Abstract
The very unstable (< 10 min at rt) o-quinone (5) derived from the vicinal diphenol anticancer drug combretastatin A-1 (1) has been obtained by careful oxidation with NaIO4 and tetrabutylammonium bromide in water/dichloromethane. Immediate reaction with phenylenediamine (6) allowed o-quinone 5 to be trapped as the stable phenazine derivative (7). For further confirmation, 5 was also captured as a dimethoxyphenylenediamine-derived phenazine (11). Both phenazines 7 and 11 significantly inhibited (ED50 ~ 0.2 μg/mL) growth of the murine P388 lymphocytic leukemia cell line and provided a new SAR insight in the combretastatin series of naturally occurring anticancer drugs.
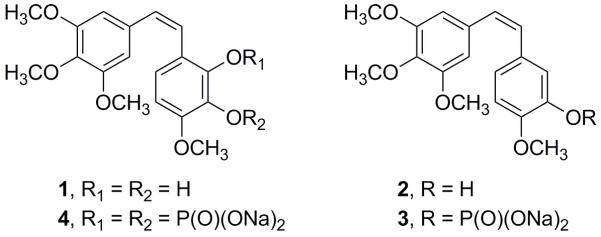
In 1987 we reported2 the isolation and synthesis of combretastatin A-1 (1, Figure 1), a reasonably abundant (0.9% w/w yield) antineoplastic constituent of the South African tree Combretum caffrum. While initial in vivo anticancer evaluations gave encouraging results, it soon became apparent that 1 was unstable relative to the lead anticancer constituent of C. caffrum, combretastatin A-4 (2, Figure 1).3 An extended series of experiments aimed at uncovering practical prodrugs of the sparingly soluble (in water) 2 and the unstable 1 culminated in the syntheses of sodium combretastatin A-4 phosphate (3, Zybrestat,™ Figure 1),4 presently in phase II human cancer clinical trials5 with phase III trials under way, and of sodium combretastatin A-1 diphosphate (4, aka OXI-4503 as the potassium salt, Figure 1),6a for which clinical development is under way.6b
Figure 1.
Structures of combretastatin A-1 (1) and A-4 (2) and the phosphate prodrugs of A-4 (3) and A-1 (4).
Selection of 3 and 4 (as the potassium salt) for development to clinical trials was based on extensive preclinical experiments that pointed to both as outstanding cancer antiangiogenic/vascular disrupting anticancer drug candidates combined with a number of other promising properties that indicated considerable mechanistic and metabolic pathway differences.7 For example, in SCID mice given the MHEC5-T hemangioendothelioma and a single dose of 4 at 100 mg/kg, tumor blood flow was reduced by 50% in 1 h and by 24 h was decreased over 90% with only partial recovery (27-35%) at 72 h. Very significantly, by 12 h, the tumor blood vessels were completely destroyed. Indeed, at 1 - 3 h, morphological changes and initiation of apoptosis had already begun, accompanied by an increase in vascular permeability favoring an increase in the retention time of companion drug(s). The terminal result as with 3 is massive tumor cell necrosis.7 As indicated in the example first noted, 4 has shown excellent potential in preclinical studies leading to tumor regression