Abstract
The high potency of clostridial neurotoxins relies predominantly on their neurospecific binding and specific hydrolysis of SNARE proteins. Their multi step mode of mechanism can be ascribed to their multi-domain three-dimensional structure. The C-terminal HCC-domain interacts subsequently with complex polysialo-gangliosides such as GT1b and a synaptic vesicle protein receptor via two neighbouring binding sites resulting in highly specific uptake of the neurotoxins at synapses of cholinergic motoneurons. After its translocation the enzymatically active light chain specifically hydrolyses specific SNARE proteins, preventing SNARE complex assembly and thereby blocking exocytosis of neurotransmitter.
Keywords: protease, SNARE, synaptic vesicle, neurotransmission, synaptotagmin, ganglioside, botulinum neurotoxin
Introduction
The family of clostridial neurotoxins (CNTs) consists of tetanus neurotoxin (TeNT) and the seven botulinum neurotoxin serotypes (BoNT/A–G) and represents the most toxic agents known. The median lethal dose is below 1 ng per kg of body weight (Gill, 1982). The disease tetanus is caused by germination of Gram-positive, anaerobic spore-forming Clostridium tetani in infected tissue lesions, thereby producing and releasing TeNT into the blood stream. In contrast, botulism is evoked by ingestion of acid resistant BoNT progenitor toxins, generated by various strains of C.botulinum, C.butyricum and C.barati, and subsequent transcytosis of this complex or the released BoNT through the intestinal epithial barrier (Bigalke and Shoer, 2000). The CNTs reach the motoneurons via circulation and specifically bind to unmyelinated areas of nerve terminals (Dolly et al., 1984). Here, BoNTs inhibit acetylcholine release followed by flaccid paralysis while TeNT is transported retrogradely to inhibitory neurons and blocks release of glycine or γ-aminobutyric acid which results in spastic paralysis.
The crystal structures of the BoNT/A and B holotoxins (Lacy et al., 1998; Swaminathan and Eswaramoorthy, 2000) revealed that most likely all CNTs are composed of four functionally independent domains that perform individual tasks in the multi-step intoxication process (Fig. 1). All CNTs are produced as ~150 kDa single chain (sc) proteins. They are posttranslationally proteolysed into a ~100 kDa heavy chain (HC) and a ~50 kDa light chain (LC). Both chains remain associated by a single disulfide bridge, noncovalent interactions and an HC derived peptide loop wrapping around the LC. The HCs are responsible for neurospecific binding, uptake and translocation of the LCs into the cytosol. Following cell attachment, internalisation via receptor-mediated endocytosis brings the BoNTs into the synaptic vesicles. Here, the acidic environment eliminates repulsive electrostatic interactions between the largely α-helical amino-terminal half of the HC, the HN-domain and the membrane, allowing its penetration into the membrane, without triggering detectable structural changes (Galloux et al., 2008). At the same time the LC is partially unfolded (Koriazova and Montal, 2003). Translocation of LC by HC can be observed in real time as an increase of channel conductance. The HC channel is occluded by the LC during transit, then unoccluded after completion of translocation and release of LC (Fischer and Montal, 2007b). Upon reduction of the disulfide bond, the LC functions as a zinc dependent endopeptidase in the cytosol (Fischer and Montal, 2007a; Schiavo et al., 1990).
Figure 1.
Schematic representation of the four domain structure of the single chain 150 kDa clostridial neurotoxins (bottom) and the corresponding crystal structure of BoNT/B (top; modified from 1EPW.pdb). The clostridial neurotoxins are posttranslationally proteolysed within the loop segment into LC and HC which remain linked together covalently by a disulfide bridge and non-covalently by a “belt” wrapping around the LC. The C-terminal half of the HC-fragment, the HCC-domain is responsible for neurospecific binding to complex polysialo-gangliosides and a synaptic vesicle protein receptor, and subsequent uptake, the HN-domain translocates the LC into the cytosol where the latter acts as metalloprotease.
Gangliosides as receptors for CNTs
The specific binding to peripheral nerve endings at the neuromuscular junction solely involves the 50 kDa C-terminal half of the HC, the HC-fragment (Evinger and Erichsen, 1986; Fishman and Carrigan, 1987; Lalli et al., 1999; Simpson, 1984a, b, 1985) and complex polysialo-gangliosides, glycosphingolipids that are found particularly in membranes of neuronal cells (Simpson and Rapport, 1971; van Heyningen and Miller, 1961). The interaction of gangliosides with CNTs was investigated for TeNT and several serotypes of BoNTs in extensive studies (Halpern and Neale, 1995; Yowler and Schengrund, 2004). These studies revealed that the disialo-carbohydrate structure as found in GD1b is essential for the binding of most of the CNTs. Furthermore, TeNT, BoNT/A, B, C, E, and F have affinities in the upper nM range in various in vitro binding assays with immobilized polysialo-gangliosides, whilst CNTs have much higher affinity (KD = 1.2 nM) to synaptosomes that are similar to neuronal tissue. At the cellular level, the cleavage of sialic acid residues by neuraminidase treatment of cultured cells isolated from spinal cord (Bigalke et al., 1986) and adrenergic chromaffin cells (Marxen et al., 1989) reduced BoNT/A potency as well as TeNT action (Critchley et al., 1986). Conversely, bovine chromaffin cells lacking complex polysialo-gangliosides were rendered sensitive to TeNT and BoNT/A by incubation with gangliosides (Marxen and Bigalke, 1989; Marxen et al., 1991). In addition, a monoclonal antibody to GT1b antagonized the action of BoNT/A on rat superior cervical ganglion neurons (Kozaki et al., 1998). The inhibition of ganglioside biosynthesis with fumonisin in primary spinal cord neurons and with D,L-threo-l-phenyl-2-hexadecanoylamino-3-morpholino-propanol-HCl (PPMP) in the neuroblastoma cell line Neuro2a resulted in insensitivity to TeNT and BoNT/A, respectively (Williamson et al., 1999; Yowler et al., 2002). Employing a genomic approach, mice, deficient in NAcGal-transferase thus only expressing Lac-Cer, GM3 and GD3, resisted treatment with TeNT and BoNT/A, B and E (Bullens et al., 2002; Kitamura et al., 1999) whereas GD3-synthase knock-out mice expressing only Lac-Cer, GM3, GM2, GM1 and GD1a are solely resistant to TeNT, but kept their sensitivity towards BoNT/A, B and E (Kitamura et al., 2005). A combination of both gene knock outs resulted in GM3-only mice which display i.a. high resistance towards BoNT/B and G (Rummel et al., 2007). Also, GM3-synthase knock-out mice theoretically expressing only Lac-Cer are insensitive to BoNT/C1 (Tsukamoto et al., 2005). Hence, complex polysialo-gangliosides such as GD1a, GD1b and GT1b mediate the first cell contact of CNT and play an important role in their specific binding to neuronal cells.
A protein is the second receptor for CNTs
The discrepancy in affinity between binding of CNTs to isolated gangliosides and neuronal tissue prompted predictions of a second receptor component. The protease-sensitive binding of BoNT/A and TeNT to rat brain synaptosomes (Dolly et al., 1982; Kitamura, 1976; Lazarovici and Yavin, 1986; Pierce et al., 1986) resulted in a dual receptor model. First, polysialo-gangliosides were considered to accumulate CNTs on the plasma membrane surface. Then, CNTs would simply stay on the surface until binding is accomplished to their thinly distributed protein receptor(s) or move laterally within the membrane while still bound to low affinity receptors thereby increasing the chance of contact with the protein receptor. Simultaneous interaction with ganglioside and protein receptor would then be considered as high affinity binding and set the stage for the subsequent specific step of endocytosis (Montecucco, 1986; Niemann et al., 1991).
Several studies demonstrated accelerated uptake of TeNT (Simpson, 1985) and BoNT/A (Black and Dolly, 1986) upon electrical stimulation into hemidiaphragm preparations as well as of BoNT/A and E upon K+ stimulation into spinal cord neurons (Keller et al., 2004). As a consequence, increased nerve stimulation resulted in an earlier onset of neurotransmitter blockade on upon application of BoNT/A (Hughes and Whaler, 1962; Simpson, 1980) and TeNT (Schmitt et al., 1981). As nerve stimulation causes increased rates of exo- and endocytosis of synaptic vesicles, one can hypothesise that synaptic vesicle proteins, which, upon neurotransmitter release, become temporarily exposed on the cell surface within the synaptic cleft, are involved in the binding and uptake of CNTs.
