Summary
The study of protein-protein interactions is a powerful approach to uncover the molecular function of gene products associated with human disease. Protein-protein interaction data are accumulating at an unprecedented pace owing to interactomics projects, although it has been recognized that a significant fraction of these data likely represents false positives. During our studies of Biogenesis of Lysosome-related Organelles Complex-1 (BLOC-1), a protein complex involved in protein trafficking and containing the products of genes mutated in Hermansky-Pudlak syndrome, we faced the problem of having too many candidate binding partners to pursue experimentally. In this work, we have explored ways of efficiently gathering high-quality information about candidate binding partners and presenting the information in a visually friendly manner. We applied the approach to rank 70 candidate binding partners of human BLOC-1 and 102 candidates of its counterpart from Drosophila melanogaster. The top candidate for human BLOC-1 was the small GTPase encoded by the RAB11A gene, which is a paralog of the Rab38 and Rab32 proteins in mammals and the lightoid gene product in flies. Interestingly, genetic analyses in D. melanogaster uncovered a synthetic sick/lethal interaction between Rab11 and lightoid. The data-mining approach described herein can be customized to study candidate binding partners for other proteins or possibly candidates derived from other types of “omics” data.
Introduction
The postgenomic era is witnessing a blossom of so-called system-biology “omics” approaches to understand the function of genes through studies on their products (transcripts and/or proteins) at an unprecedented large scale. Among them are the “interactomics” approaches aimed at elucidating the network of protein-protein interactions that occur in vivo (von Mering et al 2002; Gandhi et al 2006). Considering the extensive success in understanding the molecular function of proteins through the study of individual protein-protein interactions, the expectation for the impact of the field of interactomics to biology – and eventually to medicine – is very high.
However, at least two main drawbacks have been recognized. First, intrinsic limitations of each interactomics approach can result in large numbers of false-negative and false-positive results. While the problem of false negatives tends to be minimized because negative results are typically not reported, one must consider that not all positive interactions being reported will turn out to be “real” (to occur in vivo and be of biological significance). In the case of the yeast-two-hybrid (Y2H) system, which so far has been the method most extensively used to study the interactomes of organisms other than yeast, false-positive rates of 50% or higher have been estimated (Deane et al 2002). Consequently, follow-up experimentation is always required to validate interactions of interest. The second drawback, which is common to other systems biology approaches, is the potential of “data overload” caused by an unprecedented wealth of experimental observations. This has led to a proliferation of successful bioinformatics strategies to filter, organize and extract useful information from the experimental data (von Mering et al 2002; Giot et al 2003; Rual et al 2005; Stelzl et al 2005; Camargo et al 2007; Gandhi et al 2006).
We have recently faced a combination of the two problems mentioned above, i.e., having to pursue experimentally too many candidate binding partners resulting from Y2H projects, during our studies on biogenesis of lysosome-related organelles complex-1 (BLOC-1). BLOC-1 is a stable protein complex that in mammals comprises eight known subunits: pallidin, muted, cappuccino, dysbindin, snapin, BLOC subunit 1 (BLOS1), BLOS2 and BLOS3 (Fig. 1A; for a recent review see Raposo and Marks 2007). Mutations in the DTNBP1 gene encoding dysbindin and the BLOC1S3 gene encoding BLOS3 cause Hermansky-Pudlak syndrome (HPS) type 7 (HPS-7) and HPS-8, respectively (Li et al 2003; Morgan et al 2006). All types of HPS, including the two associated with BLOC-1 deficiency, follow an autosomal-recessive mode of inheritance and are characterized by partial loss of pigmentation in hair, skin and eyes (i.e., oculocutaneous albinism) and prolonged bleeding times due to platelet storage pool deficiency (reviewed by Wei 2006). Both clinical manifestations arise from defects in the biogenesis of so-called “lysosome-related” organelles, namely melanosomes and platelet dense granules (Raposo and Marks 2007). The other known types of HPS are associated with deficiencies in another three protein complexes: HPS-3, -5 and -6 are due to mutations in the HPS3, HPS5 and HPS6 genes encoding subunits of BLOC-2, HPS-1 and -4 diseases arise from mutations in the HPS1 and HPS4 genes encoding subunits of BLOC-3, and HPS-2 is due to mutations in the AP3B1 gene encoding a subunit of adaptor protein-3 (AP-3) (Di Pietro and Dell’Angelica 2005; Wei 2006). While the molecular role of AP-3 as a sorting-signal-decoding device mediating intracellular protein trafficking between endosomes and lysosomes (or between endosomes and lysosome-related organelles) is well established, the molecular functions of the BLOCs remain poorly understood (Di Pietro and Dell’Angelica 2005; Raposo and Marks 2007). Nevertheless, BLOC-1 was localized in melanocytes to early-endosome-associated tubules and found to facilitate the trafficking of tyrosinase-related protein 1 and the Menkes disease protein, ATP7A, to maturing melanosomes (Di Pietro et al 2006; Setty et al 2007; Setty et al 2008).
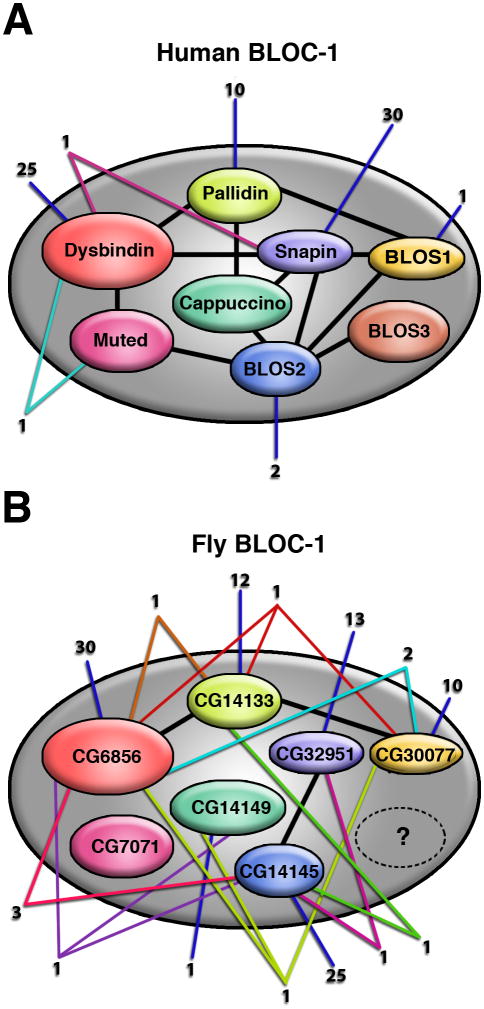
Fig. 1.
Schematic representations of the subunit composition of BLOC-1 from humans (A) and the corresponding orthologues encoded by the genome of Drosophila melanogaster (B). Thick black lines denote published experimental evidence for binary inter-subunit interactions. Numbers connected by blue lines represent unique binding partners described for individual BLOC-1 subunits, and numbers connected by lines of other colors denote candidate binding partners shared by two or more subunits.
