Abstract
IL-21 is a pleiotropic cytokine that is required for normal immunoglobulin production. We previously showed that IL-21 was elevated in BXSB-Yaa mice with systemic lupus erythematosus. These mice also have elevated IL-10 levels, and we now show that IL-21 induces IL-10 mRNA and protein, suggesting unexpected immunosuppressive activities for IL-21. Indeed, Th1 priming with IL-21 leads to accumulation of cells with immunosuppressive activity, and IL-21 over-expression decreases specific antibody production after immunization in an IL-10-dependent fashion. Moreover, we show that IL-21 signaling is required for maximal induction of IL-10 by IL-6 or IL-27. Overall, our data indicate that IL-21 regulates immune responses at least in part by inducing IL-10 and reveal unanticipated immunosuppressive actions for this cytokine.
Introduction
T cell differentiation is a highly regulated event, leading to the production of committed effector cells that mediate the elimination of a broad range of pathogens. Three major T cell effector lineages have been described thus far: the Th1, Th2 and Th17 lineages. The differentiation of each of these is controlled in part by lineage-specific transcription factors that orchestrate and reinforce specific effector programs (1, 2), with each of these T cell populations producing a characteristic array of cytokines that mediate effector function not only of T cells but also of B cells and antigen-presenting cells.
Regulatory mechanisms have evolved to limit the functional activity of these effector T cells. Some of these involve specialized regulatory subsets comprising both natural and induced T regulatory cells (3). Additionally, each of these T cell lineages is also capable of self-regulation. IL-10 is a central cytokine involved in this process, suppressing the production of inflammatory cytokines and inhibiting the function of antigen-presenting cells, thereby diminishing T cell responses to antigen (4). Thus, IL-10 is a critical negative regulator of a range of pathophysiological responses. Although its production from T cells was initially reported to be restricted to the Th2 lineage, it is now clear that IL-10 is also produced by Th1 and Th17 cells and that it can limit the inflammatory effector responses of these cells (5, 6), underscoring the importance of understanding the mechanisms for controlling IL-10 production in these effector populations.
IL-21 is a type I cytokine that is produced by antigen-stimulated CD4+ T cells as well as NK T cells, but its target populations include both lymphoid and non-lymphoid populations, including T, B, NK, and myeloid cells (7). IL-21 signals through a heterodimeric receptor containing IL-21R (8, 9) plus the common cytokine receptor γ chain, γc (7, 10, 11), which is mutated in humans with X-linked severe combined immunodeficiency (12) and also is shared by the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15 (13). Like IL-10, expression of IL-21 was initially reported to be Th2 specific (14), but subsequent studies demonstrated that IL-21 is also produced by Th1, Th2, and Th17 CD4+ T cell subsets, thus having the potential to act as an immunoregulatory cytokine in the context of each of these effector populations (15-19). Although the role of IL-21 in the differentiation of Th17 cells remains controversial (20, 21), it is clear that IL-21 can enhance the expansion of these cells via the induction of IL-23R on Th17 cells (15).
IL-21 can potently augment both humoral and cell-mediated immunity, but it also has inhibitory effects. IL-21 is known to critically regulate immunoglobulin production (22) and to drive the differentiation of B cells to antibody-producing plasma cells (23, 24). At least part of this effect of IL-21 on immunoglobulin production involves its role in the development of T follicular helper cells that drive germinal center development (25, 26). Moreover, IL-21 can cooperate with IL-7 or IL-15 to promote CD8+ T cell expansion (27), and it promotes anti-tumor responses by CD8+ T cells and NK cells (27-31). Conversely, IL-21 exerts negative effects on lymphoid and myeloid cells, inducing B-cell apoptosis (24, 32, 33) and inhibiting dendritic cell maturation and function (34). The type of action mediated by IL-21 is presumably determined by its biological context (24), depending on the specific activation state of the target cell as well as the cytokine milieu.
In the BXSB-Yaa mouse model of systemic lupus erythematosus, serum IL-21 levels increased with age, correlating with the severity of autoimmunity (24). Additionally, IL-10 levels similarly increase (24). We now report that IL-21 is a potent regulator of IL-10 and demonstrate that IL-10 production is decreased in IL-21R knockout (KO) mice but increased in IL-21 transgenic mice. These latter mice exhibit markedly defective immunoglobulin production in response to specific immunization, consistent with a suppressive phenotype. We show that IL-21 induces IL-10 expression in normal wild type T cells, including cells that are already committed to Th1 or Tc1 differentiation. Moreover, TCR priming in the presence of IL-21 results in the accumulation of T cells with immunosuppressive activity that is dependent on IL-10, and furthermore, immunosuppressive activity is unexpectedly observed in IL-21 transgenic mice, an effect that is largely mediated by IL-10.
Materials and Methods
Mice
C57BL/6 and Balb/c mice were from The Jackson Laboratory (Bar Harbor, ME). IL-10 KO mice were from Taconic. IL-21R KO mice (22) on a C57BL/6 background (3 generations) and IL-21 transgenic mice (24) on a mixed background (129xBl6) were previously described. Mice lacking STAT3 expression in T cells (35) were generated by crossing Stat3 floxed mice (36) with CD4-Cre recombinase transgenic mice. All experiments were performed under protocols approved by the NHLBI Animal Care and Use Committee.
Th/Tc cell polarization
Splenic naive CD4+ or CD8+ T cells were isolated by positive selection with magnetic beads (Miltenyi Biotec). Cells were cultured on plate-bound anti-CD3 (2 μg/ml) + anti-CD28 (1 μg/ml) for 3 days either in the presence of IL-12 (10 ng/ml) + anti-IL-4 (11B11, 10 μg/ml) for Th1 polarization or in the presence of IL-4 (100 U/ml) + anti-IFN-γ (XMG1.2, 10 μg/ml) for Th2 polarization (antibodies from BD Biosciences). 50 ng/ml IL-21 (R&D Systems) was included where indicated. Cells were expanded in IL-2 for 3 to 5 days and re-stimulated with PMA (10 ng/ml) + ionomycin (1 μM). For Th17/Tc17 differentiation, cells were cultured for 4 days on plate-bound anti-CD3 + anti-CD28 in the presence of TGFβ (2 ng/ml), IL-6 (10 ng/ml), and both anti-IFN-γ and anti-IL-4. For intracellular cytokine production, cells were stimulated for a total of 6 h with PMA + ionomycin, in the presence of Golgi stop for the last 4 h, fixed using the Cytofix/Cytoperm kit (BD Biosciences), and stained with antibodies to IL-10 (JES5−16E3), IFN-γ (XMG1.2) (BD Biosciences) and IL-17A (EBiosciences). AE7, a Th1 cell line (37), kindly provided by Dr. Ronald H. Schwartz, was activated by antigen in the presence of APCs, and then expanded in IL-2, rested overnight before stimulation with PMA + ionomycin for 24 hrs.
