Summary
Seasonal and circadian rhythms control fundamental physiological processes including neural excitability and synaptic plasticity that can lead to the periodic modulation of motor behaviors like social vocalizations. Parental male midshipman fish produce three call types during the breeding season: long duration (min to >1 h) advertisement `hums', frequency and amplitude modulated agonistic `growls' (s), and very brief (ms) agonistic `grunts' produced either singly or repetitively as `grunt trains' for up to several minutes. Fictive grunts that establish the temporal properties of natural grunts are readily evoked and recorded in vivo from vocal occipital nerve roots at any time of day or year by electrical microstimulation in either the midbrain periaqueductal gray or a hindbrain vocal pre-pacemaker nucleus. Now, as shown here, the longer duration fictive growls and hums can also be elicited, but are restricted to the nocturnal reproductive season. A significant drop in call threshold accompanies the fictive growls and hums that are distinguished by their much longer duration and lower and more regular firing frequency. Lastly, the long duration fictive calls are dependent upon increased stimulation time and intensity and hence may result from activity-dependent changes in the vocal motor circuit that are themselves modulated by seasonal and circadian rhythms.
Keywords: teleost, vocalization, pattern generator, circadian rhythm, seasonal plasticity
INTRODUCTION
Synchronizing seasonal and circadian patterns of behavior with relevant social and abiotic cues must ultimately depend upon plasticity in the morpho-physiological properties of neural networks (Herzog, 2007; Panda, 2008). The avian song control system has been a model for periodic anatomical and functional plasticity in the adult central nervous system across the longest time span, the reproductive year (e.g. Arnold et al., 1976; Ball et al., 2004; Brenowitz, 2004; Meitzen et al., 2007; Park et al., 2005). Birdsong has also been studied to demonstrate state-dependent auditory activity during the 24 h sleep/wake cycle (Dave et al., 1998; Schmidt and Konishi, 1998), as well as the role of sleep in song learning (Shank and Margoliash, 2008). However, while many animals clearly exhibit daily as well as seasonal patterns of vocal production, few organisms have allowed the comprehensive exploration of a single rhythmic behavior that extends from the broadest neural and neuroendocrine cycles to the oscillating activity of a dedicated circuit. Now, as shown here, a teleost fish, the plainfin midshipman (Porichthys notatus Girard 1854, family Batrachoididae), presents a seasonally and diurnally rhythmic vocal behavior readily accessible to neurophysiological and behavioral study.
Essentially the entire life history of the adult midshipman fish is characterized by dramatic patterns of seasonal and daily periodicity in reproductive behavior. From non-reproductive winters spent in deep waters off the Pacific coastline, they migrate to tidal spawning grounds in the spring where males excavate nests under rocks from which to attract females and guard offspring (Bass, 1996). Nesting males court females primarily at night with long duration (∼350 ms to >1 h) advertisement calls known as `hums' that are generated by the rhythmic contraction of muscles attached to the walls of the swim bladder (Fig. 1Ai,Aiia) (Bass et al., 1999; Brantley and Bass, 1994; Ibara et al., 1983). The multiharmonic hum has a highly stable fundamental frequency around 100 Hz at ambient temperatures that does not vary across the duration of the call (Fig. 1Aiia; Fig. 2Ai) and shows almost no amplitude modulation (Fig. 1Aiia). The hum contrasts sharply with the very brief (50–100 ms), higher frequency (∼110 Hz) and broadband agonistic grunt produced singly by nesting parental males (type I), an alternative male morphotype (type II) that either sneaks or satellite spawns, and females. During agonistic encounters with other males, the grunt is also produced repetitively as a `grunt train' by nesting males at rates of 1.5–3 Hz for as long as several minutes (Fig. 1Aiib; Fig. 2Aii) (Bass et al., 1999; Brantley and Bass, 1994; Cohen and Winn, 1967; McKibben and Bass, 1998). A second agonistic call, the `growl', is exclusive to the nest-building males and most frequently recorded at night (Bass et al., 1999). Growls are the most complex call; they overlap hums in duration (∼200 ms to 5 s) and are reiterative sequences of grunt- and hum-like signals (Fig. 1Aiic,d; Fig. 2Aiii). Only the nesting, type I male morph employs all call types and thus has been the focus of the present study.
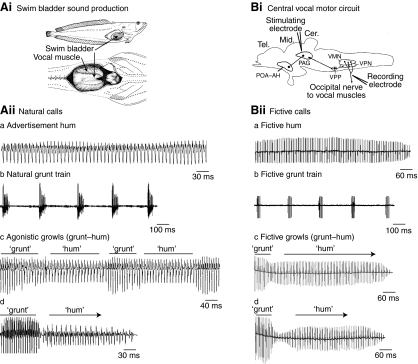
Fig. 1.
Natural and fictive vocalizations of midshipman fish (Porichthys notatus). Note that time scales differ between natural and fictive calls to allow visualization of more complex waveforms in natural calls. (Ai) Vocalizations are produced by the simultaneous contraction of a pair of vocal muscles attached to the lateral walls of the swim bladder [modified from Bass et al. (Bass et al., 2008)]. (Aii) Representative natural calls of parental, type I male. (Aiia) The advertisement hum has sound pulses produced at a highly regular frequency for the entire duration of the call, ∼400 ms to >1 h. (Aiib) Agonistic grunt trains are repetitions of brief grunts at a rate of 1.5–3 Hz. (Aiic,d) Agonistic growls are the most complex vocalization with amplitude and frequency modulation. They are an amalgam of brief grunts (∼50–150 ms) and longer duration, multiharmonic hums and range from 300 ms to several seconds in duration. The grunt portion of the call in Aiid is clipped in the original recording because of the proximity of the fish to the hydrophone. (Bi) Sagittal view of the central network responsible for vocal production [modified from Bass and McKibben (Bass and McKibben, 2003)] (for details, see Bass et al., 1994; Goodson and Bass, 2002). Stimulation in the midbrain periaqueductal gray (PAG), which receives afferents from the forebrain preoptic area and anterior hypothalamus (POA–AH) and projects to the hindbrain/spinal vocal pattern generator (VPP–VPN–VMN), evokes fictive vocalizations that are recorded from the occipital nerves that innervate each vocal muscle. (Biia) Fictive hums also have a regular discharge frequency with average durations of 400 ms to 1 s. (Biib) Fictive grunt trains are repetitions of fictive grunts, like the natural call. (Biic,d) The fictive growl or `grunt–hum' averages 400–800 ms in duration. Other abbreviations for Bi: Cer., cerebellum; Mid., midbrain; Tel., telencephalon; VMN, vocal motor nucleus; VPN, vocal pacemaker nucleus; VPP, vocal prepacemaker nucleus.
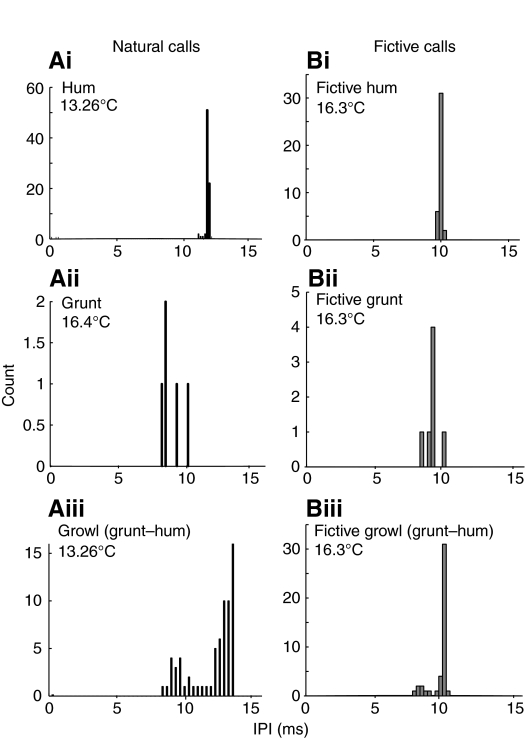
Fig. 2.
Interpulse intervals (IPIs) for individual natural and fictive vocalizations. Shown here are the IPIs for representative examples of each call type studied. Recording temperatures are indicated since the repetition rate of natural sound pulses and the discharge frequency of the vocal motor volley/fictive call are temperature sensitive (Bass and Baker, 1991; Brantley and Bass, 1994). (Ai) The IPI of a natural hum (1 s duration) centers tightly on 12 ms. (Aii) A natural grunt (∼50 ms duration) with an average IPI of 8.5 ms. (Aiii) A natural growl (800 ms duration) can have considerable frequency modulation but with a bimodal distribution: the shorter, faster grunt-like portion of this growl has an IPI of ∼9 ms, while the longer, more regular hum-like portion averages 13 ms. (Bi) Like the natural hum, a fictive hum (400 ms duration) can have an extremely regular IPI (briefer at ∼10 ms than the natural one at ∼12 ms because of the higher recording temperature). (Bii) The IPI of a fictive grunt averages 8.5 ms (like the natural one because of similar recording temperatures). (Biii) The IPIs of this fictive growl or `grunt–hum' (470 ms duration), like the natural one, are bimodally distributed (between 8.5 and 10 ms, which is also briefer than the natural call because of the higher temperature).
