Abstract
Introduction
Corporal veno-occlusive dysfunction (CVOD), which usually is associated with a loss of smooth muscle cells (SMC) and an increase in fibrosis within the corpora cavernosa, can be induced by an injury to the cavernosal nerves. The corporal tissue expresses inducible nitric oxide synthase (iNOS), presumably as an anti-fibrotic and SMC-protective response.
Aims
We studied the temporal relationship in the corpora between the expression of iNOS, other histological and biochemical changes, and the development of CVOD, after bilateral cavernosal nerve resection (BCNR) in the rat.
Methods
Rats underwent either BCNR or sham operation. Cavernosometry was performed 1, 3, 7, 15, 30, and 45 days (n=8/groups) after surgery. Penile tissue sections were subjected to Masson trichrome staining for SMC and collagen, and immunodetection for alpha smooth muscle actin, iNOS, neuronal NOS (nNOS), endothelial NOS (eNOS), proliferating cell nuclear antigen (PCNA), and TUNEL. Quantitative western blot analysis was done in homogenates.
Main outcome measures
Time course on the development of fibrosis and CVOD
Results
Following BCNR, CVOD was detectable 30 days later and it became more pronounced by 45 days. In contrast, the SMC/collagen ratio in the BCNR corpora was reduced at 7 days and bottomed at 30 and 45 days, due in part to the reduction of SMC, presumably caused by an increase in apoptosis peaking at 3 days. PCNA also peaked at 3 days but then decayed. nNOS was reduced early (3-7 days) and disappeared at 30 days, whereas eNOS was not affected. iNOS was induced at day 3, and steadily increased peaking at 30 days.
Conclusions
CVOD develops in the BCNR rat as a result of the early loss of corporal SMC by the neuropraxia-induced apoptosis, which the initial cell replication response cannot counteract, followed by fibrosis. The time course of iNOS induction supports the antifibrotic role of iNOS.
Keywords: fibrosis; erectile dysfunction, smooth muscle, nerve sparing, radical prostatectomy, penis, nitric oxide; cGMP, collagen, inducible nitric oxide synthase, apoptosis
INTRODUCTION
Despite the use of nerve-sparing surgical techniques during radical pelvic surgery in men, the cavernosal nerves still appear to be somewhat susceptible to injury during the surgical procedure as evidenced by persistent and relatively high rates of erectile dysfunction in the immediate post operative period following such nerve sparing techniques (1-4). The primary reason for this surgically induced impotence is corporal veno-occlusive dysfunction (CVOD) or venous leakage (5-8) which becomes manifest whenever there is a decrease in the content of corporal smooth muscle cells (SMC) (9). When this occurs, the remaining corporal smooth muscle mass is unable to achieve sufficient relaxation to attain the high intracorporeal pressures which are necessary for the passive occlusion of the veins that egress the corporal bodies as they traverse underneath and through the tunica albuginea of the penis.
We have previously demonstrated in the rat, in a model of cavernosal nerve resection, that CVOD is apparent at 45 days after the neural injury (10-13). This functional impairment was associated with a decrease in the SMC mass and an increase in collagen content in the corporal tissue. In addition, we also observed a concomitant increase in the expression of the inducible nitric oxide synthase (iNOS) following bilateral cavernosal nerve resection (BCNR). Since we have shown in other experimental injury models that the upregulation of iNOS post-injury, presumably via the synthesis of NO, can act as an anti-fibrotic defense mechanism against the development of fibrosis, we then hypothesized that the iNOS may be acting in a similar manner on the corporal tissue in this BCNR model. The evidence to support this hypothesis comes from our finding that the long-term continuous oral administration of a PDE5 inhibitor, which is known to upregulate the action of nitric oxide, not only prevented both the BCNR-induced CVOD and the loss of the corporal SMC mass (10,11,12) normally seen following this type of injury, but there was the unexpected finding that the PDE5 inhibitors also enhanced replication of the corporal SMC themselves.
However, even though it has been well established that CVOD develops after BCNR and that iNOS expression is increased in the corporal tissue, the temporal relationship between these processes have never been fully elucidated. The aim of this study was to determine: a) whether the development of the histological and biochemical changes that occur after BCNR precedes the onset of the CVOD, and b) when and how long does iNOS induction occur following such a neural injury. These observations would help establish the time frame of when to initiate treatment with PDE5 inhibitors following cavernosal nerve damage in order to achieve the optimum anti-apoptotic and anti-fibrotic effect of these drugs.
MATERIALS AND METHODS
Animal treatments
Five month-old male Fisher 344 rats (Harlan Sprague-Dawley, San Diego, CA) were randomly divided into sham operated and BCNR groups. Animals were sacrificed at 1, 3, 7, 15, 30 and 45 days after surgery (n=8 each group). BCNR was performed as previously described (9-12). Animals were operated under aseptic conditions and isoflurane anesthesia. In supine position, a midline incision was done, the pelvic cavity was opened, and the bladder and prostate were located. Under an operating microscope, the major pelvic ganglion and its inflow and outflow nerve fibers were identified after removing the fascia and fat on the dorsolateral lobe of the prostate. The main branch of the cavernosal nerve is the largest efferent nerve which runs along the surface of the prostatic wall. Above the main branch there are another four to six small efferent fibers which also run towards the membranous urethra, considered as ancillary branches of the CN. In order to recognize the main cavernosal nerve, stimulation with an electrode to induce penile erection was applied. In the sham-operated group both cavernosal nerves were identified but not resected. In BCNR, the main cavernosal nerves and ancillary branches were resected by removing a 5-mm segment. This procedure mainly eliminates the nitrergic NANC stimulation to the corporal smooth muscle that elicits its relaxation during penile erection, while also interrupting some vasoconstrictor neurotransmission through coalescent adrenergic fibers in the cavernosal nerve. All animal experiments were approved by the IACUC at our institution.