RNA analysis
Total RNA was isolated using TRIzol (Invitrogen). First strand cDNAs were synthesized using the Omniscript reverse transcription kit (Qiagen) and oligo-dT. Quantitative real time PCR was performed on a 7900H sequence detection system (Applied Biosystems). Real time primers and Taqman probes were from Applied Biosystems. RNA was normalized relative to expression of Rpl7.
Retroviral transduction
Retroviral supernatants were produced from 293T cells co-transfected with pCL-Eco plus either pRV-GFP or pStat3C-GFP (38), which encodes a constitutively-active STAT3, using Lipofectamine (Invitrogen). One day later, cells were changed to complete RPMI-1640 medium and incubated at 32°C. Retrovirus-containing supernatant was harvested 48 and 72 h after transfection. T cells were isolated from WT Balb/c mice and activated with 5 μg/ml plate bound anti-CD3 + 1 μg/ml anti-CD28 for 24 h. Activated T cells were transduced with viral supernatant in the presence of 8 μg/ml polybrene (Sigma) by spinning at 2,500 rpm for 45 min at 30°C. Cells were further activated with 2 μg/ml anti-CD3 + 1 μg/ml anti-CD28, and a second retroviral transduction was performed 24 h later. Cells were grown in complete RPMI medium containing 100 U/ml IL-2 for 2 days, stimulated with 50 ng/ml IL-21 for 24 h, incubated with PMA + ionomycin for 6 h and with Golgi stop for the last 4 h, fixed with Cytofix/Cytoperm, and stained with PE-anti-IL-10 (BD Bioscience). Transduced (GFP+) cells that expressed IL-10 were quantified.
In vitro co-culture assay
OT-1 CD8+ T cells were labeled with CFSE (Molecular Probes) and stimulated with splenic APC that had been depleted of T cells, irradiated (3000 rad), and pulsed with the SIINFEKL peptide that is specific for the OT-1 TCR. These primed OT-1 cells were co-cultured at a ratio of 2:1 with Th1 cells that had been primed in the presence or absence of 50 ng/ml IL-21 along with anti-IL-10R (clone 1B1.3a; 10 μg/ml) or an isotype-matched control mAb (clone R3−34) (BD Bioscience). Th1 cells were restimulated with anti-CD3 + anti-CD28 in either the presence of absence of IL-21 on the day prior to the co-culture. Vα2+ CD8+ T cells were stained with APC-anti-CD25 (3C7) (BD Bioscience) at 24 h, and CFSE dilution was monitored at days 2 and 3 after activation.
In vivo antibody and cytokine responses
IL-21 transgenic mice were immunized intraperitoneally with 100 μg ovalbumin emulsified with Imject alum (Pierce). Ova-specific immunoglobulin levels were measured one week later, as previously described (22).
Results
IL-21 induces IL-10 production during the primary immune response
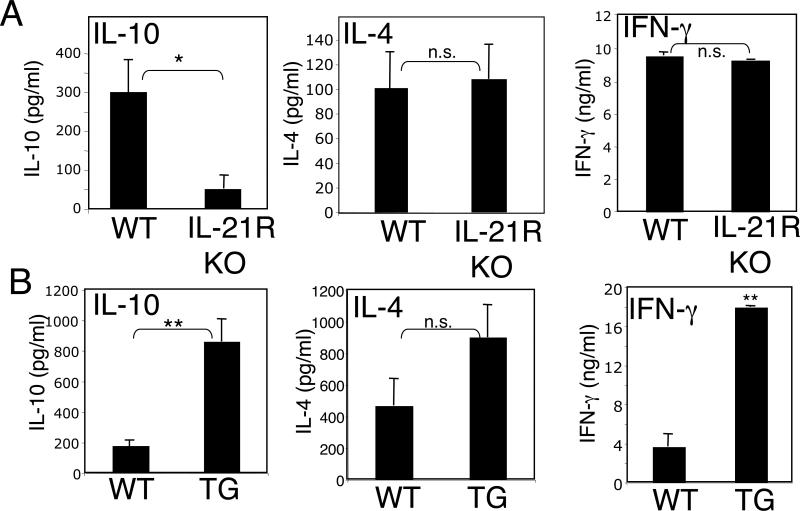
We previously observed high levels not only of IL-21 but also of IL-10 in BXSB-Yaa mice (24), which develop systemic lupus erythematosus. To investigate whether IL-21 could regulate the levels of IL-10, we first analyzed IL-10 production in CD4+ and CD8+ T cells from either IL-21R KO mice (22) or IL-21 transgenic mice (24). Splenic T cells were stimulated in vitro with anti-CD3 + anti-CD28, and levels of IL-10 production were measured. Levels of IL-10 were markedly lower in cells from IL-21R KO mice than from WT mice, although IL-4 and IFN-γ levels were not affected (Fig. 1A). Conversely, IL-10 production was increased in cells from IL-21 transgenic mice (Fig. 1B). Interestingly, IL-4 and IFN-γ production by the IL-21 TG cells was also higher than from control cells (Fig. 1B).
Figure 1.
IL-21 induces IL-10 expression. (A and B) Splenic T cells from either IL-21R KO mice and their littermate controls (A) or from IL-21 TG mice and their littermate controls (B) were purified by negative selection and stimulated in vitro with anti-CD3 (2 ug/ml) + anti-CD28 (1 ug/ml) for 48 h. Secreted IL-10, IL-4, and IFNγ were measured by ELISA using specific kits (BD PharMingen). Shown is an experiment representative of three individual experiments. *P < 0.01; **P < 0.001. The genetic backgrounds for the IL-21R KO mice and IL-21 transgenic mice differ (see Methods).