The synaptic vesicle membrane protein synaptotagmin (Syt) (Geppert et al., 1991; Perin et al., 1991) was identified to be the protein receptor for BoNT/B in rat brain synaptosomes employing cross-linking experiments (Nishiki et al., 1994; Nishiki et al., 1993). The current 13 isoforms of the Syt family are supposed to trigger vesicular fusion upon Ca2+ entry (Chapman, 2002; Südhof, 2002). The recombinant isoforms Syt-I and Syt-II reconstituted in GD1a or GT1b endowed liposomes (Nishiki et al., 1996a) as well as Syt-II stably expressed in CHO cells (Nishiki et al., 1996b) interacted in vitro with BoNT/B. Use of recombinant deletion mutants of Syt-II demonstrated that only the N-terminal intravesicular domain, which is extracellularly exposed upon exocytosis, plus the transmembrane domain retains BoNT/B binding activity (Kozaki et al., 1998). Later, the Syt-I and Syt-II mediated entry of BoNT/B was confirmed by means of loss-of-function and gain-of-function studies employing PC12 cells. Furthermore, results of GST-pull-down assays narrowed the BoNT/B binding segment of Syt-I and Syt-II down to the 20 juxtamembrane amino acids of the intravesicular domain. The corresponding 20mer peptide neutralised the toxicity of BoNT/B in mice when administered together with gangliosides. Neither binding of BoNT/A and E to Syt-I and Syt-II nor their uptake were observed (Dong et al., 2003). Shortly after, Rummel et al. demonstrated that BoNT/G interacts with the identical juxtamembrane segments of Syt-I and Syt-II in vitro. A dramatic decrease in the BoNT/G activity at mice phrenic nerve (MPN) hemidiaphragm preparations upon preincubation with the intravesicular domains of Syt-I and Syt-II revealed that these molecules also act in vivo as protein receptors for BoNT/G. However, none of the remaining CNTs bound to Syt-I and Syt-II (Rummel et al., 2004a).
The 12 transmembrane domain synaptic vesicle glycoprotein 2 (SV2) was identified as protein receptor for BoNT/A. In in vitro experiments the isoform SV2C displays the highest affinity to BoNT/A, followed by SV2A and SV2B (Dong et al., 2006). BoNT/A interacts with the 125 amino acid long luminal domain (referred to as domain “four”) of SV2 which inhibits the neurotoxicity of BoNT/A using the MPN assay (Mahrhold et al., 2006). Subsequently, it was shown that BoNT/A and B associate with detergent resistant synaptic vesicle protein complexes consisting of SV2, Syt-I, synaptophysin, synaptobrevin 2, and the vacuolar proton pump vATPase (Baldwin and Barbieri, 2007). Very recently it was demonstrated, that the N-glycosylation of the luminal domain 4 of SV2A and SV2B enables the binding to and the uptake of BoNT/E into hippocampal neurons (Dong et al., 2008). The protein receptors of the remaining BoNT serotypes are still awaiting their identification.
The diverse sites of action of BoNTs and TeNT, leading to truly opposite symptoms, are i.a. caused by different modes of internalization at the presynaptic terminal. Whereas BoNT is taken up in motoneurons via SV recycling, TeNT binds to GD1b or GT1b and glycosylphosphatidylinositol-(GPI)-anchored glycoproteins associated in rafts (Herreros et al., 2000; Munro et al., 2001) and uses a clathrin-mediated pathway for its entry. In NGF differentiated PC12 cells the GPI-anchored glycoprotein Thy-I was determined as binding partner of TeNT HC-fragment (Herreros et al., 2001). This specialized clathrin-and AP-2–dependent uptake mechanism does not require the endocytotic adaptor protein epsin1 (Deinhardt et al., 2006a). While BoNT containing endosomal compartments are acidified by the vATPase in the presynapse, TeNT travels in various vesicles with neutral lumen inside the axon of motoneurons towards the spinal cord (Bohnert and Schiavo, 2005). Rab5 is essential for an early step in TeNT sorting but is absent from axonally TeNT transporting vesicles which are marked by the small GTPase Rab7, p75NTR, TrkB and BDNF (Deinhardt et al., 2006b).
Characterisation of binding sites in CNTs
The crystal structure of the TeNT HC-fragment revealed that it is composed of two domains, an N-terminal lectin-like jelly-roll domain (HCN, residues 865–1110) and a C-terminal β-trefoil domain (HCC, residues 1110–1315) (Knapp et al., 1998; Umland et al., 1997). Deletion mutagenesis studies showed that the TeNT HCC-domain binds to gangliosides and neuronal cells even more efficiently than the complete HC-fragment (Halpern and Loftus, 1993), whereas the HCN-domain does not bind at all (Figueiredo et al., 1995). Although the HCN-domain displays a lectin-like fold, no carbohydrate binding to this segment has been observed. Currently, it is unknown what role if any the HCN-domain plays during intoxication. Hypotheses suggest a function as a rigid, complex spacer between HN- and HCC-domain as well as an involvement in the translocation process.
An early crosslinking experiment employing 125I-azido-GD1b and the TeNT HC-fragment led to radiolabeling of H1293 in the proximity of a large cavity within the HCC-domain (Shapiro et al., 1997). The neighbourhood of H1293 to the ganglioside binding pocket was confirmed in a mutagenesis study showing reduced in vitro binding of the TeNT HC mutant H1293A to isolated ganglioside GT1b (Sinha et al., 2000). The mutation of the TeNT residue Y1290, forming the bottom of this cavity, to phenylalanine, serine and alanine also reduced affinity to GT1b as well as binding to synaptosomal membranes (Sutton et al., 2001). In computer-aided docking studies employing small molecules for inhibition of TeNT ganglioside binding, this cavity was chosen out of 52 others as the ganglioside binding site. Into this pocket the anticancer drug doxorubicin, an anthracycline antibiotic, was docked thereby competitively inhibiting the binding of the TeNT HC-fragment to liposome-integrated GT1b (Lightstone et al., 2000). Cocrystallization of the TeNT HC-fragment and four carbohydrate subunits of GT1b revealed four distinct binding sites, including the one in the proximity of H1293, where lactose interacts with the residues D1222, T1270, S1287, W1289, Y1290 and G1300. A separate site comprising R1226 as the key residue coordinated either a molecule of N-acetylgalactosamine (NAcGal) or sialic acid. Two additional sites were identified in cocrystals with galactose (Gal) or NAcGal (Emsley et al., 2000). However, the latter two sites are unlikely to function as binding pockets for polysialo gangliosides, due to insufficient space (Gal) or a high flexibility of the carbon backbone (NAcGal). Isaac et al. refined their cocrystallisation approach by using a synthetic GT1b-β analogue lacking the ceramide portion. Indeed, the terminal disaccharide Galβ3GalNAcβ bound to the lactose binding site next to H1293 while the disialic acid branch of another GT1b-β molecule interacted with the sialic acid binding site comprising R1226 (Fotinou et al., 2001). Cocrystallisation of a TeNT HC-fragment with disialyllactose detected this GD3 derivative bound to the sialic acid binding site (Jayaraman et al., 2005). Mutation of residues D1222, H1270 and W1289 in the lactose binding site led to reduced binding of TeNT HC-fragment to GT1b in surface plasmon resonance experiments and NGF differentiated PC12 cells (Louch et al., 2002). Finally, the physiological importance of the lactose binding site in TeNT was demonstrated by the application of corresponding, recombinant full length TeNT mutants using the MPN assay leading to a 350 fold reduction in neurotoxicity in case of the single amino acid mutation W1289L (Rummel et al., 2003). Furthermore, these experiments demonstrated that the sialic acid binding site is essential for TeNT action, since the mutant TeNT R1226F only retained 1.4 % activity in the MPN assay. Mass spectroscopy experiments indicated simultaneous binding of two molecules GT1b to the TeNT HC-fragment, but no ganglioside mediated crosslinking was observed (Rummel et al., 2003). Based on these results the ganglioside specificities of the individual lactose and sialic acid binding sites were refined (Chen et al., 2008). Although binding of a ganglioside to the sialic acid binding pocket was shown, it is conceivable that there is subsequent substitution by or a direct interaction with the reported GPI anchored glycoproteins. The binding of the tripeptide YEW to the sialic acid site of TeNT supports this assumption (Jayaraman et al., 2005). At present, it is still unclear whether the two ganglioside binding sites of TeNT are a peculiarity of this neurotoxin and whether it relates to its retrograde intraaxonal transport.