The rhythmic properties of midshipman vocalizations are determined by the activity of a vocal pattern generator that shares evolutionary origins with comparable networks in tetrapods (Bass and Baker, 1990; Bass et al., 1994; Bass et al., 2008). The pattern generator includes a rostral, hindbrain vocal pre-pacemaker nucleus (VPP, formerly the ventral medullary nucleus) that projects to paired columns of vocal pacemaker neurons (VPN) that lie ventrolateral to the paired vocal motor nuclei (VMN) found along the midline of the caudal medulla and the rostral spinal cord (Fig. 1Bi). Vocal motoneurons receive input from the pacemaker neurons that set their discharge frequency, the subsequent contraction rate of the muscles and either the fundamental frequency of harmonic vocalizations or the pulse repetition rate (PRR) of non-harmonic vocalizations (Bass and Baker, 1990) [see Cohen and Winn (Cohen and Winn, 1967) and Skoglund (Skoglund, 1961) for one-to-one correspondence between each complex action potential in the nerve volley, muscle contraction and sound pulse in midshipman and the closely related toadfish, Opsanus tau].
Vocal motoneurons can be activated in a neurophysiological preparation of midshipman and toadfish by electrical microstimulation in the forebrain's preoptic area–anterior hypothalamus (POA–AH) (Fig. 1Bi), the midbrain's periaqueductal gray (PAG) (Fig. 1Bi) and the hindbrain's VPP (Fig. 1Bi) (Bass and Baker 1990; Goodson and Bass, 2000a; Goodson and Bass, 2000b; Goodson and Bass, 2002; Kittelberger et al., 2006; Remage-Healey and Bass, 2004; Remage-Healey and Bass, 2006). Electrical microstimulation in each of the above regions can produce a rhythmic vocal motor volley known as a fictive vocalization that is readily monitored with electrodes placed on ventral occipital nerve roots that form the vocal nerve innervating the ipsilateral vocal muscle (see Fig. 1Bi) (Bass and Baker, 1990). Surgical isolation of the hindbrain–spinal region containing the VPP–VPN–VMN circuitry further shows that this region alone can produce and modulate the duration of fictive grunts with discharge frequencies independent of the stimulus frequency (Bass and Baker, 1990; Remage-Healey and Bass, 2004; Remage-Healey and Bass, 2006; Kittelberger et al., 2006). Thus, the firing pattern of the vocal motor circuit directly determines easily quantified temporal properties such as the fundamental frequency/PRR and duration of natural calls that together with amplitude modulation (AM) can be used to characterize fictive calls. Midshipman fish behaviorally discriminate and neurally encode vocalizations that vary in duration, frequency and AM; hence, the behavioral saliency of these neuro-behavioral traits (Bass and McKibben, 2003).
Vocalizations play a crucial role in the seasonal reproductive behaviors of midshipman and toadfish as they do in songbirds and anurans (Bass and McKibben, 2003; Kelley and Brenowitz, 2002). Because of the one-to-one correlation between the temporal features of the vocal motor volley/fictive call and natural calls, the fictive in vivo preparation becomes a reliable measure of the probability of the fish producing each type of natural vocalization in a particular physiological condition. The simplest and briefest fictive call type, the grunt, has been electrically and neurochemically (glutamate) evoked from midshipman at any time of day or year (Bass and Baker, 1990; Goodson and Bass, 2000a; Remage-Healey and Bass, 2004; Weeg et al., 2005; Kittelberger et al., 2006). However, long duration fictive calls with firing patterns suggestive of natural growls and hums have only been occasionally recorded (Goodson and Bass, 2000b). Now for the first time, using a new stimulation paradigm, we show that long duration fictive growls and hums can indeed be readily evoked in parental males, but almost exclusively at night and only when they are in reproductive condition. Similarly, long duration fictive grunt trains have now been evoked for the first time in reproductive males, although they can occur either during the day or at night. Thus, as shown here, the seasonally and nocturnally dependent vocal behaviors of midshipman fish are clearly supported by periodic changes of basal activity in the vocal motor system. With this analysis of the state dependence of long duration fictive calls and their comparison with the natural calls, we can further dissect how either system or local circuit changes in neurophysiology ultimately dictate the natural rhythmicity of a behavior.
MATERIALS AND METHODS
During April–August 2007–2008, type I males (12–20 cm, standard length) were hand collected from nest sites in the intertidal zone of Washington State and California and shipped within 6–72 h to Cornell University where they were housed on a 14 h light (L):10 h dark (D) light cycle with lights out at 17:00 h Eastern Standard Time (EST). Type I males were unambiguously identified on the basis of their body size and coloration upon collection as well as visual inspection of their vocal muscle and testes (Bass, 1996). A subset of males were collected in July and August and shifted to a winter photoperiod of 10 h L:14 h D in October. By this time in the autumn, this group had reverted to a non-reproductive state with either partially or fully regressed gonads, reflecting duration in captivity as well as a response to natural shifts in photoperiod (Sisneros et al., 2004a; Sisneros et al., 2009). The gonadosomatic indices (GSIs, ratio of gonad mass to body mass–gonad mass) were determined for a subset of males at the end of neurophysiological experiments following deep anesthetization in 0.025% benzocaine (Sigma, St Louis, MO, USA). The mean (±s.e.m.) GSIs for reproductive and non-reproductive males were, respectively, 1.84±0.21 and 0.77±0.12 (N=6 animals per group). All methods were approved by the Institutional Animal Care and Use Committee at Cornell University.
Neurophysiological experiments
The fictive vocalization preparation used here has been thoroughly described elsewhere (Bass and Baker, 1990; Goodson and Bass, 2000a; Remage-Healey and Bass, 2004). Briefly, brain and rostral spinal cord with occipital nerve roots were exposed by dorsal craniotomy under general anesthesia with 0.025% benzocaine (Sigma) and a local injection at the wound site of a long-lasting analgesic (0.25% bupivacaine; Abbot Laboratories, Chicago, IL, USA) with 0.01 mg ml–1 epinephrine (adrenaline; International Medication Systems, El Monte, CA, USA). After surgery, fish were immobilized with an intramuscular injection of pancuronium bromide (0.5 mg kg–1, Astra Pharmaceutical, Westborough, MA, USA) and stabilized in a Plexiglas tank with aged, chilled (16–17°C) saltwater perfused through the mouth. One hour after surgery, an insulated tungsten electrode (125 μm diameter, 8 deg. tip angle, 5 MΩ impedance, 20 μm exposed tips; A-M Systems, Sequim, WA, USA) was used to evoke the vocal/occipital nerve motor volley (fictive vocalization) through delivery of 40 brief (30 ms) trains of 200 Hz stimuli (0.1 ms pulse width, 50–75 μA positive current) at 1 s–1 via a WPI stimulus isolation unit (Model 850 S, World Precision Instruments, Sarasota, FL, USA) to either the midbrain PAG region which connects to the hindbrain pattern generator or the hindbrain VPP region that projects to the VPN–VMN circuit (Fig. 1Bi). The same low current intensity was used for all fish at all time points, whether it was at or above the call threshold for each individual. When only the threshold current (minimum current to elicit a call) was used, the probability of evoking long duration calls was much reduced (see Results). Well-documented surface landmarks and depth measurements based on previous mapping studies of the vocal motor system provided guides for electrode placement (Goodson and Bass, 2002; Kittelberger et al., 2006; Remage-Healey and Bass, 2004). As noted earlier, fictive vocalizations reflect the firing properties of the VPN–VMN circuit that directly determines a natural call's duration and fundamental frequency (harmonic call)/pulse repetition rate (non-harmonic call); hence, its designation as a fictive call/vocalization. Fictive calls were recorded unilaterally from an occipital nerve with an extracellular electrode (Teflon-coated silver wire with exposed ball tip; 50–100 μm diameter) and digitized using MATLAB software designed by Dr Bruce Land (Department of Neurobiology and Behavior, Cornell University). The two sides of the brain fire together so that a unilateral recording represents bilateral synchrony of the descending vocal motor volley (Bass and Baker, 1991) that leads to the natural, simultaneous contraction of the paired vocal muscles (Skoglund, 1961; Cohen and Winn, 1967).
