Abstract
The magnitude of metabolic activation is greatly underestimated in autoradiographic studies using [1- or 6-14C]glucose compared to parallel assays with [14C]deoxyglucose indicating that most of the label corresponding to the additional [14C]glucose consumed during activation compared to rest is quickly released from activated structures. Label could be lost by net release of [14C]lactate from brain or via lactate exchange between blood and brain. These possibilities were distinguished by comparison of glucose and lactate specific activities in arterial blood and brain before, during, and after generalized sensory stimulation and during spreading cortical depression. Over a wide range of brain lactate concentrations, lactate specific activity was close to the theoretical maximum, i.e., half that of [6-14C]glucose, indicating that exchange-mediated dilution of lactate is negligible and that efflux of [14C]lactate probably accounts for most of the label loss. Low lactate dilution also indicates that dilution of glutamate C4 fractional enrichment in [13C]glucose studies, currently ascribed predominantly to lactate exchange, arises from other unidentified pathways or factors. Alternative explanations for glutamate dilution (presented in Supplementary Material) include poorly-labeled amino acid pools and oxidative metabolism of minor substrates in astrocytes to first dilute the astrocytic glutamine pool, followed by dilution of glutamate via glutamate-glutamine cycling.
Keywords: lactate, [14C]glucose, sensory stimulation, spreading depression, astrocyte, glutamine
Rapid uptake and metabolism in brain of labeled glucose and its analogs are cornerstones of brain imaging in vivo and functional metabolic studies, and commonly-used procedures have been designed to assay the initial, irreversible step of glycolysis or the oxidative pathways. The [14C]deoxyglucose (DG) and [18F]fluorodeoxyglucose methods to calculate local rates of glucose utilization (CMRglc) take advantage of the intracellular trapping and metabolic stability of the DG-6-phosphate produced by hexokinase (Sokoloff et al., 1977). In magnetic resonance spectroscopic (MRS) studies, glucose oxidation rates are calculated from measured rates of incorporation of label from [13C]glucose into specific carbon atoms of TCA cycle-derived amino acids and metabolic modeling (Mason and Rothman, 2004; Henry et al., 2006). Unfortunately, the generation and the fate of labeled diffusible metabolites of glucose, particularly lactate, during brain activation are not readily evaluated by either methodology alone.
Evidence for increased formation and rapid release of labeled metabolites of glucose from brain during activation arose from autoradiographic studies using [6-14C]glucose and [14C]DG in parallel. Relative trapping of labeled products of [6-14C]glucose in activated compared to resting tissue was much too low compared to [14C]DG, and the calculated magnitude of increased CMRglc was underestimated by at least 50% with [14C]glucose (Collins et al., 1987; Lear and Ackermann 1988, 1989, 1991; Ackermann and Lear 1989; Adachi et al., 1995; Cruz et al., 1999; Cruz et al., 2007). Increased [14C]lactate production and release was suggested to account for these discordant results. Label loss could arise from net release of labeled lactate to blood and other brain regions and from blood-brain barrier-mediated exchange of labeled brain lactate for unlabeled lactate in blood. These two possibilities can be distinguished by assay of brain lactate specific activity relative to that of brain glucose. Net release would not alter the relative specific activity of brain lactate, whereas lactate exchange and generation of unlabeled lactate via glycogenolysis would dilute the labeled lactate pool and reduce the relative specific activity of brain lactate (Fig. 1). Dilution of lactate specific activity occurs in brain of suckling rats (Cremer and Heath, 1974), but was not detected in resting adult rat brain (Hawkins et al., 1974). However, lactate exchange is thought to underlie dilution of the fractional enrichment of C4 of glutamate during continuous infusion of [1- or 6-13C]glucose in MRS assays (Mason et al., 1992, 1995; Hyder et al., 1996; see Discussion). To evaluate potential contributions of lactate release and lactate exchange, results of our previous studies (see Supplementary Material) were re-analyzed to compare lactate and glucose specific activities in arterial plasma and brain before, during, and after brain activation under normal and pathophysiological conditions.
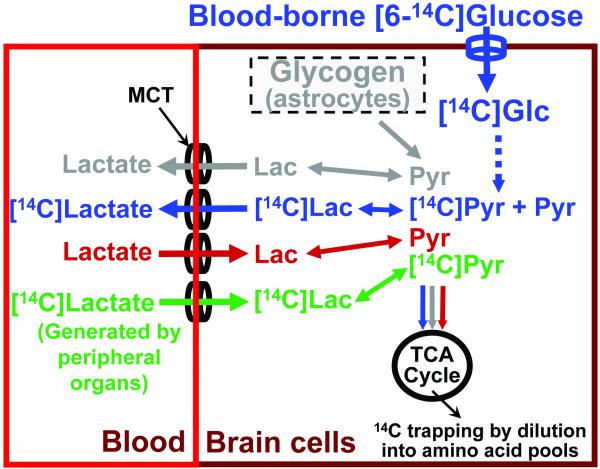
Fig. 1. Exchange and dilution fluxes can influence brain lactate specific activity.