as a single agent that also attacks the remaining tumor rim cancer cells.5,7 The mechanism of action differs (as was originally presumed, 4 forms an o-quinone intermediate in vivo)7a from that of 3 and both have been shown to improve the effectiveness of, for example, Avastin and other anticancer drug combinations. A Phase Ib clinical trial of the 3/Avastin combination began in early 2006. The first Phase I clinical trial of 4 (as the potassium salt) was initiated in 2005, supported by the use of extensive blood studies and MRI and PET scans to gain further insights into the mechanism of action.7
Results and Discussion
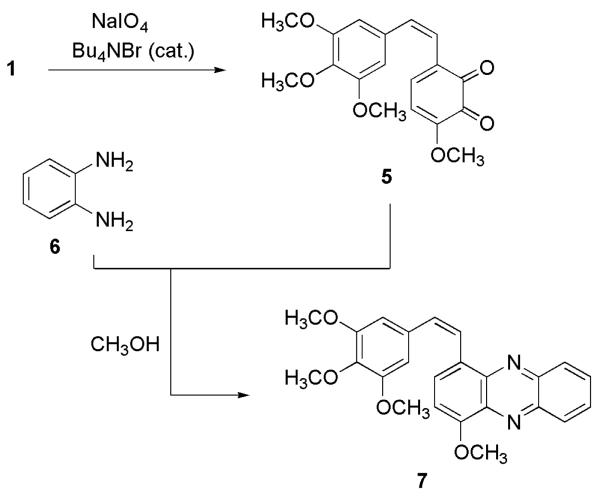
Early (ca. 1990) in our chemical studies of 1, it seemed likely that the easily observed color changes and rapid degradation of this vicinal phenol were due to oxidation reactions leading to the corresponding, and quite unstable, o-quinone 5.8 In order to better understand the in vivo metabolism of 1, we have first succeeded in preparing and trapping the very transient 5 as a phenazine derivative (Figure 2). Prior to oxidation of 1, a number of oxidative reagents were investigated using a more readily available diphenol. Thus, catechol was subjected to oxidative procedures using NaIO4/DCM/Bu4NBr,9 Ag2O/EtOH,10 Ag2O/EtOH/sonication,10 11a and NaIO4/H2O.11b-d The products from these reactions suggested that the NaIO4/DCM/Bu4NBr procedure might be useful with 1. The 1H NMR analysis of the crude product from catechol indicated the presence of the o-quinone with signals at δ 5.88 and δ 3.07 as multiplets, along with other products.
Figure 2.
Synthesis of phenazine 7 from CA1 (1).
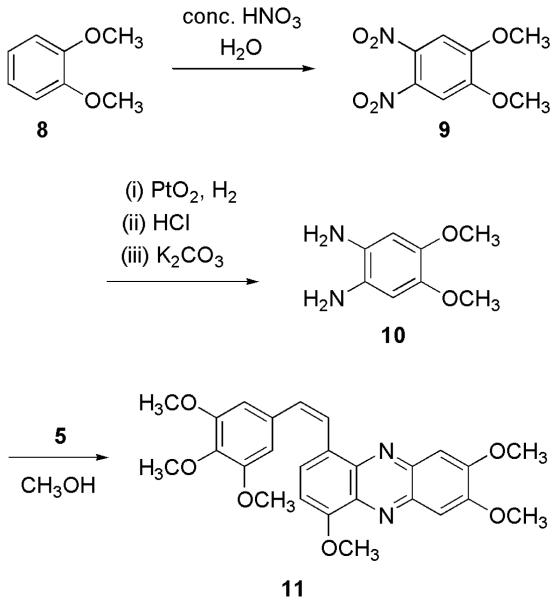
Oxidation of 1 with NaIO4/DCM/Bu4NBr gave evidence of 5 in solution, but it proved to be too unstable to isolate. Although its existence was indicated by a blue color, this rapidly changed to black, as observed with the catechol oxidation. To confirm formation of 5, trapping it as a pyrazine derivative11c,d proved to be very useful. By this route, 1 was oxidized to 5 by use of the NaIO4 technique (Figure 2).9 After 10 min, diamine 6 was added. Separation of the crude reaction mixture led to pyrazine 7 in 25% yield. For added confirmation that 5 had been prepared, albeit in transient fashion, the oxidation of 1 was repeated a number of times and the resulting o-quinone allowed to react with diamine 10 prepared (via 9, Figure 3) from veratrole (8).12 As expected, compound 11 was formed. Additional combretastatin SAR information was derived from the o-quinone experiments when phenazines 7 and 11 were found to inhibit significantly (ED50 ~ 0.2 μg/mL growth of the P388 lymphocytic leukemia cell line.
Figure 3.
Synthesis of phenazine 11.