Dynamic Infusion Cavernosometry (DIC)
Cavernosometry was performed as previously described (10-12,14). Briefly, basal intracavernosal pressure (ICP) was recorded, and 0.1 .ml papaverine (20 mg/ml) was administered through a cannula into the corpora cavernosa. The ICP during tumescence was recorded as “ICP after papaverine”. Saline was then infused through another cannula, increasing infusion rate by 0.05 ml/min every 10 seconds, until the ICP reached 80 mmHg (“maintenance rate”). The “drop rate” was determined by recording the fall in ICP within the next 1 minute after the infusion was stopped.
Histochemistry and immunohistochemistry
After cavernosometry, animals were sacrificed and the skin-denuded penile shafts were fixed overnight in 10% buffered formalin, washed, and stored in alcohol (70%) at 4°C until processed for paraffin embedded tissue sections (5 um). Adjacent tissue sections were used for: a) Masson trichrome staining for collagen (blue) and SMC (red); c) immunodetection with: 1) monoclonal antibodies against α-smooth muscle actin (ASMA) as a SMC marker (Sigma kit, Sigma Diagnostics, St Louis, MO) and proliferating cell nuclear antigen (PCNA) as marker of cell proliferation (Chemicon, Temecula, CA); 2) polyclonal antibody against iNOS (15) (Calbiochem, La Jolla, CA); 3) monoclonal antibody against eNOS (16) (Calbiochem); 4) monoclonal antibody against nNOS (17) (Calbiochem). The specificity of the antibodies was validated by western blot.
Sections were then incubated with biotinylated anti-Mouse IgG (ASMA PCNA, eNOS, nNOS) or biotinylated anti-Rabbit IgG (iNOS), respectively, followed by ABC complex (Vector labs, Temecula, CA) and 3,3'diaminobenzidine (Sigma) (PCNA and iNOS), or with the ASMA Sigma kit (ASMA) and 3-amino-9-ethylcarbazole. TUNEL assay was performed as described (10-13) by applying the Apoptag peroxidase detection assay (Chemicon), with TdT enzyme and anti-digoxigenin-conjugated peroxidase, and 3,3'diaminobenzidine/H2O2. Sections were counterstained with hematoxylin. Negative controls in the immunohistochemical detections were done by replacing the first antibody with IgG isotype. The negative control for TUNEL was by substituting buffer for the TdT enzyme. Testicular tissue sections were used as positive control.
Quantitative image analysis
Quantitative image analysis (QIA) was performed by computerized densitometry using the ImagePro 4.01 program (Media Cybernetics, Silver Spring, MD), coupled to an Olympus BHS microscope equipped with an Olympus digital camera (11-15). For Masson staining, 40× magnification pictures of the penis comprising half of the corpora cavernosa were analyzed for SMC (stained in red) and collagen (stained in blue), and expressed as SMC/collagen ratio. For ASMA and iNOS staining, only the corpora cavernosa were analyzed in a computerized grid and expressed as % of positive area vs. total area of the corpora cavernosa. For PCNA and TUNEL determinations, the number of positive cells at 400X was counted and results were expressed as a % of positive cells/total cells in the corpora cavernosa. In all cases, two fields at 40x (both sides of the corpora cavernosa) or 8 fields at 400x, were analyzed per tissue section, with at least 4 matched sections per animal and 8 animals per group.
Western blot analysis
Penile tissue homogenates (100 mg tissue) were obtained in T-PER (PIERCE, Rockford, IL) and protease inhibitors (3 μM leupeptin, 1 μM pepstatin A, 1mM phenyl methyl sulfonyl fluoride), and centrifuged at 10,000 g for 5 min. Supernatant proteins (30-50 μg) were subjected to western blot analyses (17-20) by 7-10 % Tris-HCl polyacrylamide gel electrophoresis (PAGE) (Bio-Rad, Hercules, CA) in running buffer (Tris/Glycine/SDS). Proteins were transferred overnight at 4°C to nitrocellulose membranes in transfer buffer (Tris/glycine/methanol) and the next day, the non-specific binding was blocked by immersing the membranes into 5% non-fat dried milk, 0.1% (v/v) Tween 20 in PBS for 1hour at room temperature. After several washes with washing buffer (PBS Tween 0.1%), the membranes were incubated with the primary antibodies for 1 hour at room temperature monoclonal antibodies were as follows: a) ASMA, as described above (1/1,000) (Calbiochem, La Jolla, CA); b) glyceraldehide-3-phosphate dehydrogenase (GAPDH) (1/10,000) (Chemicon International, Temecula, CA); and c) PCNA (Chemicon International).The washed membranes were incubated for 1 hour at room temperature with 1/3,000 dilution (anti-mouse), followed by a secondary antibody linked to horseradish peroxidase. After several washes, the immunoreactive bands were visualized using the ECL plus western blotting chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ). The densitometric analyses of the bands were performed with Image J (NIH, Bethesda, MD). A positive control was run throughout all gels for each antibody to standardize for variations in exposures and staining intensities. Negative controls were performed omitting the primary antibody. Band intensities were determined by densitometry and corrected by the respective intensities for a housekeeping protein, glyceraldehyde phosphate dehydrogenase (GAPDH), upon reprobing.
Statistical analysis
Values were expressed as mean ± SEM. The normality distribution of the data was established using the Wilk-Shapiro test. Multiple comparisons were analyzed by a two factor (time and treatment) analysis of variance (two way ANOVA), followed by post-hoc comparisons with the Bonferroni test, according to the GraphPad Prism V 4.1. Differences were considered significant at p < 0.05.