The lactose binding site is characterised by the presence of the peptide motif H.....SXWY.....G and it is conserved among the majority of CNTs. This cavity displays the typical features necessary for carbohydrate interaction found also in other protein toxins such as ricin and cholera toxin. An aromatic residue, preferable tryptophan or tyrosine, supplies the surface for the hydrophobic face of the sugar ring. Polar residues like glutamate, serine or asparagine are oppositely located to interact with the sugar hydroxyl groups. Cocrystallisation studies with BoNT/B and sialyllactose or doxorubicin, respectively, suggested that the lactose binding site is the ganglioside binding pocket in BoNTs (Eswaramoorthy et al., 2001; Swaminathan and Eswaramoorthy, 2000). Detailed mutational analyses defined the contribution of various residues within the homologous lactose binding pocket of BoNT/A and B. Again, the mutations of the aromatic key residues, W1266L and W1262L in BoNT/A and B, respectively, lead to dramatic reductions of neurotoxicity using the MPN assay (Rummel et al., 2004b). In contrast to TeNT, mass spectroscopy data revealed the binding of only a single GT1b molecule to the HC-fragment of BoNT/A and B, which according to the binding data seems to bind in a different mode within the lactose site (Rummel et al., 2004b). Now, these physiological data was confirmed by a cocrystal of a synthetic GT1b and the HC-fragment of BoNT/A (Stenmark et al., 2008). Recently, the tryptophan residue 1268 of BoNT/G was shown to play a key role in ganglioside interaction (Rummel et al., 2007). Shortly thereafter, the conserved tryptophan of the ganglioside binding pocket in BoNT/C1 was identified as W1258 (Tsukamoto et al., 2008).
Since BoNT do not possess a second carbohydrate binding site, the question arises, whether protein receptors such as Syt-II for BoNT/B bind in a pocket that is homologous to the sialic acid binding site within the HCC-domain of TeNT. The different affinities of HC-fragment hybrids consisting of HCN- and HCC-domains of BoNT/B strains Okra and 111, respectively, to GT1b/Syt-II endowed liposomes pointed into that direction (Ihara et al., 2003). The use of isolated HCC-domains of BoNT/B and G exhibiting an interaction with their protein receptor Syt-II further supported this hypothesis (Rummel et al., 2004a). Computer-based surface analysis of potential Syt binding sites in the HCC-domain of BoNT/B followed by detailed mutational analyses in the sialic acid site corresponding area in BoNT/B and G revealed reduced binding of Syt-I and Syt-II in GST-pull-down assays as well as drastically reduced neurotoxicity of full length BoNT/B and G mutants in the MPN assay (Rummel et al., 2007). In parallel, two independent co-crystal structures revealed that the intraluminal Syt-II peptide binds in an α-helical conformation to the sialic acid site corresponding area within the HCC-domain of BoNT/B (Chai et al., 2006; Jin et al., 2006). Although both binding sites are in close proximity (Fig. 2) they function independently and do not require pre-formation of a ganglioside/protein receptor complex (Rummel et al., 2007). Moreover, mutants of BoNT/B with both the ganglioside and Syt binding sites (individually or in combination) deactivated do not exhibit appreciable toxicity excluding any significant contributions of other cell surface molecules to binding and entry of BoNTs (Rummel et al., 2007). Taken together, these results support the dual-receptor concept nearly 20 years after it had been proposed.
Figure 2.
The protein receptor binding site (amino acids highlighted in orange stick drawing) occupied by the α-helical 20mer peptide of synaptotagmin II is located in close proximity to the ganglioside binding site (amino acids highlighted in green stick drawing) within in the HCC-domain (red ribbon) of the BoNT/B HC-fragment (2nm1.pdb)(Jin et al., 2006).
CNT LC Proteases
BoNT and TeNT LCs are amongst the most selective proteases known (Oost et al., 2003). Primary sequence and structural analysis of LCs suggest that their enzymatic mechanism is related to that of other Zn2+-metalloproteases (Agarwal et al., 2004; Breidenbach and Brunger, 2005; Lacy et al., 1998; Rao et al., 2005; Swaminathan and Eswaramoorthy, 2000), but the structural basis of SNARE target selectivity is unusual. Remarkably, the LCs do not appear to recognize a consensus site, or even have rigorous requirements for particular side chains flanking the scissile bond (Schmidt and Bostian, 1997). Also, the LCs generally require long stretches of their target SNAREs for optimal efficiency (Cornille et al., 1997; Foran et al., 1994; Schmidt and Bostian, 1995, 1997; Vaidyanathan et al., 1999; Yamasaki et al., 1994). Indeed, point mutations in SNARE regions remote from the scissile bond can dramatically reduce LC efficiency (Breidenbach and Brunger, 2004; Pellizzari et al., 1996; Rossetto et al., 1994; Schmidt and Bostian, 1995). The cleavage-site selectivity of CNT-LCs is very high. For example, the scissile bond in SNAP-25 for BoNT/A (Q197-R198) is shifted by exactly one residue compared to that for BoNT/C1 (R198-A199). BoNT/C1 cleaves only one of two identical neighboring peptide bonds (K253-A254 and K260-A261) in syntaxin-1A (Schiavo et al., 1995).
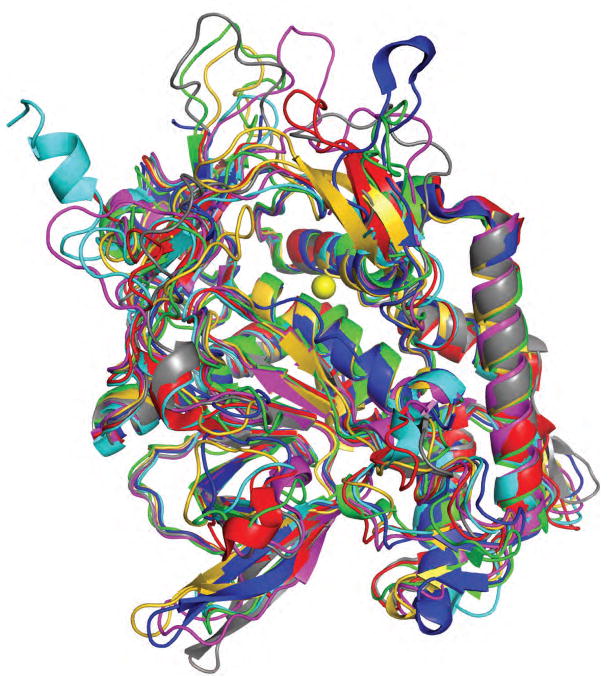
The apo structures of all members of the family of CNT-LCs are now available: BoNT/A LC (Breidenbach and Brunger, 2004; Burnett et al., 2007; Lacy et al., 1998; Segelke et al., 2004), BoNT/B LC (Swaminathan and Eswaramoorthy, 2000), BoNT/C1 LC (Jin et al., 2007), BoNT/D LC (Arndt et al., 2006), BoNT/E LC (Agarwal et al., 2004), BoNT/F LC (Agarwal et al., 2005), BoNT/G LC (Arndt et al., 2005), and TeNT LC (Breidenbach and Brunger, 2005; Rao et al., 2005) (Fig. 3). The structural differences among the CNT-LCs are mostly limited to solvent-exposed loops and potential substrate interaction sites. The striking similarity of LC active sites naturally leads to the question of which LC features are determinants of substrate selectivity. Furthermore, none of the LCs efficiently cleave truncated substrate peptides less than 20–30 residues. Rather, unusually long stretches of the substrates are required for optimal cleavage (Cornille et al., 1997; Foran et al., 1994; Schmidt and Bostian, 1995, 1997; Vaidyanathan et al., 1999). In general, long sequences that are located N-terminal of the scissile bonds appear to be important for cleavage, as revealed by mutagenesis studies with synaptobrevin 2 and SNAP-25 (Binz et al., 1994; Sikorra et al., 2006; Yamasaki et al., 1994). For example, the optimal portion of SNAP-25 required for maximally-efficient cleavage by BoNT/A spans residues 146–202 (Chen and Barbieri, 2006; Vaidyanathan et al., 1999). Other CNTs require 30–60 residue stretches of their substrates for efficient cleavage, regardless of scissile-bond location (Cornille et al., 1997; Foran et al., 1994; Yamasaki et al., 1994). Moreover, point mutations in SNAREs far-remote from the scissile bond can dramatically influence the proteolysis efficiency (Breidenbach and Brunger, 2004; Pellizzari et al., 1996; Rossetto et al., 1994; Sikorra et al., 2008). Comparison of structures of wildtype BoNT/A in different crystal forms revealed significant conformational variability of some of surface loops, especially near the active site of the LC (Burnett et al., 2007).
Figure 3. Superposition of the LC protease structures from all seven serotypes of BoNTs and TeNT.
BoNT/A LC (PDB code 2SIG, green) (Burnett et al., 2007), BoNT/B LC (1EPW, magenta, rmsd = 1.6 Å) (Swaminathan and Eswaramoorthy, 2000), BoNT/C1 LC (2QN0, gold, rmsd = 2.2 (Jin et al., 2007), BoNT/D LC (2FPQ, blue, rmsd = 1.3 Å) (Arndt et al., 2006), BoNT/E LC (1T3A, cyan, rmsd = 1.6 Å) (Agarwal et al., 2004), BoNT/F LC (2A8A, red, rmsd = 1.9 Å) (Agarwal et al., 2005), BoNT/G LC (1ZB7, orange, rmsd = 2.1 Å) (Arndt et al., 2005), and TeNT LC (1Z7H, grey, rmsd = 1.7 Å) (Breidenbach and Brunger, 2005). The specified atomic root mean square differences are for all Cα atoms with respect to the BoNT/A LC structure. The Zn2+ is shown as a yellow sphere. Despite their different substrate specificities, CNT-LCs display high structural similarity.
