.02-04-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farsad K, et al. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J Cell Biol. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MC, et al. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–17. doi: 10.1016/j.cell.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci U S A. 1998;95:11199–204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinhard C, Schweikert M, Wieland FT, Nickel W. Functional reconstitution of COPI coat assembly and disassembly using chemically defined components. Proc Natl Acad Sci U S A. 2003;100:8253–7. doi: 10.1073/pnas.1432391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bremser M, et al. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 35.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–55. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 36.Kitstakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J. Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stamnes M, Schiavo G, Stenbeck G, Sollner TH, Rothman JE. ADP-ribosylation factor and phosphatidic acid levels in Golgi membranes during budding of coatomer-coated vesicles [published erratum appears in Proc Natl Acad Sci U S A 1999 Mar 2;96(5):2569] Proc Natl Acad Sci U S A. 1998;95:13676–80. doi: 10.1073/pnas.95.23.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serafini T, et al. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- 39.Presley JF, et al. Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature. 2002;417:187–93. doi: 10.1038/417187a. [DOI] [PubMed] [Google Scholar]
- 40.Kweon HS, et al. Golgi enzymes are enriched in perforated zones of golgi cisternae but are depleted in COPI vesicles. Mol Biol Cell. 2004;15:4710–24. doi: 10.1091/mbc.E03-12-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freyberg Z, et al. Intracellular localization of phospholipase D1 in mammalian cells. Mol Biol Cell. 2001;12:943–55. doi: 10.1091/mbc.12.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonazzi M, et al. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat Cell Biol. 2005;7:570–80. doi: 10.1038/ncb1260. [DOI] [PubMed] [Google Scholar]
- 43.Baldanzi G, et al. Diacylglycerol kinase-alpha phosphorylation by Src on Y335 is required for activation, membrane recruitment and Hgf-induced cell motility. Oncogene. 2008;27:942–56. doi: 10.1038/sj.onc.1210717. [DOI] [PubMed] [Google Scholar]
- 44.Pathre P, et al. Activation of phospholipase D by the small GTPase Sar1p is required to support COPII assembly and ER export. Embo J. 2003;22:4059–69. doi: 10.1093/emboj/cdg390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trucco A, et al. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–81. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.