RESULTS
Alterations in the SMC/collagen ratio in the corpora cavernosa precede the onset of CVOD following BCNR
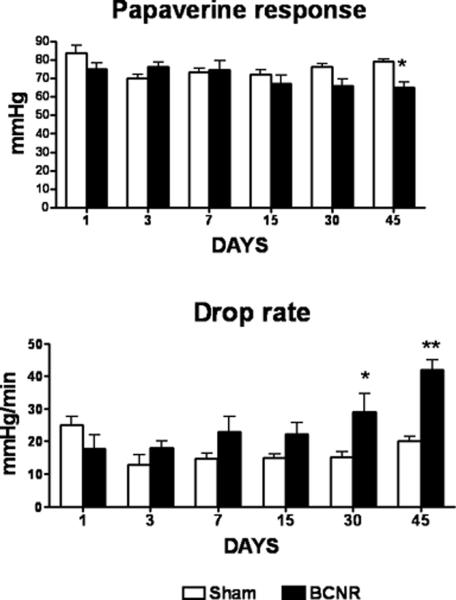
DIC was performed at 1, 3, 7, 15, and 30 days after cavernosal nerve injury in order to determine when CVOD occurs post-BCNR. DIC values for the 45 day time period were taken from one of our previous papers with identical sets of BCNR- and sham-operated rats (11). However, in all the subsequent figures for histological observations the representative micrographies for 15 and 45 days are omitted to reduce space. Figure 1 (top) shows that the peak ICP following papaverine injection was not significantly affected by BCNR during the observed 30 days post-injury, although at 45 days after surgery the value was significantly reduced. The drop rate, however, began to slowly increase by 7 days but only became significant by 30 days and markedly progressed by 45 days post-injury.
Figure 1. Time course of effect of bilateral cavernosal nerve resection on the erectile function of the rat measured by pharmacological and infusion cavernosometry.
Top: Response of the intracavernosal pressure to papaverine; Bottom: Response of the intracavernosal pressure to the interruption of saline infusion. SHAM: sham-operated animals; BCNR: animals subjected to nerve resection and killed at 1, 3, 7,15, 30 and 45 days after surgery.*: p<0.05; **: p<0.01; ***P<0.001
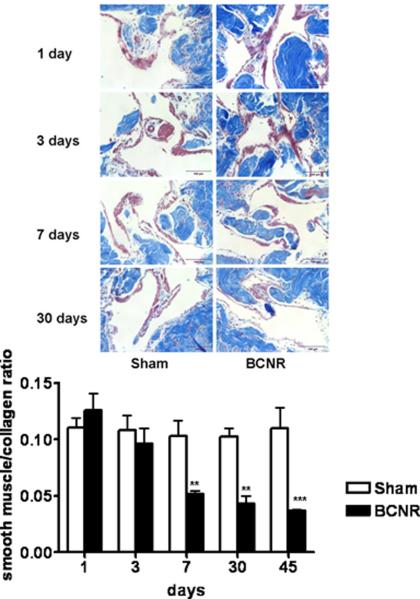
Evaluation of the smooth muscle and collagen content within the corpora was then performed in cross-sections of the penile tissue harvested from the animals following performance of DIC. Fig. 2 top shows that there does not appear to be any obvious visual changes in the Masson trichrome staining for collagen and SMC on representative micrographs in the sham groups through out the experiment. However, a progressive intensification of the collagen deposition (stained in blue) and a reduction in the smooth muscle (stained in red) started to be visualized at day 7 after BCNR. When QIA was performed (bottom), an alteration in the SMC/collagen ratio is detected as early as day 3 post injury, which becomes significantly severe by day 7 and remains so for the remainder of the study. The red staining of the SMC was easily differentiated from the red blood cells which were not considered in the QIA determinations.
Figure 2. Time course of the effect of bilateral cavernosal nerve resection on the smooth muscle/collagen ratio in the rat corpora cavernosa.
Penile corpora cavernosa tissue sections from the rat groups presented on Figure 1 were stained with Masson trichrome. Top: representative pictures (200X, Bar=50 μm). Bottom: QIA. SHAM: sham-operated animals; BCNR: animals subjected to nerve resection killed at 1, 3, 7 and 30 days after surgery. ** P<0.01, ***: p<0.001
Picrus sirius red assays and observation under polarized microscope were done in adjacent sections to the ones used for Masson in order to discriminate the collagen III/I ratio. We have found that at the time points 7 and 30 there is an increase in collagen III/I ratio towards more production of collagen III, whereas at 45 days the ratio is inverted to more collagen I than III (not shown). This difference could be due to the fact that the rate of collagen III synthesis is much faster than collagen I
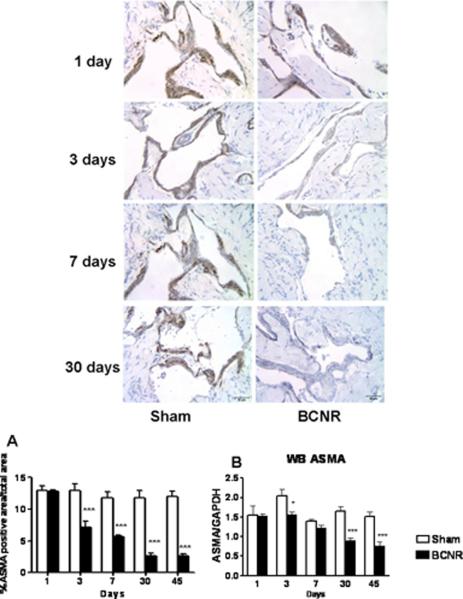
A second procedure to estimate SMC content based on the immunohistochemical determination of ASMA, an accepted marker of SMC in the corpora cavernosa, was also performed. Fig. 3, top shows a considerable reduction with time in ASMA staining in the BCNR group as compared to the sham group as early as 3 days after BCNR that progressively worsens at 30 and 45 days. The respective reductions in ASMA content determined by QIA (bottom, A) were 40%, 76%, and 78% at 7, 30 and 45 days, respectively, post injury. The expression of ASMA in the sham-operated group remained unchanged throughout the experiment. When western blot analysis of ASMA expression in homogenates of penile shaft tissue (bottom, B) was performed, it paralleled the immunohistochemical measurements. Collectively, these results suggest that the histological changes induced by BCNR precede, as expected, the functional impairment of vasculogenic erectile response, and that the earliest event is SMC loss rather than collagen deposition. This is based on the fact that the reduction in ASMA+ cells is rather considerable at a period (3 days) when the SMC/collagen ratio has only slightly decreased.