As part of our efforts aimed at elucidating the molecular mechanism of BLOC-1 function, we have focused our attention onto direct protein-protein interactions reported in the literature, either as the focus of individual studies (reviewed by Li et al 2007; see also Felten et al 2007; Nian et al 2007; Mistry et al 2007; Suzuki et al 2007; Bao et al 2008; Granata et al 2008; Sun et al 2008) or as part of large sets of interactomics data (Rual et al 2005; Stelzl et al 2005; Camargo et al 2007). In all of these cases, the initial – or only – experimental evidence was obtained by Y2H analysis. In the case of human BLOC-1, the number of candidate binding partners for one or more of its subunits added up to 70 (Fig. 1A). The existence of a BLOC-1 counterpart in the fruit fly, Drosophila melanogaster, was predicted by the presence in its genome of recognizable orthologues for seven of the eight subunits of the mammalian complex (Falcón-Pérez et al 2007); for the products of these seven fly genes the total number of candidate binding partners derived from large-scale Y2H analyses (Giot et al 2003; Formstecher et al 2005) was 102 (Fig. 1B). No homologues of BLOC-1 subunits have been found in the genome of the yeast, Saccharomyces cerevisiae.
The above numbers of candidate binding partners for human and Drosophila BLOC-1 would exceed our ability to pursue them experimentally, especially if one considers that multiple approaches would be required to test whether each putative interaction might occur in vivo and be relevant to the function of BLOC-1 in intracellular protein trafficking. Various methods have been described to assess the reliability of interactions within large sets of Y2H data (Deane et al 2002; Goldberg and Roth 2003), or to attempt to reduce the very high false-positive rates (~90%) of in silico predictions of protein-protein interactions (Mahdavi and Lin 2007; Scott and Barton 2007). These methods were designed to assess simultaneously thousands of putative interactions, hence they rely on either global properties of the dataset (e.g., small-world network properties) or scoring criteria that tend to be simplistic as they are restricted to information that can be gathered automatically (e.g., co-occurrence of keywords in GeneOntology descriptions, existence of paralogs reported to interact with each other).
In this work, we have explored ways to efficiently gather high-quality information about the candidate binding partners of BLOC-1 subunits from humans and flies, and to rank the candidates and present the information in a visually friendly manner. For the top candidate resulting from this analysis, the endosomal Ras-related GTPase Rab11, follow-up experimental work uncovered an unexpected genetic interaction with the product of the fly gene lightoid, which is the orthologue of the Ruby gene defective in a rat model of HPS (Oiso et al 2004) and encodes a Rab protein implicated in the biogenesis of lysosome-related organelles (Ma et al 2004; Wasmeier et al 2006). The possibility of applying a similar data-mining approach to analyze other subsets of “omics” data is discussed.
Methods
Literature and database searches
Literature searches for candidate binding partners of human BLOC-1 were performed by using all alternative names of each BLOC-1 subunit as keywords in PubMed (http://www.pubmed.gov) and subsequently browsing the abstracts of all resulting papers. In addition, the supplementary materials of four papers reporting large-scale human protein-protein interaction studies (Rual et al 2005; Stelzl et al 2005; Camargo et al 2007; Ewing et al 2007), and a publicly available database of Y2H data generated by the Alliance for Cell Signaling (http://www.afcs.org), were searched using the subunit names as keywords. Candidate binding partners for BLOC-1 subunits from D. melanogaster were identified by searching the Drosophila Interactions Database (http://www.droidb.org) using the names of subunit-encoding genes (Fig. 1B). Information about official gene symbol, chromosome number, protein name and reported or proposed function was gathered from the Entrez Gene database at the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene). Patterns of gene expression were inferred from the analysis of Expression Sequence Tag counts available through the NCBI UniGene database (http://ncbi.nlm.nih.gov/UniGene/). Information about reported or proposed functions for S. cerevisiae orthologues was obtained from the Saccharomyces Genome Database (http://www.yeastgenome.org/). When available, information about the presence in human proteins of regions with predicted propensity to adopt coiled-coil folds or transmembrane domains was gathered from the Human Protein Reference Database (HPRD) (http://www.hprd.org/) (Mishra et al 2006).
Protein sequence analyses
Sequence analyses of candidate binding partners were carried out using the reference amino acid sequences downloaded from the NCBI Entrez Gene database; if more than one isoform were predicted (owing to alternative splicing of the encoding gene), the longest protein sequence was used. Homology searchers for readily recognizable orthologues in D. melanogaster and S. cerevisiae (for human proteins) or in H. sapiens and S. cerevisiae (for fly proteins) from the non-redundant protein sequence database were carried out using the Gapped-BLASTP algorithm (Altschul et al 1997) with default parameters as available at the NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Information about predicted functional domains was obtained from the conserved-domain search tool available at NCBI as part of the BLASTP server. In the cases of Drosophila proteins, or of human proteins where no predictions of coiled-coil or transmembrane regions were available at the HPRD, predictions of such regions were carried out at the Network Protein Sequence Analysis Tools server (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_server.html) using default parameters (Combet et al 2000).
Candidate ranking
The information gathered about candidate binding partners of BLOC-1 subunits was organized in a table using Microsoft® Excel 2004 for Mac® Version 11.2, were each row represented a candidate binding partner and the columns corresponded to various scoring criteria. A color-code was adopted whereby green, yellow and red at each column position represented “encouraging”, “less encouraging” or “discouraging” information about the candidate, respectively. White color was used to denote lack of information or information that was too general to be considered either encouraging or discouraging. Further details about the color-based scoring system are available in Supplementary Table 1. In order to rank the candidates, the color code was converted into numerical values using a custom-made Macro tool (available upon request), and the sum of all derived values was calculated for each row and used to sort the rows (in descending order) using the Data AutoFilter tool of Excel.
Genetic experiments in flies
Flies were reared at 25°C in a designated room with automatic 12-h light/12-h dark cycles, using standard food and following conventional fly husbandry procedures (Greenspan 1997). The following D. melanogaster lines were obtained from the Bloomington Drosophila Stock Center at Indiana University (Bloomington, IN, USA): y2 wy2 g2 (stock 192), ltd1 (stock 338) and y1 w*; P{w+mC=lacW}Rab11j2D1/TM3, Sb1 (stock 12148). Wild-type Canton-S flies and lines carrying the modified chromosome FM7 as well as the chromosome balancers CyO and TM3, Sb1 were kind gifts from David E. Krantz (University of California, Los Angeles, CA, USA). The g2 line was derived from y2 wy2 g2 by multiple outcrosses into Canton-S and was kindly provided by Anne F. Simon (University of California, Los Angeles, CA, USA). For some experiments, the ltd1 line was partially “cantonized” by three outcrosses into the genetic background of Canton-S.
Results
Ranking of candidate binding partners of human BLOC-1 subunits
Literature and databases searches for candidate binding partners of BLOC-1 resulted in a total of 68 gene products reported to interact with individual subunits, and two gene products reported to interact with dysbindin and another subunit (Fig. 1A). Twenty seven of these candidates resulted from small-scale Y2H screenings (reviewed by Li et al 2007; see also Felten et al 2007; Nian et al 2007; Mistry et al 2007; Suzuki et al 2007; Bao et al 2008; Granata et al 2008; Sun et al 2008), while the rest of them were found as part of large-scale Y2H projects (Rual et al 2005; Stelzl et al 2005; Camargo et al 2007; no candidates were found in a large-scale mass spectrometry study reported by Ewing et al 2007). In order to select the most promising candidates for experimental analyses, we sought to rank them according to a number of specific criteria that would be relevant to the likelihood that a given candidate would interact with BLOC-1 in vivo and participate in its role in intracellular protein trafficking between endosomes, lysosomes and related organelles. Because none of these criteria would constitute an absolute requirement for a candidate to be considered further, we reasoned that the combination of all criteria (i.e., the sum of all scores) would represent our best estimate of how promising each candidate would be. To allow for rapid visual analysis, for each criterion we used green and red colors to represent “encouraging” or “discouraging” information, respectively; yellow was used to represent information that was not as encouraging as the one labeled with green, and white was used to represent lack of information or information that was too vague to be considered either encouraging or discouraging (see Supplementary Table 1 for details about the color code for each criterion).