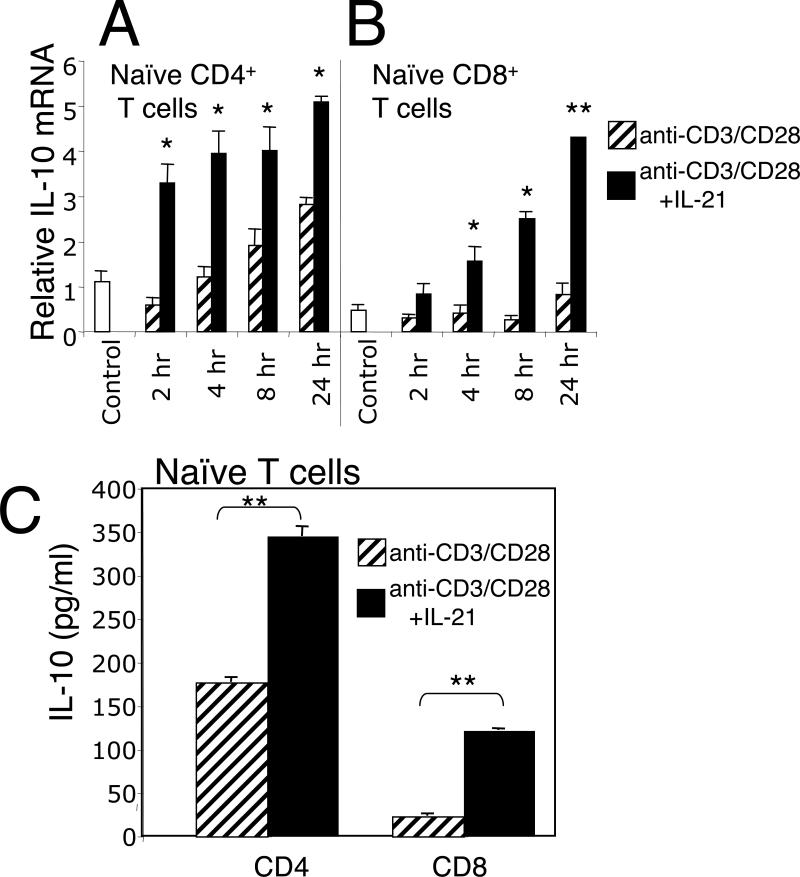
To further investigate the ability of IL-21 to induce IL-10 expression, we next measured IL-10 mRNA levels in freshly isolated wild type CD4+ and CD8+ T cells. Stimulation of CD4+ T cells with anti-CD3 + anti-CD28 induced IL-10 mRNA (Fig. 2A), but the addition of IL-21 increased the potency of this response at all time points examined (Fig. 2A). In contrast to its effect on CD4+ T cells, anti-CD3 + anti-CD28 induced little IL-10 mRNA in CD8+ T cells, but again the addition of IL-21 markedly increased IL-10 mRNA levels (Fig. 2B). Analysis of secreted IL-10 protein levels in naive CD4+ and CD8+ T cells confirmed that IL-10 production was induced by anti-CD3 + anti-CD28 in CD4+ T cells and was further enhanced by the addition of IL-21, whereas anti-CD3 + anti-CD28 alone induced only a low level of IL-10 production in CD8+ T cells, but the inclusion of IL-21 significantly induced IL-10 secretion (Fig. 2C). Interestingly, IL-21 did not induce IL-10 expression in naïve CD4+ or CD8+ T cells (data not shown). Three other γc family cytokines (IL-2, IL-7, IL-15) were evaluated either alone or in combination with IL-21 for their ability to induce IL-10 in the primary response of CD4+ or CD8+ T cells. Of these cytokines, only IL-21 significantly induced IL-10 (Suppl. Fig.1). This is potentially explained by the fact that IL-2, IL-7, and IL-15 primarily activate STAT5 proteins whereas IL-21 favors STAT3.
Figure 2.
IL-21 induces IL-10 mRNA in CD4+ and CD8+ T cells. (A and B) Splenic CD4+ or CD8+ T cells from Balb/c mice were stimulated with anti-CD3 (2 ug/ml) + anti-CD28 (1 ug/ml) without or with IL-21 for the indicated times and IL-10 mRNA levels measured by real-time PCR. The control refers to cells not exposed to either anti-CD3+ anti-CD28 or to IL-21. IL-21 alone did not induce IL-10 mRNA above control levels in either CD4+ or CD8+ T cells (not shown). (C) Naive CD4+ or CD8+ T cells were stimulated with anti-CD3 + anti-CD28 in the absence or presence of IL-21 for 48 h, at which point culture supernatants were assayed for IL-10 by ELISA (BD Pharmingen). Data are representative of three individual experiments. *P < 0.05; **P < 0.01.
IL-21 induction of IL-10 is mediated by STAT3
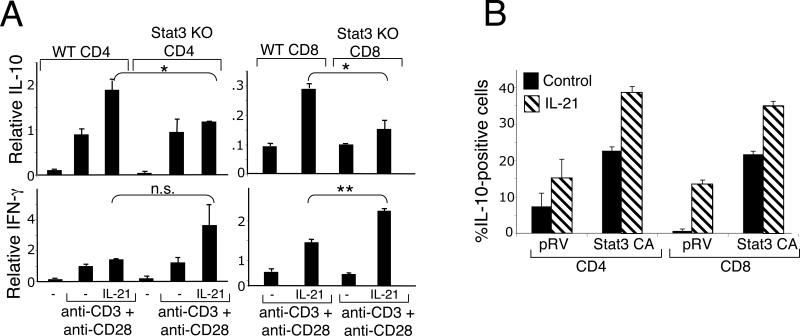
Because IL-21 activates STAT3 (10, 35, 39), we next investigated whether STAT3 is required for IL-21-induced IL-10 expression using conditional KO mice that lack expression of STAT3 in T cells (35, 36). We compared IL-10 mRNA induction in WT and Stat3 KO CD4+ T cells (Fig. 3A, left upper panel) and CD8+ T cells (Fig. 3A, right upper panel). As is evident, similar levels of IL-10 were observed in both WT and Stat3 KO T cells that were stimulated only with anti-CD3 (2 ug/ml) + anti-CD28 (1 ug/ml)(concentrations that induce maximal expression of IL-10); however, the IL-21-boosted expression that was evident in WT cells was markedly diminished in Stat3 KO T cells (Fig. 3A, upper panels). This indicates that there are at least two mechanisms for inducing expression of the IL-10 gene: one that is TCR-dependent but independent of STAT3 and another that is IL-21-dependent and mediated by STAT3. In contrast to IL-10, IFN-γ mRNA induction by anti-CD3 + anti-CD28 was not diminished and in fact was even augmented by IL-21 in Stat3 KO CD4+ (Fig. 3A, left lower panel) and CD8+ (Fig. 3A, right lower panel) T cells. The importance of STAT3 for IL-21-mediated induction of IL-10 expression was further evaluated by using a constitutively activated form of STAT3 (38). As compared to a control retrovirus, transduction of a virus encoding constitutively-activated STAT3 increased the number of IL-10-producing CD4+ and CD8+ T cells, and this was further increased by the addition of IL-21 (Fig. 3B). This additional effect of IL-21 may reflect its ability to also activate other signaling pathways, including, for example, MAP kinase and PI 3-kinase coupled pathways (35).
Figure 3.
IL-21-induced expression of IL-10 is mediated by STAT3. (A) IL-21-mediated induction of IL-10 is diminished in CD4+ and CD8+ splenic T cells from Stat3 KO mice (upper panels), whereas IFN-γ is not diminished (lower panels). CD4+ and CD8+ splenic T cells were isolated from Stat3 KO mice or WT littermates and stimulated with anti-CD3 (2 ug/ml) + anti-CD28 (1 ug/ml) in the absence or presence of IL-21 for 6 h. Relative IL-10 and IFN-γ mRNA levels were quantified by real-time PCR. *P < 0.05; **P<0.01 (B) Constitutively activated STAT3 expression augments basal and IL-21-induced IL-10 protein expression. CD4+ and CD8+ T cells from Balb/c mice were transduced with a retrovirus encoding a constitutively activated form of STAT3. Cells were stimulated overnight with anti-CD3 (2 ug/ml) + anti-CD28 (1 ug/ml) without or with IL-21 and stained intracellularly with antibodies to IL-10. FACS analysis of gated GFP-positive CD4+ or CD8+ T cells is shown. Data shown are representative of three individual experiments.