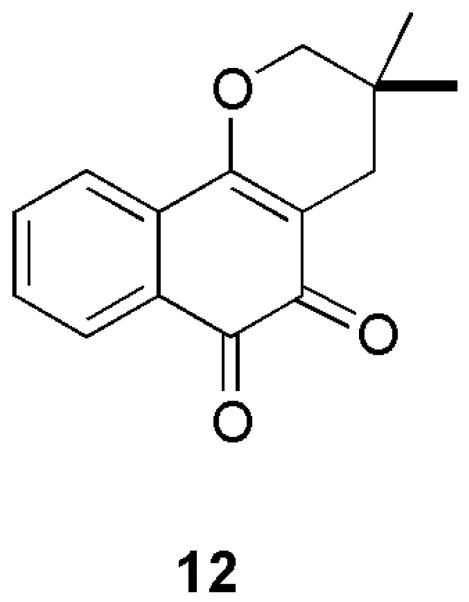
In summary, the rather remarkable instability of 5 derived from 1 that can be attributed to its very extended π-electron system may certainly account for the complex array of metabolic products produced in vivo as well as the difference in anticancer activity of 4 in comparison to 3.7 Some of those considerations may apply to rhinacanthone (12), an o-quinone antineoplastic constitutent of the traditional medicinal (cancer, heptatitis and others) plant Rhinacanthus nasutus.13
Experimental Section
General Experimental Procedures
Melting points are uncorrected and were determined with an Electrothermal 9100 melting point apparatus. The IR spectra were obtained using a ThermoNicolet Avatar 360 Series FT-IR. The 1H NMR and 13C NMR spectra were recorded on Varian Gemini 300 and Varian Unity 500 instruments using CDC13(TMS internal reference) as solvent. High-resolution APCI+ (atmospheric pressure chemical ionization) mass spectra were obtained with a Jeol JMS-LCmate mass spectrometer. Elemental analyses were determined by Galbraith Laboratories, Inc. (Knoxville, TN). Ether refers to diethyl ether and Ar to argon gas. All solvents were redistilled. All chemicals were purchased from either Sigma-Aldrich Chemical Company (Milwaukee, WI) or Acros Organics (Fisher Scientific, Pittsburgh, PA) and used as received. Reactions were monitored by thin-layer chromatography using Analtech silica gel GHLF Uniplates and were developed with either phosphomolybdic acid (10% w/w solution in ethanol) or iodine and visualized employing either long (366 nm) or short wave (254 nm) UV irradiation. Solvent extracts of aqueous solutions were dried over anhydrous magnesium sulfate or sodium sulfate. Where appropriate, the crude products were purified by silica gel chromatography using gravity (70-230 mesh ASTM) silica gel from E. Merck (Darmstadt, Germany).
3-Methoxy-6[2-(3,4,5-trimethoxyphenyl)-Z-ethylene]-[1,2]benzoquinone (5)
To a stirred solution of vicinal phenol 1 (0.10 g, 0.301 mmol) in DCM (5 mL) at rt was added a solution of NaIO4 (83.8 mg, 0.361 mmol) in H2O (1.0 mL) and tetrabutylammonium bromide (10 mg, 0.03 mmol). After 10 min the reaction mixture turned blue. After separation of the phases, the organic phase was dried and concentrated in vacuo. The blue coloration over a period of 5-10 min changed to black, indicating decomposition of 5.