Figure 3. Time course of the effect of bilateral cavernosal nerve resection on the smooth muscle cell content in the rat corpora cavernosa.
Penile corpora cavernosa sections adjacent to those presented on Fig. 2 were immunostained for ASMA as an SMC marker. Top: 40X, Bar=50 μm). Middle: quantitative image analysis. ***: p<0.001
BCNR results in a decrease in nNOS, an increase in iNOS and no change in eNOS content in the corpora
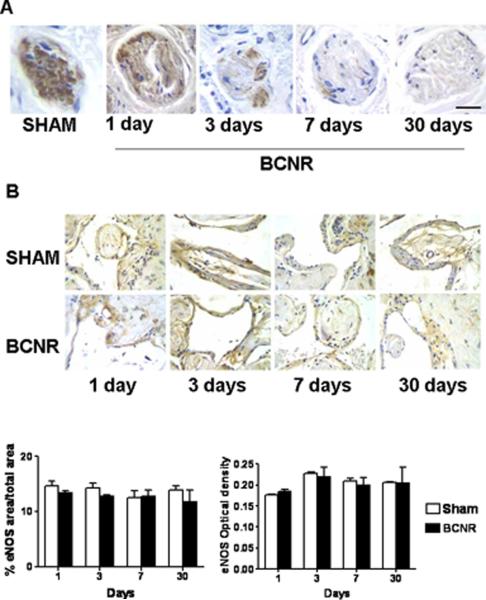
Since BCNR causes damage to the axons of the cavernosal nerve, Fig. 4 A confirms by immunohistochemistry with an antibody selective for nNOS a decrease in nNOS staining, that is seen in cross sections of the cavernosal nerve as early as 24 hours following the nerve injury. This antibody does not cross-react with eNOS and iNOS. Because the decrease in staining intensity was so evident, no quantitative determination was deemed necessary to corroborate the visual inspection. In contrast, no changes were appreciable in the immunohistochemical detection of eNOS, which was constrained to the endothelium lining of the corpora cavernosa lacunar spaces or cisternae (Fig. 4 B). This was confirmed by QIA.
Figure 4. Time course of nNOS expression after nerve resection in cavernosal nerve terminals and of eNOS in the corporal enothelium.
A. Penile sections sections adjacent to those presented on Fig. 2 were immunostained for nNOS. Magnification: 400X, Bar=50 μm). B. Sections adjacent to those presented on Fig. 2 were immunostained with an eNOS antibody. The expression of eNOS is not altered by nerve resection. Top: 400X, Bar=50 μm). Bottom: quantitative image analysis.
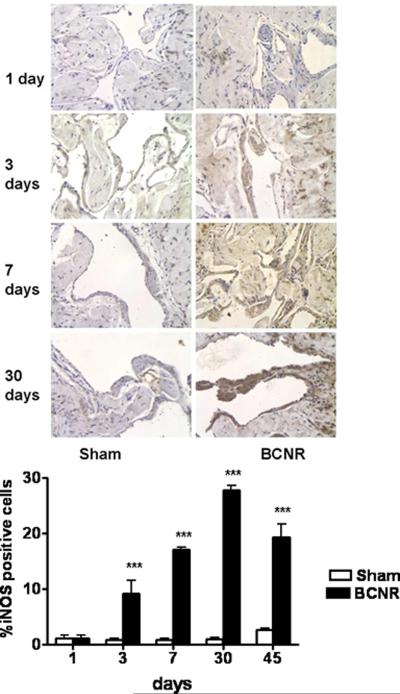
In an even more marked contrast to nNOS decrease, iNOS immunostaining in the corpora of the BCNR rats started to increase by 10-fold at day 3, and continued to remain high throughout the study period, while it stayed almost undetectable in the sham-operated animals at all time periods (Fig. 5 top). The quantitative determination indicated that iNOS expression reached a peak at 30 days when there was about a 50-fold increase over both the sham operated and the pre-injury values (bottom).
Figure 5. Time course of the effect of bilateral cavernosal nerve resection on the expression of iNOS in the penile corpora cavernosa.
Penile corpora cavernosa sections adjacent to those presented in Fig 2 were subjected to immunodetection for iNOS. Top: representative pictures (200X, Bar=50 μm). Bottom: quantitative image analysis. ***p<0.001.
The reduction in SMC occurring after BCNR is due to an early peak of apoptosis that initially is compensated by increased cell proliferation but later on predominates over this process
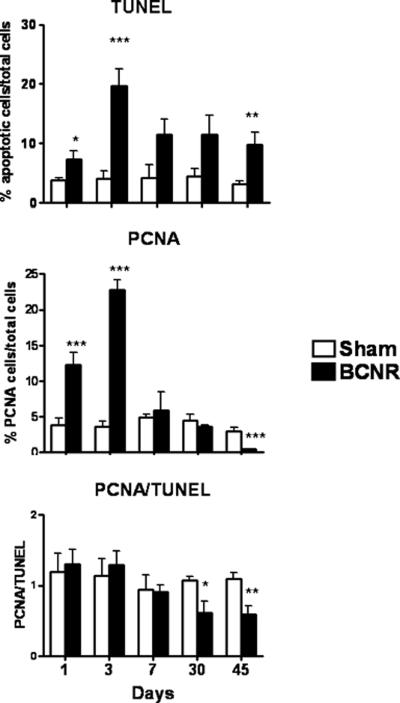
TUNEL immunodetection assay revealed that by 1 day, and more so at 3 days following BCNR, there was a marked increase in apoptosis of cells in the corpora (not shown) and this was confirmed by QIA which showed that the peak of apoptosis occurred at 3 days with a 5-fold increase in the apoptotic index in the BCNR animals. This was followed by a gradual reduction, but still showing an over 2-fold higher apoptotic index at 45 days after BCNR (Fig 6 top).