The maximal specific activity (SA) of total brain [14C]pyruvate (Pyr) and [14C]lactate (Lac) derived from blood-borne 1- or 6-14C-labeled glucose (Glc) is half that of [14C]glucose because only one of the two pyruvate molecules produced from each glucose molecule is labeled (blue text and arrows). The relative specific activity (RSA) of pyruvate and lactate compared to that of glucose can be reduced below the theoretical maximum of 0.5 due to (i) degradation of unlabeled astrocytic glycogen to generate unlabeled pyruvate (gray text and arrows) and (ii) uptake into brain of unlabeled lactate or pyruvate from blood (red text and arrows) via monocarboxylic acid transporters (MCT, black). Labeled lactate in blood generated by peripheral organs (green text and arrows) could raise or lower the brain lactate SA, depending on the plasma lactate SA relative to that in brain. Net release of [14C]lactate (blue line) will not alter the brain lactate/brain glucose RSA, whereas influx of low SA lactate (red and green lines in) would reduce brain lactate SA. Exchange (blue line out, red and green lines in) of labeled lactate generated in brain for unlabeled or poorly-labeled lactate in blood would also reduce brain lactate SA while maintaining a constant brain lactate concentration. Any release of [14C]lactate from brain will cause a proportionate underestimation of calculated glucose utilization rates due to incomplete trapping of 14C metabolites; label trapping occurs mainly via dilution into the large tricarboxylic acid (TCA) cycle-derived amino acid (Glu, Gln, Asp) pools.
RESULTS
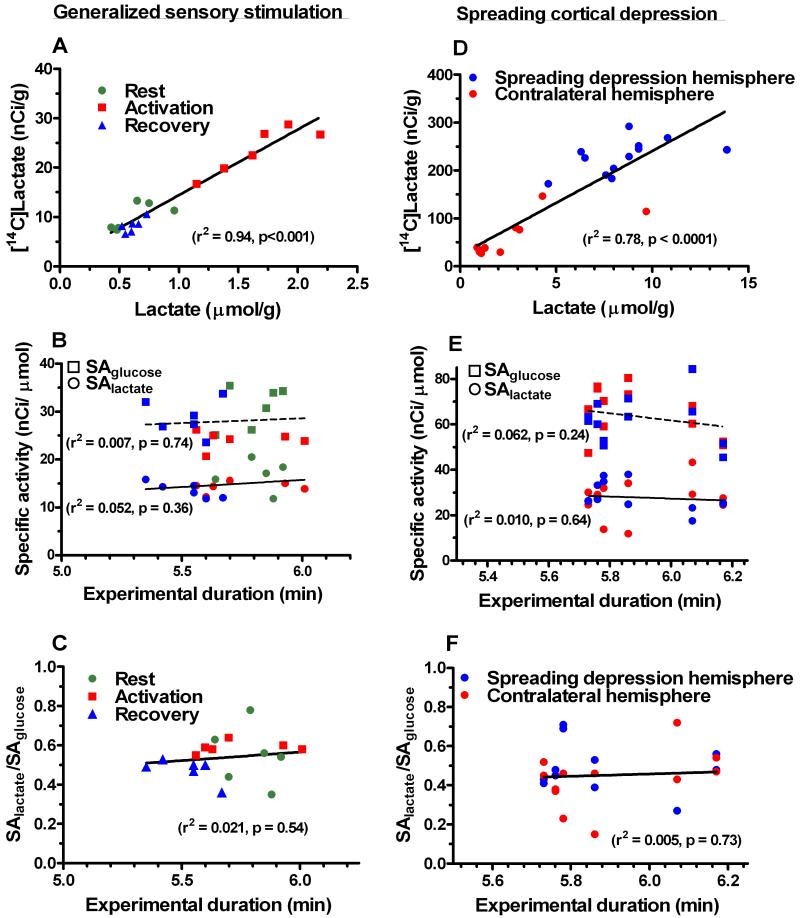
During sensory stimulation (Fig. 2A) and during spreading cortical depression (Fig. 2D), the concentration of 14C-labeled lactate in brain rose in direct proportion to the increase in the level of unlabeled lactate over a wide range of brain lactate level, extending from normal (0.5 μmol/g) to pathophysiological levels (14 μmol/g). The specific activities (SA) of brain glucose and lactate showed no time dependence over the narrow range of duration (5-6 min) of the [6-14C]glucose labeling period (Figs. 2B, E). The ratios of SA brain lactate to SA brain glucose (i.e., relative specific activity, RSA, in each rat) were also stable during rest, somatosensory activation, and recovery from stimulation (Fig. 2C) and during spreading depression (Fig. 2F).
Fig. 2. Levels of 14C-labeled and unlabeled lactate in brain and lactate relative specific activities.
Values are from individual rats sampled during rest, sensory stimulation, or recovery from generalized sensory stimulation (A, B, C; Dienel et al., 2002), or during unilateral K+-induced spreading cortical depression or in the contralateral cerebral cortex (D, E, F; Adachi et al., 1999). Labeled and unlabeled lactate levels (A, D), specific activities (SA) of brain glucose (squares) and lactate (circles) (B, E), and ratios of specific activities of lactate to those for glucose (C, F) were determined in each animal. Color codes in B are the same as in A and C, and those in E correspond to D and F. Linear regression lines were calculated for each data set.
The mean lactate/glucose RSAs in adult rats were close to the theoretical maximum of 0.5 at each of the three activity stages (i.e., rest, activation, and recovery) in normal rats and in the K+-treated and untreated hemispheres in rats with unilateral spreading depression (Fig. 2; Supplementary Table 1). Lactate/glucose RSAs were similar during conditions when both glycolytic flux and lactate levels in cerebral cortex are normal (at rest and recovery from stimulation), elevated (during sensory stimulation), and markedly increased (during spreading cortical depression). The overall mean (± SD) lactate/glucose RSAs under normal (0.54 ± 0.10, n=18) and pathophysiological (0.45 ± 0.14, n=24) conditions do not differ from 0.5 (P>0.5; t test) and they are 3-4-fold higher than that in suckling rats (i.e., 0.13; Supplementary Table 1).