1-Methoxy-4-[(3,4,5-trimethoxyphenyl)-(Z)-vinyl]phenazine (7)
The preceding oxidation was repeated with diphenol 1 (0.10 g, 0.301 mmol) in DCM (5 mL) with an aqueous solution (l mL) of NaIO4 (83.8 mg, 0.361 mmol) and tetrabutylammonium bromide (10 mg, 0.03 mmol). The reaction mixture turned blue over 10 min. FT-IR analysis yielded a characteristic carbonyl stretch at 1739 cm-1, indicating formation of 5. The phases were separated and the organic phase was concentrated in vacuo and the resultant residue immediately dissolved in methanol (10 mL) and stirred at 0 °C. At that point 1,2-phenylenediamine (6, 50.0 mg, 0.46 mmol) was added. After stirring for an additional 2 h, the condensation was terminated by addition of NaHCO3 (10 mL, sat. aq.) and the product was extracted with EtOAc (3 × 30 mL). The combined organic extract was dried and concentrated in vacuo, and the oily residue was purified twice by silica gel chromatography (gravity; 1:1 EtOAc-n-hexane) to afford phenazine 7, which crystallized from acetone-n-hexane as a red solid (30 mg, 25% from 1): mp 116-118 °C; IR (neat) vmax 2927, 2854, 1623, 1586, 1503, 1462, 1244, 1127, 1006, 762 cm-1; 1H NMR (300 MHz) δ 3.53 (3H, s, OCH3), 3.55 (9H, s, 3 × OCH3), 6.41 (1H, d, J = 2.1 Hz, H-2R′), 6.53 (1H, d, J = 8.1 Hz, H-2), 6.67 (2H, brs, CH=CH), 6.79 (1H, d, J = 8.1 Hz, H-3), 6.93 (1H, d, J = 2.1 Hz, H-6R′), 7.77 (2H, m, H-10, H-11), 8.35 (1H, d, J = 7.5 Hz, H-9), 8.45 (1H, d, J = 7.5 Hz, H-6); 13C NMR (500 MHz) δ 154.7, 150.9, 150.7, 142.2, 142.0, 141.7, 140.7, 136.4, 129.8, 129.7, 129.5, 129.3, 129.1, 129.0, 127.4, 125.0, 106.7, 104.1, 103.8, 56.4, 56.2, 56.0, 55.8; HRMS (APCI+) m/z 403.1658 [M + H]+ (calcd for C24H23N2O4, 403.1659); anal. C 71.29%, H 5.79%, calcd for C24H22N2O4, C 71.63%, H 5.51%.
1,2-Dimethoxy-4,5-dinitrobenzene (9)
Concentrated HNO3 (10 mL) and H2O (10 mL) were combined in a 50 mL 3-neck round bottom flask equipped with an Ar inlet, condenser, and addition funnel. The reaction mixture was then cooled to 0 °C and veratrole (8, 4.34 g, 0.032 mol, 4.0 mL) was added dropwise with vigorous stirring over 1 h to produce a yellow slurry, which was then heated to 60 °C to liberate NO2. Once gas evolution had ceased (4 h), the yellow solution was poured onto ice water (100 mL), and the yellow precipitate was isolated by vacuum filtration. The solid was then washed with NaHCO3 (2 × 200 mL, sat. aq.) and H2O (2 × 200 mL). Crystallization from ethanol (hot) provided dinitrobenzene 9 (5.33 g, 75%) as yellow needles: mp 127-129 °C [lit.12a mp 130-131 °C]; IR (neat) vmax 3072, 2989, 1590, 1517, 1370, 1328, 1280, 1231, 1047, 876, 786 cm-1; 1H NMR (300 MHz) δ 4.02 (6H, s, 2 × OCH3), 7.34 (2H, s, ArH).
1,2-Dimethoxy-4,5-diaminobenzene (10)
To a solution of dinitrobenzene 9 (1.0 g, 4.4 mmol) in EtOAc-EtOH (9:1, 10 mL) at rt was added Pt2O (0.1 g) in a heavy-walled hydrogenation flask. The flask was purged (4 ×) with H2 followed by hydrogenation at 50 psi for 12 h. The black suspension was then removed by filtration (vacuum) of the solution phase through a bed of celite into a hydrochloric acid saturated solution of EtOAc-EtOH (9:1, 20 mL). The solvent was removed in vacuo and the residue crystallized from ethanol to afford the dihydrochloride salt of 10 (0.32 g, 27%) as colorless needles: 1H NMR (300 MHz) K 3.88 (6H, s, 2 × OCH3), 6.96 (2H, s, ArH).
To a solution of diamine 10 dihydrochloride (2.0 g, 8.3 mmol) in methanol (100 mL) at rt was added K2CO3 (2.29 g, 16.6 mmol). The mixture changed in color from pink to yellow. The solution was filtered (under Ar) and evaporated to dryness. The resultant orange solid was triturated with methanol (3 ×) to furnish diamine 10 as an orange solid, which was used immediately without characterization to avoid decomposition.