LC – Substrate Interactions
The structure of a BoNT/A LC -·SNAP-25 complex (PDB ID 1XTG) (Breidenbach and Brunger, 2004) for the first time provided molecular insights into the basis of LC substrate selectivity (Fig. 4). To date, this is the only structure of a complex between a CNT-LC and its substrate. A previous report of the structure of a complex between BoNT/B-LC and synaptobrevin 2 (Hanson and Stevens, 2000) is not supported by the experimental data (Breidenbach and Brunger, 2004; Rupp and Segelke, 2001) and the corresponding Protein Data Bank deposition has been withdrawn (PDB ID 1F83). Remarkably, SNAP-25 wraps around most of the LC’s circumference; the extensive interface between the enzyme and its substrate is not restricted to the active site. Moreover, in contrast to the contiguous helical conformation observed in the ternary SNARE complex (Sutton et al., 1998), SNAP-25 adopts three distinct types of secondary structure upon binding to BoNT/A. The N-terminal residues of SNAP-25 (147–167) form an α-helix, the C-terminal residues (201–204) form a distorted β-strand, and residues in between are mostly extended (Breidenbach and Brunger, 2004). Mutagenesis and kinetics experiments demonstrated that the N-terminal α-helix and the C-terminal β-sheet are critical for an efficient substrate binding and cleavage, and are termed α- and β-exosites, respectively. The structure confirmed the existence of such exosites which had been postulated before based on biochemical experiments (Rossetto et al., 1994; Washbourne et al., 1997).
Figure 4. Structure of the BoNT/A·LC-SNAP-25 complex.
The protease component of BoNT/A (gray) forms an extended interface with the C-terminal core domain of SNAP-25 (green). Multiple sites of enzyme-substrate interaction remote from the catalytic Zn2+ (magenta sphere) and associated nucleophile (blue sphere) extend around most of the toxin’s circumference, imparting the protease with exquisite specificity. SNAP-25 is unstructured in the absence of a binding partner but adopts a mix of α-helix, β-sheet, and extended conformations when complexed with BoNT/A.
The highly unusual extended enzyme–substrate interface used by BoNT/A serves to properly orient its conformationally variable SNARE target such that the scissile peptide bond is placed within close proximity of the catalytic motif of the enzyme. Notably, many of the interactions that impart substrate specificity occur on the face of the protease that is opposite to its active site (α-exosite), and the C-terminus of the substrate (β-exosite) induces a conformational change in the active site pocket, probably rendering the protease competent for catalysis. The multi-site binding strategy used by BoNT/A LC accounts for the extreme selectivity of this enzyme. The structure of the BoNT/A LC -·SNAP-25 complex vividly illustrates the extent of substrate that must be available for efficient proteolysis to occur. SNAREs exhibit considerable conformational variability; they can exist as monomeric components with little secondary structure, as partially structured SNARE complexes or subcomplexes, or in complex with regulatory factors (Brunger, 2006).
The structural and enzyme kinetics studies of the BoNT/C1-LC have provided further information regarding the toxin-substrate interaction (Jin et al., 2007). BoNT/C1-LC is unique among all BoNTs in that it exhibits dual specificity toward both syntaxin 1A and SNAP-25. Interestingly, while both BoNT/A and BoNT/C1 cleave SNAP-25, the scissile bond is shifted by only a single residue (Q197-R198 for BoNT/A and R198-A199 for BoNT/C1). Structural modeling revealed that the remote α-exosite that was previously identified in the complex of BoNT/A-LC and SNAP-25 is structurally conserved in BoNT/C1. Single site mutations in the predicted α-exosite of BoNT/C1 had a significant but less severe effect on SNAP-25 cleavage in comparison to that of BoNT/A, suggesting that this region plays a less stringent role on substrate discrimination. Such a “promiscuous” substrate-binding strategy by the α-exosite could account for its dual substrate specificity. As a crucial supplement to the function of the remote α-exosite, the scissile-bond proximal exosites probably ensure the correct register for hydrolysis. This includes the β-exosite as observed on BoNT/A and key residues surrounding the scissile peptide bond. A small, distinct pocket (S1′) found near the active site of BoNT/C1 potentially ensures the correct register for the cleavage site by only allowing Ala as the P1′ residue for both SNAP- 25 and syntaxin 1A. Mutations of this SNAP-25 residue dramatically reduced enzymatic activity of BoNT/C1 (Jin et al., 2007). The S1′ pocket is significantly larger in BoNT/A LC allowing it to accommodate the arginine residue as the P1′ residue as revealed by the crystal structures of the inhibitor L-arginine hydroxamate (ArgHX) with wild-type BoNT/A-LC (Silvaggi et al., 2007) as well as an inactive double-mutant of BoNT/A LC (Fu et al., 2006).
The crystal structure of the BoNT/A-LC·SNAP-25 complex revealed a small loop (residues 183–190) that detaches from the surface of BoNT/A-LC and separates the α-exosite from the active site. This loop may be able to accommodate the necessary “slack” for the cleavage-site register shift between BoNT/A and BoNT/C1 while maintaining the approximate position of the α-exosite. Consistent with this notion, there is little effect on substrate cleavage upon insertion of up to three extra residues in this loop (Jin et al., 2007). The divided roles for substrate discrimination among different exosites could provide some flexibility of the precise scissile bond position while ensuring high overall substrate specificity.
LC – Inhibitor Interactions and Implications for Drug Development
For many years, complexes between LC proteases and inhibitors resisted attempts at co-crystallization. Over the past two years, dramatic progress has been made resulting in co-crystal structures of several inhibitor-BoNT/A LC complexes (Fu et al., 2006; Kumaran et al., 2008a; Kumaran et al., 2008b; Silvaggi et al., 2007; Silvaggi et al., 2008; Zuniga, 2008). Co-crystal structures with small hydroxamate compounds and tetrapeptides, all including an Arg moiety, have now uniquely identified the P1′ binding pocket of the LC protease (Fu et al., 2006; Kumaran et al., 2008b; Silvaggi et al., 2007). However, the identification of the other substrate binding pockets is still questionable since available structures of larger pseudo-peptides resulted in backbone conformations that are very different from that of native substrate (SNAP-25) (Zuniga, 2008).
As an example we discuss here the crystal structure of a complex between an active form of BoNT/A LC and a pseudopeptide inhibitor that mimics the seven-residue Q197RATKML203 sequence of the 206-residue SNAP-25 near the cleavage site (Zuniga, 2008). This inhibitor, referred to as I1, is the most potent non-zinc-chelating, non- hydroxamate-based antagonist reported to date, with a Ki = 41 nM. When tested against LCs of BoNT/B, D, E, F, and thermolysin, I1 did not display any detectable inhibition, indicating that its inhibitory action is BoNT/A specific. Surprisingly, the co-crystal structure revealed a 310 helical backbone conformation for the peptide inhibitor bound to the active site of BoNT/A LC which is in sharp contrast to the extended conformation of bound SNAP-25 observed in the BoNT/A LC[E224Q,Y366F]:SNAP-25 complex (Fig. 5). The inhibitor induces binding pockets in BoNT/A LC which are found neither in the apo BoNT/A LC nor in the BoNT/A LC[E224Q,Y366F]:SNAP-25 complex (Fig. 6).
Figure 5. Comparison of the I1 conformation with that of SNAP-25.
Cartoon representation of the backbone of BoNT/A LC-bound inhibitor I1 (panel A) and the Q197-L203 fragment in BoNT/A LC-bound SNAP-25 (panel B). The backbones of the corresponding BoNT/A LC structures have been superimposed to produce the shown orientations of I1 and the SNAP-25 fragment. However, for clarity, the corresponding BoNT/A LC structures are not shown. The identity of the residues in both molecules is indicated by their one-letter code, with the exception of residues DAB and DNP-DAB in I1. Side chains of all residues are shown as sticks. N, C, O and S atoms are colored in blue, cyan, red, and yellow, respectively. The Zn2+ is displayed as a grey sphere. Both backbones display the same N- to C-terminus directionality indicated by the solid vertical arrow. The dashed vertical line indicates the presence of additional SNAP-25 residues on both ends of the Q197-L203 fragment, which have been omitted for clarity.
Figure 6. Induced-fit binding of I1 to the BoNT/A active site.
View of the binding cleft of the BoNT/A LC (tan surface) in complex with inhibitor I1 (grey sticks). Inhibitor residues are indicated. The induced binding pockets are evidenced by the steric clashes between the apo BoNT/A LC surface (magenta) with some of the inhibitor residues (tryptophan, threonine and leucine) upon superposition (indicated by black ovals).