Neurophysiological and statistical analysis
Previously, fictive vocalization preparations performed during the day typically evoked grunts with 15 brief (30 ms) stimulus trains presented at one-second intervals (1 s–1) at each of several time-points over the course of 120 min (Goodson and Bass, 2000a; Goodson and Bass, 2000b; Goodson and Bass, 2002; Remage-Healey and Bass, 2004; Remage-Healey and Bass, 2006; Remage-Healey and Bass, 2007). However, it was found here during pilot studies with reproductive males that they were highly responsive to a longer stimulation time at night, consistent with the time that they mainly produce long duration calls (Bass et al., 1999; Brantley and Bass, 1994; Ibara et al., 1983). Thus, if the number of stimulus trains was increased to 40 at every recording, long duration calls could be readily evoked from some males by 60 min post-baseline recordings. Hence, the first set of studies in this investigation delivered 40 brief stimulus trains at 1 s–1 at eight time-points (baseline/0, 5, 15, 30, 45, 60, 90, and 120 min) to different groups of reproductive and non-reproductive males at different times of the day.
It was also found during the course of these first experiments that at the 120 min time point, presentation of an additional 60 stimulus trains at 1 s–1, continuous with the initial 40, had an especially robust effect on the ability to evoke long duration calls at night in reproductive males. We subsequently tested reproductive males in the day and non-reproductive males day and night in the same way. To further evaluate the effect of the prolonged stimulation on evoking long calls before any slower physiological changes were incurred during the 120 min experiment, we compared these results with those of a separate group of reproductive animals that received 100 s of stimulation trains at baseline.
The minimum current or threshold for evoking fictive calls, call duration and the ratio of the number of fictive growls/grunts were averaged for each time point (5–120 min) and normalized against the baseline (0) of each fish. As reported in the Results, natural and fictive growls are a hybrid of grunt- and hum-like calls. For duration measurements of grunt–hums, the duration of the initial grunt-like response (≥3 pulses) and any subsequent response (≥3 pulses) were added for the complete value but did not include the silent gap between the two. The repetition rates of the motor volley that mimics the fundamental frequency of natural calls were determined by the peak-to-peak interval between compound action potentials or `interpulse interval' (IPI).
Call duration, grunt–hum probability and threshold change (reported as means with s.e.m.) were analyzed in JMP (7.0) using repeated-measures ANOVA followed by planned individual contrast post-hoc tests for between subjects comparisons from 30 to 120 min. Statistical analysis of baseline grunt duration, based on comparisons of mean values between each study group (see Results), was performed in Graphpad Prism (5.0) with a one-way ANOVA followed by Tukey's post-hoc tests. To expand the database for this analysis beyond the number of animals comprising the experimental groups (3–6) in the main body of this study, we included values from a larger sample size of animals treated identically at baseline (20 brief stimulus trains at 1 s–1 rather than the 40 stimulus trains at 1 s–1 used throughout the remainder of the study). A one-way ANOVA followed by Tukey's post-hoc tests was also used for duration change (log transformed) after presenting 100 stimulus trains (values normalized against the first 20 s of stimulation). Comparison of IPIs between fictive call types produced by the same fish was performed in Graphpad Prism (5.0) with paired t-tests, while unpaired t-tests were used for comparisons between the IPIs of fictive calls and the fundamental frequencies of natural calls. The IPI/frequency of a particular call type from any single fish is highly consistent, thus an average of 40 calls is not significantly different from one. A general linear mixed model was used to evaluate differences in duration between fictive grunts and growls, and between fictive grunts and natural grunts in order to account for a greater variation in call duration measured from individual fish. Statistical comparisons were always based on the mean values obtained for each animal in a group, not on the total call number for all animals in the group.
Photoperiod manipulation
We wanted to determine whether the nocturnal dependence of the male's fictive grunt–hums and hums either reflected an endogenous rhythm or was dependent upon external light cues. Thus, reproductive type I males shipped to the lab in either July or August 2008 were subjected to 24 h of either dark or light for 5 days after an initial exposure for 1–5 days to the 14 h L:10 h D cycle. These animals were then tested for the ability to produce long duration fictive calls. Taking advantage of the midshipman's typical lack of feeding during the first 1–2 weeks of acclimation to captive conditions (Sisneros et al., 2009), food was withheld from these animals so as not to confound the effect of the photoperiod regime with food entrainable rhythms. Of the six fish in each treatment group, three were tested between 11:00 and 12:00 h EST of the circadian day, while three were tested after 18:00 h EST of the circadian night. Subjects of night experiments and all 24 h D fish were exposed to 30 min of white light during surgery with eyes covered, after which the rest of the neurophysiology experiment was conducted in red light only, which does not inhibit the nocturnal behavioral activity of midshipman fish (see McKibben and Bass, 1998).
Sound recordings
Recordings of midshipman vocalizations (courtesy of Margaret Marchaterre, Department of Neurobiology and Behavior, Cornell University) were made directly from spawning sites in the intertidal zone of Brinnon, Washington using hydrophones (Bioacoustics Research Program, Cornell Laboratory of Ornithology, Ithaca, NY) placed directly adjacent to nests, which are excavations under large rocks (see Bass, 1996; Bass et al., 1999; Bass and Clark, 2003). Since the fundamental frequency/pulse repetition rate of natural harmonic (hums and growls)/non-harmonic (grunts) calls and the discharge frequency of fictive calls vary directly with ambient temperature (Bass and Baker, 1991; Brantley and Bass, 1994; McKibben and Bass, 1998), temperature was also recorded (temperature loggers from DataLoggers, Onset Computer, Pocasset, MA, USA). All sound recordings were made between dusk and dawn when spawning and vocal activity peak (Brantley and Bass, 1994; Bass et al., 1999). Recordings were digitized at 2 kHz and 16-bit resolution and waveforms visualized and analyzed using Raven Pro 1.3 (Bioacoustics Program, Cornell Laboratory of Ornithology).
RESULTS
As demonstrated in earlier studies and repeated here, brief fictive grunts can be evoked any time of year or day by electrical microstimulation in either midbrain or hindbrain vocal nuclei and predict the temporal properties of the natural call (see individual grunts in Fig. 1Aiib; Fig. 1Biib; and Introduction). We now show using a new stimulation paradigm (see Materials and methods, `Neurophysiological and Statistical Analysis') that the long duration, fictive hum and growl (Fig. 1Biia,c,d) are almost exclusively evoked from parental males during the scotophase of the reproductive season (14 h L:10 h D housed animals), reflecting the nocturnal occurrence of the natural calls during the spawning season (Brantley and Bass, 1994; Bass et al., 1999). Below, we address first seasonal and diurnal differences in fictive call duration, frequency (measured as interpulse intervals) and call threshold. We then present a more detailed analysis of the temporal properties of long duration fictive and natural calls, revealing the dramatic and combined effects of reproductive state, time of day and stimulation time on call type and probability. We conclude with the effects of photoperiod manipulation on fictive call production.
Diurnal and seasonal changes in call duration and frequency
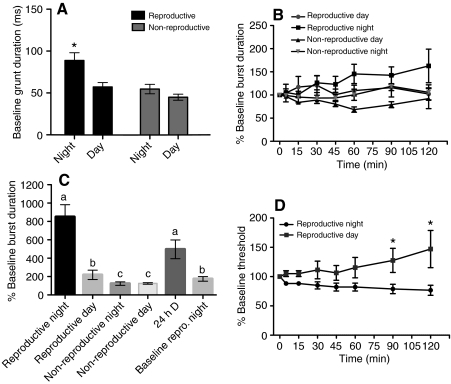
The mean baseline fictive grunt duration of reproductive and non-reproductive males reflects seasonal and daily changes in basal vocal motor excitability. Baseline fictive grunt values were determined for separate day and night groups of reproductive and non-reproductive males (Fig. 3A). Mean grunt duration of reproductive males tested at night was significantly greater (P<0.05) than for all other groups (reproductive night: 88.79±9.28 ms; reproductive day: 57.16±5.33 ms; non-reproductive night: 54.7±5.63 ms; non-reproductive day: 44.96±3.66 ms; N=10 animals/group, 20 calls/animal).
Fig. 3.
Diel and seasonal variation in fictive calls. (A) A night versus day comparison of mean baseline grunt duration in reproductive and non-reproductive males. Asterisk, reproductive males at night have a significantly higher fictive grunt duration than all other groups (see text). (B) Call duration change over 120 min with 40 stimulus trains at each recording. Reproductive males were housed on a 14 h L:10 h D cycle, and non-reproductive in 10 h L:14 h D cycle (all N=3). There was a significant, overall effect of reproductive state (see text). (C) 100 stimulus trains (1 s–1) at baseline in reproductive males at night versus 100 stimulus trains at 120 min in all groups (N=5 for reproductive night; 3 each for reproductive day, non-reproductive night and non-reproductive day; 6 each for 24 h D and baseline reproductive night). Letters (a, b, c) denote significant differences (see text). (D) The call threshold stimulus current significantly decreases in reproductive (14 h L:10 h D) males at night, but rises during the day (asterisks indicate significant differences, see text).