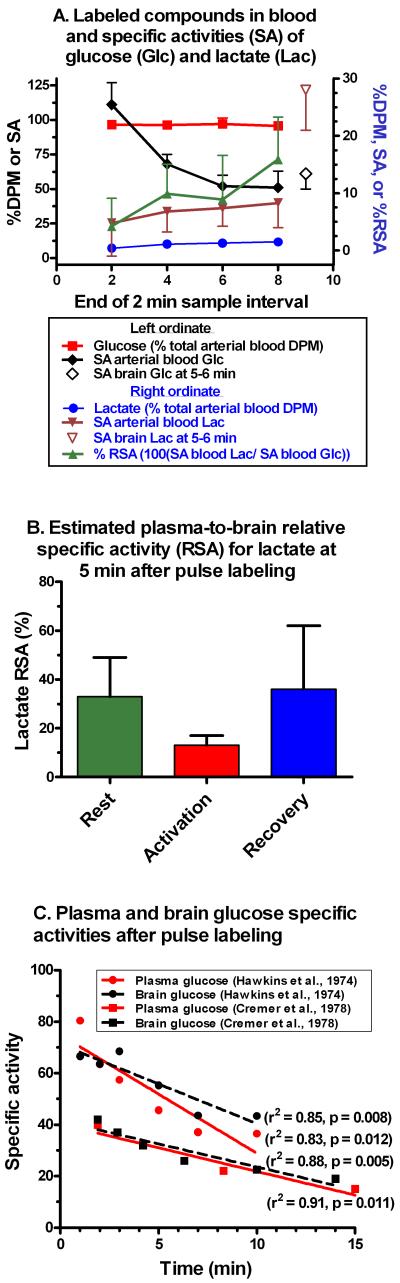
[14C]Lactate is generated by peripheral organs and released to blood after pulse intravenous labeling with [6-14C]glucose but it is present in very low amounts corresponding to 1.1 ± 0.5% (mean ± SD, n=20) of the total dpm in whole arterial blood throughout the 8 min labeling period (circles, Fig. 3A). [14C]Glucose accounted for 96.3 ± 0.6% of the 14C (squares, Fig. 3A), and small amounts of other labeled compounds are also detectable (Cruz et al., 1999). Low labeling of blood and plasma lactate (~1-2% of total 14C at 5-30 min after an intravenous injection of [U-14C]glucose) compared to [14C]glucose (>96% at 5-10 min, >90% at 15-30 min) is also observed in normal fed or fasted rats, alcohol-treated, diabetic, and eviscerated rats (Flock et al.,1969; 1971). Thus, blood [14C]lactate may arise from various tissues (e.g., muscle, not necessarily liver); the percent 14C recovered in glucose and lactate is stable under various conditions.
Fig. 3. Glucose and lactate specific activities in blood and brain.
(A) Percent of total 14C dpm recovered in purified glucose (Glc; squares) and purified lactate (Lac; circles) in each of the four two-minute timed arterial blood samples (0-2, 2-4, 4-6, and 6-8 min) drawn from each of five conscious rats with bilateral K+-induced spreading cortical depression that were given intravenous bolus injections of [6-14C]glucose (Cruz et al., 1999). For each of these arterial blood samples the following are also plotted: glucose SA (nCi/μmol; filled diamonds), lactate SA (nCi/μmol; filled inverted triangles), and the relative specific activity (RSA) of blood lactate SA to that of blood glucose SA (triangles); the SA ratios were calculated from respective values in each rat and the mean RSA at each time point is expressed as percent, i.e., 100(SA lactate/SA glucose). Brain metabolites were not analyzed in this arteriovenous difference study (Cruz et al., 1999), but they were measured in our preceding study of brain glucose utilization during unilateral spreading cortical depression (Adachi et al., 1995) that used an identical pulse labeling procedure. For comparison to blood values, mean brain glucose SA (open diamond) and brain lactate SA (open inverted triangle) determined at 5-6 min (n=12/group; Fig. 2E) are included (for clarity, brain values are offset from the true sampling time). Values are means; vertical bars represent 1 SD (if not visible, the SD is smaller than the symbol). Note that the legend text color code identifies the y axis corresponding to different data sets; black text denotes the left ordinate and blue text identifies the right ordinate data sets. (B) Plasma lactate SA was not determined in our generalized sensory stimulation study in which brain SAs were assayed (Fig. 2B). To compare plasma lactate SA to brain lactate SA in these animals, plasma lactate specific activities were calculated from measured lactate levels in the last plasma sample at kill time, measured total 14C in the same plasma sample and the estimated 14C in blood lactate (i.e., using the mean [14C]lactate concentration of 1.1 ± 0.5% over the 8 min experimental interval; circles, Fig. 3A). Each plasma lactate SA was then expressed as a percentage of the measured brain lactate SA in the same animal (i.e., %RSA; n=6/group). (C) Arterial plasma and brain glucose specific activities were plotted from the studies by Hawkins et al. (1974) who reported normalized SAs and Cremer et al. (1978) (units are 10-3 x dpm/μmol).