Figure 6. Time course of the effect of bilateral cavernosal nerve resection on the cell turnover in the rat corpora cavernosa.
Penile corpora cavernosal sections adjacent to those presented on the preceding figures were subjected to TUNEL and PCNA staining. Top: QIA for TUNEL. Middle: QIA for PCNA; Bottom: The ratio between the total area occupied by cells undergoing cell replication (PCNA+) and the apoptotic index obtained above was established for each animal, and then used to calculate means+/-SEM. * p:<0.05; **: p<0.01; ***p<0.001
When cell proliferation was measured by immunohistochemistry for PCNA, there was an intensification of cell proliferation at 1 and 3 days post BCNR but this level was subsequently reduced by day 7 post injury to basal levels (Fig. 6 middle). QIA showed that, as in the case of apoptosis the cell proliferation peak occurred at 3 days, with a similar 5-fold increase in PCNA staining, which decreased thereafter. Interestingly, at 30 and 45 days post BCNR, the PCNA values in the BCNR groups were lower than in the control sham-operated animals. Because of the initial stimulation of cell replication, the ratio between the proliferation and apoptotic indexes in the corpora (bottom) remains around a value of 1 until 7 days after BCNR, with no significant differences between BCNR and the sham operated rats. However, at both 30 and 45 days there is a considerable reduction in PCNA due to the predominance of cell death over cell proliferation. This agrees with the time course for SMC content in Fig. 3.
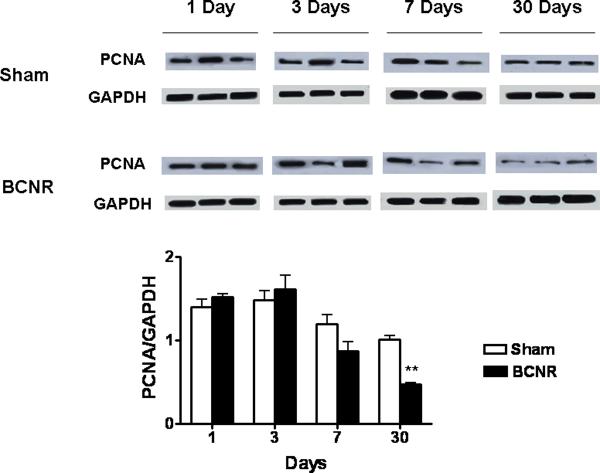
The western blot analysis of PCNA expression in total penile shaft homogenates (Fig. 7) confirmed the decrease in PCNA staining seen by immunohistochemistry in the tissue sections of the corpora cavernosa of BCNR rats (Figure 6, middle panel). However, the levels of PCNA in the homogenates of the penile shaft (Fig 7) were inconsistently high at the two earliest time periods, probably reflecting the presence of tunical and corpus spongiosum tissue (not considered in the analysis of the tissue sections of Figure 7).
Figure 7. Corroboration of the PCNA immunostaining by western blot.
Homogenates from corpora cavernosa tissue were subjected to western blot analysis with the same antibody used for figure 6. ** P<0.01
DISCUSSION
The current results clarify the sequential events that lead to the development of CVOD in the rat following cavernosal nerve damage. The assumption is that CVOD or venous leakage occurs because the SMC mass in the corpora is impacted in such a way that it cannot achieve sufficient relaxation to attain an intracorporeal pressure high enough that can compress the subtunical veins as they egress from the tunica albuginea of the penis. Normally this is evident by a decrease in the SMC content together with an increase in tissue fibrosis within the corpora.
The absolute amount of corpora smooth muscle, that only drops significantly at day 30, but not at 7, approaching the value at day 45, appears to be more critical for corporal compliance and venous occlusion than the smooth collagen content ratio that falls down earlier. This interpretation would explain the fact that the significant increase in drop rate occurs at day 30 but not at 7 days, thus implying that a certain threshold in the corporal smooth muscle content combined with collagen deposition may be needed below which the functional impairment would become evident.
The absence of a parallel significant decrease in the papaverine response at day 30 (despite the trend seen on Fig. 1) may be due to the relatively high papaverine dosage (100 ul of 20 mg/ml solution, which is approximately 5 mg/kg. B.W) used in this study. This may be excessive to detect a marginal CVOD, based on the erectile response to the drug. However, we have recently conducted a papaverine dose/response titration curve during DIC in the rat, and we have found that 15 mg/ml of papaverine is an optimal concentration (or 3.8 mg/kg) for performing DIC, and this intracorporal dose will be used in the future.
Therefore, if the hypothesis of the decrease in the SMC content together with an increase in tissue fibrosis within the corpora is correct, then apoptosis should occur first followed by an observed decrease in the corporal smooth muscle content in combination with an increase in tissue fibrosis before CVOD becomes evident. Indeed, in our animal model of BCNR, the process of apoptosis is apparent 24 hours following the neural injury, an observation that has been previously reported by others (21-24). What our data does show for the first time is that this apoptotic process peaks around 3 days following BCNR and, although there is a slight decrease from this peak level seen after day 3, the level of apoptosis continues to remain elevated up to the end of the experiment which was 45 days after BCNR.
The data confirm the observation of previous investigators (20-23) that programmed cell death is apparent as early as 1 day after the onset of the neural injury. In addition, while the peak for these pro-apoptotic processes occurs by day 3 following the neural injury, there also appears to be a considerable increase in cell proliferation within the trabecular tissue around the cisternae, a finding that has not been previously reported and one that may represent an attempt by the tissue itself to counteract apoptosis. Thereafter, cell proliferation, by drastically declining already at 7 days, becomes insufficient to counteract the much slower decline in apoptosis. As a result, the imbalance between both processes manifests at 30 days, agreeing with the earliest period where there is a net loss of SMC. Since the ratio of the SMC to collagen decreases significantly, and rather drastically at 7 days after BCNR, but the content of the SMC decreases much earlier, at 3 days, which coincides with the peak in apoptosis, it may be concluded that collagen deposition is intensified after the SMC loss, and that therefore the reduction of the cellular compartment precedes the onset of fibrosis. It is the net loss of SMC what appears to trigger the first manifestation of CVOD that occurs 30 days after BCNR.
