Abstract
Dendritic cells are best known as antigen-presenting cells that initiate adaptive immune responses. Three new papers suggest that basophils initiate allergen- and helminth-driven CD4+ T helper type 2 responses by functioning as antigen-presenting cells in draining lymph nodes.
Although the cellular and molecular mechanisms that regulate the development of T helper type 1 cell (TH1 cell), interleukin 17 (IL-17)-producing T helper cell (TH-17 cell) and regulatory T cell responses are fairly well understood, the specific cellular mediators and factors that control the initiation of TH2 responses are still highly debated. Although it is certain that dendritic cells (DCs), pattern-recognition receptors and cytokines secreted by DCs are key in the initiation and expansion of most effector and regulatory T cell classes, the relative importance of activated DCs and Toll-like receptor signaling in the development of TH2 effector responses is less clear. In this issue of Nature Immunology, three papers demonstrate that DCs are not required for the generation of CD4+ TH2 responses to protease allergens1, helminthic parasites2 or antigen-immunoglobulin E (IgE) complexes in vivo3. Instead, all three groups identify the major histocompatibility complex (MHC) class II-positive IL-4-producing basophil as the ‘professional’ antigen-presenting cell (APC) that is both necessary and sufficient for the generation of type 2 immunity.
Studies have suggested that DCs adopt a fairly limited activation profile when exposed to TH2-inducing allergens and helminths4. They also fail to produce IL-4, the key driver of CD4+ TH2 cell responses. Therefore, attention has focused on identifying the accessory cells that provide the early innate source of IL-4 and soluble mediators that ‘instruct’ DC-mediated TH2 differentiation. Proposed sources of IL-4 have included eosinophils, mast cells, basophils and natural killer T cells, as well as autocrine IL-4 from CD4+ T cells5-8. Additional cytokine cofactors have also been identified, including IL-21, IL-25, IL-33 and thymic stromal lymphopoietin, which invariably augment development of TH2 responses by modulating the activation status of DCs and other APCs9-12. The long-standing view of TH2 differentiation has revolved around this basic theory, which suggests CD4+ TH2 cell development is driven by DCs that present antigen in the context of MHC class II and by extrinsic cellular and secreted factors that modify DC maturation and provide an early source of IL-4 (refs. 13,14).
Sokol and colleagues investigate the mechanisms that regulate the development of TH2 responses after exposure to papain, a cysteine protease hydrolase enzyme from papaya that breaks down complex proteins and thus mimics the activity of proteases secreted by many TH2-promoting helminth parasites. Although basophils are found mainly in the blood and peripheral tissues, they are rapidly recruited to the lymph nodes during a primary response to papain and in response to the soluble antigens of Schistosoma mansoni eggs15. Once in the lymph nodes, the basophils secrete IL-4 and thymic stromal lymphopoietin, and depletion studies suggest that basophils are critically involved in the generation of antigen-specific TH2 responses. The conclusion reached was that basophils function as accessory cells for DC-mediated TH2 differentiation because DCs are also rapidly recruited to the lymph nodes. Nevertheless, the specific identify of the APC population was unclear in those studies15. In the manuscript presented here, Sokol and colleagues show that, unexpectedly, DCs are in fact not required for the development of papain-induced TH2 responses1. Although papain-primed DCs initiate the development of TH2 responses in vivo, they are not able to induce CD4+ TH2 cells in vitro unless basophils are included in the culture. Surprisingly, these authors discover that DCs are not even necessary, as basophils alone support the robust proliferation of naive T cells. Thus, unlike the in vitro generation of TH1 and TH-17 responses, for which DCs, antigen and Toll-like receptor signals are sufficient, TH2 responses exploit a distinct basophil-dependent but DC-independent mechanism. These findings are unexpected, as basophils have been thought to be MHC class II negative; however, these authors show very convincingly that some activated basophils express MHC class II. Basophils also have the molecular ‘machinery’ required to function as APCs, as shown by in vitro MHC class II-blocking studies. The TH2 cells generated are also papain specific. These findings collectively provide evidence that basophils can function as professional APCs, at least for the generation of TH2 responses in vitro.