The SA of glucose purified from arterial blood (filled diamonds, Fig. 3A) fell with time after the pulse injection but it always greatly exceeded that of lactate, which rose 1.7-fold over the same interval (filled inverted triangles, Fig. 3A). The lactate/glucose RSA in arterial blood, expressed as percent, rose gradually from 4% at 2 min to 16% at 6-8 min (triangles, Fig. 3A), but was well below the lactate/glucose RSA (~50%) in brain (Fig. 2; Supplementary Table 1). At 5-6 min after pulse labeling, blood and brain glucose SA’s were similar (filled and open diamonds, respectively, Fig. 3A), whereas blood lactate SA was only 29% of that in brain (filled and open inverted triangles, respectively, Fig. 3A). In normal conscious rats assayed during rest, generalized sensory stimulation, and recovery from stimulation, estimated plasma lactate SA was <36% that of brain lactate (Fig. 3B) during all three stages of activity. Thus, any lactate uptake into brain from blood would reduce the brain lactate SA.
Plasma glucose specific activities fall after the initial peak following an intravenous pulse injection of [14C]glucose (Fig. 3A), and if brain glucose SA fell faster than that of brain lactate, the calculated lactate/glucose RSA (Fig. 2C, F) may be inflated, minimizing the apparent dilution of lactate SA. Rates of change in brain glucose SA were, therefore, estimated using data from two published studies (Fig. 3C). Plasma and brain glucose SAs were linear functions of time over the range of about 2-15 min. Brain values were similar to or slightly higher than those in plasma because, after the initial peak, [14C]glucose is cleared more rapidly from plasma than from brain. When the slopes of the regression lines were divided by their respective y-axis intercepts (i.e., the zero time value), the fractional decreases with time of plasma glucose SA were -4.5 and -6.1 percent per min in the Cremer et al. and Hawkins et al. studies, respectively, whereas brain glucose SA decreased at a calculated rate of about -4.3 percent per min in both studies. Thus, if the lag between changes in glucose SA and lactate SA were one min, the brain lactate/brain glucose RSA would be overestimated by <5%.
DISCUSSION
Low lactate exchange and its implications
The results show that dilution of brain lactate/glucose RSA during brief pulse-labeling assays due to blood lactate uptake or blood-brain lactate exchange (Fig. 1) is negligible (Fig. 2C, F; Supplementary Table 1). Any glycogen-derived unlabeled lactate that remained in brain would also reduce lactate SA in tissue extracts, suggesting that pyruvate and lactate formed from glycogen were quickly oxidized without mixing with glycolytic pools labeled by [14C]glucose and/or the lactate was rapidly released from activated brain. Insignificant inflation of the lactate/glucose RSA arising from a brief lag between labeling of brain lactate by brain glucose (Fig. 3C) is consistent with the following evidence for rapid turnover of glycolytic intermediates. (i) The brain levels of glycolytic intermediates between glucose-6-phosphate and pyruvate are low, totaling about 0.4 μmol/g (Siesjö, 1978; Veech, 1980; McIlwain and Bachelard, 1985). (ii) The overall resting rate of glucose utilization in rat cerebral cortex is ~1 μmol/g/min (Sokoloff et al., 1977), corresponding to a triose flux of 2 μmol/g/min (two trioses are formed per glucose), which rises during activation. (iii) The turnover time (concentration divided by flux) of a triose present at ~0.1 μmol/g (e.g., pyruvate, 0.09 μmol/g; Veech et al., 1973) is ~3 sec in resting brain. (iv) Equilibration of labeled pyruvate with lactate in brain is rapid. At 10 sec after injection of [2-14C]pyruvate into a carotid artery of pentobarbital-anesthetized adult rats about 40% of the label is recovered in brain lactate (Cremer et al., 1978). Thus, [14C]glucose quickly labels lactate.
Adult rat brain has a much higher maximal glucose transport capacity across the blood-brain barrier compared to lactate (Cremer et al., 1979; Simpson et al., 2007), and influx of labeled [14C]glucose therefore has a higher impact on brain lactate SA than influx of plasma [14C]lactate as long as blood glucose SA greatly exceeds blood lactate SA and blood lactate levels are relatively low. The predominant influence of labeled blood glucose on brain lactate labeling would also be expected in MRS studies when continuous infusions are used to maintain high, constant levels of [13C]glucose in blood. In contrast to adults, suckling mammals have high endothelial monocarboxylic acid transporter (MCT) levels (Cremer et al., 1979; Vannucci and Simpson, 2003), and brain lactate SA dilution ascribed to lactate exchange is readily detected (Cremer and Heath, 1974; Cremer, 1980; Supplementary Table 1). Uptake of [14C]lactate into brain is also greater in suckling rats leading to a higher brain lactate/blood lactate SA ratio (0.36; Cremer and Heath, 1974; Cremer, 1980) compared to adults (0.26 ± 0.07; Konitzer and Voigt, 1977). Together, the above findings indicate that blood-derived glucose has the greatest influence on adult brain lactate SA under various conditions (Figs. 2; 3A, 3B). Predicted dilutional fluxes for lactate based on rates of unidirectional uptake of labeled lactate into brain may be overestimated, since there is likely be some backflow of the label prior to its mixing with the brain pools and its metabolism within brain.
High lactate specific activity and rapid lactate labeling strongly support the conclusion that net lactate release, not lactate exchange, explains most (but not necessarily all) of the label loss during metabolic labeling with [14C]glucose during brain activation (See Introduction). Release as lactate of most of the additional glucose consumed during activation compared to rest also indicates that lactate shuttling from astrocytes to neurons, if any, is a minor aspect of brain energetics during functional activation. If lactate were shuttled and locally oxidized, label would enter the large amino acid pools and be registered in autoradiographic assays. In spite of the capability for lactate oxidation by neurons and astrocytes in adult rat brain (O’Neal and Koeppe, 1966; Konitzer and Voigt, 1977; Zielke et al., 2007), lactate release predominates during activation, presumably to maintain increased glycolytic flux. Because glucose and lactate transport to and from the brain are equilibrative processes, there is no energy cost associated with fuel transport. As long as glucose supply is adequate to sustain brain function, lactate release from brain for utilization by other organs does not represent a ‘waste’ of an energy-rich fuel. Instead, rapid lactate clearance from activated cells is a key aspect of increased fluxes through reversible reactions (lactate dehydrogenase and MCTs) driven by concentration gradients.