The reduction of nitrergic nerve terminals that are clearly distinguishable from the dorsal nerve and may be ascribed topologically to the cavernosal nerve, is evident as early as 1 day after BCNR. This suggests that Wallerian nerve degeneration exacerbated throughout the 45 day-period is most likely responsible for the changes observed in the corpora cavernosa SMC. Most interestingly and somewhat surprisingly was, the lack of changes in the content of eNOS, thereby suggesting that the endothelium is not considerably affected by BCNR. This indicates that: a) eNOS-dependent endothelial dysfunction may not be elicited by neuropraxia and is not involved in CVOD, that appears to result mainly from corporal SMC loss and fibrosis; and b) in the absence of nNOS, eNOS cannot per se produce sufficient nitric oxide as to sustain the papaverine-induced production of cGMP caused by the unspecific PDE inhibition exerted by the drug (25). However, since neither endothelial function nor eNOS activity has been determined, it is not possible to rule out a possible functional impairment of the endothelium after BCNR despite unaltered expression of eNOS.
Perhaps the most intriguing observation is the time course of iNOS induction by BCNR, which seems to follow the nNOS decrease in the nitrergic nerves but peaks at 30 days. This is long after apoptosis has reached a maximum at 3 days, thus ruling out the possibility that this cell death is triggered by nitric oxide from iNOS, a compound that is usually considered as pro-apoptotic (26,27). However, there is evidence that nitric oxide can in fact be anti-apoptotic according to tissue and physiological conditions (28). Alternatively, this sustained increase of iNOS expression may be responsible for the observed reduction of the compensatory cell proliferation in the corpora after BCNR, based on the fact that both nitric oxide and cGMP are considered to be antiproliferative for the SMC in the arterial media (29). However, this possibility appears to be ruled out by our previous results with L-NIL, an inhibitor of iNOS activity (11,15). At least at 45 days after BCNR, a steady iNOS inhibition by daily oral L-NIL significantly reduced the SMC/collagen ratio, thus suggesting that iNOS is acting protecting the SMC or inhibiting collagen deposition which would be in agreement with the cardioprotective effects of nitric oxide, cGMP, and iNOS on cardiomyocytes during ischemia reperfusion pre- or post-conditioning (30-32). iNOS may not only be produced by smooth muscle cells, since macrophages, and interstitial fibroblasts are also known to express this protein upon induction. No co-localization studies for iNOS and ASMA were performed in this work, and therefore it cannot be ruled out that iNOS synthesis in the corpora occurs also in cell types other than the smooth muscle cells. In addition, it is not surprising that a steady increase in iNOS would occur in the presence of a sustained decline in the overall content of the putative cell type where iNOS is induced, since iNOS expression is due to transcriptional stimulation that by a steady increase within each cell and the cumulative production of nitric oxide can substantially exceed the rate of cell loss.
However, since CVOD and fibrosis do develop in BCNR despite the steady iNOS production, this process is apparently insufficient to counteract the factors that trigger “corporal dystrophy” (fibrosis and SMC loss), a term that we propose as analogous to skeletal muscle dystrophy.
This leads to the fundamental question regarding which factors triggered by the neuropraxia are responsible for causing corporal SMC dystrophy. The most likely is the interruption of the secretion of neurotrophins which in addition to their effects on neural tissue (33,34) are postulated to stimulate smooth muscle hyperplasia, particularly in the respiratory airways and the intestine (35,36). This depletion may cause the down-regulation of SMC proliferation triggered by a spontaneous defense mechanism against neuropraxia. Conversely, the induction of cytokine release, mainly TNFα and TGFβ1, that are pro-apoptotic and fibrotic factors and activate the proteasome ubiquitin proteolytic pathway, is a recognized feature of Wallerian degeneration (37), and it underlies, at least in part, the skeletal muscle atrophy subsequent to denervation (38,39). However, the lack of neuromotor discharge and activity may also be an essential factor in this atrophy.
Irrespective of the mechanism that triggers fibrosis and SMC loss subsequent to cavernosal nerve damage, three things became obvious through this work. First, that is the early histopathological impairment within the corpora smooth muscle that leads later to the functional impairment, CVOD. It may require a certain threshold in the smooth muscle/collagen ratio that below that the functional impairment become evident Therefore in the clinical setting an early therapeutical intervention to reduce apoptosis of the corporal SMC or sustain their initial proliferation response, would be warranted, e.g., immediately after radical prostatectomy. Secondly, since iNOS induction appears to be an endogenous antifibrotic and protective response on the smooth muscle, the early therapy may be based on pharmacological agents that mimic this process, such as the continuous long-term administration of PDE5 inhibitors we have studied in rats (10-13,33,40), or of nitric oxide generators (41,42), or in men for a combination of both types of compounds (43). Such a therapeutic modality may be accompanied with neurotrophin administration such as BDNF (34) in an attempt to restore the anabolic signals to the smooth muscle that endogenous neurotrophic factors are no longer mediating. Thirdly, since our experimental model of BCNR, where the cavernosal nerves are both completely resected, may not be very representative of the cavernosal nerve injury that may occur with pelvic surgery in which the nerves may only be partially damaged, it is possible that axonal regeneration, which does not seem to occur after BCNR within the time course of this study, may occur with radical pelvic surgery particularly with the nerve sparing procedures. As such, the treatments described above to prevent the histological changes in the corpora may also be efficacious in stimulating axonal regeneration.
Acknowledgement
This study was supported by Award Number SC1NS064611 from the National Institute of Neurological Disorder and Stroke (NINDS) and National Institute of General Medicine (NIGMS) (M.G.F) and by a grant from Department of Defense (PC061300), and in part by grants from the National Institutes of Health (R01DK-53069 and G12RR-03026) (N.G.C).