1,7,8-Trimethoxy-4-[2-(3,4,5-trimethoxyphenyl)-(Z)-vinyl]phenazine (11)
Diphenol 1 (0.10 g, 0.30 mmol) in DCM (5 mL) was oxidized to 5 with NaIO4 (83.8 mg, 0.34 mmol) and tetrabutylammonium bromide (10 mg, 0.03 mmol) in water as described above. As usual, after 10 min, the reaction mixture turned blue and FT-IR analysis yielded the expected carbonyl stretch at 1739 cm-1. As summarized above (see 7), the organic phase was concentrated in vacuo, the residue was immediately dissolved in methanol (10 mL) and stirred at 0 °C, and diamine 10 (77 mg, 0.46 mmol) was added. Two hours later, the condensation was terminated by addition of NaHCO3 (10 mL, sat. aq.) and the mixture was extracted with EtOAc (3 × 30 mL). The combined organic extract was dried and concentrated in vacuo, and the oily residue was separated (2 ×) by silica gel chromatography (gravity; 1:1 EtOAc-n-hexane) to yield phenazine 11 as crystals from acetone-n-hexane (0.04 g, 29% from 1): mp 141-142 °C; IR (neat) vmax 2928, 2851, 1625, 1589, 1506, 1466, 1241, 1123, 1009, 764 cm-1; 1H NMR (300 MHz) δ 3.55 (3H, s, OCH3), 3.57 (9H, s, 3 × OCH3), 3.66 (6H, s, 2 × OCH3), 6.49 (1H, d, J = 2.1 Hz, H-2′), 6.63 (1H, d, J = 8.1 Hz, H-2), 6.77 (2H, brs, CH=CH), 6.89 (1H, d, J = 8.1 Hz, H-3), 6.99 (1H, d, J = 2.1 Hz, H-6′), 7.79 (1H, s, H-9), 7.84 (1H, s, H-6) ppm; 13C NMR (500 MHz) δ 156.8, 156.7, 145.4, 150.9, 150.7, 140.0, 139.0, 138.9, 138.7, 138.4, 129.5, 129.0, 127.6, 126.0, 106.9, 105.2, 105.0, 104.1, 103.8, 56.7, 56.5, 56.4, 56.2, 56.0, 55.8; HRMS (APCI+) m/z 463.1797 [M + H]+ (calcd for C26H27N2O6, 463.1869); anal. C 67.41%, H 5.79%, calcd for C26H26N2O6, C 67.52%, H 5.67%.
Acknowledgement
We are pleased to thank for financial assistance grants R01 CA90441-01-05 and 2R56 CA090441-06A1 awarded by the Division of Cancer Treatment and Diagnosis, National Cancer Institute, DHHS; the Arizona Disease Control Research Commission; the Robert S. Dalton Endowment Fund; Dr. Alec D. Keith; the J. W. Kieckhefer Foundation; the Margaret T. Morris Foundation; and the Caitlin Robb Foundation. Other very helpful assistance was provided by Drs. John C. Knight, Jean-Charles Chapuis, and Michael Hoard and by Felicia Craciunescu and Michael Dodson.
References and Notes
- (1).Contribution 552 in the series Antineoplastic Agents: for part 551, see Pettit GR, Numata A, Iwamoto C, Usami Y, Yamada T, Ohishi H, Cragg GM. J. Nat. Prod. 2006;69:332–337. doi: 10.1021/np058075+.