Reproductive males at night showed a subtle but significant effect (P<0.05) of reproductive state on the duration of fictive calls evoked over the 120 min stimulus trial (Fig. 3B; the data at each time point are the average of 40 fictive calls evoked by 40 stimulus trains). Fig. 4Ai,ii shows 20 s segments of representative stimulus trials to better illustrate the time-dependent shifts in the temporal properties of fictive calls. Increased call duration was mainly dependent upon an increase in duration of an initial short latency, grunt-like response (Fig. 4Ai,ii). However, by 120 min, a much lower amplitude, but typically longer duration component sometimes followed the initial grunt-like response (Fig. 4Aii, see Materials and methods for determination of total duration). Reproductive males tested in the daytime were less affected by stimulation than animals at night, but still showed signs of being more responsive than either day or night non-reproductive fish.
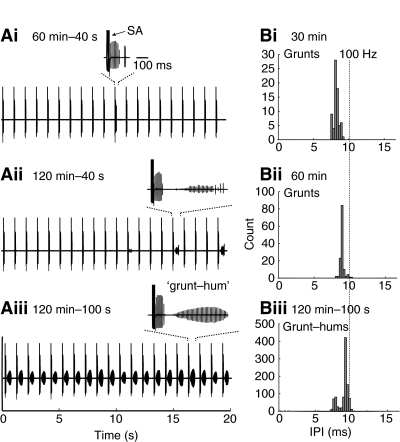
Fig. 4.
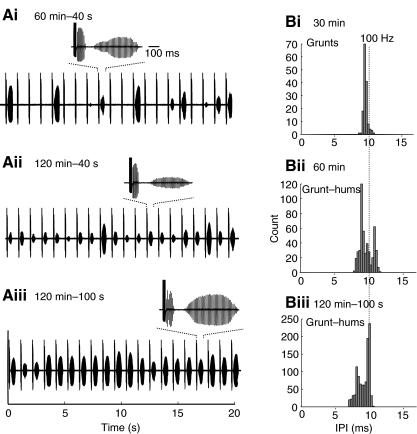
Fictive call stimulus–response trains from a reproductive male housed in 14 h L: 10 h D and tested at night. (Ai–iii) Example of the time- and stimulation-dependent progression of fictive grunts to longer duration calls. Shown here are 20 s excerpts from 40 stimulus trains with stimulus artifacts (S.A., Ai) shown at 1 s intervals followed by the fictive call (see text for details). (Bi–iii) As fictive grunts transition to fictive grunt–hums and duration increases, mean firing frequency (20 calls, one male) decreases. Recording temperature, 16.3°C.
When a stimulus trial of 100 stimulus trains (1 s–1) was presented at 120 min, call duration increased dramatically in reproductive males at night compared with both reproductive males in the day and to non-reproductive males (day and night), coincident with the evocation of long duration growl-like calls (P<0.05) (Fig. 3C; Fig. 4Aiii). Most of the long duration calls had two components as sometimes observed with 40 stimulus trains at the 120 min time point (see above). However, the grunt-like part of the response was typically followed by a long hum-like response: the combined response resembling natural growls (Fig. 4Aiii; also see Fig. 1Aiid). Two of six reproductive males tested at night and given 100 stimulus trains at baseline were able to produce long duration calls as opposed to five of five at 120 min, suggesting both short term and long term activity-dependent changes in the vocal motor circuit. Non-reproductive males (day or night) increased call duration significantly less than all other groups (P<0.05) (Fig. 3C), reflecting the absence of fictive growls and hums. At night, reproductive males tested at 120 min produced significantly more long duration calls than either the reproductive males tested during the day at 120 min or the reproductive males tested during the night at baseline (Fig. 3C). Thus, long duration call production peaked in the group that permitted both short term and long term, activity-dependent changes to occur in vocal circuits already primed by a nocturnal, reproductive condition.
The IPI, which reflects the fictive call's discharge frequency, was also increased in the longer duration calls evoked with 100 stimulus trains at 120 min. The IPI analysis is shown in Fig. 2Bi–iii for single calls in comparison with single natural calls and in Fig. 4Bi–iii for a mean of 20 fictive calls to show cumulative results. The shift from grunts to growls that was potentiated by the 100 stimulus trains (Fig. 4A) was accompanied by the appearance of a bimodal distribution of IPIs, composed of the growl's faster grunt-like and slower hum-like components (Fig. 4B).
In sum, the facilitation of vocal motor excitability, as reflected in the increased production of long duration calls, depended upon reproductive state, time of day and degree of stimulation.
Diurnal and seasonal changes in call threshold
As shown above, fictive growls and hums are distinguished from grunts by their physical attributes (duration, frequency, amplitude modulation) and by the time of day and year at which they can be evoked. Fictive growls and hums are also distinguished by a decreased response threshold (minimum current to elicit a call), and a paradoxical dependence on increased stimulation intensity. Amongst reproductive males, there was a conspicuous and significant drop (25–40%) in call threshold (P=0.036) at night, compared with the rise seen during the day (Fig. 3D) (90 min P=0.01 and 120 min P=0.0006) that paralleled the steady, time-dependent increase in duration and IPIs (Fig. 4). While fictive grunts can follow a stimulus at the very low threshold current, fictive growls and hums are elicited from reproductive males at night (and to a much lesser degree during the day) with a slightly elevated stimulation current (25–50 μA above threshold). Thus, in spite of the decrease in burst threshold and this evidence for the vocal circuit's heightened excitability, the likelihood of evoking the longer calls with every stimulus pulse was still greater if the current remained slightly above threshold.
Fine temporal properties of fictive and natural calls
As we noted earlier, the fictive growl was designated as a `grunt–hum' due to its hybrid nature, namely a grunt-like beginning followed by a longer, hum-like portion with damped amplitude at either end. Thus, both natural and fictive growls have two distinguishable parts that are either continuous or separated by a sudden, brief change in amplitude (Fig. 1Aiic,d,Biic,d; also see Fig. 4Aiii) and exhibit a bimodal distribution of IPIs (Fig. 2Aiii,Biii; also see Fig. 4Biii). The duration and mean frequency of natural growls can range broadly even in one animal (e.g. 542 ms to 8 s; 59–116 Hz; N=10 calls), with durations that obviously exceed our fictive recordings. However, naturally brief growls (e.g. Fig. 1Aiid) appear to be a fundamental unit or pattern for the longer calls and the fictive growl is its neural correlate. For reproductive males tested at night and presented with 100 stimulus trains at 120 min, the mean duration of fictive growls (444.67±41.67 ms, N=6 animals, 5 calls/animal) was significantly longer than that of the grunts evoked at baseline from the same fish (67.36±6.81 ms; N=6 animals, 5 calls/animal; P<0.0001). The mean frequency (at 16.4°C) of the hum-like portions of the fictive growls was significantly lower than that of the grunts (mean grunt frequency=106.84±1.81 Hz; mean hum frequency=97.88±0.53 Hz; P=0.003).
Fictive hums alone, although rarely produced de novo (one animal, 3 calls, 1140±332.21 ms, continuous through two stimulus trains with little amplitude modulation), resembled brief natural hums in IPIs (Fig. 2Ai,Bi) [differences in recording temperatures can account for different absolute values for IPIs of both natural and fictive calls (see Brantley and Bass, 1994; Bass and Baker, 1991)]. The more common hum-like portions of fictive growls also had a very regular, low firing frequency (97.88±0.53 Hz; N=7 animals, 5–15 calls/animal) that was not significantly different (P=0.87) from that of the natural hum (mean fundamental frequency=97.44±2.76 Hz 6; N=5 animals, 1 call/animal; same recording temperature). IPIs strikingly differentiated all fictive and natural hums from even the longest fictive or natural grunts (∼200 ms), which exhibit a higher, irregular IPI (Fig. 2Ai,ii,Bi,ii) [see also Brantley and Bass (Brantley and Bass, 1994); Bass et al. (Bass et al., 1999) and Bass and Clark (Bass and Clark, 2003) for natural grunts]. The distribution of IPIs (∼10 ms) in all fictive hums, either singular or part of a grunt–hum, was the tightest of any of the natural or fictive calls (Fig. 2Bi). Fictive hums and the hum-like portions of fictive growls were also similar to brief natural hums in duration [see Brantley and Bass (Brantley and Bass, 1994) for hums as brief as 370 ms]. However, a statistical comparison is not warranted because the duration of naturally produced hums is highly context dependent (A.H.B. and M. Marchaterre, unpublished observations) while the evoked correlates are strictly electrophysiological phenomona that reflect the state of the pattern generator.
Unlike the fictive hums and growls, fictive grunt trains were easily triggered during both night and day trials but, like hums and growls, only in reproductive males. Natural grunt trains consist of individual grunts repeated at a rate of 1.5–3 Hz that can persist for several minutes (Fig. 1Aiib; see Introduction). After the 120 min recording period, free-running grunt trains were readily triggered with 3–20 s of stimulus trials in the hindbrain VPP region (Fig. 1Bi) and continued independently for more than 5 min without further stimulation, mimicking the natural call with a mean grunt repetition rate of 1.9±0.1 Hz (N=5 animals, one grunt train/animal). For individual grunts within the trains, the pulse repetition rate averaged 113±2.17 Hz, with a mean grunt duration of 46.56±7 ms (N=5 animals, 5 grunts/animal). This was not significantly different from the intra-grunt frequency (P=0.13) and duration (P>0.89) of grunts from natural grunt trains (mean frequency=108.52±1.563 Hz; mean duration=47±1.98 ms; N=5 animals, 5 grunts/animal). While fictive growls and hums could only be evoked from the midbrain's PAG region, which projects to the VPP (Fig. 1B1), grunt trains could only be evoked with stimulation in the VPP or VPN region.