Abbreviations
- ASMA
α-smooth muscle actin
- BCNR
bilateral cavernosal nerve resection
- CVOD
corporal veno-occlusive dysfunction
- DIC
dynamic infusion cavernosometry
- GAPDH
glyceraldehide-3-phosphate dehydrogenase
- ICP
intracavernosal pressure
- iNOS
NOS II, inducible nitric oxide synthase
- PCNA
proliferating cell nuclear antigen
- QIA
quantitative image analysis
- SMC
smooth muscle cells
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Levinson AW, Pavlovich CP, Ward NT, Link RE, Mettee LZ, Su LM. Association of surgeon subjective characterization of nerve sparing quality with potency following laparoscopic radical prostatectomy. J Urol. 2008;179:1510–1514. doi: 10.1016/j.juro.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 2.Madeb R, Golijanin D, Knopf J, Vicente I, Erturk E, Patel HR, Joseph JV. Patient-reported validated functional outcome after extraperitoneal robotic-assisted nerve-sparing radical prostatectomy. JSLS. 2007;11:443–448. [PMC free article] [PubMed] [Google Scholar]
- 3.Ayyathurai R, Manoharan M, Nieder AM, Kava B, Soloway MS. Factors affecting erectile function after radical retropubic prostatectomy: results from 1620 consecutive patients. BJU Int. 2008;101:833–836. doi: 10.1111/j.1464-410X.2007.07409.x. [DOI] [PubMed] [Google Scholar]
- 4.Briganti A, Salonia A, Gallina A, Chun FK, Karakiewicz PI, Graefen M, Huland H, Rigatti P, Montorsi F. Management of erectile dysfunction after radical prostatectomy in 2007. World J Urol. 2007;25:143–148. doi: 10.1007/s00345-007-0148-9. [DOI] [PubMed] [Google Scholar]
- 5.Luo H, Goldstein I, Udelson D. A three-dimensional theoretical model of the relationship between cavernosal expandability and percent cavernosal smooth muscle. J Sex Med. 2007;4:644–5. doi: 10.1111/j.1743-6109.2007.00492.x. discussion 651-55. [DOI] [PubMed] [Google Scholar]
- 6.McCullough A, Woo K, Telegrafi S, Lepor H. Is sildenafil failure in men after radical retropubic prostatectomy (RRP) due to arterial disease? Penile duplex Doppler findings in 174 men after RRP. Int J Impot Res. 2002;14:462–465. doi: 10.1038/sj.ijir.3900909. [DOI] [PubMed] [Google Scholar]
- 7.Broderick GA. Evidence based assessment of erectile dysfunction. Int J Impot Res. 1998;10(Suppl 2):S64–73. discussion S77-S79. [PubMed] [Google Scholar]
- 8.Nikoobakht M, Saraji A, Meysamie A. Preoperative corporal biopsy as a predictor of postoperative results in venoocclusive erectile dysfunction. Urol J. 2005;2:160–164. [PubMed] [Google Scholar]
- 9.Hu WL, Hu LQ, Li SW, Zheng XM, Tian BC. Expression of transforming growth factor-beta1 in penile tissue from rats with bilateral cavernosal nerve ablation. BJU Int. 2004;94:424–428. doi: 10.1111/j.1464-410X.2004.04969.x. [DOI] [PubMed] [Google Scholar]
- 10.Kovanecz I, Rambhatla A, Ferrini MG, Vernet D, Sanchez S, Rajfer J, Gonzalez-Cadavid NF. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008;101:203–210. doi: 10.1111/j.1464-410X.2007.07223.x. [DOI] [PubMed] [Google Scholar]
- 11.Kovanecz I, Rambhatla A, Ferrini M, Vernet D, Sanchez S, Rajfer J, Gonzalez-Cadavid NF. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impot Res. 2008;20:202–212. doi: 10.1038/sj.ijir.3901612. [DOI] [PubMed] [Google Scholar]
- 12.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68:429–35. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Rambhatla A, Kovanecz I, Ferrini M, Gonzalez-Cadavid NF, Rajfer J. Rationale for phosphodiesterase 5 inhibitor use post-radical prostatectomy: experimental and clinical review. Int J Impot Res. 2008;20:30–34. doi: 10.1038/sj.ijir.3901588. [DOI] [PubMed] [Google Scholar]
- 14.Davila HH, Rajfer J, Gonzalez-Cadavid NF. Corporal veno-occlusive dysfunction in aging rats: evaluation by cavernosometry and cavernosography. Urology. 2004;64:1261–1266. doi: 10.1016/j.urology.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Ferrini MG, Davila H, Valente EG, Gonzalez-Cadavid NF, Rajfer J. Aging-related induction of inducible nitric oxide synthase (iNOS) is vasculo-protective in the arterial media. Cardiovascular Res. 2004;61:796–805. doi: 10.1016/j.cardiores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Mazza ON, Angerosa M, Becher E, Toblli JE. Differences between Candesartan and Hydralazine in the protection of penile structures in spontaneously hypertensive rats. J Sex Med. 2006;3:604–611. doi: 10.1111/j.1743-6109.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 17.Magee T, Zeller CB, Ferrini M, Davila H, Vernet D, Burnett AL, Rajfer J. González-Cadavid NFA protein inhibitor of NOS (PIN) is expressed in the rat and mouse penile nerves and co-localizes with penile neuronal NOS (PnNOS) Biol Reprod. 2002;68:478–488. doi: 10.1095/biolreprod.102.007310. [DOI] [PubMed] [Google Scholar]
- 18.Nolazco G, Kovanecz I, Vernet D, Gelfand RA, Tsao J, Ferrini MG, Magee T, Rajfer J, Gonzalez-Cadavid NF. Effect of muscle-derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 2008;101:1156–1164. doi: 10.1111/j.1464-410X.2008.07507.x. [DOI] [PubMed] [Google Scholar]
- 19.Artaza JN, Singh R, Ferrini MG, Braga M, Tsao J, Gonzalez-Cadavid NF. Myostatin promotes a fibrotic phenotypic switch in multipotent C3H 10T1/2 cells without affecting their differentiation into myofibroblasts. J Endocrinol. 2008;196:235–249. doi: 10.1677/JOE-07-0408. [DOI] [PubMed] [Google Scholar]