Dilution of glutamate C4 and glutamine C4 labeling in [13C]glucose studies
Glutamate C4 is labeled by [1- or 6-13C]glucose during the first turn of the TCA cycle, and the measured steady state fractional enrichment of glutamate C4 was lower than expected based on measured enrichment of [1-13C]glucose (Mason et al., 1992, 1995). This overall dilution was attributed mainly to lactate influx, based on careful consideration of rates of lactate uptake into brain (i.e., 0.05-0.09 μmol/g/min; see Discussion in Supplementary Material) and estimates of fluxes of other potential contributory pathways (Mason et al., 1992, 1995). In anesthetized, 24h-fasted rats, half of the 30% glutamate C4 dilution was ascribed to lactate exchange, the other half to ketone body utilization, to give an overall dilution rate equivalent to ~25% of the calculated TCA cycle rate; because ketone body levels are negligible in fed rats or after short-term fasting, most glutamate C4 dilution has been linked to lactate, with minor calculated contributions (≤1%) by the pentose phosphate shunt pathway and brain proteolysis (Hyder et al., 1996). Subsequently, glutamine C4 fractional enrichment was found to be ~25% less than that of glutamate C4, revealing an additional isotopic dilution in astrocytes (Shen et al., 1999). Glutamine uptake from blood to brain (0.004 μmol/g/min in rats; Smith et al., 1987) is too low to explain its dilution, and de novo glutamine synthesis during labeling studies using [1- or 6-13C]glucose does not dilute C4 (see below).
Glutamate and glutamine C4 dilutions are observed during anesthesia and under resting and activating conditions in 13C MRS studies and must be taken into account when calculating glucose oxidation rates. Calculated dilutional fluxes for lactate (Vdil-lac) and glutamine (Vdil-gln) in brain of anesthetized-ventilated rats are substantial (0.15 and 0.14 μmol/g/min, respectively) compared to the calculated neuronal TCA cycle flux of 0.52 μmol/g/min (de Graaf et al., 2003). In awake rats, Vdil-gln was estimated as 0.16-0.22 μmol/g/min, with a calculated rate of glucose oxidation of about 0.93 μmol/g/min (Öz et al., 2004). Recently, Shen et al. (2008) emphasized the influence of the glutamine C4 dilution on calculated glutamate-glutamine cycle rate and also reviewed in detail the possible sources for the glutamate and glutamine C4 dilutions. The lactate dilution factor represents the overall glutamate C4 dilution from all sources, presumably dominated by lactate (Shen et al., 2008). Because lactate dilution was not detectable after pulse labeling of adult rats (Fig. 2; Supplementary Table 1) and net and unidirectional lactate uptake into adult human brain corresponds to a small fraction (~2.6 and 10%, respectively) of estimated neuronal glucose oxidation (See Supplementary Material), other pathways or factors must contribute substantially to the ~30% reduction of glutamate C4 fractional enrichment.
Alternative explanations for glutamate C4 dilution
Higher-than-recognized fluxes in alternative pathways, astrocytic oxidation of minor substrates, and compartmentation of amino acid pools may differentially contribute to glutamate and glutamine dilutions under various conditions. For example, during brain activation, dilution via the pentose phosphate shunt pathway may exceed previous calculated estimates. Decarboxylation of [1-14C and 1-13C]glucose and re-entry of downstream unlabeled compounds into the glycolytic pathway would dilute glutamate and glutamine C4. The pentose shunt pathway has high capacity in adult brain and its flux is markedly enhanced in vitro by oxidative stress and catecholamine neurotransmitters. During acoustic activation of conscious rats, pentose shunt flux in tonotopic bands in the inferior colliculus increases 2.3-fold; this contributes to loss of label in autoradiographic studies using [1-14C]glucose but not those using [6-14C]glucose (Cruz et al., 2007; see Supplementary Material).
Astrocytic oxidation of minor substrates
In the absence of significant lactate SA dilution, an alternative hypothesis to explain the neuronal glutamate C4 dilution is that dilution of glutamine C4 in astrocytes may be the predominant factor, followed secondarily by dilution of the larger neuronal glutamate pool via the glutamine-glutamate cycle (Fig. 4). Dilution of glutamine C4 can arise from many processes, including pentose phosphate shunt activity, glutamine exchange across the blood-brain and blood-CSF barriers, and oxidative metabolism of unlabeled minor substrates by astrocytes (Fig. 4; see Supplementary Material). Preferential oxidative substrates for astrocytes include glycogen-derived pyruvate, specific amino acids generated by proteolysis or taken up from blood, blood-borne short- and long-chain fatty acids, and endogenous lipid turnover. Of interest is the observation by Tuček and Cheng (1974) that intracisternal injections of glucose, pyruvate, and acetoacetate yield glutamine relative specific activities greater than one (contrasting values <1 when given intravenously), indicating that this route favors their utilization via the ‘small’ (astrocytic) glutamate compartment. Astrocytes appear to have greater access to extracellular glucose, pyruvate, and acetoacetate, whereas these substrates are oxidized via the ‘large’ (mainly neuronal) glutamate pool when entering brain from blood.