Ten different criteria were applied to prioritize candidate binding partners of human BLOC-1 subunits (Fig. 2). The first three corresponded to experimental evidence found in the original article describing an interaction between a BLOC-1 subunit and a given candidate gene. The criteria were based on three commonly used types of protein-protein interaction assays: Y2H data, affinity-pulldown assay, and coimmunoprecipitation. The Y2H data were considered encouraging (i.e., green color) if resulting from a small-scale screening (assuming that the authors had a valid reason to select a given interaction partner out of several prey constructs that might have led to expression of reporter genes) or if deemed to be of high confidence by a large-scale Y2H project. The affinity-pulldown data were considered most encouraging if a recombinant form of the binding partner was able to pull-down the native BLOC-1, not just an isolated subunit in recombinant form or overexpressed in cells by transfection. Likewise, the coimmunoprecipitation data were considered most encouraging if involving native BLOC-1 as opposed to a transiently overexpressed subunit. Such stringency level for these two criteria, i.e., considering most encouraging only those positive pulldown and coimmunoprecipitation results involving the entire BLOC-1 complex, stemmed from our own study (Nazarian et al 2006) on the previously reported interaction of the dysbindin subunit of BLOC-1 with α- and β-dystrobrevins (Benson et at 2001). In that study, we had found that dysbindin can interact with the dystrobrevins when isolated from the complex (i.e., in the context of the Y2H or in recombinant form) but not in the context of native BLOC-1, likely because the region of dysbindin that can bind dystrobrevins in vitro is engaged in multiple inter-subunit interactions within BLOC-1 and not available for dystrobrevin binding in vivo (Nazarian et al 2006). Although immunofluorescence colocalization is another criterion often used to validate protein-protein interactions, inspection of the relevant literature led us to exclude it from our analyses. This is because BLOC-1 subunits have been reported to “colocalize” with various binding partners at dissimilar locations such as the plasma membrane (Benson et al 2001; Benson et al 2004), cytoplasm (Fukui et al 2005), both plasma membrane and cytoplasm (Yuan et al 2006; Mistry et al 2007), the perinuclear region (Rüder et al 2005), the Golgi complex (Wolff et al 2006) and even inside nuclei (Felten et al 2007; Nian et al 2007).
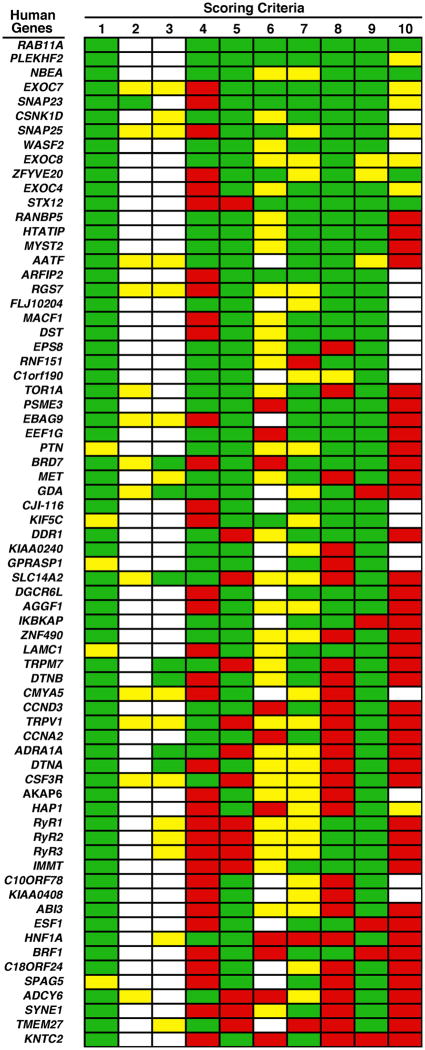
Fig. 2.
Ranking of candidate binding partners for human BLOC-1 subunits. Candidates are listed using the official names of the encoding human genes, with the top position in the list representing the first place in the ranking. Scoring criteria: 1, confidence level on the Y2H interaction (green is for high confidence in a large-scale Y2H project or isolated from an small-scale Y2H screen); 2, interaction detected by affinity pulldown (green is for a positive result obtained using native BLOC-1); 3, interaction detected by coimmunoprecipitation (green is for a positive result obtained using native BLOC-1); 4, predicted regions with propensity to fold into coiled-coils (green is for their absence from candidate); 5, predicted transmembrane regions (green is for their absence from candidate); 6, predicted functional domains (green is for the prediction of at least one domain previously implicated in protein/membrane trafficking); 7, expression pattern (green is for ubiquitous expression); 8, homology to fruit fly proteins (green is for the presence of a recognizable orthologue encoded by the genome of D. melanogaster); 9, homology to yeast proteins (green is for failure to detect a clearly recognizable homologue encoded by the genome of S. cerevisiae); 10, proposed biological function (green is for a role in protein/membrane trafficking on endosomes or lysosomes).
The information for scoring criteria 4–10 was obtained from databases and, upon initial training, could be gathered at a rate of ~6 min per candidate. Criteria 4 and 5 corresponded to the prediction of coiled-coil-forming and transmembrane regions, respectively, and high-quality information was found readily available at HPRD for most human proteins. Coiled-coil-forming regions are involved in protein-protein interactions but are also notorious for their tendency to give false-positive results in the Y2H system. Because all BLOC-1 subunits except for BLOS3 (for which no candidate binding partner has been described) contain coiled-coil-forming domains, we decided that not finding these regions in the candidate binding partners would be encouraging and finding them would be discouraging. Similarly, for transmembrane regions we decided to consider their presence discouraging, in part because of concerns about false positives in the Y2H and the expectation that, by analogy to well-known membrane trafficking pathways, most components of the pathway in which BLOC-1 functions will turn out to be peripheral membrane proteins. Criterion 6 was based on information about structural or functional domains predicted for the candidate binding partners. Here, conserved functional domains specifically related to protein/membrane trafficking, also known as vesicle-mediated trafficking, were considered most encouraging. Criterion 7 was based on mRNA expression patterns as inferred from “virtual dot blots” available at the UniGene database. Because all BLOC-1 subunits are expressed ubiquitously (reviewed by Di Pietro and Dell’Angelica 2005; Wei 2006) and evidence for a role of BLOC-1 in protein trafficking within non-specialized cells has been obtained (Di Pietro et al 2006; Salazar et al 2006), detection of the candidate’s transcript in a wide variety of tissues and cell types was considered most encouraging. Criteria 8 and 9 were based on the ability to detect homologues of the candidate binding partner in D. melanogaster and S. cerevisiae, respectively. Because BLOC-1 subunit orthologues can be found in the former but not in the latter, we scored as most encouraging detecting a homologue of the human candidate binding partner in D. melanogaster (with E-value < 10−4) and not finding it in S. cerevisiae. For the sake of time, the search for homologues was carried out in a single BLASTP round using the non-redundant protein sequence database, and subsequently using an in-built tool to restrict the viewing of results to proteins from the only two species of interest. If a homologue was found in S. cerevisiae through the BLASTP search, information about its potential function was gathered from the Saccharomyces Genome Database, and if it was related to protein trafficking the result was considered less encouraging (yellow) than not finding such a homologue (green) but more than finding a homologue with an unrelated function (red).