IL-21 induces IL-10 expression during polarization of Th1 and Tc1 cells
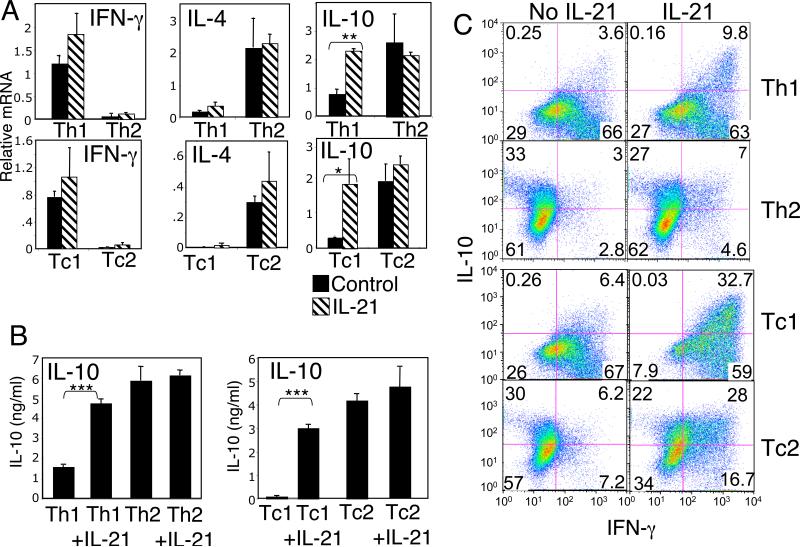
Because IL-10 is known to suppress inflammatory responses (4), we next investigated whether IL-21 could influence IL-10 production during either Th1/Tc1 or Th2/Tc2 in vitro polarization. Naive CD4+ or CD8+ T cells were cultured in Th1/Tc1 or Th2/Tc2 polarizing conditions (see Methods) in the absence or presence of exogenous IL-21 for 7 days, and then re-stimulated with PMA + ionomycin. IL-21 induced only a slight increase in IFN-γ in Th1 or Tc1 cells, whereas a larger increase in IL-10 mRNA was observed in these cells (Fig. 4A, right panels). Similarly, IL-21 increased IL-10 protein in Th1 and Tc1 cells (Fig. 4B). In contrast, in Th2 or Tc2 cells, which express a high basal level of IL-10 mRNA and protein, IL-21 did not significantly induce IL-10 expression (Fig. 4A and 4B). Flow cytometric analysis revealed that stimulation of Th1 or Tc1 cells with IL-21 led to an increase in cells co-producing IL-10 and IFN-γ (Fig. 4C, upper right quadrants) but not of cells producing only IL-10 (Fig. 4C, upper left quadrants), indicating that the induction of IL-10 was occurring in already committed Th1 or Tc1 cells. Although IL-21 did not significantly increase the total amount of IL-10 mRNA or protein produced by Tc2 cells, flow cytometry revealed that IL-21 led to an increase in the number of Tc2 cells co-producing IL-10 and IFN-γ (Fig. 4C, upper right quadrants of Tc2 and represented graphically in Suppl. Fig.2).
Figure 4.
IL-21 induces IL-10 mRNA and protein expression in Th1 and Tc1 cells. (A and B) Naive CD4+ or CD8+ T cells were polarized under either Th1/Tc1 or Th2/Tc2 conditions in the absence or presence of IL-21 and then expanded in IL-2. In (A), polarized cells were then stimulated with PMA + ionomycin for 6 h and relative levels of IFN-γ, IL-4, and IL-10 mRNA were quantified by real-time PCR. In (B), Th1/Tc1 and Th2/Tc2 polarized cells were re-stimulated with anti-CD3 + anti-CD28 for 48 h in the absence or presence of IL-21, and levels of IL-10 secreted in culture supernatants were measured by ELISA. Statistically significant differences, when present, are indicated by asterisks: *P < 0.05; **P < 0.01; ***P < 0.001. (C) Th1/Tc1 and Th2/Tc2 cells that had been polarized either in the absence or presence of IL-21 were re-stimulated for 6 h with PMA + ionomycin in the presence of Golgi stop for the last 4 h. Intracellular levels of IL-10 and IFN-γ were assessed by flow cytometry. The quadrants were determined based on staining with isotype control antibodies. Data shown are representative of three individual experiments.
IL-21 upregulates IL-10 production during the induction of Th17/Tc17 polarized cells
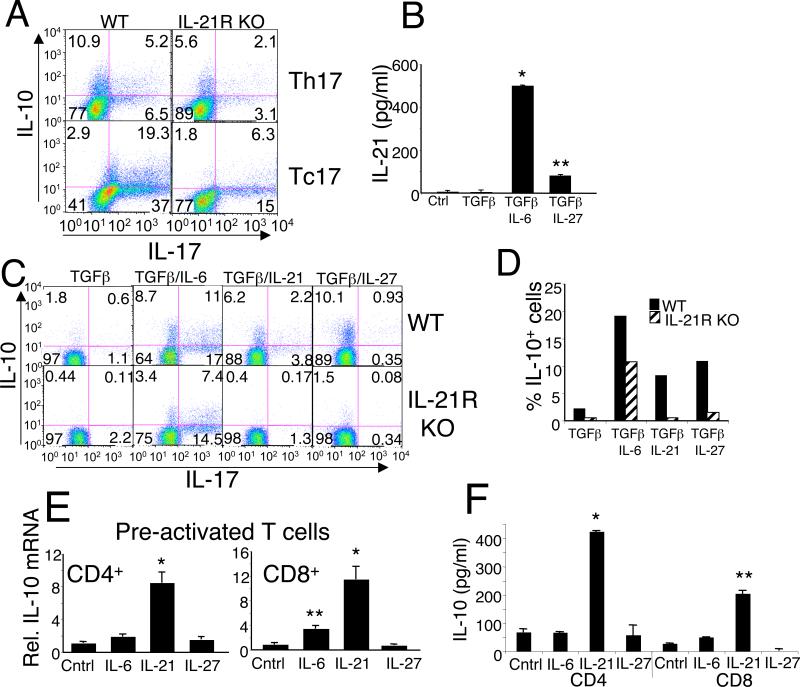
Th17 cells can be generated in vitro by stimulating naive CD4+ T cells with anti-CD3 + anti-CD28 in the presence of TGFβ and IL-6, and these conditions lead to the generation of CD4+ T cells co-producing IL-17 and IL-10 (5). To determine whether IL-21 could also influence the IL-10 production by Th17 cells, naive CD4+ or CD8+ T cells from WT or IL-21R KO mice were similarly primed under Th17/Tc17 conditions for 4 d and intracellular cytokines were evaluated (Fig. 5A). Priming of WT cells under these conditions led to the generation/expansion of cells producing IL-10 alone or IL-17 alone as well as cells producing both cytokines. T cells from IL-21R KO mice that were primed under Th17/Tc17 conditions exhibited diminished numbers of cells producing both IL-10 and IL-17 and of cells producing each individual cytokine.