Photoperiod manipulation
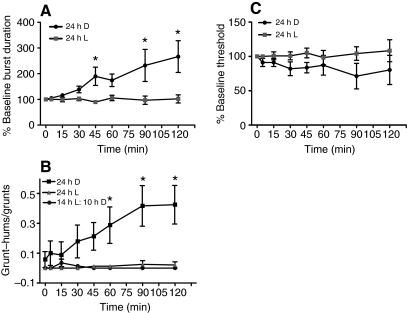
After housing reproductive males in a 24 h D cycle for 5 days, there was a significant effect of phototreatment (P=0.0075). The low frequency/long duration calls could be readily evoked during both the natural day and night (day/night test groups were thus pooled) and only required 40 stimulus trains as opposed to 100 s for the 14 h L:10 h D fish (Fig. 5A; Fig. 6Ai,ii; compare with Fig. 4Ai–iii). This resulted in an increasingly significant time×phototreatment effect on call duration at 45 (P=0.01), 90 (P=0.001) and 120 min (P=0.0001) compared with 24 h L (Fig. 5A). Fig. 6Ai,ii shows traces from a 24 h D fish from which fictive grunt–hums were already evoked by 30–60 min post-baseline. As in 14 h L:10 h D reproductive males, 100 stimulus trains presented at 120 min further potentiated the response (Fig. 6Aiii; also see Fig. 3C). There was also an increasingly significant effect of time×phototreatment (P=0.0037) on the proportion of grunt–hums to grunts after 40 stimulus trains in the 24 h D group compared with the 24 h L and 14 h L:10 h D groups (Fig. 5B) (60 min, P=0.007; 90 min, P=0.0001; 120 min, P=0.00007). Thus, instead of the 24 h D treatment revealing an endogenous circadian rhythm in vocal excitability, increasing at night and decreasing during the day in conjunction with their natural behavior, the constant darkness appeared to tonically facilitate vocal motor output.
Fig. 5.
Photoperiod-dependent plasticity of fictive calls. (A) Call duration change in reproductive males subjected to 24 h of darkness (24 h D) versus 24 h of light (24 h L) (N=6 animals/group). Asterisks indicate significant differences (see text). (B) The ratio of fictive grunt–hums to grunts in reproductive males housed in 24 h D, 24 h L and 14 h L:10 h D (same animals as in `reproductive night' test group). Asterisks indicate significant differences (see text). (C) There was an insignificant trend for call threshold in 24 h D males (day/night pooled) to fall, while call threshold in 24 h L (pooled) animals rose (see text).
Fig. 6.
Photoperiod-dependent plasticity of stimulus–response trains. (Ai–iii) Examples of the time- and stimulation-dependent progression of fictive grunts to longer duration calls from a reproductive male housed in 24 h D and tested during the circadian day. (Bi–iii) Although fictive grunt–hums appeared earlier in 24 h D males compared with 14 h L:10 h D animals (compare with Fig. 4Ai,Bii), the overall firing frequency (mean 20 bursts, one male) started low and increased slightly by 120 min (shown here for one animal tested during the circadian night). Recording temperature, 16.3°C.
IPIs also changed gradually with time, but tended to start longer in 24 h D fish and then decreased slightly rather than showing the increase found in 14 h L:10 h D males (see Fig. 6Bi,ii for shift in mean IPI between the 30 and 60 min records in a 24 h D male tested at night; compare with Fig. 4Bi,ii). In concert with these results, 5 days of 24 h L had the opposite effect: fictive vocal output was suppressed day and night such that the probability of inducing long duration calls was nearly eliminated. When call threshold was compared between all 24 h D and all 24 h L males, there was a non-significant trend (P>0.05) for threshold to fall in 24 h D males and to rise in 24 h L males similar to the day/night contrast found in 14 h L:10 h D housed animals (Fig. 5C, compare with Fig. 3D). While the results suggested that light exposure may directly affect excitability in the circuit, the question remains whether there is also a persistent endogenous rhythm in call threshold. The number of animals tested so far was too small to reveal any significant differences between the day and night groups in each phototreatment.
DISCUSSION
The current study emphasizes that it is as important to consider endogenous or environmentally driven biological rhythms when investigating the neurophysiology of a behavior as when studying the natural behavior itself. Agonistic grunts, the most elemental midshipman vocalization emitted by males and females, are neither diurnally nor seasonally dependent and reflect a minimal activation of the vocal motor system. By contrast, the much longer growls and hums are temporally confined to the spawning season and produced by parental males mainly at night. Likewise, the probability of eliciting fictive growls and hums in a neurophysiological preparation is much greater in reproductive males at night, while fictive grunts are evoked at any time of day. Clearly, neurophysiological preparations other than those involving the extensively studied circadian pacemaker, the suprachiasmatic nucleus, are subject to diurnal and seasonal changes (see below). By paying attention to naturally occurring behavioral rhythms, the full potential of a neural network, as in the midshipman vocal motor system, is revealed.
Long duration fictive vocalization in midshipman fish
The evocation of fictive growls and hums, the neural correlates of the natural, long duration calls used during the breeding season, depends upon reproductive state and time of day. These calls are accompanied by several distinct neurophysiological changes that reflect the altered state of the vocal motor system at night in a reproductive male. First, either shortly preceding or in tandem with the evocation of the first fictive growl or hum, the call threshold drops by as much as 40% as these longer calls increase in number and length. Second, in addition to the significant (up to 1000%) increase from baseline duration with added stimulation, the firing rate concomitantly falls. Third, fictive calls become more regular in their IPIs, also like natural hums and the hum-like parts of growls. Interestingly, even though the fictive grunt threshold significantly decreases in conjunction with the first fictive hums, the kindling and evocation of these long duration calls still rely upon a greater current intensity. This might reflect the recruitment of neurons with lower input resistance, as in those exhibiting more electrotonic coupling necessary for synchronous firing (Christie et al., 1989; Christie and Jelinek, 1993).
All of the above characteristics – duration increase, frequency and call threshold decrease, and firing rate constancy – may be considered the outcome of short term (40–100 stimulus trains at 1 s–1) and long term (120 min trials), activity-dependent plasticity in the vocal motor circuit. Furthermore, this network or cellular plasticity is itself susceptible to seasonal and daily modulation, such that prolonged stimulation (100 stimulus trains) in a reproductive male during the day evokes only a small fraction of the number of fictive hums that can be elicited from another male at night. However, if the stimulation is not increased from 40 to 100 stimulus trains, the potential to produce long duration calls from reproductive males is not entirely revealed in any group. This strongly suggests that activity-dependent plasticity in a circuit emerges from behaviorally relevant network activity, or electrical stimulation of sufficient duration to mimic naturally occurring network activation (Buchanan, 1996; Parker and Grillner, 1999). Future experiments need to further explore the interaction between short and long term activity-dependent changes that give rise to the vocal circuit plasticity studied here. However, these initial studies clearly reveal the dramatic effects of increased stimulation on the probability of evoking long duration calls. Similarly, with prolonged stimulation in the motor cortex of monkeys, muscle twitches evolve into complex movements reflecting natural behaviors (Graziano et al., 2005).
In contrast with previous studies in midshipman (see Introduction), the current experiments increased the number of stimulus trains from 15 to 40 (at 1 s–1) during each stimulus trial, but did not increase the duration of the individual stimulus trains (30 ms). This may be one reason why the recorded fictive hum rarely exceeded 1 s, while parental males will hum without pause for up to an hour. In comparison, it is remarkable that the spontaneous fictive grunt train fired independently for many minutes in reproductive animals after only 3–20 s of hindbrain stimulation. It would suggest that rhythmic, oscillatory-like output from the hindbrain vocal circuit can produce the grunt train, while the hum relies upon added upstream drive from the midbrain PAG and the forebrain's POA-AH that is a major integration site for neuroendocrine and vocal mechanisms (Goodson and Bass, 2002).
The induction of different classes of long duration calls also shows site specificity, namely stimulation in the midbrain PAG region for growls and hums and the hindbrain VPP region for grunt trains. In general, the results are consistent with earlier studies showing that multiple sites in the vocal motor system can modulate the activity pattern of the pacemaker–motoneuron circuit [see Results and other reviews by Goodson and Bass (Goodson and Bass, 2002) and Kittelberger et al. (Kittelberger et al., 2006)]. However, the current study shows, for the first time in the teleost fictive call preparation, the site-dependent induction of vocal patterns that reflect the greatest divergence in vocal patterning. These new results are further consistent with studies of the vocal brainstem in mammals, including primates (e.g. Fenzl and Schuller, 2005; Jurgens and Hage, 2007).