Finally, the last criterion was based on the functions reported or proposed for the candidates. We first performed a pilot analysis focusing on over a dozen of candidates, for which the original literature was scanned and read either completely or, in cases with too many original research articles, through a selection of recent reviews. The collected information was then used as a reference to assess the potential quality of functional information readily available in various databases. Although our analysis was neither quantitative nor extensive enough to provide a definitive comparison of the quality of different databases, in our opinion it was the combination of the Summary sections in the NCBI Entrez Gene database and the GeneOntology terms (also available from the same database) that captured more efficiently the information of what is known or predicted about the function of most human proteins analyzed. We considered most encouraging those descriptions about a function in protein/membrane trafficking (or vesicle-meditated protein transport) with reference to endosomes or lysosomes, less encouraging similar descriptions without specific references to endosomes or lysosomes, and discouraging those descriptions about unrelated functions such as transcription or translation.
In order to rank the candidates, the color code was converted into numerical values using a rather simple rule: green = +2, yellow = +1, white = 0 and red = −1. The only exception was the criterion 10, for which the above values were doubled (green = +4, yellow = +2, white = 0, red = −2) to give extra weight to the gathered information about the candidate’s function. As mentioned above, two of the candidates (CK1δ and β-dystrobrevin) had been reported to interact with more than one BLOC-1 subunit (Benson et al 2001; Li et al 2003; Wolff et al 2006; Yin et al 2006).
Although we first considered the possibility of giving extra weight to candidates interacting with multiple BLOC-1 subunits, we also gave consideration to a counterargument whereby these multiple interactions could reflect a tendency of “sticky” proteins to give multiple false positives. Hence, we adopted the conservative approach of giving to the candidate only the best of the two scores obtained when analyzed with each interacting BLOC-1 subunit separately. The resulting ranking is shown in Fig. 2, and additional information is provided in Supplementary Table 2. At the top of the ranking is the product of the RAB11A gene, which is a small GTPase of the Rab family of Ras-related proteins. In particular, the RAB11A gene product, Rab11, is associated with recycling endosomes and has been shown to play key roles in protein and membrane trafficking events during development (Ullrich et al 1996; Prekeris et al 2000; Pelissier et al 2003; Riggs et al 2003; Alone et al 2005; Giansanti et al 2007). Rab11 is also a paralog of Rab38 and Rab32, which are two highly related Rab family members with restricted expression and roles in the biogenesis of melanosomes (Wasmeier et al 2006). At second and third places are the proteins phafin 2 and neurobeachin, respectively. Fourth in the ranking is the product of the EXO70 gene, which is a subunit of the exocyst complex implicated in the “tethering” of exocytic vesicles at specific sites of the plasma membrane (reviewed by Munson and Novick 2006). Interestingly, another two subunits of the exocyst, encoded by the EXOC8 and EXOC4 genes, had also been reported to interact with BLOC-1 subunits in large-scale Y2H projects and herein ranked at the 9th and 11th places, respectively (Fig. 2 and Supplementary Table 2).
Ranking of candidate binding partners of subunits of Drosophila BLOC-1
We next sought to apply our ranking approach to candidate binding partners from a different species: the fruit fly D. melanogaster. Here, all interactions but two were derived from a large-scale Y2H study reported by Giot et al (2003), which also observed three of the several inter-subunit interactions observed for human BLOC-1 (Starcevic and Dell’Angelica 2004) (Fig 1A and B, black lines). Ninety-one gene products were reported to interact with a single BLOC-1 subunit, while 11 gene products were found to interact with two or three subunits (Fig. 1B). Although few of these interactions were deemed to be of high confidence according to an automatic scoring system (Giot et al 2003), careful examination of small subsets of interactions derived from large Y2H projects has revealed that even those interactions deemed to be of “low confidence” in individual datasets might turn out to be real and should not be dismissed (Gandhi et al 2006). Consequently, all candidates were included in our analysis.
We used similar scoring criteria as those described above for the human candidate binding proteins, except for the following differences. First, since the only experimental evidence for interaction of the Drosophila proteins was derived from Y2H analysis, the criteria based on affinity-pulldown and coimmunoprecipitation assays were irrelevant and were not used. Second, given that reliable predictions of coiled-coil-forming and transmembrane domains were not readily available for Drosophila proteins (as they were in HPRD for human proteins) we ran such predictions using the Network Protein Sequence Analysis Tools server. Third, no criterion based on patterns of mRNA expression was used because the available data on the expression of BLOC-1 subunits in D. melanogaster were sparse and not very consistent. Fourth, by analogy to the criterion that finding Drosophila homologues of human candidate binding proteins would be encouraging, we considered encouraging finding a human homologue of a fly candidate binding partner through a simple BLASTP search (with E-value < 10−4). Finally, given that the information gathered about the function of fly proteins was more limited than that of human proteins, instead of doubling the numerical weight of this criterion we added one more based on the function reported or proposed for the human homologue, if any, and then applied the same simple conversion rule (green = +2, yellow = +1, white = 0 and red = −1) to all criteria.
The resulting ranking of candidate binding partners of Drosophila BLOC-1 subunits is shown in Fig. 3, and further details are listed in Supplementary Table 3. There were virtually no common binding partners for human and fly BLOC-1, which is not entirely surprising given the well-documented lack of overlap between interactomic data obtained for different species or even for the same species – by different projects (reviewed by Gandhi et al 2006). However, ranked at the 7th place was the product of the CG2095 gene (Fig. 3), which is a subunit of the exocyst complex from flies and the orthologue of the human EXOC4 gene product that was ranked 11th among the human candidates (Fig. 2).
Fig. 3.