A co-crystal structure of BoNT/A LC with a weakly inhibiting (Ki=1.9 μM) heptapeptide, N-Ac-CRATKML, and that of the weakly inhibiting peptides QRATKM and RRATKM have also been recently reported (Kumaran et al., 2008a; Silvaggi et al., 2008). In the N-Ac-CRATKML complex the cysteine Sγ atom directly coordinates the Zn2+ in the protease active site. Although the backbone secondary structure and directionality of this peptide is somewhat similar to that of I1, the direct coordination to the Zn2+ results in very different peptide:protein interactions. For the N-Ac-CRATKML peptide, the N-terminal acetyl group partially occupies the S1′ site in the enzyme, resulting in an altered placement of the P1′ arginine residue, which no longer makes the salt bridge contact with N370, observed for I1. The P2′ alanine residues in the N-Ac-CRATKML, QRATKM and RRATKM inhibitors lack many of the interactions of the corresponding tryptophan sidechain in I1, explaining in part the superior potency of I1 compared to these other inhibitors. This key interaction induced by I1 tryptophan provides novel, specific details to exploit in future structure-based discovery and design investigations.
The mechanism for peptide cleavage employed by the BoNT/A LC is believed to be similar to that of thermolysin, as supported by several structural and mutagenesis studies (Agarwal et al., 2004; Binz et al., 2002; Li et al., 2000; Swaminathan et al., 2004). In this model, the geometry of the zinc ion coordination in the active site changes upon SNAP-25 binding, such that the catalytic water is displaced by the carbonyl oxygen in the P1 position (i.e. Q197) of the substrate and placed in proximity to the carboxylate group of the putative ‘proton shuttle’, E224 (panel A). As a result, the catalytic water makes two hydrogen bonds with E224 in a bidentate manner. From this position, the oxygen atom in the catalytic water remains weakly coordinated to the zinc, resulting in the penta-coordination of the metal ion. Hence, E224 acts as a general base to generate a hydroxide ion that is oriented to nucleophilically attack the carbonyl carbon of the scissile peptide bond. This tetrahedral intermediate state is stabilized by putative interactions provided by the zinc ion and the hydroxyl group of Y366. Finally, the collapse of this tetrahedral intermediate and the E224-mediated transfer of two protons onto the scissile amide generate a stable amino group that then leaves the active site. In this model, the catalytic water, together with the ‘proton shuttle’ E224, are of critical importance for catalytic hydrolysis of the peptide bond in the SNAP-25 substrate.
The question arises why pseudopeptides such as I1 are not cleaved by wildtype BoNT/A LC. The structure of the BoNT/A LC:I1 complex shows that the carbonyl oxygen of P1 DNP-DAB is indeed acting as the fourth zinc-coordinating group in the complex, instead of the catalytic water molecule (Lacy et al., 1998), a feature that has been previously reported for small molecule hydroxamate inhibitors in complex with the BoNT/A LC (Fu et al., 2006; Silvaggi et al., 2007). In addition, the complex structure also confirms the proposed hydrogen bonding between the hydroxyl group of the Y366 side chain in the BoNT/A LC, and the P1 carbonyl oxygen. Nonetheless, the P1 N-terminal amino group forms a hydrogen bond with the carboxylate group of E224, the putative ‘proton shuttle’ residue in the BoNT/A LC (Li et al., 2000), effectively abrogating any interaction between E224 and a potential catalytic water molecule. Thus, E224 is clearly not positioned for a potential nucleophilic attack on the scissile carbonyl. In addition to this proton shuttle ‘blocking’ effect, the putative scissile amide in position P1′ in the inhibitor is distant from the E224 carboxylate group, further hampering any hydrolytic event with the scissile bond. Thus, the I1 inhibitor effectively disrupts the catalytic mechanism of BoNT/LC by displacement of the catalytic water molecule and the E224 proton shuttle.
The co-crystal structure of I1 with BoNT/LC provides a new paradigm for peptidomimetic inhibitor binding in the BoNT/A LC substrate cleft and rationalizes the SAR of related derivatives (Schmidt and Bostian, 1997; Schmidt et al., 1998). This structural knowledge can serve as the platform for designing more potent analogs with therapeutic viability.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal R, Binz T, Swaminathan S. Structural analysis of botulinum neurotoxin serotype F light chain: implications on substrate binding and inhibitor design. Biochemistry. 2005;44:11758–11765. doi: 10.1021/bi0510072. [DOI] [PubMed] [Google Scholar]
- Agarwal R, Eswaramoorthy S, Kumaran D, Binz T, Swaminathan S. Structural analysis of botulinum neurotoxin type E catalytic domain and its mutant Glu212-->Gln reveals the pivotal role of the Glu212 carboxylate in the catalytic pathway. Biochemistry. 2004;43:6637–6644. doi: 10.1021/bi036278w. [DOI] [PubMed] [Google Scholar]
- Arndt JW, Chai Q, Christian T, Stevens RC. Structure of botulinum neurotoxin type D light chain at 1.65 A resolution: repercussions for VAMP-2 substrate specificity. Biochemistry. 2006;45:3255–3262. doi: 10.1021/bi052518r. [DOI] [PubMed] [Google Scholar]
- Arndt JW, Yu W, Bi F, Stevens RC. Crystal structure of botulinum neurotoxin type G light chain: serotype divergence in substrate recognition. Biochemistry. 2005;44:9574–9580. doi: 10.1021/bi0505924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MR, Barbieri JT. Association of botulinum neurotoxin serotypes a and B with synaptic vesicle protein complexes. Biochemistry. 2007;46:3200–3210. doi: 10.1021/bi602396x. [DOI] [PubMed] [Google Scholar]
- Bigalke H, Muller H, Dreyer F. Botulinum A neurotoxin unlike tetanus toxin acts via a neuraminidase sensitive structure. Toxicon. 1986;24:1065–1074. doi: 10.1016/0041-0101(86)90133-9. [DOI] [PubMed] [Google Scholar]
- Bigalke H, Shoer LF. Clostridial Neurotoxins. In: Aktories K, Just I, editors. Bacterial Protein Toxins. Berlin, Heidelberg, New York: Springer-Verlag; 2000. pp. 407–444. [Google Scholar]
- Binz T, Bade S, Rummel A, Kollewe A, Alves J. Arg(362) and Tyr(365) of the botulinum neurotoxin type a light chain are involved in transition state stabilization. Biochemistry. 2002;41:1717–1723. doi: 10.1021/bi0157969. [DOI] [PubMed] [Google Scholar]
- Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Sudhof TC, Jahn R, Niemann H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem. 1994;269:1617–1620. [PubMed] [Google Scholar]
- Black JD, Dolly JO. Interaction of 125I-labeled botulinum neurotoxins with nerve terminals. I. Ultrastructural autoradiographic localization and quantitation of distinct membrane acceptors for types A and B on motor nerves. J Cell Biol. 1986;103:521–534. doi: 10.1083/jcb.103.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert S, Schiavo G. Tetanus toxin is transported in a novel neuronal compartment characterized by a specialized pH regulation. J Biol Chem. 2005;280:42336–42344. doi: 10.1074/jbc.M506750200. [DOI] [PubMed] [Google Scholar]
- Breidenbach MA, Brunger AT. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature. 2004;432:925–929. doi: 10.1038/nature03123. [DOI] [PubMed] [Google Scholar]
- Breidenbach MA, Brunger AT. 2.3 A crystal structure of tetanus neurotoxin light chain. Biochemistry. 2005;44:7450–7457. doi: 10.1021/bi050262j. [DOI] [PubMed] [Google Scholar]
- Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q Rev Biophys. 2006;38:1–47. doi: 10.1017/S0033583505004051. [DOI] [PubMed] [Google Scholar]
- Bullens RW, O’Hanlon GM, Wagner E, Molenaar PC, Furukawa K, Furukawa K, Plomp JJ, Willison HJ. Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J Neurosci. 2002;22:6876–6884. doi: 10.1523/JNEUROSCI.22-16-06876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett JC, Ruthel G, Stegmann CM, Panchal RG, Nguyen TL, Hermone AR, Stafford RG, Lane DJ, Kenny TA, McGrath CF, et al. Inhibition of metalloprotease botulinum serotype A from a pseudo-peptide binding mode to a small molecule that is active in primary neurons. J Biol Chem. 2007;282:5004–5014. doi: 10.1074/jbc.M608166200. [DOI] [PubMed] [Google Scholar]