Figure 5.
IL-21 controls expression of IL-10 in Th17/Tc17 primary cells and is required for normal induction of IL-10 by IL-6 or IL-27. (A) Naive CD4+ or CD8+ T cells from WT or IL-21R KO mice were polarized in vitro in the presence of TGFβ + IL-6 for 4 days and re-stimulated for 6 h with PMA + ionomycin in the presence of Golgi stop for the last 4 h. Intracellular levels of IL-10 and IL-17 were assessed by flow cytometry. The quadrants were determined based on staining with isotype control antibodies. (B) WT CD4+ T cells were stimulated under neutral conditions with anti-CD3 + anti-CD28 in the presence or absence of TGFβ and IL-6 or IL-27 for 4 days, at which time culture supernatants were assayed by ELISA for IL-21. (C) CD4+ T cells from either WT or IL-21R KO mice were stimulated with anti-CD3 + anti-CD28 + anti-IFNγ + anti-IL-4 in the presence of TGFβ with either IL-6, IL-21, or IL-27 for 4 days and then re-stimulated for 6 h with PMA + ionomycin in the presence of Golgi stop for the last 4 h. Intracellular levels of IL-10 and IL-17 were assessed by flow cytometry. (D) The percentage of IL-10 positive cells in (c) are represented in a bar graph. (E) CD4+ and CD8+ T cells were pre-activated with anti-CD3 + anti-CD28 for 48 h, washed and rested overnight, and were re-stimulated without or with IL-6, IL-21 or IL-27 for 6 h. RNA was then isolated and relative IL-10 mRNA levels quantified by real-time PCR. (F) Parallel culture supernatants were assayed at 48 h for IL-10 by ELISA. *P<0.01; **P<0.05.
Several groups have reported that IL-6 and IL-27 can induce IL-10 (5, 40-42). We hypothesized that this induction might be mediated, at least in part, via the induction of IL-21, given our demonstration above that IL-21 can potently induce IL-10. We therefore investigated the effects of IL-6 and IL-27 on the production of IL-21. CD4+ T cells were stimulated with anti-CD3 + anti-CD28 alone or with TGFβ,TGFβ + IL-6, or TGFβ + IL-27 for 72 h, and levels of secreted IL-21 were measured. IL-6 potently induced IL-21 (Fig. 5B), consistent with previous observations (15-17). We also found that IL-27 induced IL-21 secretion by CD4+ T cells, although less potently than did IL-6 (Fig. 5B). We next evaluated the contribution of IL-21 signaling to the induction of IL-10 by using IL-21R KO CD4+ T cells. Cells were stimulated with anti-CD3 + anti-CD28 and TGFβ without or with the addition of IL-6, IL-21, or IL-27 for 4 days, and intracellular levels of IL-10 and IL-17 were then evaluated as described in the Methods (Fig. 5C and 5D). All three cytokines substantially enhanced the production of IL-10 by WT cells (Fig. 5C, upper panels). As expected, the effect of IL-21 was not observed in IL-21R KO T cells, but the number of IL-10 producing cells induced by IL-27 was also greatly reduced in IL-21R KO T cells (Fig. 5C lower panels, and Fig. 5D), indicating that much of the induction of IL-10 by IL-27 in fact occurred via the induction of IL-21. There was also a partial decrease in IL-6-induced IL-10 expression in the IL-21R KO mice. When we compared the effects of IL-21, IL-6, and IL-27 on the induction of IL-10 in T cells pre-activated with anti-CD3 + anti-CD28 for 48 hrs but where TGFβ was not added, interestingly, IL-21 was more potent than either IL-6 or IL-27 in its ability to induce IL-10 mRNA (Fig. 5E) and protein (Fig. 5F) expression in both CD4+ and CD8+ T cells.
IL-21 can induce IL-10 production in previously committed Th1, Tc1 or Tc17 cells
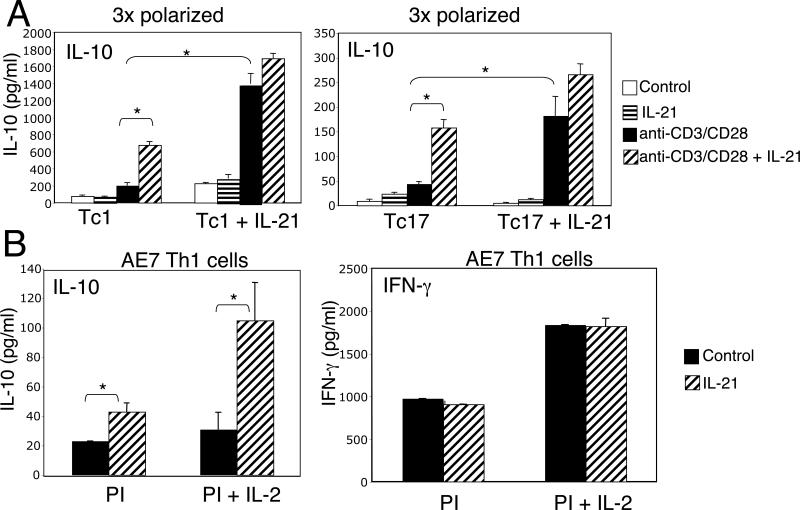
Above, we showed that IL-21 could increase IL-10 production when it was present at the time of antigen stimulation under either neutral or polarizing conditions. We next investigated whether IL-21 could also augment IL-10 expression in cells that were terminally committed to lineages in which IL-10 production is greatly diminished. We first subjected CD8+ T cells (which unlike CD4+ T cells do not produce IL-21) to three rounds of Tc1 or Tc17 polarization. As shown in Fig. 6A, IL-21 enhanced TCR-induced IL-10 production in these already committed cells (left set of bars in each panel). Interestingly, cells that were initially polarized in the presence of IL-21 had a much higher response to TCR stimulation alone (compare the solid bar in the right half of each panel to that in the left half of each panel).
Figure 6.