Fig. 4. Glutamate and glutamine dilutional fluxes and oxidation of minor substrates in astrocytes and neurons.
Metabolism of blood-borne labeled glucose (and other substrates, blue box) labels the ‘large’ (neuronal, blue) glutamate (Glu) compartment and yields a glutamine (Gln)/glutamate relative specific activity (RSA = SAGln/SAGlu) <1. In contrast, many other substrates (red box) given intravenously or intracerebrally are preferentially oxidized via the ‘small’ (astrocytic, red) glutamate compartment and yield a Gln/Glu RSA >1. If the true lactate dilution (Vlac-dil) is very low, conceivably the dilution of glutamate fractional enrichment may arise secondary to glutamine dilution. Glutamine dilution is ascribed to glutamine exchange (Vdil-gln) but may also include oxidative metabolism of various minor substrates in astrocytes. If the true glutamine dilution in astrocytes is large compared to the true lactate dilution in neurons, oxidation of minor unlabeled compounds in astrocytes may first dilute the astrocytic glutamine pool, followed by dilution of the neuronal glutamate pool via the glutamate-glutamine cycle (black). Note that various compounds enter the TCA cycle at different points (not shown for clarity). See Discussion and Supplementary Material for more details.
Estimates of potential dilutional pathway fluxes involving compounds preferentially oxidized by astrocytes are summarized below; details and references are provided in the Supplementary Material. Leucine and glutamine influx into rat brain can generate maximal C4 dilutional fluxes of ~0.042 and 0.004 μmol/g/min, respectively. Proteolytic generation of amino acids that enter the TCA cycle as α-ketoglutarate or acetyl CoA (glutamate, glutamine, serine, glycine, alanine, leucine, threonine, cysteine, proline) could dilute C4 at maximal overall rate of ~0.06 μmol/min/g. Anaplerotic CO2 fixation by astrocytic pyruvate carboxylase incorporates unlabeled CO2 into glutamine C1 and the ‘new’ glutamine would incorporate label from C1 or C6 glucose into C4 plus C2, i.e., C4 is not diluted. Unlabeled carbon from oxidation of other amino acids (e.g., aspartate, valine, isoleucine) derived from uptake from blood or brain proteolysis (totaling ~0.02 μmol/min/g) can enter the TCA cycle as succinyl CoA or oxaloacetate and dilute glutamine C1-C3; this dilution may influence interpretation of fractional enrichments of isotopomers generated during the second and third turns of the TCA cycle. Short- and long-chain fatty acids are preferentially oxidized by astrocytes and enter the TCA cycle as acetyl CoA at estimated rates of ~0.1 μmol/min/g. Together, amino acid and fatty acid oxidation could contribute to glutamine dilution at a rate of ~0.1-0.2 μmol/min/g, approximating the Vdil-gln determined in MRS studies; pentose shunt flux would increase this dilution rate.
Dilution of neuronal glutamate C4 secondary to dilution of astrocytic glutamine C4 would contribute to (i) the observed lag in labeling of C4 glutamine compared to C4 glutamate by [1- or 6-13C]glucose (cycling of unlabeled neurotransmitter glutamate also contributes to glutamine dilution) and (ii) underestimation of astrocytic oxidation rates and energy demands that may exceed their recently-recognized high rates of oxidative metabolism (Hertz et al., 2007). High astrocytic energy demand during functional activation is supported by the ability of acutely-isolated astrocytes from adult brain oxidize glucose (Lovatt et al., 2007), stimulation of their respiration by extracellular K+ (Hertz, 1966), and mitochondrial enrichment in fine perisynaptic astrocytic processes compared to nearby neuropil (Lovatt et al., 2007).
Amino acid compartmentation
When brain slices were incubated with [13C]glucose, lactate labeling approached the theoretical maximum of 50%, whereas the fractional enrichment of glutamate C4 was less than 20% under ‘resting’ and depolarizing conditions and that of glutamine C4 was <10%; amino acid pools relatively inaccessible to metabolic labeling were suggested to explain the low enrichment (Badar-Goffer et al., 1992). Compartmentation of amino acids is supported by the finding that exogenous unlabeled glutamine did not displace labeling of glutamate or glutamine C4 by [1-13C]glucose in brain slices, but it reduced glutamate and glutamine C4 labeling by [3-13C]lactate (Rae et al., 2003 and references cited therein). In slice studies, unlabeled lactate derived from endogenous glucose and glycogen would be released during slice preincubation, and lactate generated during the [13C]glucose labeling period and released to the medium or superfusate should have the same enrichment as lactate in the slice. Thus, lactate uptake or exchange would make little, if any, contribution to C4 glutamate and glutamine dilution in slices; effects of compartmentation and other pathways discussed above must predominate.
Concluding comments
Exchange reactions, minor pathway fluxes, preferential substrate oxidation in astrocytes, and segregated metabolic pools may contribute to amino acid dilution in astrocytes and neurons. Lactate dilution is small and the glutamate C4 dilution would be clarified if re-named, e.g., Vdil-glu. The potential influence of (i) a predominant glutamine dilution in astrocytes to cause a secondary dilution of glutamate and (ii) metabolically inaccessible amino acid pools need to be assessed in modeling studies. A better understanding of label release and dilutional pathways is necessary to improve determination of the energy and neurotransmitter-related fluxes in astrocytes and neurons during rest, functional activation, and disease.