During the in vivo generation of TH1 or TH-17 responses, DCs encounter pathogens in peripheral tissues, where they sample foreign antigens, become activated and then migrate to the lymph nodes, where they present antigen in the context of MHC class II to naive T cells (Fig. 1). Sokol et al. seek to determine whether basophils use a similar mechanism to initiate TH2 responses or require DCs to escort papain into the draining lymph node, where antigen encounter occurs1. To answer these questions, they design a clever set of experiments in which they inject papain into the ear pinna of mice and then excise the ear at either 2 h or 24 h after injection. If DCs are needed to capture and deliver antigen to lymph nodes, rapid excision of the injection site would ablate the development of the TH2 response in the draining lymph node. Interestingly, they find no difference in TH2 development at 2 h and 24 h, which suggests that migratory DCs are not involved and that soluble proteins such as papain are being delivered directly to the lymph node. They also show that basophils can endocytose, process and present soluble antigens, but unlike DCs, they are not good at processing particulate antigens. They hypothesize that because most TH2-inducing antigens from helminth parasites are excretory or secretory proteins, this mechanism would be ideally suited for the generation of TH2 responses to large extracellular eukaryotic pathogens. In support of that hypothesis, Perrigoue et al. show that MHC class II-positive DCs are not required for the generation of protective CD4+ TH2 cell-dependent immunity to the gastrointestinal nematode parasite Trichuris muris2. Similar to the studies by Sokol and colleagues1, the results of Perrigoue et al. show that IL-4-producing basophils are MHC class II positive and can promote the MHC class II-dependent differentiation of antigen-specific CD4+ TH2 cells in vitro2. More importantly, depletion of basophils in vivo with a monoclonal antibody (Mar-1) specific for the receptor FcεRI considerably impairs immunity to T. muris, which suggests that basophils facilitate the development of protective TH2 immunity, thus extending the findings of Sokol et al. regarding papain to a complex TH2-promoting pathogen.
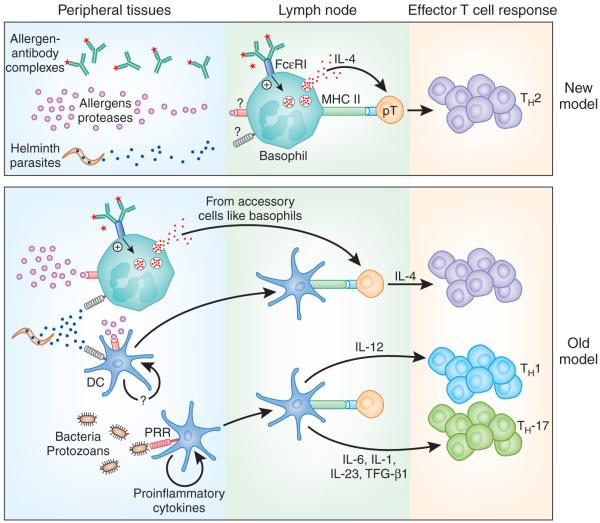
Figure 1.
A new paradigm for the initiation of type 2 immunity. In the present model (bottom), DCs serve as the main professional APCs for the development of antigen-specific CD4+ T cell responses. During the development of TH1 and TH-17 responses, DCs are activated in the periphery by various pattern-recognition receptors (PRR) and migrate to the draining lymph nodes, where they present antigen to naive T cells in the context of MHC class II (MHC II). This ‘DC1’ population secretes specific cytokines, such as IL-12, that ‘instruct’ CD4+ TH1 responses or cytokines such as IL-1, IL-6 and IL-23, which participate in the differentiation of TH-17 cells. In contrast to TH1- and TH-17-promoting antigens, TH2-inducing allergens, antigen-IgE immune complexes and helminth-derived secreted proteins activate an alternative APC-designated ‘DC2’ that requires an exogenous source of IL-4 to direct TH2 development. In the revised model (top), DCs are not required for the development of antigen-specific TH2 cell responses, because basophils can function as professional APCs. In contrast to the ‘DC2’ population, basophils also produce IL-4 when stimulated by TH2-inducing antigens in the draining lymph nodes. Consequently, accessory cells are no longer required for the initiation of TH2 responses in this model. TGF, transforming growth factor.
Basophils isolated from the spleens of mice infected with the intestinal nematode Strongyloides venezuelensis are also MHC class II positive, as shown by Yoshimoto et al.3. Splenic basophils from infected mice also secrete IL-4 and, in agreement with the other two studies1,2, these cells are able to induce the development of antigen-specific TH2 cells in vitro in the absence of DCs3. Yoshimoto et al.3 show that IL-4-deficient basophils are not functional, demonstrating that the production of IL-4 by MHC class II-positive basophils is critical for TH2 differentiation. Interestingly, basophils from naive mice have the same TH2-inducing ability, so IgE-primed basophils do not seem to be important. Nevertheless, enhanced TH2 responses result when antigen-IgE complexes are included in the culture, which suggests that antigen-specific IgE augments the development of antigen-specific TH2 responses, perhaps by facilitating antigen uptake. Yoshimoto et al.3 also discover that basophils express the lymph node-homing receptor CD62L, which indicates that basophils have the necessary ‘machinery’ to enter secondary lymphoid tissues where TH2 responses are initiated. Perhaps most importantly, however, they determine that IL-3 can induce HLA-DR expression on a subset of human basophils. Thus, these important findings may not be restricted to the mouse.