Unlike teleosts, call patterning in tetrapods depends upon the integration of vocal and respiratory mechanisms (Bass and Baker, 1997; Wild, 2004; Zornik and Kelley, 2008). Like studies in toadfishes and other vocal teleosts (Bass and Baker, 1990; Bass and Baker, 1991; Barber and Mowbray, 1956; Packard, 1960; Skoglund, 1961), recordings of vocal motor volleys in frogs (in this case from a laryngeal branch of the vagus nerve) essentially show a 1:1 correspondence between each complex potential, muscle contraction and sound pulse (Yamaguchi and Kelley, 2000). In vitro studies of isolated brain preparations from the terrestrial frog Lithobates pipiens [formerly Rana pipiens (see Frost, 2007)] identify two `semi-independent' call pattern generators, one at isthmal levels and one (`the classical respiration generator') at caudal hindbrain–spinal levels (Schmidt, 1992). Recent in vitro studies in Xenopus laevis, a fully aquatic frog with a vocal circuit like that of terrestrial species (see Zornik and Kelley, 2007; Zornik and Kelley, 2008), show that bath application of serotonin can evoke fictive responses that mimic the temporal properties of natural vocalizations (Rhodes et al., 2007). In vitro brain stimulation studies of frogs have been less conclusive. As Zornik and Kelley point out, the temporal properties of the electrically evoked responses are typically not independent of the stimulus frequency (Zornik and Kelley, 2008), in contrast to studies like the current one of vocal fish (Fig. 4A; Fig. 6A; also see Introduction). Rather, in studies of Xenopus, each electrical stimulus pulse evokes a single complex potential in the nerve; responses that mimic a natural call have only occasionally been obtained (see Rhodes et al., 2007). The nuances of evoking fictive calls with electrical microstimulation in frogs and in terrestrial vertebrates in general are likely dependent, in part, on a more complex call circuitry that involves the integration of respiratory rhythms (Bass and Baker, 1997; Zornik and Kelley, 2008).
Steroid- and melatonin-dependent rhythmicity
What allows the observed neurophysiological changes in fictive calling to occur in a night-time but not a noon-time brain, let alone in a reproductive versus a non-reproductive animal? No doubt gonadal hormones play an enormous role in the seasonal cycles of vocal activity, or any number of other rhythmic behaviors. Indeed, increases in the degree of temporal encoding of the higher harmonics of male hums by the peripheral auditory system of female midshipman fish during the reproductive season can be induced in non-reproductive females with either testosterone or estradiol treatments over a period of about 3–4 weeks (Sisneros et al., 2004b). The seasonal rhythmicity in vocal neurophysiology reported here is also reminiscent of the steroid-dependent, morphometric changes in vocal nuclei in songbirds (e.g. Arnold et al., 1976; Ball et al., 2004; Brenowitz, 2004) and midshipman fish (Forlano and Bass, 2005a; Forlano and Bass, 2005b; Bass and Forlano, 2008). As in songbirds, plasma levels of steroid hormones cycle with reproductive state in midshipman, while androgen and estrogen receptors are found in the midshipman's vocal control system in conjunction with the expression of brain aromatase, which converts testosterone to estradiol (reviewed by Bass and Remage-Healey, 2008; Forlano et al., 2006).
While intramuscular injections of androgens in midshipman fish increase the probability of evoking longer duration grunts, they do not evoke fictive growls and hums with the temporal attributes described here (Remage-Healey and Bass, 2004). Thus, other aspects of reproductive state and time of day are apparently key factors in the natural production of long duration calls during the breeding season. In songbirds as well, there is evidence for testis-independent effects on song production (without accounting for centrally synthesized neurosteroids), since both sham-operated and castrated sparrows under long day conditions have enlarged song control nuclei, and exogenous melatonin decreases the size of telencephalic vocal nuclei (Bernard et al., 1997; Bentley et al., 1999). Finally, the basal rate of the electric organ discharge (EOD) of weakly electric fish increases at night independent of water temperature or breeding status, although EOD rate in breeding males coupled with females is still the greatest (Silva et al., 2007; Stoddard et al., 2007). Thus, steroid hormones, with their effect on the morphology as well as synaptic and intrinsic firing properties of neurons, may be necessary, but not sufficient, for the maximum upregulation of seasonally dependent vocal behaviors.
Diurnal changes in neuronal activity have been documented in brain regions less typically linked to the motor components of reproductive behaviors, such as the hippocampus (Barnes et al., 1977; Chaudhury et al., 2005). Excitatory postsynaptic potentials (EPSPs) in response to perforant pathway stimulation, recorded in vivo in rats and monkeys at different times of day, were as much as 30% larger in the dark phase than the light phase of nocturnal rats, while the opposite effect was observed in diurnal monkeys. Barnes and colleagues (Barnes et al., 1977) hypothesized a circadian cycle of synaptic transmission in the hippocampus that covaries with natural behavioral fluctuations, while Chaudhury and colleagues (Chaudhury et al., 2005) concluded that an endogenous circadian oscillator modulates long term potentiation in the mouse hippocampus.
Sometimes such rhythmic changes in behavior and neural systems can be directly controlled by melatonin binding to regionally abundant receptors (Whitfield-Rucker and Cassone, 2000; Gahr and Kosar, 1996; Musshoff et al., 2002; Rosenstein and Cardinali, 1990; Wan et al., 1999). For example, melatonin applied to brain slices of the avian vocal circuit decreases firing rate in a telencephalic vocal nucleus where the inhibitory G protein-coupled melatonin 1 b receptor is expressed (Jansen et al., 2005). In teleost fish, melatonin is rhythmically secreted from the retina and pineal gland in intact and isolated preparations under various light conditions (Bolliet et al., 1996; Cahill, 1996; Migaud et al., 2007). Our exposure of the midshipman to 24h D or 24h L for 5 days produced neurophysiological results that correlate with the light-manipulated in vivo melatonin rhythm found in several temperate teleost species [Migaud et al., unpublished observations reported in Martinez-Chavez et al. (Martinez-Chavez et al., 2008)] and in one subtropical species, the common dentex (Pavlidis et al., 1999). Common dentex (Dentex dentex) acclimatized to 12 h L:12 h D and thereafter exposed to 24 h D did not exhibit an endogenous melatonin rhythm (low in the day, high at night); rather, levels were maintained as high as during the natural night-time. If melatonin naturally enhances vocal circuit function in the common dentex at night and 24 h D stimulates tonically high levels as found in common dentex, then it may explain our ability to as easily elicit fictive growls and hums from the 24 h D treated fish tested during the circadian day as during the circadian night. Likewise, 24 h L can inhibit melatonin production (and rhythmicity) altogether (Martinez-Chavez et al., 2008), thus explaining the almost complete loss of long duration fictive calling in our 24 h L fish during both natural day and night. Future studies in midshipman need to assess shifting melatonin levels through natural and manipulated photo regimes to more directly investigate the above scenarios. Given the extensive GABAergic innervation of the vocal motor nucleus (Marchaterre et al., 1989), and the evidence for melatonin modulation of GABAergic activity in mammalian cortex (Musshoff et al., 2002; Wan et al., 1999), an interaction between this hormone and levels of inhibition in the vocal motor circuit may contribute to the transition from short grunts to long duration, lower frequency hums.
Future studies in midshipman need to assess shifting melatonin levels through natural and manipulated photo regimes to more directly investigate the above scenarios. This will include further evaluation of fluctuating fictive call threshold during natural day and night of both photo regimes. At this point, with a limited number of animals tested, there was only a trend for a persistent call threshold rhythm: lower in the natural night compared with day in constant darkness (24 h D), but not apparent in constant light (24 h L).
Concluding comments
The mechanisms underlying the observed neurophysiological changes in the production of long duration fictive growls and hums from parental male midshipman fish likely include a periodic modulation of both excitatory and inhibitory activity in one or more vocal nuclei, as well as modulation of ion channels [e.g. for the SCN (see Pennartz et al., 2002; Teshima et al., 2003; Meredith et al., 2006)]. Such natural fluctuations could be the downstream effects of steroidal and/or non-steroidal (e.g. melatonin) hormone activation of either local membrane or nuclear receptors, or even the product of local oscillating clock gene transcription. Midshipman fish now offer the opportunity to integrate the physiological mechanisms underlying stereotyped, oscillatory-like vocalizations with the prevailing rhythms that shape them. Lastly, given the shared origins of vocal pattern generators in fish and tetrapods (Bass et al., 2008), the functional principles revealed by these and other studies will prove informative to the vocal systems of vertebrates in general.