Supplementary Material
ACKNOWLEDGEMENT
This project was supported, in part, by NIH grant NS36728.
Abbreviations
- CMRglc
local cerebral rate of glucose utilization
- Glc
glucose
- Lac
lactate
- DG
2-deoxy-D-glucose
- MCT
monocarboxylic acid transporter
- MRS
magnetic resonance spectroscopy
- SA
specific activity
- RSA
relative specific activity
REFERENCES
- Ackermann RF, Lear JL. Glycolysis-induced discordance between glucose metabolic rates measured with radiolabeled fluorodeoxyglucose and glucose. J. Cereb. Blood Flow Metab. 1989;9:774–785. doi: 10.1038/jcbfm.1989.111. [DOI] [PubMed] [Google Scholar]
- Adachi K, Cruz NF, Sokoloff L, Dienel GA. Labeling of metabolic pools by [6-14C]glucose during K(+)-induced stimulation of glucose utilization in rat brain. J Cereb Blood Flow Metab. 1995;15:97–110. doi: 10.1038/jcbfm.1995.11. [DOI] [PubMed] [Google Scholar]
- Badar-Goffer RS, Ben-Yoseph O, Bachelard HS, Morris PG. Neuronal-glial metabolism under depolarizing conditions. A 13C-n.m.r. study. Biochem. J. 1992;282:225–30. doi: 10.1042/bj2820225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RC, McCandless DW, Wagman IL. Cerebral glucose utilization: Comparison of [14C]deoxyglucose and [6-14C]glucose quantitative autoradiography. J. Neurochem. 1987;49:1564–1570. doi: 10.1111/j.1471-4159.1987.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Cremer JE. Measurement of brain substrate utilization in adult and infant rats using various 14C-labeled precursors. In: Passonneau JV, Hawkins RA, Lust WD, Welsh FA, editors. Cerebral Metabolism and Neural Function. Williams & Wilkins; Baltimore: 1980. pp. 300–308. [Google Scholar]
- Cremer JE, Heath DF. The estimation of rates of utilization of glucose and ketone bodies in the brain of the suckling rat using compartmental analysis of isotopic data. Biochem. J. 1974;142:527–44. doi: 10.1042/bj1420527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer JE, Sarna GS, Teal HM, Cunningham VJ. Amino acid precursors: Their transport into brain and initial metabolism. In: Fonnum F, editor. Amino acids as chemical transmitters; Proceedings of the NATO Advanced Study Institute on Amino Acids as Chemical Transmitters; Plenum Press, New York. 1977; 1978. pp. 669–689. held in Oslo, Norway. [Google Scholar]
- Cremer JE, Cunningham VJ, Pardridge WM, Braun LD, Oldendorf WH. Kinetics of blood-brain barrier transport of pyruvate, lactate and glucose in suckling, weanling and adult rats. J. Neurochem. 1979;33:439–45. doi: 10.1111/j.1471-4159.1979.tb05173.x. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Adachi K, Dienel GA. Metabolite trafficking during K+-induced spreading cortical depression: Rapid efflux of lactate from cerebral cortex. J. Cereb. Blood Flow Metab. 1999;19:380–392. doi: 10.1097/00004647-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Ball KK, Dienel GA. Functional imaging of focal brain activation in conscious rats: Impact of [(14)C]glucose metabolite spreading and release. J. Neurosci. Res. 2007;85:3254–3266. doi: 10.1002/jnr.21193. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Brown PB, Mason GF, Rothman DL, Behar KL. Detection of [1,6-13C2]-glucose metabolism in rat brain by in vivo 1H-[13C]-NMR spectroscopy. Magn. Reson. Med. 2003;49:37–46. doi: 10.1002/mrm.10348. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Wang RY, Cruz NF. Generalized sensory stimulation of conscious rats increases labeling of oxidative pathways of glucose metabolism when the brain glucose-oxygen uptake ratio rises. J. Cereb. Blood Flow Metab. 2002;22:1490–1502. doi: 10.1097/01.WCB.0000034363.37277.89. [DOI] [PubMed] [Google Scholar]
- Flock EV, Tyce GM, Owen CA., Jr. Glucose metabolism in brains of diabetic rats. Endocrinology. 1969;85:428–37. doi: 10.1210/endo-85-3-428. [DOI] [PubMed] [Google Scholar]
- Flock EV, Tyce GM, Owen CA., Jr. Glucose metabolites in blood of normal and ethanol-treated rats. Mayo Clin. Proc. 1971;46:391–9. [PubMed] [Google Scholar]
- Hawkins RA, Miller AL, Cremer JE, Veech RL. Measurement of the rate of glucose utilization by rat brain in vivo. J. Neurochem. 1974;23:917–23. doi: 10.1111/j.1471-4159.1974.tb10743.x. [DOI] [PubMed] [Google Scholar]
- Henry PG, Adriany G, Deelchand D, Gruetter R, Marjanska M, Öz G, Seaquist ER, Shestov A, Uğurbil K. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magn. Reson. Imaging. 2006;24:527–39. doi: 10.1016/j.mri.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hertz L. Neuroglial localization of potassium and sodium effects on respiration in brain. J Neurochem. 1966;13:1373–87. doi: 10.1111/j.1471-4159.1966.tb04300.x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J. Cereb. Blood Flow Metab. 2007;27:219–49. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hyder F, Chase JR, Behar KL, Mason GF, Siddeek M, Rothman DL, Shulman RG. Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc. Natl. Acad. Sci. U S A. 1996;93:7612–7. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide K, Schmalbruch IK, Quistorff B, Horn A, Secher NH. Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. J. Physiol. 2000;522:159–64. doi: 10.1111/j.1469-7793.2000.t01-2-00159.xm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konitzer K, Voigt S. Metabolism of blood-borne lactate in rat brain in vivo. Acta Biol. Med. Ger. 1977;36:1049–59. [PubMed] [Google Scholar]