- (2).Pettit GR, Singh SB, Niven ML, Hamel E, Schmidt JM. J. Nat. Prod. 1987;50:119–131. doi: 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- (3)(a).Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Experientia. 1989;45:209–211. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]; (b) Pettit GR, Singh SB, Boyd MR, Hamel E, Pettit RK, Schmidt JM, Hogan F. J. Med. Chem. 1995;38:1666–1672. doi: 10.1021/jm00010a011. [DOI] [PubMed] [Google Scholar]
- (4)(a).Pettit GR, Temple C, Narayanan VL, Varma R, Simpson MJ, Boyd MR, Rener GA, Bansal N. Anti-Cancer Drug Des. 1995;10:229–309. [PubMed] [Google Scholar]; (b) Pettit GR, Rhodes MR. Anti-Cancer Drug Des. 1998;13:183–191. [PubMed] [Google Scholar]
- (5)(a).Lippert JW., III. Bioorg. Med. Chem. 2007;15:605–615. doi: 10.1016/j.bmc.2006.10.020. [DOI] [PubMed] [Google Scholar]; (b) Mariotti A, Perotti A, Sessa C, Rüegg C. Expert Opin. Investig. Drugs. 2007;16:451–465. doi: 10.1517/13543784.16.4.451. [DOI] [PubMed] [Google Scholar]; (c) Vincent L, Kermani P, Young LM, Cheng J, Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ, Bohlen P, Chaplin DJ, May C, Rafi S. J. Clin. Invest. 2005;115:2992–3006. doi: 10.1172/JCI24586. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Deshpande HA, Gettinger SN, Sosa JA. Curr. Opin. Oncol. 2008;20:19–24. doi: 10.1097/CCO.0b013e3282f28373. [DOI] [PubMed] [Google Scholar]
- (6)(a).Pettit GR, Lippert JW., III. Anti-Cancer Drug Des. 2000;15:203–216. [PubMed] [Google Scholar]; (b) Patterson DM, Ross P, Koetz B, Saleem A, Stratford M, Stirling J, Padhani A, Asselin MC, Price P, Rustin GJ. Mol. Cancer. Ther. 2007;6:3436S–3436S. [Google Scholar]
- (7)(a).Folkes LK, Christlieb M, Madej E, Stratford MRL, Wardman P. Chem. Res. Toxicol. 2007;20:1885–1894. doi: 10.1021/tx7002195.Salmon HW, Siemann DW. Clin. Cancer Res. 2006;12:4090–4094. doi: 10.1158/1078-0432.CCR-06-0163.Sheng Y, Hua J, Pinney KG, Garner CM, Kane RR, Prezioso JA, Chaplin DJ, Edvardsen K. Int. J. Cancer. 2004;111:604–610. doi: 10.1002/ijc.20297.Chaplin DJ, Edvardsen K, Pinney KG, Prezioso JA, Wood M. PCT Int. Appl. WO 2004078126 A2, 2004101
- (8)(a).Haines AH. Methods for the Oxidation of Organic Compounds. Vol. 2. Academic Press; London: 1988. pp. 305–438. [Google Scholar]; (b) Sackett DL. Pharmacol. Ther. 1993;59:163–228. doi: 10.1016/0163-7258(93)90044-e. [DOI] [PubMed] [Google Scholar]
- (9).Pieken WA, Kozarich JW. J. Org. Chem. 1989;54:510–512. [Google Scholar]
- (10).Garrido JMPM, Delerue-Matos C, Borges F, Macedo TRA, Oliveira-Brett AM. J. Chem. Soc., Perkin Trans. 2. 2002:1713–1717. [Google Scholar]
- (11)(a).Hall CA, III, Cuppett SL, Dussault P. J. Agric. Food Chem. 1998;46:1303–1310. [Google Scholar]; (b) Adler E, Falkehag I, Smith B. Acta Chem. Scand. 1962;16:529–540. [Google Scholar]; (c) Tazaki H, Taguchi D, Hayashida T, Nebeta K. Biosci. Biotechnol. Biochem. 2001;65:2613–2621. doi: 10.1271/bbb.65.2613. [DOI] [PubMed] [Google Scholar]; (d) Nohta H, Mitsui A, Ohkura Y. Anal. Chim. Acta. 1984;165:171–176. [Google Scholar]
- (12)(a).Ehrlich J, Bogert MT. J. Org. Chem. 1947;12:522–534. doi: 10.1021/jo01168a006. [DOI] [PubMed] [Google Scholar]; (b) Dave KJ, Riley CM, Vander Velde D, Stobaugh JF. J. Pharm. Biomed. Anal. 1990;8:307–312. doi: 10.1016/0731-7085(90)80043-o. [DOI] [PubMed] [Google Scholar]
- (13).Kongkathip N, Kongkathip B, Siripong P, Sangma C, Luangkamin S, Niyomdecha M, Pattanapa S, Piyaviriyagul S, Kongsaeree P. Bioorg. Med. Chem. 2003;11:3179–3191. doi: 10.1016/s0968-0896(03)00226-8. [DOI] [PubMed] [Google Scholar]