- 20.Cantini LP, Ferrini MG, Vernet D, Magee TR, Qian A, Gelfand RA, Rajfer J, Gonzalez-Cadavid NF. Profibrotic Role of Myostatin in Peyronie's Disease. J Sex Med. 2008;5:1607–1622. doi: 10.1111/j.1743-6109.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 21.Klein LT, Miller MI, Buttyan R, Raffo AJ, Burchard M, Devris G, Cao YC, Olsson C, Shabsigh R. Apoptosis in the rat penis after penile denervation. J Urol. 1997;158:626–630. [PubMed] [Google Scholar]
- 22.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;169:1175–1179. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 23.Lysiak JJ, Yang SK, Klausner AP, Son H, Tuttle JB, Steers WD. Tadalafil increases Akt and extracellular signal-regulated kinase 1/2 activation, and prevents apoptotic cell death in the penis following denervation. J Urol. 2008;179:779–785. doi: 10.1016/j.juro.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod. 2007;76:19–28. doi: 10.1095/biolreprod.106.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maggi M, Filippi S, Ledda F, Magini A, Forti G. Erectile dysfunction: from biochemical pharmacology to advances in medical therapy. Eur J Endocrinol. 2000;143:143–154. doi: 10.1530/eje.0.1430143. [DOI] [PubMed] [Google Scholar]
- 26.Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007;27:2524–2531. doi: 10.1161/ATVBAHA.107.151167. [DOI] [PubMed] [Google Scholar]
- 27.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 28.Choi BM, Pae HO, Jang SI, Kim YM, Chung HT. Nitric oxide as a pro-apoptotic as well as anti-apoptotic modulator. J Biochem Mol Biol. 2002;35(1):116–126. doi: 10.5483/bmbrep.2002.35.1.116. 31. [DOI] [PubMed] [Google Scholar]
- 29.Muhs A, Heublein B, Schletter J, Herrmann A, Rüdiger M, Sturm M, Grust A, Malms J, Schrader J, von der Leyen HE. Preclinical evaluation of inducible nitric oxide synthase lipoplex gene therapy for inhibition of stent-induced vascular neointimal lesion formation. Hum Gene Ther. 2003;14:375–383. doi: 10.1089/104303403321208970. [DOI] [PubMed] [Google Scholar]
- 30.Cohen MV, Downey JM. Cardioprotection: spotlight on PKG. Br J Pharmacol. 2007;152:833–834. doi: 10.1038/sj.bjp.0707453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol. 2007;152:855–869. doi: 10.1038/sj.bjp.0707409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q, Guo Y, Tan W, Ou Q, Wu WJ, Sturza D, Dawn B, Hunt G, Cui C, Bolli R. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-kappa B dependent pathway. Circulation. 2007;116:1577–1584. doi: 10.1161/CIRCULATIONAHA.107.689810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagoda G, Jin L, Lehrfeld TJ, Liu T, Burnett AL. FK506 and sildenafil promote erectile function recovery after cavernous nerve injury through antioxidative mechanisms. J Sex Med. 2007;4:908–916. doi: 10.1111/j.1743-6109.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin G, Bella AJ, Lue TF, Lin CS. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part 2. J Sex Med. 2006:821–827. doi: 10.1111/j.1743-6109.2006.00292.x. discussion 828-829. [DOI] [PubMed] [Google Scholar]
- 35.Rochlitzer S, Nassenstein C, Braun A. The contribution of neurotrophins to the pathogenesis of allergic asthma. Biochem Soc Trans. 2006;34:594–599. doi: 10.1042/BST0340594. [DOI] [PubMed] [Google Scholar]
- 36.Groneberg DA, Rabe KF, Fischer A. Novel concepts of neuropeptide-based drug therapy: vasoactive intestinal polypeptide and its receptors. Eur J Pharmacol. 2006;533:182–194. doi: 10.1016/j.ejphar.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 37.Palin K, Cunningham C, Forse P, Perry VH, Platt N. Systemic inflammation switches the inflammatory cytokine profile in CNS Wallerian degeneration. Neurobiol Dis. 2007 Dec 23; doi: 10.1016/j.nbd.2007.11.012. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Midrio M. The denervated muscle: facts and hypotheses. A historical review Eur J Appl Physiol. 2006;98:1–21. doi: 10.1007/s00421-006-0256-z. [DOI] [PubMed] [Google Scholar]
- 39.Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr Opin Clin Nutr Metab Care. 2001;4:183–190. doi: 10.1097/00075197-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Ferrini MG, Kovanecz I, Sanchez S, Vernet D, Davila HH, Rajfer J, Gonzalez-Cadavid NF. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod. 2007;76:915–923. doi: 10.1095/biolreprod.106.059642. [DOI] [PubMed] [Google Scholar]
- 41.Vernet D, Ferrini MG, Valente E, Magee TR, Bou-Gharios G, Rajfer J, Gonzalez-Cadavid NF. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie's fibrotic plaque and in its rat model. Nitric Oxide. 2002;7:262–276. doi: 10.1016/s1089-8603(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 42.Valente EG, Ferrini MG, Vernet D, Qian A, Rajfer J, Gonzalez-Cadavid NF. PDE L-arginine and PDE inhibitors counteract fibrosis in the Peyronie's fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–244. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Rajfer J, Gore JL, Kaufman J, Gonzalez-Cadavid N. Case report: Avoidance of palpable corporal fibrosis due to priapism with upregulators of nitric oxide. J Sex Med. 2006;3:173–176. doi: 10.1111/j.1743-6109.2005.00090.x. [DOI] [PubMed] [Google Scholar]