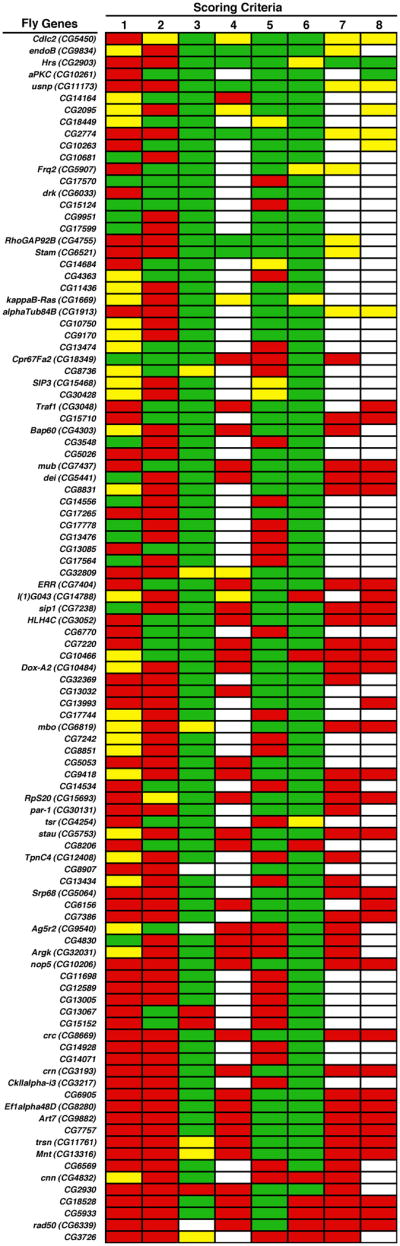
Ranking of candidate binding partners for subunits of BLOC-1 from flies. Candidates are listed using the official names of the encoding genes from D. melanogaster (alternative names between parentheses), with the top position in the list representing the first place in the ranking. Scoring criteria: 1, confidence level on the Y2H interaction (green is for high confidence); 2, predicted regions with propensity to fold into coiled-coils (green is for their absence from candidate); 3, predicted transmembrane regions (green is for their absence from candidate); 4, predicted functional domains (green is for the prediction of at least one domain previously implicated in protein/membrane trafficking); 5, homology to human proteins (green is for the presence of a recognizable orthologue encoded by the human genome); 6, homology to yeast proteins (green is for failure to detect a clearly recognizable homologue encoded by the genome of S. cerevisiae); 7, proposed biological function of the fly protein (green is for a role in protein/membrane trafficking on endosomes or lysosomes); 8, proposed biological function of the human orthologue, if any (green is for a role in protein/membrane trafficking on endosomes or lysosomes).
Genetic interactions in flies
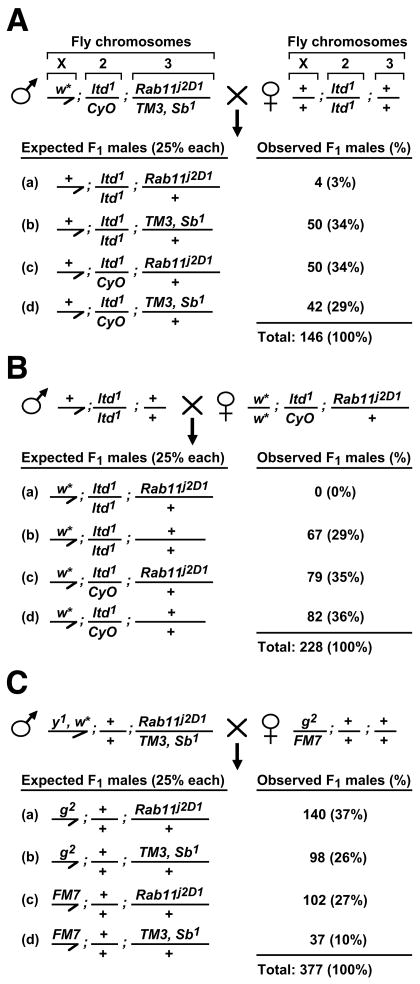
We next attempted to pursue experimentally the top candidate binding partner of human BLOC-1, the Rab11 GTPase. Preliminary affinity-pulldown assays, using recombinant Rab11 expressed in bacteria and native BLOC-1 from bovine brain cytosol, have so far yielded no significant interaction (Rodriguez-Fernandez and Dell’Angelica, unpublished results). However, additional experiments will be required to rule out the possibility that our negative results may have been a consequence of the experimental conditions used; for example, the interaction of small GTPases such as Rab11 with other proteins is known to depend strictly on their binding to GDP or GTP (Prekeris et al 2000; Jagoe et al 2006) and differences in the protein-binding abilities of soluble and membrane-associated BLOC-1 have been documented (Di Pietro et al 2006). In an alternative approach, we sought for evidence of genetic interaction between a mutant allele of Rab11 in flies and mutations in components of the molecular machinery that is conserved between humans and flies and required for the biogenesis of lysosome-related organelles in both species. Unfortunately, direct genetic interaction between Rab11 and BLOC-1 could not be tested in flies, because so far no mutant lines deficient in Drosophila BLOC-1 have been reported in the literature or made available at public repositories. Consequently, we focused on genes like those encoding subunits of the AP-3 complex, which are required for the biogenesis of melanosomes and platelet dense granules in humans as well as for the biogenesis of eye pigment granules in flies. Actually, the association of AP-3 subunit mutations with HPS in human (Dell’Angelica et al 1999) was demonstrated only after the role of Drosophila AP-3 in eye pigment granule biogenesis was discovered (Ooi et al 1997; Simpson et al 1997). Other reasons for focusing on AP-3 were the reported physical and functional interactions between this complex and BLOC-1 in mammals (Di Pietro et al 2006, Salazar et al 2006). Another relevant example involves two Rab proteins, Rab38 and Rab32, which are required for proper biogenesis of melanosomes in mammals (Wasmeier et al 2006); mutations in the Rab38-encoding gene were documented for rat models of HPS (Oiso et al 2004), and mutations in the only Drosophila ortholog of Rab38 and Rab32, lightoid, were shown to cause defects in the biogenesis of fly eye pigment granules (Ma et al 2004). Because Rab11 is paralog of lightoid, the possibility of partial functional overlap between them deserved consideration. Consequently, we performed fly genetic analyses to test whether the Rab11j2D1 mutant allele, which causes lethality in homozygous form owing to essential roles of Rab11 in cytokinesis and tissue development (Pelissier et al 2003; Riggs et al 2003; Alone et al 2005; Giansanti et al 2007), could enhance the eye pigmentation defects of flies deficient in AP-3 (i.e., homozygous for the g2 mutant allele) or in the Rab38/Rab32 ortholog (i.e., homozygous for the ltd1 mutant allele). The eye pigmentation of flies homozygous for g2 and heterozygous for Rab11j2D1 was indistinguishable from that of homozygous g2 flies (data not shown). Unexpectedly, almost no fly homozygous for ltd1 and carrying a single copy of the Rab11j2D1 allele survived to young adulthood. Thus, upon a fly crossing designed to yield about 25% of male flies homozygous for ltd1 and heterozygous for Rab11j2D1, only four male flies with this genotype out of more than a hundred (3%) were recovered within 24 hours after eclosion (Fig. 4A). Similar results were obtained using a different mutant allele of Rab11, Rab1193Bi (data not shown). As an attempt to rule out effects caused by other loci, we outcrossed the ltd1 allele into the genetic background of Canton-S and performed a new set of crosses searching for flies homozygous for ltd1 and heterozygous for Rab11j2D1; this time, however, the number of males with this genotype was zero (Fig. 4B). On the other hand, male flies homozygous for g2 and heterozygous for Rab11j2D1 were viable; actually, they were observed in excess of the theoretical 25% frequency owing to detrimental effects of FM7 and TM3 chromosomes on viability (Fig. 4C). Taken together, these results demonstrated a synthetic sick/lethal interaction between Rab11 and lightoid, likely due to partially overlapping functions of the encoded Rab proteins.
Fig. 4.