IL-21 augments IL-10 expression even in committed Tc1 or Tc17 primary cells and in Th1 committed AE7 cells. (A) CD8+ T cells were subjected to three rounds of polarization under either Tc1 or Tc17 conditions in either the absence or presence of IL-21 and were then stimulated with or without anti-CD3/CD28 in either the absence or presence of IL-21 for 24 h, at which point IL-10 levels were measured in culture supernatants. (B) AE7 cells were TCR stimulated and were then expanded in IL-2, rested overnight and were then re-stimulated with PMA + ionomycin in either the presence or absence of IL-21 and IL-2. Culture supernatants were assayed by ELISA at 24 h for IL-10 and IFN-γ. Data shown are representative of three separate experiments. *P < 0.05.
We next investigated whether IL-21 could induce IL-10 production even in a Th1 committed cell line, AE7. Although PMA + ionomycin alone induced relatively little IL-10 in this cell line, the addition of IL-21 significantly increased the amount of IL-10 secreted protein (Fig. 6B). In cells treated with PMA + ionomycin + IL-2, which is required for long-term viability of these cells, IL-21 induced approximately 4-fold more IL-10 than in cells not receiving the IL-21. Although the IL-10 locus is not normally transcriptionally active in these committed lineages (43), our results indicate that even in this context, IL-21 could augment IL-10 mRNA levels.
Th1 cells primed in the presence of IL-21 exhibit a marked IL-10-mediated inhibitory effect on antigen-induced IL-2Rα expression and cell-cycle progression
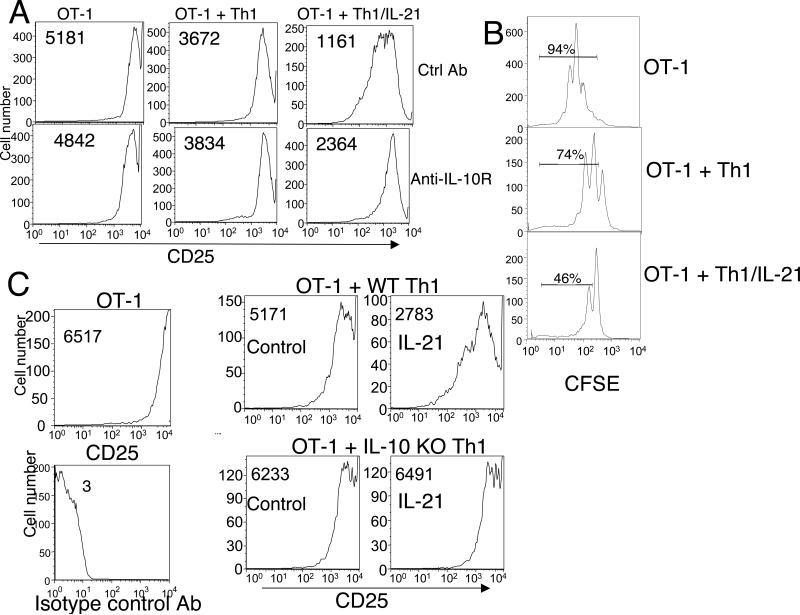
IL-10-producing cells can inhibit the proliferation and/or activation of other cellular subsets (3). We therefore examined the functional consequences of IL-21-induced expression of IL-10 using TCR transgenic OT-1 CD8+ T cells that were activated using splenic APC that had been pulsed with the SIINFEKL peptide, which is recognized by the OT-1 TCR. As expected, activated OT-1 cells exhibited high expression of the IL-2 receptor α chain (CD25) (Fig. 7A, left upper panel). The addition of Th1 cells modestly decreased this expression (Fig. 7A, middle upper panel), but Th1 cells polarized in the presence of IL-21 had an even greater inhibitory effect (Fig. 7A, right upper panel). Analogous inhibitory effects on cell cycle progression were observed when CFSE dilution was examined, with IL-21-primed Th1 cells having a greater inhibitory effect on CFSE dilution than Th1 cells not exposed to IL-21 (Fig. 7B). The slight effect of Th1 cells polarized in the absence of IL-21 may reflect competitive growth effects resulting from the addition of these cells in a 2:1 CD8:CD4 ratio. The addition of an antibody to IL-10R partially reversed the inhibition induced by priming with IL-21 (Fig. 7A, lower right panel versus upper right panel). Consistent with this finding, as compared to WT Th1 cells, IL-10 KO Th1 cells primed with IL-21 did not inhibit OT-1 activation as assessed by IL-2Rα (CD25) expression (Fig. 7C, compare lower right to upper right panel), confirming that the suppressive effect of IL-21 was indeed dependent on IL-10. The addition of IL-10 to OT-1 cells primed with peptide/APC also diminished the induction of CD25, confirming that IL-10 can mediate this suppressive effect independent of the presence of T helper cells (Suppl. Fig. 3).
Figure 7.
Th1 cells polarized in the presence of IL-21 inhibit the activation of naive CD8+ T cells, and this inhibition is IL-10-dependent. Naive OT-1 cells were activated with peptide/APC in the absence or presence of Th1 cells that were primed in the presence or absence of IL-21. (A) CD25 surface expression on gated Vα2+ OT-1 cells was measured by flow cytometry at 48 h in cells treated either with a control Ab (upper panels) or anti-IL-10R (lower panels). MFIs are indicated in each profile. (B) Proliferation of OT-1 cells was assessed by CFSE dilution at 48 h after peptide/APC stimulation. Shown is the effect of the addition of Th1 cells primed in the absence vs. presence of IL-21. The percentage of cells in the indicated region is shown. MFIs were 96, 237, and 351 for OT-1, OT-1 + Th1, and OT-1 + Th1/IL-21, respectively. These proliferation assays were performed in the absence of exogenous IL-2. (C) CD25 surface expression on OT-1 cells from WT mice (upper two panels) or IL-10 KO mice (lower two panels) was measured at 48 h without co-culture (left panel) or after co-culture with Th1 cells (middle panels) or Th1 cells polarized with IL-21 (right panels). MFIs are indicated in each profile. Shown is an experiment representative of three individual experiments.