- Chai Q, Arndt JW, Dong M, Tepp WH, Johnson EA, Chapman ER, Stevens RC. Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature. 2006;444:1096–1100. doi: 10.1038/nature05411. [DOI] [PubMed] [Google Scholar]
- Chapman ER. Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- Chen C, Baldwin MR, Barbieri JT. Molecular basis for tetanus toxin coreceptor interactions. Biochemistry. 2008;47:7179–7186. doi: 10.1021/bi800640y. [DOI] [PubMed] [Google Scholar]
- Chen S, Barbieri JT. Unique substrate recognition by botulinum neurotoxins serotypes A and E. J Biol Chem. 2006;281:10906–10911. doi: 10.1074/jbc.M513032200. [DOI] [PubMed] [Google Scholar]
- Cornille F, Martin L, Lenoir C, Cussac D, Roques BP, Fournie-Zaluski MC. Cooperative exosite-dependent cleavage of synaptobrevin by tetanus toxin light chain. J Biol Chem. 1997;272:3459–3464. doi: 10.1074/jbc.272.6.3459. [DOI] [PubMed] [Google Scholar]
- Critchley DR, Habig WH, Fishman PH. Reevaluation of the role of gangliosides as receptors for tetanus toxin. J Neurochem. 1986;47:213–222. doi: 10.1111/j.1471-4159.1986.tb02852.x. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Berninghausen O, Willison HJ, Hopkins CR, Schiavo G. Tetanus toxin is internalized by a sequential clathrin-dependent mechanism initiated within lipid microdomains and independent of epsin1. J Cell Biol. 2006a;174:459–471. doi: 10.1083/jcb.200508170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006b;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Dolly JO, Black J, Williams RS, Melling J. Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature. 1984;307:457–460. doi: 10.1038/307457a0. [DOI] [PubMed] [Google Scholar]
- Dolly JO, Williams RS, Black JD, Tse CK, Hambleton P, Melling J. Localization of sites for 125I-labelled botulinum neurotoxin at murine neuromuscular junction and its binding to rat brain synaptosomes. Toxicon. 1982;20:141–148. doi: 10.1016/0041-0101(82)90183-0. [DOI] [PubMed] [Google Scholar]
- Dong M, Liu H, Tepp WH, Johnson EA, Janz R, Chapman ER. Glycosylated SV2A and SV2B Mediate the Entry of Botulinum Neurotoxin E into Neurons. Mol Biol Cell. 2008 doi: 10.1091/mbc.E08-07-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Richards DA, Goodnough MC, Tepp WH, Johnson EA, Chapman ER. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J Cell Biol. 2003;162:1293–1303. doi: 10.1083/jcb.200305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- Emsley P, Fotinou C, Black I, Fairweather NF, Charles IG, Watts C, Hewitt E, Isaacs NW. The structures of the HC-fragment of tetanus toxin with carbohydrate subunit complexes provide insight into ganglioside binding. J Biol Chem. 2000;275:8889–8894. doi: 10.1074/jbc.275.12.8889. [DOI] [PubMed] [Google Scholar]
- Eswaramoorthy S, Kumaran D, Swaminathan S. Crystallographic evidence for doxorubicin binding to the receptor-binding site in Clostridium botulinum neurotoxin B. Acta Crystallogr D Biol Crystallogr. 2001;57:1743–1746. doi: 10.1107/s0907444901013531. [DOI] [PubMed] [Google Scholar]
- Evinger C, Erichsen JT. Transsynaptic retrograde transport of fragment C of tetanus toxin demonstrated by immunohistochemical localization. Brain Res. 1986;380:383–388. doi: 10.1016/0006-8993(86)90241-6. [DOI] [PubMed] [Google Scholar]
- Figueiredo D, Turcotte C, Frankel G, Li Y, Dolly O, Wilkin G, Marriott D, Fairweather N, Dougan G. Characterization of recombinant tetanus toxin derivatives suitable for vaccine development. Infect Immun. 1995;63:3218–3221. doi: 10.1128/iai.63.8.3218-3221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Montal M. Crucial Role of the Disulfide Bridge between Botulinum Neurotoxin Light and Heavy Chains in Protease Translocation across Membranes. J Biol Chem. 2007a;282:29604–29611. doi: 10.1074/jbc.M703619200. [DOI] [PubMed] [Google Scholar]
- Fischer A, Montal M. Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. Proc Natl Acad Sci U S A. 2007b;104:10447–10452. doi: 10.1073/pnas.0700046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman PS, Carrigan DR. Retrograde transneuronal transfer of the C-fragment of tetanus toxin. Brain Res. 1987;406:275–279. doi: 10.1016/0006-8993(87)90792-x. [DOI] [PubMed] [Google Scholar]
- Foran P, Shone CC, Dolly JO. Differences in the protease activities of tetanus and botulinum B toxins revealed by the cleavage of vesicle-associated membrane protein and various sized fragments. Biochemistry. 1994;33:15365–15374. doi: 10.1021/bi00255a017. [DOI] [PubMed] [Google Scholar]
- Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, Sinha KA, Fairweather NF, Isaacs NW. The crystal structure of tetanus toxin HC- fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J Biol Chem. 2001;276:32274–32281. doi: 10.1074/jbc.M103285200. [DOI] [PubMed] [Google Scholar]
- Fu Z, Chen S, Baldwin MR, Boldt GE, Crawford A, Janda KD, Barbieri JT, Kim JJ. Light chain of botulinum neurotoxin serotype A: structural resolution of a catalytic intermediate. Biochemistry. 2006;45:8903–8911. doi: 10.1021/bi060786z. [DOI] [PubMed] [Google Scholar]
- Galloux M, Vitrac H, Montagner C, Raffestin S, Popoff MR, Chenal A, Forge V, Gillet D. Membrane interaction of botulinum neurotoxin A T domain: The belt region is a regulatory loop for membrane interaction. J Biol Chem. 2008 doi: 10.1074/jbc.M802557200. [DOI] [PubMed] [Google Scholar]
- Geppert M, Archer BT, 3rd, Sudhof TC. Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J Biol Chem. 1991;266:13548–13552. [PubMed] [Google Scholar]
- Gill DM. Bacterial toxins: a table of lethal amounts. Microbiol Rev. 1982;46:86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern JL, Loftus A. Characterization of the receptor-binding domain of tetanus toxin. J Biol Chem. 1993;268:11188–11192. [PubMed] [Google Scholar]
- Halpern JL, Neale EA. Neurospecific binding, internalization, and retrograde axonal transport. Curr Top Microbiol Immunol. 1995;195:221–241. doi: 10.1007/978-3-642-85173-5_10. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Stevens RC. Cocrystal structure of synaptobrevin-II bound to botulinum neurotoxin type B at 2.0 A resolution. Nat Struct Biol. 2000;7:687–692. doi: 10.1038/77997. [DOI] [PubMed] [Google Scholar]
- Herreros J, Lalli G, Montecucco C, Schiavo G. Tetanus toxin fragment C binds to a protein present in neuronal cell lines and motoneurons. J Neurochem. 2000;74:1941–1950. doi: 10.1046/j.1471-4159.2000.0741941.x. [DOI] [PubMed] [Google Scholar]
- Herreros J, Ng T, Schiavo G. Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons. Mol Biol Cell. 2001;12:2947–2960. doi: 10.1091/mbc.12.10.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R, Whaler BC. Influence of nerve-ending activity and of drugs on the rate of paralysis of rat diaphragm preparations by Cl. botulinum type A toxin. J Physiol. 1962;160:221–233. doi: 10.1113/jphysiol.1962.sp006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara H, Kohda T, Morimoto F, Tsukamoto K, Karasawa T, Nakamura S, Mukamoto M, Kozaki S. Sequence of the gene for Clostridium botulinum type B neurotoxin associated with infant botulism, expression of the C-terminal half of heavy chain and its binding activity. Biochim Biophys Acta. 2003;1625:19–26. doi: 10.1016/s0167-4781(02)00537-7. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Eswaramoorthy S, Kumaran D, Swaminathan S. Common binding site for disialyllactose and tri-peptide in C-fragment of tetanus neurotoxin. Proteins. 2005;61:288–295. doi: 10.1002/prot.20595. [DOI] [PubMed] [Google Scholar]
- Jin R, Rummel A, Binz T, Brunger AT. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature. 2006;444:1092–1095. doi: 10.1038/nature05387. [DOI] [PubMed] [Google Scholar]
- Jin R, Sikorra S, Stegmann CM, Pich A, Binz T, Brunger AT. Structural and Biochemical Studies of Botulinum Neurotoxin Serotype C1 Light Chain Protease: Implications for Dual Substrate Specificity(,) Biochemistry. 2007;46:10685–10693. doi: 10.1021/bi701162d. [DOI] [PubMed] [Google Scholar]
- Keller JE, Cai F, Neale EA. Uptake of botulinum neurotoxin into cultured neurons. Biochemistry. 2004;43:526–532. doi: 10.1021/bi0356698. [DOI] [PubMed] [Google Scholar]