Synthetic sick/lethal interaction between mutant alleles of the fly genes Rab11 and lightoid encoding related Rab GTPases. (A) Male flies carrying a null-mutant allele of the white gene (w*) on chromosome X, a copy of a null-mutant allele of the lightoid gene (ltd1) over a second-chromosome balancer (CyO, which induces a “curly-wing” phenotype) and a copy of a mutant allele of Rab11 (Rab11j2D1) over a third-chromosome balancer (TM3, Sb1, which induces a “short-and-thick-hair” phenotype) were crossed with virgin females homozygous for the ltd1 mutation. (B) male flies homozygous for the ltd1 mutation were crossed with virgin females homozygous for the w* mutation on chromosome X and carrying a copy of ltd1 over the second-chromosome balancer CyO and a single copy of the Rab11j2D1 allele on the third chromosome. (C) male flies carrying mutations in the yellow (y1) and white (w*) genes on chromosome X and heterozygous for the Rab11j2D1 allele over the third-chromosome balancer (TM3, Sb1) were crossed with virgin females heterozygous for a mutant allele of the garnet gene (g2) over a modified X-chromosome, FM7 (which in males leads to abnormally small eyes). Shown in all panels (A–C) are the four possible genotypes expected for the males in the progeny at a theoretical frequency of 25% each, as well as the absolute numbers (and percentages) of adult male flies observed within 24 hours after eclosion. Notice in (A) and (B) the significantly low numbers of male flies that survived to adulthood when homozygous for ltd1 and carrying a single copy of the Rab11j2D1 allele.
Discussion
The goal of this work was to find ways of obtaining high-quality information to prioritize candidate binding partners, in cases where the number of reported interactions exceeds the capacity of individual laboratories to perform all of the necessary validation experiments. Such is the situation that we have faced through our studies of BLOC-1, for which 70 candidate binding partners have been found in humans and 102 in flies – mostly by large-scale Y2H projects. Bearing in mind that a large proportion of Y2H data represents false positives (Deane et al 2002; von Mering et al 2002; Gandhi et al 2006), the assumption that all interactions reported for BLOC-1 may be “real” appears unwarranted; rather, many of them are probably not worthy to be pursued experimentally. We suspect that researchers working on other proteins of medical relevance may be facing a similar dilemma. For example, over 280 candidate binding partners have been described for DISC1, the product of a gene that is truncated upon a chromosomal translocation strongly associated with psychiatric disease and for which the molecular function remains poorly understood (Camargo et al 2007).
Various methods have been described for the global assessment of large sets of interactomics data (Deane et al 2002; Giot et al 2003; Goldberg and Roth 2003; Rual et al 2005; Stelzl et al 2005; Camargo et al 2007; Mahdavi and Lin 2007; Scott and Barton 2007). Some approaches to assess the reliability of Y2H data rely on the existence of paralogs shown to interact with each other (Deane et al 2002); although successful for many proteins, in the case of BLOC-1 only two of its subunits display homology to other human sequences, and for them virtually no interaction data are available (data not shown). Other approaches give weight to finding the corresponding interaction between the orthologues from another species; again such an idea has been successful for several proteins (e.g., Gandhi et al 2006) yet it cannot be applied to binding partners of BLOC-1 because the only protein shared by the lists of human and fly candidate binding partners (the Sec8 protein encoded by human EXOC4 and Drosophila CG2095) was reported to interact with dysbindin in humans and BLOS2 in flies (Supplementary Tables 2 and 3). Our approach is unique in that it “customizes” the scoring criteria according to prior knowledge by the researcher about characteristics of the candidates that he/she would find encouraging to pursue with experimental work. We believe that a customized approach can be very powerful when focusing on candidate binding partners of well-characterized proteins or of proteins with unique properties (e.g., tissue-specific expression, well-established localization to a specific cellular compartment). On the other hand, we recognize that the choice of criteria is intrinsically arbitrary, which could adversely affect the usefulness of the ranking. For example, some researchers might disagree with our choice to consider encouraging the absence of predicted coiled-coil-forming and transmembrane domains in the candidate’s primary structure. Nevertheless, it is worth emphasizing that no single criterion is sufficient to completely exclude a candidate from further consideration. For example, 6 of the top 12 human candidates do contain coiled-coil-forming regions, as compared to a total of 35 out of 70 candidates in the entire list, and the human candidate ranked 12th does contain a transmembrane domain. Finally, despite our best efforts, some of the information gathered about the candidates may be inaccurate. For example, practical reasons led us to restrict our search for experimental evidence to only the first article reporting the interaction, although for a few candidate binding partners (e.g., SNAP25; Ilardi et al 1999) subsequent work have brought the original findings into question (Vites et al 2004). In addition, some of the candidates for which failure to detect a yeast homologue in a BLASTP search was considered encouraging do contain orthologues in yeast (e.g., the exocyst subunits encoded by EXOC7 and EXOC4; Munson and Novick 2006) that probably would have been detected by more sensitive but time-consuming algorithms such as PSI-BLAST. These limitations notwithstanding, we find the data-mining approach, and the idea of summarizing the information using a color code, potentially very useful. For example: we have previously invested significant amounts of resources and time to pursue experimentally the reported interactions between the dysbindin subunit of BLOC-1 and the dystrobrevins (Benson et al 2001), with negative results (Nazarian et al 2006); in retrospect, the current ranking of the two dystrobrevins (encoded by the DTNB and DTNA genes) to the 45th and 51st places would have discouraged us from pursuing these interactions in particular.
At the top of the ranking of human candidates was the product of the RAB11A gene. Rab11 is a small GTPase associated with a subset of endosomes known as recycling endosomes, which accumulate at a perinuclear region of the cell and play important roles in the sorting of proteins for recycling to the plasma membrane as well as in asymmetric distribution of signaling molecules during mitosis (Ullrich et al 1996; Prekeris et al 2000; Emery et al 2005). Moreover, Rab11 is required for normal cytokinesis, and for development of various tissues in flies (Pelissier et al 2003; Riggs et al 2003; Alone et al 2005; Giansanti et al 2007). Consistent with these important functions, homozygous mutations in the only Rab11 gene in flies cause lethality as embryos or early larvae (Alone et al 2005). This is in contrast with homozygous null mutations in lightoid, the only fly orthologue of both Rab32 and Rab38, which result in viable flies that display specific defects in the biogenesis of a lysosome-related organelle: the fly eye pigment granule (Ma et al 2004). Likewise, mutations in the Rab38-encoding gene in mice and rats result in viable animals with defective biogenesis of melanosomes, and the rat mutant is considered an animal model of HPS (Loftus et al 2002; Osio et al 2004). At first sight, one may conclude that Rab32/Rab38/lightoid, and not Rab11, would be the key Rab protein for lysosome-related organelles and with which BLOC-1 might interact. However, genetic analyses in flies have suggested that lightoid is unlikely to be the only Rab involved in this process. Thus, the pigmentation phenotype of homozygous null ltd1 is not as severe as those of other eye color mutants, and enhancement of the phenotype was observed for double mutants simultaneously deficient in lightoid and AP-3 (Ma et al 2004) or in lightoid and BLOC-2 (Falcón-Pérez et al 2007). These considerations led us to evaluate the possibility that Rab11, which is a paralog of Rab32/Rab38/lightoid, could have some degree of functional overlap with the latter. Our results did provide evidence for functional overlap, but in an unexpected manner: while ltd1 homozygous flies and Rab11j2D1 heterozygous flies were viable as adults and fertile, flies that were both ltd1 homozygous and Rab11j2D1 heterozygous barely survived to young adulthood. This synthetic sick/lethal effect leads us to speculate that these two related Rab protein may indeed have overlapping functions, for instance by interacting with common effector proteins, although such overlap would extend to some of the essential functions of Rab11. Further work will be required to understand the molecular basis for this intriguing genetic interaction, and the possible involvement of BLOC-1 in this process.