IL-21 suppression of in vivo immunoglobulin induction is mediated in part by IL-10
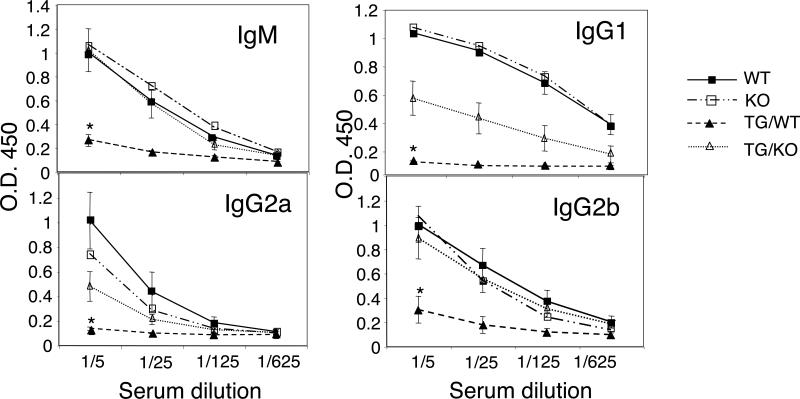
Even though IL-21 induces terminal differentiation of B cells and is required for a normal immune response (22, 24), the elevated levels of IL-10 produced by IL-21 transgenic cells suggested that these mice might unexpectedly exhibit an immunosuppressive phenotype in the context of an immune response. To investigate this possibility, we immunized the mice intraperitoneally with ovalbumin and measured primary serum immunoglobulin responses at day 7. As shown in Fig. 8, ovalbumin-specific IgM, IgG1, IgG2a, and IgG2b were substantially lower in the IL-21 transgenic mice (TG/WT) than in wild-type littermates (WT). To determine whether this decreased response was a result of IL-21-mediated IL-10 induction, the IL-21 transgenic mice were crossed onto the IL-10 KO mouse background (TG/KO). These mice had similar OVA-specific IgM and IgG2b responses to those of WT mice, showing that deleting IL-10 reversed the suppressive effect of IL-21 transgenic expression on production of these immunoglobulins. OVA-specific IgG1 and IgG2a responses were also reversed in the absence of IL-10, albeit only partially. Thus, we show that IL-21 can suppress antigen-induced immunoglobulin production and that IL-10 production plays an important role in mediating this immunosuppressive effect of IL-21. The fact that the IgG1 and IgG2a effect were only partially reversed on the IL-10 KO background suggests that other factors may also contribute to the immunosuppressive effects of IL-21.
Figure 8.
IL-21 transgenic mice exhibit diminished production of specific antibody in response to primary immunization with ovalbumin, and this inhibition is partially dependent on the presence of IL-10. IL-21 transgenic mice and non-transgenic littermates on IL-10 KO and WT backgrounds were immunized i.p. with 100 ug ovalbumin in alum. At day 7 after primary immunization, serum levels of OVA-specific immunoglobulin isotypes were assessed by ELISA. Data are mean ± SEM from three experiments (2−4 animals per experiment). Statistical significance was determined using Student's t test. *The statistical difference between TG/WT and TG/IL-10 KO was P < 0.001 for IgM, P < 0.002 for IgG1, P < 0.02 for IgG2a, and ) < 0.009 for IgG2b.
Discussion
IL-21 is a pleiotropic cytokine with an array of actions on T, B, NK, and myeloid cells (11). IL-10 is produced by multiple cell types, and like IL-21, it has pleiotropic effects on multiple lineages. Among its actions, IL-10 has immunosuppressive properties related to its direct effects on antigen-presenting cells and its ability to inhibit the production of multiple cytokines and chemokines (4, 44), but it also has stimulatory effects, such as its abilities to augment the number of CD8+ T cells during a primary response (45) and the proliferation of B cells activated via the B-cell receptor and costimulatory signals (4, 44).
We have now shown that IL-21 increases IL-10 expression in TCR-stimulated naive CD4+ and CD8+ T cells and potently induces IL-10 in pre-activated T cells. A previous study showed that IL-21 could augment anti-CD40-induced B cell production of IL-10, but the total production of IL-10 was small and was associated with a 4−5 fold expansion of B cells (46). Our results in T cells are direct and not dependent on proliferative amplification of target cells. IL-21 has also been shown to augment IL-10 production in long-term NK cell cultures that were stimulated with either IL-2 or IL-15 and this augmentation was accompanied by an increased functional maturation of NK cells (47). Thus, although IL-21 augments IL-10 production in B cells and NK cells, this production correlated with positive effects on differentiation or proliferation rather than with immunosuppressive effects as we observed in T cells. Interestingly, although we show that IL-21 can induce IL-10 in several different T helper lineages, T follicular helper cells isolated from immunized mice were shown to produce IL-21 but did not produce IL-10 (25). The absence of IL-10 production by these cells may be important in germinal center proliferation and expansion of memory B cells, as IL-10 has been found to inhibit memory B cell proliferation and favor differentiation to plasma cells (48).
These results identify a novel mechanism by which IL-10 production can be induced and amplified in both naive and activated T cells. Although IL-6 has been shown to directly induce IL-10 in naive CD4+ T cells (5, 41), this induction is partially dependent on the intermediate induction of IL-21, which can then either directly induce IL-10 production or further amplify the IL-6-induced IL-10 production. Although both IL-6 and IL-21 induce IL-10 in a STAT3-mediated manner during the primary induction of naive T cells, there are differences in the ability of these two cytokines to induce IL-10 in already activated T cells, suggesting potential differences in downstream signaling events via these cytokines. Moreover, we show that IL-27-induced IL-10 production in naive CD4+ T cells stimulated via the TCR is largely dependent on the intermediate induction of IL-21, providing a likely mechanism for the observation that IL-27 suppresses autoimmune inflammation via IL-10 (40). Interestingly, as compared to IL-6 and IL-27, in preactivated T cells, IL-21 is the most potent inducer of IL-10 production.
In cells polarized using Th17 conditions (TGFβ + IL-6), there was an increase in cells that produce only IL-10 and in cells producing both IL-10 and IL-17, but each of these populations was diminished when IL-21R KO cells were used. Recently, it was shown that Th17 polarization results in the generation of cells containing two major subpopulations that separately produce either IL-21 or IL-17 (49). IL-10 was produced by each of these subpopulations but again levels were diminished when these cells were derived from IL-21R KO mice, indicating a key role for IL-21 in the production of IL-10.
The functionality of the IL-21/IL-10 connection was demonstrated by the augmented inhibitory activity of Th1 cells primed with IL-21; the inhibitory activity of IL-21 correlated with its induction of IL-10 and was diminished by an antibody to IL-10R. Furthermore, when IL-10 KO Th1 cells were primed with IL-21, no inhibitory activity was observed, establishing a critical role for IL-10 in mediating the immunosuppressive activities of IL-21. IL-10 is produced by multiple lineages that function as suppressors, including natural Treg cells (3). However, we have found no differences in either the number or the suppressive activity of Treg cells in IL-21R KO mice. The induction of IL-10 by IL-21 in already differentiated effector T cell populations may be of importance in the control of inflammation, as IL-10 production by IFNγ-producing CD4+ T cells has been shown to play a major role in host protection from inflammation in several infectious disease models (6, 50). We have examined the responses of IL-21R KO mice to Toxoplasma gondii and found no difference in either the degree of infection or in the production of IFN-γ/IL-10 double producer cells (unpublished observations), indicating that there are redundant pathways controlling the production of IL-10.