LIST OF ABBREVIATIONS
- AM
amplitude modulation
- D
dark
- EOD
electric organ discharge
- EPSP
excitatory postsynaptic potential
- GABA
gamma Aminobutyric acid
- GSIs
gonadosomatic indices
- IPI
interpulse interval
- L
light
- PAG
periaqueductal gray
- POA–AH
preoptic area–anterior hypothalamus
- PRR
pulse repetition rate
- SCN
suprachiasmatic nucleus
- VMN
vocal motor nucleus
- VPN
vocal pacemaker nucleus
- VPP
vocal pre-pacemaker nucleus (formerly the ventral medullary nucleus)
We thank Dr Bruce Land (Cornell University) for designing the MATLAB data acquisition software, Margaret Marchaterre for recording and providing the natural sounds, Francoise Vermeylen of the Cornell Statistical Consulting Unit for advice on statistics, and Drs Bruce Johnson and Boris Chagnaud for helpful comments on the manuscript. Support from a Cornell University Fellowship and NIH predoctoral training grants 5-T32-MH15793 and GM007469 (T.K.R.), and NSF IOB 0516748 (A.H.B.). Deposited in PMC for release after 12 months.
References
- Arnold, A. P., Nottebohm, F. and Pfaff, D. W. (1976). Hormone concentrating cells in vocal control and other areas of the brain of the zebra finch (Poephila guttata). J. Comp. Neurol. 165, 487-511. [DOI] [PubMed] [Google Scholar]
- Ball, G. F., Auger, C. J., Bernard, D. J., Charlier, T. D., Sartor, J. J., Riters, L. V. and Balthazart, J. (2004). Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann. NY Acad. Sci. 1016, 586-610. [DOI] [PubMed] [Google Scholar]
- Barber, S. B. and Mowbray, W. H. (1956). Mechanism of sound production in the sculpin. Science 124, 219. [DOI] [PubMed] [Google Scholar]
- Barnes, C. A., McNaughton, B. L., Goddard, G. V., Douglas, R. M. and Adamec, R. (1977) Circadian rhythm of synaptic excitability in rat and monkey central nervous system. Science 197, 91-92. [DOI] [PubMed] [Google Scholar]
- Bass, A. H. (1996). Shaping brain sexuality. Am. Sci. 84, 352-363. [Google Scholar]
- Bass, A. H. and Baker, R. (1990). Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified cells. J. Neurobiol. 21, 1155-1168. [DOI] [PubMed] [Google Scholar]
- Bass, A. H. and Baker, R. (1991). Adaptive modification of homologous vocal control traits in teleost fishes. Brain Behav. Evol. 38, 240-254. [DOI] [PubMed] [Google Scholar]
- Bass, A. H. and Baker, R. (1997). Phenotypic specification of hindbrain rhombomeres and the origins of rhythmic circuits in vertebrates. Brain Behav. Evol. 50, 3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, A. H. and Clark, C. (2003). The physical acoustics of underwater sound communication. In Acoustic Communication: Springer Handbook of Auditory Research (ed. A. M. Simmons, R. Fay and A. Popper), pp. 15-64, New York: Springer.
- Bass, A. H. and Forlano, P. M. (2008). Neuroendocrine mechanisms of alternative reproductive tactics: the chemical language of social plasticity. In Alternative Reproductive Tactics: An Integrative Approach (ed. R. F. Oliveira, M. Taborsky and J. Brockmann), pp. 109-131, Cambridge: Cambridge University Press.
- Bass, A. H. and McKibben, J. R. (2003). Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog. Neurobiol. 69, 1-26. [DOI] [PubMed] [Google Scholar]
- Bass, A. H. and Remage-Healey, L. H. (2008). Central pattern generators for social vocalization: androgen-dependent neurophysiological mechanisms. Horm. Behav. 53, 659-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, A. H., Marchaterre, M. A. and Baker, R. (1994). Vocal-acoustic pathways in a teleost fish. J. Neurosci. 14, 4025-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, A. H., Bodnar, D. and Marchaterre, M. A. (1999). Complementary explanations for exiting phenoypes in an acoustic communication system. In The Design of Animal Communication (ed. M. D. Hauser and M. Konishi), pp. 493-514. Cambridge, MA: The MIT Press.
- Bass, A. H., Gilland, E. H. and Baker, R. (2008). Evolutionary origins of social vocalization in a vertebrate hindbrain-spinal compartment. Science 321, 417-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley, G. E., Van't Hof, T. J. and Ball, G. F. (1999). Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc. Natl. Acad. Sci. USA 96, 4674-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, D. J., Wilson, F. E. and Ball, G. F. (1997). Testis-dependent and independent effects of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea). Brain Res. 760, 163-169. [DOI] [PubMed] [Google Scholar]
- Bolliet, V., Lapointe, M. A. and Falcon, F. J. (1996). Rhythmic melatonin secretion in different teleost species: an in vitro study. J. Comp. Physiol. 165, 677-683. [DOI] [PubMed] [Google Scholar]
- Brantley, R. K. and Bass, A. H. (1994). Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus Girard (Teleostei, Batrachoididae). Ethology 96, 213-232. [Google Scholar]
- Brenowitz, E. A. (2004). Plasticity of the adult avian song control system. Ann. NY Acad. Sci. 1016, 560-585. [DOI] [PubMed] [Google Scholar]
- Buchanan, J. T. (1996). Lamprey spinal interneurons and their roles in swimming activity. Brain Behav. Evol. 48, 287-296. [DOI] [PubMed] [Google Scholar]
- Cahill, G. M. (1996). Circadian regulation of melatonin production in cultured zebrafish pineal and retina. Brain Res. 708, 177-181. [DOI] [PubMed] [Google Scholar]
- Chaudhury, D., Wang, L. M. and Colwell, C. S. (2005). Circadian regulation of hippocampal long-term potentiation. J. Biol. Rhythms 20, 225-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, M. J. and Winn, H. E. (1967). Electrophysiological observations on hearing and sound production in the fish, Porichthys notatus. J. Exp. Zool. 165, 355-370. [DOI] [PubMed] [Google Scholar]
- Christie, M. J. and Jelinek, H. F. (1993). Dye-coupling among neurons of the rat locus coeruleus during postnatal development. J. Neurosci. 56, 129-137. [DOI] [PubMed] [Google Scholar]
- Christie, M. J., Williams, J. T. and North, R. A. (1989). Electrical coupling synchronizes subthreshold activity in locus coeruleus neurons in vitro from neonatal rats. J. Neurosci. 9, 3584-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave, A. S., Yu, A. C. and Margoliash, D. (1998). Behavioral state modulation of auditory activity in a vocal motor system. Science 282, 2250-2253. [DOI] [PubMed] [Google Scholar]
- Fenzl, T. and Schuller, G. (2005). Echolocation call and communication calls are controlled differentially in the brainstem of the bat Phyllostomus discolor. BMC Biol. 3, 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano, P. M. and Bass, A. H. (2005a). Steroid regulation of brain aromatase expression in glia: female preoptic and vocal motor nuclei. J. Neurobiol. 65, 50-58. [DOI] [PubMed] [Google Scholar]
- Forlano, P. M. and Bass, A. H. (2005b). Seasonal plasticity of brain aromatase mRNA expression in glia: divergence across sex and vocal phenotypes. J. Neurobiol. 65, 37-49. [DOI] [PubMed] [Google Scholar]
- Forlano, P. M., Schlinger, B. S. and Bass, A. H. (2006). Brain aromatase: new lessons from non-mammalian vertebrates. Front. Neurendocrinol. 27, 247-274. [DOI] [PubMed] [Google Scholar]
- Frost, D. R. (2007). Amphibian species of the world: http://research.amnh.org/herpetology/amphibia/index.php. New York: American Museums of Natural History.
- Gahr, M. and Kosar, E. (1996). Identification, distribution, and developmental changes of a melatonin binding site in the song control system of the zebra finch. J. Comp. Neurol. 367, 308-318. [DOI] [PubMed] [Google Scholar]
- Goodson, J. L. and Bass, A. H. (2000a). Forebrain peptide modulation of sexually polymorphic vocal motor circuitry. Nature 403, 769-772. [DOI] [PubMed] [Google Scholar]
- Goodson, J. L. and Bass, A. H. (2000b). Vasotocin innervation and modulation of vocal-acoustic circuitry in the teleost Porichthys notatus. J. Comp. Neurol. 422, 363-379. [DOI] [PubMed] [Google Scholar]
- Goodson, J. L. and Bass, A. H. (2002). Vocal-acoustic circuitry and descending vocal pathways in teleost fish: convergence with terrestrial vertebrates reveals conserved traits. J. Comp. Neurol. 448, 298-322. [DOI] [PubMed] [Google Scholar]
- Graziano, M. S. A., Aflalo, T. and Cooke, D. F. (2005). Arm movements evoked by electrical stimulation in the motor cortex of monkeys. J. Neurophysiol. 94, 4209-4223. [DOI] [PubMed] [Google Scholar]
- Herzog, E. D. (2007). Neurons and networks in daily rhythms. Nat. Rev. Neurosci. 8, 790-802. [DOI] [PubMed] [Google Scholar]
- Ibara, R. M., Penny, L. T., Ebeling, A. W., van Dykhuizen, G. and Cailliet, G. (1983). The mating call of the plainfin midshipman fish, Porichthys notatus. In Predators and Prey in Fishes (ed. D. L. G. Noakes, D. G. Lindquist, G. S. Helfman and J. A. Ward), pp. 205-212. The Hague, Netherlands: Dr W. Junk.