Although all three groups show that MHC class II-positive basophils can initiate TH2 differentiation in vitro in the absence of other professional APCs, it is important to confirm this mechanism in vivo. To do this, all three groups use similar and complimentary approaches to rule out the possibility that DCs are involved. Sokol et al.1 and Perrigoue et al.2 both use the CD11c-diphtheria toxin receptor mouse model in which delivery of diphtheria toxin effectively depletes the mice of all CD11c-expressing cells16. Sokol et al. show that although depletion of DCs blocks TH1 differentiation, it has no effect on papain induced TH2 responses1. Similarly, Perrigoue et al. find that these mice do not have diminished development of protective TH2 immunity after T. muris infection2. Both groups also take the opposite approach by restricting MHC class II expression to DCs17. Here again, although MHC class II-positive DCs are adequate for the development of TH1 responses, they are not sufficient for the development of TH2 responses in vivo. Although IL-4 producing basophils are recruited to the lymph nodes in these studies, expression of MHC class II on basophils seems to be critical for the development of the TH2 response. Notably, however, when the mice in which MHC class II expression is restricted to DCs are infected with T. muris and treated with a neutralizing monoclonal antibody to interferon-γ, the production of TH2 cytokines is restored, which suggests that MHC class II-positive DCs can induce protective TH2 responses if the counter-regulatory TH1 response is blocked2. Thus, it seems that basophils are not strictly required for the initiation of TH2 responses. Instead, they promote TH2 differentiation by blocking DC-induced TH1 responses, at least during the development of T. muris-induced TH2 responses. Finally, basophil adoptive-transfer studies presented by both Sokol et al.1 and Yoshimoto et al.3 confirm that MHC class II-positive basophils are sufficient for the initiation of TH2 immunity in vivo.
Although the combined results from all three papers convincingly show that basophils can function as professional APCs and trigger TH2 differentiation both in vitro and in vivo, studies over the past 15-20 years suggest that a variety of mediators, cell types and mechanisms are involved in the development of polarized CD4+ TH2 cell responses. Consequently, it will be necessary to determine whether all antigen-specific TH2 responses are initiated by this basophil-dependent mechanism or whether specific DC subsets or other APC populations trump basophils in some circumstances. In the studies presented here, basophils trump DCs because in addition to functioning as professional APCs, they also produce the key TH2-differentiating cytokine IL-4. Future studies will need to determine whether IL-4-producing, MHC class II-positive basophils are required simply for the initiation of TH2 responses or whether they are also critical in the maintenance of chronic TH2 responses. This information will be particularly useful because it might indicate whether targeting basophils would be beneficial in the treatment of persistent TH2-mediated diseases such as allergy and asthma. It would also be helpful to understand how basophils recognize specific allergens, proteases and parasite products and how these mediators trigger IL-4 production. Intravital imaging of basophil-T cell interactions in the lymph node in real time, as has been done with DCs, may also show how and when basophils are recruited to the draining lymph node during the initiation of an antigen-specific immune response. In conclusion, although the enigmatic basophil has been widely ignored by immunologists, the discovery that they can function as professional APCs will probably create a flurry of interest and lead to new and exciting findings about their function in the regulation of disease.
References
- 1.Sokol CL, et al. Nat. Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrigoue JG, et al. Nat. Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimoto T, et al. Nat. Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald AS, Straw AD, Bauman B, Pearce EJ. J. Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 5.Seder RA, Paul WE, Davis MM, Fazekas de st Groth B. J. Exp. Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moqbel R, et al. J. Immunol. 1995;155:4939–4947. [PubMed] [Google Scholar]
- 7.Ying S, et al. J. Immunol. 1997;158:3539–3544. [PubMed] [Google Scholar]
- 8.Brunner T, Heusser CH, Dahinden CA. J. Exp. Med. 1993;177:605–611. doi: 10.1084/jem.177.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fort MM, et al. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 10.Pesce J, et al. J. Clin. Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YJ, et al. Annu. Rev. Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 12.Rank MA, et al. J. Allergy Clin. Immunol. 2009;116:2044–2055. [Google Scholar]
- 13.Pulendran B, et al. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldonado-Lopez R, et al. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokol CL, Barton GM, Farr AG, Medzhitov R. Nat. Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung S, et al. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemos MP, Fan L, Lo D, Laufer TM. J. Immunol. 2003;171:5077–5084. doi: 10.4049/jimmunol.171.10.5077. [DOI] [PubMed] [Google Scholar]