- Kitamura M. Binding of botulinum neurotoxin to the synaptosome fraction of rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1976;295:171–175. doi: 10.1007/BF00499451. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Igimi S, Furukawa K. Different response of the knockout mice lacking b-series gangliosides against botulinum and tetanus toxins. Biochim Biophys Acta. 2005;1741:1–3. doi: 10.1016/j.bbadis.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Takamiya K, Aizawa S, Furukawa K. Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. Biochim Biophys Acta. 1999;1441:1–3. doi: 10.1016/s1388-1981(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Knapp M, Segelke B, Rupp B. The 1.61 Ångström structure of the tetanus toxin ganglioside binding region: solved by MAD and mir phase combination. American Crystallography Association Annual Meeting; 1998. p. 90. [Google Scholar]
- Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat Struct Biol. 2003;10:13–18. doi: 10.1038/nsb879. [DOI] [PubMed] [Google Scholar]
- Kozaki S, Kamata Y, Watarai S, Nishiki T, Mochida S. Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb Pathog. 1998;25:91–99. doi: 10.1006/mpat.1998.0214. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Rawat R, Ahmed SA, Swaminathan S. Substrate binding mode and its implication on drug design for botulinum neurotoxin A. PLoS Pathog. 2008a;4:e1000165. doi: 10.1371/journal.ppat.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Rawat R, Ludivico ML, Ahmed SA, Swaminathan S. Structure- and substrate-based inhibitor design for Clostridium botulinum neurotoxin serotype A. J Biol Chem. 2008b;283:18883–18891. doi: 10.1074/jbc.M801240200. [DOI] [PubMed] [Google Scholar]
- Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat Struct Biol. 1998;5:898–902. doi: 10.1038/2338. [DOI] [PubMed] [Google Scholar]
- Lalli G, Herreros J, Osborne SL, Montecucco C, Rossetto O, Schiavo G. Functional characterisation of tetanus and botulinum neurotoxins binding domains. J Cell Sci. 1999;112(Pt 16):2715–2724. doi: 10.1242/jcs.112.16.2715. [DOI] [PubMed] [Google Scholar]
- Lazarovici P, Yavin E. Affinity-purified tetanus neurotoxin interaction with synaptic membranes: properties of a protease-sensitive receptor component. Biochemistry. 1986;25:7047–7054. doi: 10.1021/bi00370a044. [DOI] [PubMed] [Google Scholar]
- Li L, Binz T, Niemann H, Singh BR. Probing the mechanistic role of glutamate residue in the zinc-binding motif of type A botulinum neurotoxin light chain. Biochemistry. 2000;39:2399–2405. doi: 10.1021/bi992321x. [DOI] [PubMed] [Google Scholar]
- Lightstone FC, Prieto MC, Singh AK, Piqueras MC, Whittal RM, Knapp MS, Balhorn R, Roe DC. Identification of novel small molecule ligands that bind to tetanus toxin. Chem Res Toxicol. 2000;13:356–362. doi: 10.1021/tx000009e. [DOI] [PubMed] [Google Scholar]
- Louch HA, Buczko ES, Woody MA, Venable RM, Vann WF. Identification of a binding site for ganglioside on the receptor binding domain of tetanus toxin. Biochemistry. 2002;41:13644–13652. doi: 10.1021/bi020291j. [DOI] [PubMed] [Google Scholar]
- Mahrhold S, Rummel A, Bigalke H, Davletov B, Binz T. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006;580:2011–2014. doi: 10.1016/j.febslet.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Marxen P, Bigalke H. Tetanus toxin: inhibitory action in chromaffin cells is initiated by specified types of gangliosides and promoted in low ionic strength solution. Neurosci Lett. 1989;107:261–266. doi: 10.1016/0304-3940(89)90828-8. [DOI] [PubMed] [Google Scholar]
- Marxen P, Erdmann G, Bigalke H. The translocation of botulinum A neurotoxin by chromaffin cells is promoted in low ionic strength solution and is insensitive to trypsin. Toxicon. 1991;29:181–189. doi: 10.1016/0041-0101(91)90102-w. [DOI] [PubMed] [Google Scholar]
- Marxen P, Fuhrmann U, Bigalke H. Gangliosides mediate inhibitory effects of tetanus and botulinum A neurotoxins on exocytosis in chromaffin cells. Toxicon. 1989;27:849–859. doi: 10.1016/0041-0101(89)90097-4. [DOI] [PubMed] [Google Scholar]
- Montecucco C. How do tetanus and botulinum neurotoxins bind to neuronal membranes? Trends Biochem Sci. 1986;11:314–317. [Google Scholar]
- Munro P, Kojima H, Dupont JL, Bossu JL, Poulain B, Boquet P. High sensitivity of mouse neuronal cells to tetanus toxin requires a GPI-anchored protein. Biochem Biophys Res Commun. 2001;289:623–629. doi: 10.1006/bbrc.2001.6031. [DOI] [PubMed] [Google Scholar]
- Niemann H, Binz T, Grebenstein O, Kurazono H, Thierer J, Mochida S, Poulain B, Tauc L. Clostridial neurotoxins: from toxins to therapeutic tools? Behring Inst Mitt. 1991:153–162. [PubMed] [Google Scholar]
- Nishiki T, Kamata Y, Nemoto Y, Omori A, Ito T, Takahashi M, Kozaki S. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J Biol Chem. 1994;269:10498–10503. [PubMed] [Google Scholar]
- Nishiki T, Ogasawara J, Kamata Y, Kozaki S. Solubilization and characterization of the acceptor for Clostridium botulinum type B neurotoxin from rat brain synaptic membranes. Biochim Biophys Acta. 1993;1158:333–338. doi: 10.1016/0304-4165(93)90032-4. [DOI] [PubMed] [Google Scholar]
- Nishiki T, Tokuyama Y, Kamata Y, Nemoto Y, Yoshida A, Sato K, Sekiguchi M, Takahashi M, Kozaki S. The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett. 1996a;378:253–257. doi: 10.1016/0014-5793(95)01471-3. [DOI] [PubMed] [Google Scholar]
- Nishiki T, Tokuyama Y, Kamata Y, Nemoto Y, Yoshida A, Sekiguchi M, Takahashi M, Kozaki S. Binding of botulinum type B neurotoxin to Chinese hamster ovary cells transfected with rat synaptotagmin II cDNA. Neurosci Lett. 1996b;208:105–108. doi: 10.1016/0304-3940(96)12557-x. [DOI] [PubMed] [Google Scholar]
- Oost T, Sukonpan C, Brewer M, Goodnough M, Tepp W, Johnson EA, Rich DH. Design and synthesis of substrate-based inhibitors of botulinum neurotoxin type B metalloprotease. Biopolymers. 2003;71:602–619. doi: 10.1002/bip.10590. [DOI] [PubMed] [Google Scholar]
- Pellizzari R, Rossetto O, Lozzi L, Giovedi S, Johnson E, Shone CC, Montecucco C. Structural determinants of the specificity for synaptic vesicle-associated membrane protein/synaptobrevin of tetanus and botulinum type B and G neurotoxins. J Biol Chem. 1996;271:20353–20358. doi: 10.1074/jbc.271.34.20353. [DOI] [PubMed] [Google Scholar]
- Perin MS, Brose N, Jahn R, Sudhof TC. Domain structure of synaptotagmin (p65) J Biol Chem. 1991;266:623–629. [PubMed] [Google Scholar]
- Pierce EJ, Davison MD, Parton RG, Habig WH, Critchley DR. Characterization of tetanus toxin binding to rat brain membranes. Evidence for a high-affinity proteinase-sensitive receptor. Biochem J. 1986;236:845–852. doi: 10.1042/bj2360845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KN, Kumaran D, Binz T, Swaminathan S. Structural analysis of the catalytic domain of tetanus neurotoxin. Toxicon. 2005;45:929–939. doi: 10.1016/j.toxicon.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Rossetto O, Schiavo G, Montecucco C, Poulain B, Deloye F, Lozzi L, Shone CC. SNARE motif and neurotoxins. Nature. 1994;372:415–416. doi: 10.1038/372415a0. [DOI] [PubMed] [Google Scholar]
- Rummel A, Bade S, Alves J, Bigalke H, Binz T. Two carbohydrate binding sites in the H(CC)-domain of tetanus neurotoxin are required for toxicity. J Mol Biol. 2003;326:835–847. doi: 10.1016/s0022-2836(02)01403-1. [DOI] [PubMed] [Google Scholar]
- Rummel A, Eichner T, Weil T, Karnath T, Gutcaits A, Mahrhold S, Sandhoff K, Proia RL, Acharya KR, Bigalke H, Binz T. Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc Natl Acad Sci U S A. 2007;104:359–364. doi: 10.1073/pnas.0609713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel A, Karnath T, Henke T, Bigalke H, Binz T. Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J Biol Chem. 2004a;279:30865–30870. doi: 10.1074/jbc.M403945200. [DOI] [PubMed] [Google Scholar]
- Rummel A, Mahrhold S, Bigalke H, Binz T. The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol Microbiol. 2004b;51:631–643. doi: 10.1046/j.1365-2958.2003.03872.x. [DOI] [PubMed] [Google Scholar]