- Jansen, R., Metzdorf, R., van der Roest, M., Fusani, L., ter Maat, A. and Gahr, M. (2005). Melatonin affects the temporal organization of the song of the zebra finch. FASEB J. 19, 848-850. [DOI] [PubMed] [Google Scholar]
- Jürgens, U. and Hage, S. R. (2007). On the role of the reticular formation in vocal pattern generation. Behav. Brain Res. 182, 308-314. [DOI] [PubMed] [Google Scholar]
- Kelley, D. B. and Brenowitz, E. (2002). Hormonal influences on courtship behaviors. In Behavioral Endocrinology (ed. J. Becker, S. M. Breedlove, D. Crews and M. McCarthy), pp. 289-329. Cambridge, MA: MIT Press.
- Kittelberger, J., Land, B. R. and Bass, A. H. (2006). Midbrain periaqueductal gray and vocal patterning in a teleost fish. J. Neurophysiol. 96, 71-85. [DOI] [PubMed] [Google Scholar]
- Marchaterre, M., Baker, H., Baker, R. and Bass, A. H. (1989). Comparative neurochemical studies of the sonic motor system in the teleost fishes. Abstr. Soc. Neurosci. 15, 1137. [Google Scholar]
- Martinez-Chavez, C. C., Al-Khamees, S., Campos-Mendoza, A., Penman, D. J. and Migaud, H. (2008). Clock-controlled endogenous melatonin rhythms in Nile tilapia (Oreochromis niloticus niloticus) and African catfish (Clarias gariepinus). Chronobiol. Int. 25, 31-49. [DOI] [PubMed] [Google Scholar]
- McKibben, J. R. and Bass, A. H. (1998). Behavioral assessment of acoustic parameters relevant to signal recognition and preference in vocal fish. J. Acoust. Soc. Am. 104, 3520-3533. [DOI] [PubMed] [Google Scholar]
- Meitzen, J., Moore, I. T., Lent, K., Brenowitz, E. A. and Perkel, D. J. (2007). Steroid hormones act trans-synaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J. Neurosci. 27, 12045-12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith, A. L., Wiler, S. W., Miller, B. H., Takahashi, J. S., Fodor, A. A., Ruby, N. F. and Aldrich, R. W. (2006). BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat. Neurosci. 9, 1041-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud, H., Davie, A., Martinez-Chavez, C. C. and Al-Khamees, S. (2007). Evidence for different photic regulation of pineal melatonin sysnthesis in teleosts. J. Pineal Res. 43, 327-335. [DOI] [PubMed] [Google Scholar]
- Musshoff, U., Riewenherm, D., Berger, E., Fauteck, J. and Speckman, E. (2002). Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus 12, 165-173. [DOI] [PubMed] [Google Scholar]
- Packard, A. (1960). Electrophysiological observations on a sound-producing fish. Nature 187, 63-64. [DOI] [PubMed] [Google Scholar]
- Panda, S. (2008). Circadian rhythms from flies to human. Nature 417, 329-335. [DOI] [PubMed] [Google Scholar]
- Park, K. H. J., Meitzen, J., Moore, I. T., Brenowitz, E. A. and Perkel, D. J. (2005). Seasonal-like plasticity of spontaneous firing rate in a songbird pre-motor nucleus. J. Neurobiol. 64, 181-191. [DOI] [PubMed] [Google Scholar]
- Parker, D. and Grillner, S. (1999). Activity-dependent metaplasticity of inhibitory and excitatory synaptic transmission in the lamprey spinal cord locomotor network. J. Neurosci. 19, 1647-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis, M., Greenwood, L., Paalavuo, M., Mölsä, H. and Laitinen, J. T. (1999). The effect of photoperiod on diel rhythms in serum melatonin, cortisol, glucose, and electrolytes in the common dentex, Dentex dentex. Gen. Comp. Endocrinol. 113, 240-250. [DOI] [PubMed] [Google Scholar]
- Pennartz, C. M. A., de Jeu, M. T. G., Bos, N. P. A., Schaap, J. and Geurtsen, A. M. S. (2002). Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature 416, 286-290. [DOI] [PubMed] [Google Scholar]
- Remage-Healey, L. and Bass, A. H. (2004). Rapid, hierarchical modulation of vocal patterning by steroid hormones. J. Neurosci. 24, 5892-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey, L. and Bass, A. H. (2006). From social behavior to neurons: steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Horm. Behav. 50, 432-441. [DOI] [PubMed] [Google Scholar]
- Remage-Healey, L. and Bass, A. H. (2007). Plasticity in brain sexuality is revealed by the rapid actions of steroids. J. Neurosci. 27, 1114-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes, H. J., Yu, H. J. and Yamaguchi, A. (2007). Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator. J. Neurosci. 27, 1485-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosentstein, R. E. and Cardinali, D. P. (1990). Central GABAergic mechanisms as targets for melatonin activity. Neurochem. Int. 17, 373-379. [DOI] [PubMed] [Google Scholar]
- Schmidt, M. F. and Konishi, M. (1998). Gating of auditory responses in the vocal control system of awake songbirds. Nat. Neurosci. 1, 513-518. [DOI] [PubMed] [Google Scholar]
- Schmidt, R. S. (1992). Neural correlates of frog calling: production by two semi-independent generators. Behav. Brain Res. 50, 17-30. [DOI] [PubMed] [Google Scholar]
- Shank, S. S. and Margoliash, D. (2008). Sleep and sensorimotor intergration during early vocal learning in a songbird. Nature 458, 73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, A., Perrone, R. and Macadar, O. (2007). Environmental, seasonal and social modulations of basal activity in a weakly electric fish. Physiol. Behav. 90, 525-536. [DOI] [PubMed] [Google Scholar]
- Sisneros, J. A., Forlano, P. M., Knapp, R. and Bass, A. H. (2004a). Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen. Comp. Endocrinol. 136, 101-116. [DOI] [PubMed] [Google Scholar]
- Sisneros, J. A., Forlano, P. M., Deitcher, D. L. and Bass, A. H. (2004b). Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science 305, 404-407. [DOI] [PubMed] [Google Scholar]
- Sisneros, J. A., Alderks, P. W., Leon, K. and Sniffen, B. (2009). Morphometric changes associated with the reproductive cycle and behaviour of the intertidal-nesting, male plainfin midshipman Porichthys notatus. J. Fish Biol. 74, 18-36. [DOI] [PubMed] [Google Scholar]
- Skoglund, C. R. (1961). Functional analysis of swim-bladder muscles engaged in sound production of the toadfish. J. Biophys. Biochem. Cytol. 10, 187-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard, P. K., Markham, M. R., Salazar, V. L. and Allee, S. (2007). Circadian rhythms in electric waveform structure and rate in the electric fish Brachyhypopomus pinnicaudatus. Physiol. Behav. 90, 11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima, K., Kim, S. H. and Allen, C. N. (2003). Characterization of an apamin-sensitive potassium current in suprachiasmatic nucleus neurons. J. Neurosci. 120, 65-73. [DOI] [PubMed] [Google Scholar]
- Wan, Q., Man, H., Liu, F., Braunton, J., Niznik, H. B., Pang, S. F., Brown, G. M. and Wang, Y. T. (1999). Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat. Neurosci. 2, 401-403. [DOI] [PubMed] [Google Scholar]
- Weeg, M. S., Land, B. R. and Bass, A. H. (2005). Vocal pathways modulate efferent neurons to the inner ear and lateral line. J. Neurosci. 25, 5967-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Rucker, M. and Cassone, V. M. (2000). Photoperiodic regulation of the male house sparrow song control system: gonadal dependent and independent mechanisms. Gen. Comp. Endocrinol. 118, 173-183. [DOI] [PubMed] [Google Scholar]
- Wild, M. (2004). Functional anatomy of the sensorimotor control of singing. Ann. NY Acad. Sci. 1016, 438-462. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, A. and Kelley, D. B. (2000). Generating sexually differentiated vocal patterns: laryngeal nerve and EMG recordings from vocalizing male and female African clawed frogs (Xenopus laevis). J. Neurosci. 20, 1559-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornik, E. and Kelley, D. B. (2007). Breathing and calling: neuronal networks in the Xenopus laevis hindbrain. J. Comp. Neurol. 501, 303-315. [DOI] [PubMed] [Google Scholar]
- Zornik, E. and Kelley, D. B. (2008). Regulation of respiratory and vocal motor pools in the isolated brain of Xenopus laevis. J. Neurosci. 28, 612-21. [DOI] [PMC free article] [PubMed] [Google Scholar]