Other candidate binding partners that ranked close to the top should also deserve future experimentation. Second in the list of the human candidates is phafin 2, a novel protein predicted to associate with early endosomes owing to the presence of a FYVE domain. At third place is neurobeachin, a member of a family of large proteins that also includes Lyst, which is mutated in Chediak-Higashi syndrome and – like BLOC-1 – is required for normal biogenesis of lysosome-related organelles (Shiflett et al 2002). At the 4th, 9th and 11th places rank three of the eight subunits of the exocyst complex, and the orthologue of one of them ranks 7th among the candidate binding partners of Drosophila BLOC-1 subunits. Interestingly, solid evidence indicates that both mammalian and Drosophila exocyst components interact with Rab11 (Zhang et al 2004; Beronja et al 2005). Finally, ranking at the top of the candidate binding partners of Drosophila BLOC-1 are a subunit of the microtubule-associated motor, dynein, a member of the endophilin family of membrane-curvature-sensing proteins, and the Hrs subunit of the endosome-associated protein complex, ESCRT-0, which also contains the Stam subunit ranked in 19th place.
It is likely that approaches similar to that described here could be useful to researchers who face other situations with an exceedingly high number of candidate genes or proteins. For example, a single Y2H screening typically results in a large number of “colonies” representing a number of candidate binding partners. Other lists of candidates may arise from other types of “omics” approaches, e.g., genes whose transcripts are found upregulated under certain experimental conditions, or proteins identified by mass spectrometric analysis of a partially purified sample. In all of these situations, the researcher may need to rank the candidates to select those more “encouraging” for experimental analysis. We believe that our “customized” criteria approach with visually friendly presentation could be helpful also in those situations.
Supplementary Material
Suppl. Table 1 Details of the scoring criteria used to rank candidate binding partners.
Suppl. Table 2 Information on the candidate binding partners of human BLOC-1 subunits.
Suppl. Table 3 Information on the candidate binding partners of Drosophila BLOC-1 subunits.
Acknowledgments
We thank to David E. Krantz and Anne F. Simon for reagents, and to Julián A. Martínez-Agosto and Verónica T. Cheli for critical reading of the manuscript. This work was supported in part by grant HL068117 from the National Institutes of Health. I. A. Rodriguez-Fernandez was supported by National Institutes of Health training grant T32 HG002536.
Details of funding: This work was supported in part by grant HL068117 from the National Institutes of Health (NIH). I. Rodriguez-Fernandez was supported by NIH training grant T32 HG002536. The authors confirm independence from the sponsors; the content of this article has not been influenced by any sponsor.
Abbreviations
- AP-3
adaptor protein-3
- BLOC
biogenesis of lysosome related organelles complex
- BLOS
BLOC subunit
- HPRD
Human Protein Reference Database
- HPS
Hermansky-Pudlak syndrome
- NCBI
National Center for Biotechnology Information
- Y2H
yeast two hybrid
Footnotes
Details of the contributions of individual authors: I. A. Rodriguez-Fernandez: Performed data-mining analysis and fly genetic experiments, prepared the figures and supplementary tables and contributed to the writing of the main text.
E. C. Dell’Angelica: Designed the data-mining analysis and fly genetic experiments, participated in the interpretation of results and wrote most of the main text.
Name of author who serves as guarantor: Esteban C. Dell’Angelica, Ph.D., Associate Professor, UCLA Dept. Human Genetics.
Competing interest statement: The authors confirm that they have no competing interests for declaration.
Details of ethical approval: No ethical approval was required for the research studies described in this article. Neither human subjects (or derived samples) nor live vertebrate animals were used.
Patient consent statement: Does not apply.
Take-home message: An approach is described to prioritize candidate protein-binding partners in cases where the number of reported interactions involving a protein of interest exceeds the capacity to perform the necessary follow-up experiments.
References to electronic databases: Hermansky-Pudlak syndrome: OMIM #203300; Dystrobrevin-binding protein 1 (DTNBP1): OMIM #607145; Biogenesis of lysosome-related organelles complex 1 subunit 3 (BLOC1S3): OMIM #609762.
References
- Alone DP, Tiwari AK, Mandal L, Li M, Mechler BM, Roy JK. Rab11 is required during Drosophila eye development. Int J Dev Biol. 2005;49:873–879. doi: 10.1387/ijdb.051986da. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Lopez JA, James DE, Hunziker W. Snapin interacts with the Exo70 subunit of the exocyst and modulates GLUT4 trafficking. J Biol Chem. 2008;283:324–331. doi: 10.1074/jbc.M706873200. [DOI] [PubMed] [Google Scholar]
- Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276:24232–24241. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- Benson MA, Tinsley CL, Blake DJ. Myospryn is a novel binding partner for dysbindin in muscle. J Biol Chem. 2004;279:10450–10458. doi: 10.1074/jbc.M312664200. [DOI] [PubMed] [Google Scholar]
- Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol. 2005;169:635–646. doi: 10.1083/jcb.200410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, et al. Disrupted in Schizophrenia 1 interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Combet C, Blanchet C, Geourjon C, Deléage G. NPS@: Network Protein Sequence Analysis. Trends Biochem Sci. 2000;25:147–150. doi: 10.1016/s0968-0004(99)01540-6. [DOI] [PubMed] [Google Scholar]
- Deane CM, Salwinski L, Xenarios I, Eisenberg D. Protein interactions. Two methods for assessment of the reliability of high throughput observations. Mol Cell Proteomics. 2002;1:349–356. doi: 10.1074/mcp.m100037-mcp200. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in theβ3A subunit of the AP-3 adaptor. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Di Pietro SM, Dell’Angelica EC. The cell biology of Hermansky–Pudlak syndrome: recent advances. Traffic. 2005;6:525–533. doi: 10.1111/j.1600-0854.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro SM, Falcón-Pérez JM, Tenza D, et al. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA. Asymmetric Rab11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell. 2005;122:763–773. doi: 10.1016/j.cell.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Ewing RM, Chu P, Elisma F, et al. Large-scale mapping of human protein-protein interactions by mass spectrometry. Mol Syst Biol. 2007;3:89. doi: 10.1038/msb4100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcón-Pérez JM, Romero-Calderón R, Brooks ES, Krantz DE, Dell’Angelica EC. The Drosophila pigmentation gene pink (p) encodes a homologue of human Hermansky Pudlak syndrome 5 (HPS5) Traffic. 2007;8:154–168. doi: 10.1111/j.1600-0854.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Felten A, Leister P, Burgdorf S, Uhlmann L, Scheidtmann KH. Characterization of rat BLOS2/Ceap, a putative yeast She3 homolog, as interaction partner of apoptosis antagonizing transcription factor/Che-1. Biol Chem. 2007;388:569–582. doi: 10.1515/BC.2007.073. [DOI] [PubMed] [Google Scholar]