- Rupp B, Segelke B. Questions about the structure of the botulinum neurotoxin B light chain in complex with a target peptide. Nat Struct Biol. 2001;8:663–664. doi: 10.1038/90361. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Papini E, Genna G, Montecucco C. An intact interchain disulfide bond is required for the neurotoxicity of tetanus toxin. Infect Immun. 1990;58:4136–4141. doi: 10.1128/iai.58.12.4136-4141.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Shone CC, Bennett MK, Scheller RH, Montecucco C. Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J Biol Chem. 1995;270:10566–10570. doi: 10.1074/jbc.270.18.10566. [DOI] [PubMed] [Google Scholar]
- Schmidt JJ, Bostian KA. Proteolysis of synthetic peptides by type A botulinum neurotoxin. J Protein Chem. 1995;14:703–708. doi: 10.1007/BF01886909. [DOI] [PubMed] [Google Scholar]
- Schmidt JJ, Bostian KA. Endoproteinase activity of type A botulinum neurotoxin: substrate requirements and activation by serum albumin. J Protein Chem. 1997;16:19–26. doi: 10.1023/a:1026386710428. [DOI] [PubMed] [Google Scholar]
- Schmidt JJ, Stafford RG, Bostian KA. Type A botulinum neurotoxin proteolytic activity: development of competitive inhibitors and implications for substrate specificity at the S1′ binding subsite. FEBS Lett. 1998;435:61–64. doi: 10.1016/s0014-5793(98)01041-2. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Dreyer F, John C. At least three sequential steps are involved in the tetanus toxin-induced block of neuromuscular transmission. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:326–330. doi: 10.1007/BF00501314. [DOI] [PubMed] [Google Scholar]
- Segelke B, Knapp M, Kadkhodayan S, Balhorn R, Rupp B. Crystal structure of Clostridium botulinum neurotoxin protease in a product-bound state: Evidence for noncanonical zinc protease activity. Proc Natl Acad Sci U S A. 2004;101:6888–6893. doi: 10.1073/pnas.0400584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RE, Specht CD, Collins BE, Woods AS, Cotter RJ, Schnaar RL. Identification of a ganglioside recognition domain of tetanus toxin using a novel ganglioside photoaffinity ligand. J Biol Chem. 1997;272:30380–30386. doi: 10.1074/jbc.272.48.30380. [DOI] [PubMed] [Google Scholar]
- Sikorra S, Henke T, Galli T, Binz T. Substrate Recognition Mechanism of VAMP/Synaptobrevin-cleaving Clostridial Neurotoxins. J Biol Chem. 2008;283:21145–21152. doi: 10.1074/jbc.M800610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorra S, Henke T, Swaminathan S, Galli T, Binz T. Identification of the amino acid residues rendering TI-VAMP insensitive toward botulinum neurotoxin B. J Mol Biol. 2006;357:574–582. doi: 10.1016/j.jmb.2005.12.075. [DOI] [PubMed] [Google Scholar]
- Silvaggi NR, Boldt GE, Hixon MS, Kennedy JP, Tzipori S, Janda KD, Allen KN. Structures of Clostridium botulinum Neurotoxin Serotype A Light Chain complexed with small-molecule inhibitors highlight active-site flexibility. Chem Biol. 2007;14:533–542. doi: 10.1016/j.chembiol.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Silvaggi NR, Wilson D, Tzipori S, Allen KN. Catalytic features of the botulinum neurotoxin A light chain revealed by high resolution structure of an inhibitory peptide complex. Biochemistry. 2008;47:5736–5745. doi: 10.1021/bi8001067. [DOI] [PubMed] [Google Scholar]
- Simpson LL. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980;212:16–21. [PubMed] [Google Scholar]
- Simpson LL. Botulinum toxin and tetanus toxin recognize similar membrane determinants. Brain Res. 1984a;305:177–180. doi: 10.1016/0006-8993(84)91136-3. [DOI] [PubMed] [Google Scholar]
- Simpson LL. The binding fragment from tetanus toxin antagonizes the neuromuscular blocking actions of botulinum toxin. J Pharmacol Exp Ther. 1984b;229:182–187. [PubMed] [Google Scholar]
- Simpson LL. Pharmacological experiments on the binding and internalization of the 50,000 dalton carboxyterminus of tetanus toxin at the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1985;234:100–105. [PubMed] [Google Scholar]
- Simpson LL, Rapport MM. The binding of botulinum toxin to membrane lipids: sphingolipids, steroids and fatty acids. J Neurochem. 1971;18:1751–1759. doi: 10.1111/j.1471-4159.1971.tb03750.x. [DOI] [PubMed] [Google Scholar]
- Sinha K, Box M, Lalli G, Schiavo G, Schneider H, Groves M, Siligardi G, Fairweather N. Analysis of mutants of tetanus toxin Hc fragment: ganglioside binding, cell binding and retrograde axonal transport properties. Mol Microbiol. 2000;37:1041–1051. doi: 10.1046/j.1365-2958.2000.02091.x. [DOI] [PubMed] [Google Scholar]
- Stenmark P, Dupuy J, Imamura A, Kiso M, Stevens RC. Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b-insight into the toxin-neuron interaction. PLoS Pathog. 2008;4:e1000129. doi: 10.1371/journal.ppat.1000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Synaptotagmins: why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- Sutton JM, Chow-Worn O, Spaven L, Silman NJ, Hallis B, Shone CC. Tyrosine-1290 of tetanus neurotoxin plays a key role in its binding to gangliosides and functional binding to neurones. FEBS Lett. 2001;493:45–49. doi: 10.1016/s0014-5793(01)02273-6. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Eswaramoorthy S. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat Struct Biol. 2000;7:693–699. doi: 10.1038/78005. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Eswaramoorthy S, Kumaran D. Structure and enzymatic activity of botulinum neurotoxins. Mov Disord. 2004;19(Suppl 8):S17–22. doi: 10.1002/mds.20005. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Kohda T, Mukamoto M, Takeuchi K, Ihara H, Saito M, Kozaki S. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J Biol Chem. 2005;280:35164–35171. doi: 10.1074/jbc.M507596200. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Kozai Y, Ihara H, Kohda T, Mukamoto M, Tsuji T, Kozaki S. Identification of the receptor-binding sites in the carboxyl-terminal half of the heavy chain of botulinum neurotoxin types C and D. Microb Pathog. 2008;44:484–493. doi: 10.1016/j.micpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Umland TC, Wingert LM, Swaminathan S, Furey WF, Schmidt JJ, Sax M. Structure of the receptor binding fragment HC of tetanus neurotoxin. Nat Struct Biol. 1997;4:788–792. doi: 10.1038/nsb1097-788. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan VV, Yoshino K, Jahnz M, Dorries C, Bade S, Nauenburg S, Niemann H, Binz T. Proteolysis of SNAP-25 isoforms by botulinum neurotoxin types A, C, and E: domains and amino acid residues controlling the formation of enzyme-substrate complexes and cleavage. J Neurochem. 1999;72:327–337. doi: 10.1046/j.1471-4159.1999.0720327.x. [DOI] [PubMed] [Google Scholar]
- van Heyningen WE, Miller PA. The fixation of tetanus toxin by ganglioside. J Gen Microbiol. 1961;24:107–119. doi: 10.1099/00221287-24-1-107. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Pellizzari R, Baldini G, Wilson MC, Montecucco C. Botulinum neurotoxin types A and E require the SNARE motif in SNAP-25 for proteolysis. FEBS Lett. 1997;418:1–5. doi: 10.1016/s0014-5793(97)01328-8. [DOI] [PubMed] [Google Scholar]
- Williamson LC, Bateman KE, Clifford JC, Neale EA. Neuronal sensitivity to tetanus toxin requires gangliosides. J Biol Chem. 1999;274:25173–25180. doi: 10.1074/jbc.274.35.25173. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Baumeister A, Binz T, Blasi J, Link E, Cornille F, Roques B, Fykse EM, Sudhof TC, Jahn R, et al. Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J Biol Chem. 1994;269:12764–12772. [PubMed] [Google Scholar]
- Yowler BC, Kensinger RD, Schengrund CL. Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J Biol Chem. 2002;277:32815–32819. doi: 10.1074/jbc.M205258200. [DOI] [PubMed] [Google Scholar]
- Yowler BC, Schengrund CL. Glycosphingolipids-sweets for botulinum neurotoxin. Glycoconj J. 2004;21:287–293. doi: 10.1023/B:GLYC.0000046271.64647.fd. [DOI] [PubMed] [Google Scholar]
- Zuniga J, Schmidt JJ, Fenn T, Burnett JC, Arac D, Gussio R, Stafford RG, Badie SS, Bavari S, Brunger AT. A potent peptidomimetic inhibitor of botulinum neurotoxin serotype A has a very different conformation than SNAP-25 substrate. Structure. 2008 doi: 10.1016/j.str.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]