Our demonstration that transgenic expression of IL-21 leads to suppression of in vivo immunoglobulin responses underscores the complexity of B cell responses to IL-21. We previously demonstrated that IL-21 plays a critical positive role in Ig responses (22) and in plasma cell differentiation (24), but it can also mediate B cell apoptosis when the appropriate costimulation is absent (32, 33), suggesting the importance of context for the IL-21 signal. Although young (4−8 week old) IL-21 transgenic mice have higher serum Ig levels, these levels decline with age (4 months and older) (unpublished observations), suggesting that the effects of chronic IL-21 activity may alter the ability of this cytokine to function as a positive regulator of Ig production.
Importantly, our demonstration that IL-21 induces IL-10 provides an explanation for the elevated IL-10 in the BXSB-Yaa mouse model of systemic lupus erythematosus. In humans with SLE, serum levels of IL-10 are also increased (51, 52) and increased IL-21 has also been observed in a substantial fraction of humans with SLE (53). Although it is unclear whether the production of IL-10 in human SLE is a mediator of this disease or a suppressive response that limits disease activity, it is interesting that diminished disease severity resulted from the administration of IL-10 with an AAV gene delivery system in the NZM2410 mouse model of lupus (54), whereas more severe disease occurred in MRL-lpr mice on the IL-10 KO background (55). These results support the idea that the production of IL-10 in response to IL-21 may serve to suppress disease activity. Like the BXSB-Yaa mice, MRL-lpr mice also produce a higher level of IL-21 (56).
In summary, we have shown that IL-21 is a potent inducer of IL-10 and that it can mediate immunosuppressive effects. This indicates a new function for IL-21 that was unanticipated given that IL-21 is required for normal immunoglobulin production and is implicated in the development of autoimmunity. We also have identified IL-21 as a critical mediator of the induction of IL-10 by IL-27, indicating a broader role for IL-21 than previously anticipated.
Supplementary Material
Acknowledgments
We thank Drs. Ronald H. Schwartz, Amin Al-Shami, Hyok Joon Kwon, Irina Rochman, and Jian-Xin Lin for valuable discussions and/or critical comments.
Footnotes
This research was supported by the Intramural Research Program, NHLBI, NIH (W.J.L.) and NIH grant AI28900 (D.E.L.).
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 3.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 4.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 5.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 6.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spolski R, Leonard WJ. Interleukin-21: Basic Biology and Implications for Cancer and Autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 8.Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, Schrader S, Burkhead S, Heipel M, Brandt C, Kuijper JL, Kramer J, Conklin D, Presnell SR, Berry J, Shiota F, Bort S, Hambly K, Mudri S, Clegg C, Moore M, Grant FJ, Lofton-Day C, Gilbert T, Rayond F, Ching A, Yao L, Smith D, Webster P, Whitmore T, Maurer M, Kaushansky K, Holly RD, Foster D. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki K, Kikly K, Michalovich D, Young PR, Leonard WJ. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc Natl Acad Sci U S A. 2000;97:11439–11444. doi: 10.1073/pnas.200360997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 13.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 14.Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 16.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 18.Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF, Jr., Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173:68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 20.Sonderegger I, Kisielow J, Meier R, King C, Kopf M. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur. J. Immunol. 2008;38:1–6. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
- 21.Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J. Immunol. 2008;180:7097–7101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 22.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, Morse HC, 3rd, Liu C, Schwartzberg PL, Leonard WJ. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 23.Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 24.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 25.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, Colombo MP, Ferrini S. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–1547. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- 29.Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, Collins M, Dunussi-Joannopoulos K. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol. 2003;171:608–615. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- 30.Wang G, Tschoi M, Spolski R, Lou Y, Ozaki K, Feng C, Kim G, Leonard WJ, Hwu P. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–9022. [PubMed] [Google Scholar]
- 31.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 32.Jin H, Carrio R, Yu A, Malek TR. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol. 2004;173:657–665. doi: 10.4049/jimmunol.173.1.657. [DOI] [PubMed] [Google Scholar]
- 33.Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–4118. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- 34.Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–4098. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- 35.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- 37.Ashwell JD, Fox BS, Schwartz RH. Functional analysis of the interaction of the antigen-specific T cell receptor with its ligands. J Immunol. 1986;136:757–768. [PubMed] [Google Scholar]
- 38.McLemore ML, Grewal S, Liu F, Archambault A, Poursine-Laurent J, Haug J, Link DC. STAT-3 activation is required for normal G-CSF-dependent proliferation and granulocytic differentiation. Immunity. 2001;14:193–204. doi: 10.1016/s1074-7613(01)00101-7. [DOI] [PubMed] [Google Scholar]
- 39.Habib T, Senadheera S, Weinberg K, Kaushansky K. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002;41:8725–8731. doi: 10.1021/bi0202023. [DOI] [PubMed] [Google Scholar]
- 40.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 41.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 42.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 43.Im SH, Hueber A, Monticelli S, Kang KH, Rao A. Chromatin-level regulation of the IL10 gene in T cells. J Biol Chem. 2004;279:46818–46825. doi: 10.1074/jbc.M401722200. [DOI] [PubMed] [Google Scholar]
- 44.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 45.Kang SS, Allen PM. Priming in the presence of IL-10 results in direct enhancement of CD8+ T cell primary responses and inhibition of secondary responses. J Immunol. 2005;174:5382–5389. doi: 10.4049/jimmunol.174.9.5382. [DOI] [PubMed] [Google Scholar]
- 46.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 47.Brady J, Hayakawa Y, Smyth MJ, Nutt SL. IL-21 induces the functional maturation of murine NK cells. J Immunol. 2004;172:2048–2058. doi: 10.4049/jimmunol.172.4.2048. [DOI] [PubMed] [Google Scholar]
- 48.Choe J, Choi YS. IL-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma cells. Eur J Immunol. 1998;28:508–515. doi: 10.1002/(SICI)1521-4141(199802)28:02<508::AID-IMMU508>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 49.Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, Nakajima H. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4+CD25-Foxp3- Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J. Exp. Med. 2007;204:285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park YB, Lee SK, Kim DS, Lee J, Lee CH, Song CH. Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 1998;16:283–288. [PubMed] [Google Scholar]
- 52.Hagiwara E, Gourley MF, Lee S, Klinman DK. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10:interferon-gamma-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:379–385. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]
- 53.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 54.Blenman KR, Duan B, Xu Z, Wan S, Atkinson MA, Flotte TR, Croker BP, Morel L. IL-10 regulation of lupus in the NZM2410 murine model. Lab Invest. 2006;86:1136–1148. doi: 10.1038/labinvest.3700468. [DOI] [PubMed] [Google Scholar]
- 55.Yin Z, Bahtiyar G, Zhang N, Liu L, Zhu P, Robert ME, McNiff J, Madaio MP, Craft J. IL-10 regulates murine lupus. J Immunol. 2002;169:2148–2155. doi: 10.4049/jimmunol.169.4.2148. [DOI] [PubMed] [Google Scholar]
- 56.Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K. IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol. 2007;178:3822–3830. doi: 10.4049/jimmunol.178.6.3822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.