- Lear JL, Ackermann RF. Comparison of cerebral glucose metabolic rates measured with fluorodeoxyglucose and glucose labeled in the 1, 2, 3-4, and 6 positions using double label quantitative digital autoradiography. J. Cereb. Blood Flow. Metab. 1988;8:575–585. doi: 10.1038/jcbfm.1988.99. [DOI] [PubMed] [Google Scholar]
- Lear J, Ackermann RF. Why the deoxyglucose method has proven so useful in cerebral activation studies: The unappreciated prevalence of stimulation-induced glycolysis. J. Cereb. Blood Flow Metab. 1989;9:911–913. doi: 10.1038/jcbfm.1989.128. [DOI] [PubMed] [Google Scholar]
- Lear J, Ackermann R. In: Lassen N, Ingvar D, Raichle M, Friberg L, editors. Autoradiographic comparison of FDG-based and GLU-based measurements of cerebral glucose transport and metabolism: Normal and activated conditions; Brain work and mental activity, Alfred Benzon Symposium 31; Copenhagen, Munskgaard. 1991.pp. 142–152. [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J. Neurosci. 2007;27:12255–66. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Rothman DL, Behar KL, Shulman RG. NMR determination of the TCA cycle rate and alpha-ketoglutarate/glutamate exchange rate in rat brain. J. Cereb. Blood Flow Metab. 1992;12:434–47. doi: 10.1038/jcbfm.1992.61. [DOI] [PubMed] [Google Scholar]
- Mason GF, Gruetter R, Rothman DL, Behar KL, Shulman RG, Novotny EJ. Simultaneous determination of the rates of the TCA cycle, glucose utilization, alpha-ketoglutarate/glutamate exchange, and glutamine synthesis in human brain by NMR. J. Cereb. Blood Flow Metab. 1995;5:12–25. doi: 10.1038/jcbfm.1995.2. [DOI] [PubMed] [Google Scholar]
- Mason GF, Rothman DL. Basic principles of metabolic modeling of NMR (13)C isotopic turnover to determine rates of brain metabolism in vivo. Metab. Eng. 2004;6:75–84. doi: 10.1016/j.ymben.2003.10.003. [DOI] [PubMed] [Google Scholar]
- McIlwain H, Bachelard HS. Biochemistry and the Central Nervous System. 5th Ed. Churchill Livingstone; Edinburgh: 1985. [Google Scholar]
- O’Neal RM, Koeppe RE. Precursors in vivo of glutamate, aspartate and their derivatives of rat brain. J. Neurochem. 1966;13:835–47. doi: 10.1111/j.1471-4159.1966.tb05879.x. [DOI] [PubMed] [Google Scholar]
- Öz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J. Neurosci. 2004;24:11273–9. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten U, Ratcheson RA, Salford LG, Siesjö BK. Optimal freezing conditions for cerebral metabolites in rats. J. Neurochem. 1973;21:1127–1138. doi: 10.1111/j.1471-4159.1973.tb07567.x. [DOI] [PubMed] [Google Scholar]
- Rae C, Hare N, Bubb WA, McEwan SR, Bröer A, McQuillan JA, Balcar VJ, Conigrave AD, Bröer S. Inhibition of glutamine transport depletes glutamate and GABA neurotransmitter pools: further evidence for metabolic compartmentation. J. Neurochem. 2003;85:503–14. doi: 10.1046/j.1471-4159.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, Mason GF, Petroff OA, Shulman GI, Shulman RG, Rothman DL. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc. Natl. Acad. Sci. U S A. 1999;96:8235–40. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Rothman DL, Behar KL, Xu S. Determination of the glutamate-glutamine cycling flux using two-compartment dynamic metabolic modeling is sensitive to astroglial dilution. J Cereb Blood Flow Metab. 2009;29:108–18. doi: 10.1038/jcbfm.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjö BK. Brain Energy Metabolism. Wiley-Interscience, John Wiley & Sons; Chichester: 1978. [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J. Cereb. Blood Flow Metab. 2007;27:1766–91. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J. Neurochem. 1987;49:1651–8. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Tuček S, Cheng SC. Provenance of the acetyl group of acetylcholine and compartmentation of acetyl-CoA and Krebs cycle intermediates in the brain in vivo. J Neurochem. 1974;22:893–914. doi: 10.1111/j.1471-4159.1974.tb04314.x. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Simpson IA. Developmental switch in brain nutrient transporter expression in the rat. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1127–34. doi: 10.1152/ajpendo.00187.2003. [DOI] [PubMed] [Google Scholar]
- Veech RL, Harris RL, Veloso D, Veech EH. Freeze-blowing: a new technique for the study of brain in vivo. J. Neurochem. 1973;20:183–8. doi: 10.1111/j.1471-4159.1973.tb12115.x. [DOI] [PubMed] [Google Scholar]
- Veech RL. Freeze-blowing of brain and the interpretation of the meaning of certain metabolite levels. In: Passonneau JV, Hawkins RA, Lust WD, Welsh FA, editors. Cerebral metabolism and neural function. Williams and Wilkins; Baltimore: 1980. pp. 34–41. [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ, Tildon JT. Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J. Neurochem. 2007;101:9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.