- Formstecher E, Aresta S, Collura V, et al. Protein interaction mapping: A Drosophila case study. Genome Res. 2005;15:376–384. doi: 10.1101/gr.2659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K, Yang Q, Cao Y, et al. The HNF-1 target collectrin controls insulin exocytosis by SNARE complex formation. Cell Metab. 2005;2:373–384. doi: 10.1016/j.cmet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Gandhi TKB, Zhong J, Mathivanan S, et al. Analysis of the human protein interactome and comparison with yeast, worm and fly interaction datasets. Nat Genet. 2006;38:285–293. doi: 10.1038/ng1747. [DOI] [PubMed] [Google Scholar]
- Giansanti MG, Belloni G, Gatti M. Rab11 is required for membrane trafficking and actomyosin ring constriction in meiotic cytokinesis of Drosophila males. Mol Biol Cell. 2007;18:5034–5047. doi: 10.1091/mbc.E07-05-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giot L, Bader JS, Brouwer C, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- Goldberg DS, Roth FP. Assessing experimentally derived interactions in a small world. Proc Natl Acad Sci USA. 2003;100:4372–4376. doi: 10.1073/pnas.0735871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata A, Watson R, Collinson LM, Schiavo G, Warner TT. The dystonia-associated protein torsinA modulates synaptic vesicle recycling. J Biol Chem. 2008;283:7568–7579. doi: 10.1074/jbc.M704097200. [DOI] [PubMed] [Google Scholar]
- Greenspan RJ. Fly pushing: the theory and practice of Drosophila genetics. New York: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- Ilardi JM, Mochida S, Sheng ZH. Snapin: a SNARE-associated protein implicated in synaptic vesicle transmission. Nat Neurosci. 1999;2:119–124. doi: 10.1038/5673. [DOI] [PubMed] [Google Scholar]
- Jagoe WN, Lindsay AJ, Read RJ, McCoy AJ, McCaffrey MW, Khan AR. Crystal structure of rab11 in complex with rab11 family interacting protein 2. Structure. 2006;14:1273–1283. doi: 10.1016/j.str.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang Q, Oiso N, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Y, Hao C, Guo X, Cui Y, He M, He X. The BLOC interactomes form a network in endosomal transport. J Genet Genomics. 2007;34:669–682. doi: 10.1016/S1673-8527(07)60076-9. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Larson DM, Baxter LL, et al. Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci USA. 2002;99:4471–4476. doi: 10.1073/pnas.072087599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Plesken H, Treisman JE, Edelman-Novemsky I, Ren M. Lightoid and Claret: a rab GTPase and its putative guanine nucleotide exchange factor in biogenesis of Drosophila eye pigment granules. Proc Natl Acad Sci USA. 2004;101:11652–11657. doi: 10.1073/pnas.0401926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi MA, Lin Y-H. False positive reduction in protein-protein interaction predictions using gene ontology annotations. BMC Bioinformatics. 2007;8:262. doi: 10.1186/1471-2105-8-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NV, Pasha S, Johnson CA, et al. A germline mutation in BLOC1S3/Reduced Pigmentation causes a novel variant of Hermansky-Pudlak syndrome (HPS8) Am J Hum Genet. 2006;78:160–166. doi: 10.1086/499338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra GR, Suresh M, Kumaran K, et al. Human Protein Reference Database - 2006 update. Nucleic Acids Res. 2006;34:411–414. doi: 10.1093/nar/gkj141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry AC, Mallick R, Frohlich O, Klein JD, Rehm A, Chen G, Sands JM. The UT-A1 urea transporter interacts with snapin, a SNARE-associated protein. J Biol Chem. 2007;282:30097–30106. doi: 10.1074/jbc.M705866200. [DOI] [PubMed] [Google Scholar]
- Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struc Mol Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- Nazarian R, Starcevic M, Spencer MJ, Dell’Angelica EC. Reinvestigation of the dysbindin subunit of BLOC-1 (biogenesis of lysosome-related organelles complex-1) as a dystrobrevin-binding protein. Biochem J. 2006;395:587–598. doi: 10.1042/BJ20051965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nian H, Fan C, Liao S, Shi Y, Zhang K, Liu Y, Han C. RNF151, a testis-specific RING finger protein, interacts with dysbindin. Arch Biochem Biophys. 2007;465:157–163. doi: 10.1016/j.abb.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Oiso N, Riddle SR, Serikawa T, Kuramoto T, Spritz RA. The rat Ruby (R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm Genome. 2004;15:307–314. doi: 10.1007/s00335-004-2337-9. [DOI] [PubMed] [Google Scholar]
- Ooi CE, Moreira JE, Dell’Angelica EC, Poy G, Wassarman DA, Bonifacino JS. Altered expression of a novel adaptin leads to defective pigment granule biogenesis in the Drosophila eye color mutant garnet. EMBO J. 1997;16:4508–4518. doi: 10.1093/emboj/16.15.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol. 2003;13:1848–1857. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Scheller RH. A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol Cell. 2000;6:1437–1448. doi: 10.1016/s1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- Raposo G, Marks MS. Melanosomes – dark organelles enlighten endosomal membrane transport. Nat Rev Mol Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs B, Rothwell W, Mische S, et al. Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J Cell Biol. 2003;163:143–154. doi: 10.1083/jcb.200305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual JF, Venkatesan K, Hao T, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- Rüder C, Reimer T, Delgado-Martinez I, et al. EBAG9 adds a new layer of control on large dense-core vesicle exocytosis via interaction with snapin. Mol Biol Cell. 2005;16:1245–1257. doi: 10.1091/mbc.E04-09-0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G, Craige B, Styers ML, et al. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol Biol Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MS, Barton GJ. Probabilistic prediction and ranking of human protein-protein interactions. BMC Bioinformatics. 2007;8:239. doi: 10.1186/1471-2105-8-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SRG, Tenza D, Truschel ST, et al. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol Biol Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SRG, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–1147. doi: 10.1038/nature07163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett SL, Kaplan J, Ward DM. Chediak-Higashi syndrome: a rare disorder of lysosomes and lysosome related organelles. Pigment Cell Res. 2002;15:251–257. doi: 10.1034/j.1600-0749.2002.02038.x. [DOI] [PubMed] [Google Scholar]
- Simpson F, Peden AA, Christopoulou L, Robinson MS. Characterisation of the adaptor-related protein complex, AP-3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic M, Dell’Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J Biol Chem. 2004;279:28393–28401. doi: 10.1074/jbc.M402513200. [DOI] [PubMed] [Google Scholar]
- Stelzl U, Worm U, Lalowski M, et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957–968. doi: 10.1016/j.cell.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Sun J, Nie J, Hao B, et al. Ceap/BLOS2 interacts with BRD7 and selectively inhibits its transcription-suppressing effect on cellular proliferation-associated genes. Cell Signal. 2008;20:1151–1158. doi: 10.1016/j.cellsig.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Morishima S, Tanaka T, Muramatsu I. Snapin, a new regulator of receptor signaling, augments alpha1A-adrenoceptor-operated calcium influx through TRPC6. J Biol Chem. 2007;282:29563–29573. doi: 10.1074/jbc.M702063200. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vites O, Rhee J-S, Schwarz M, Rosenmund C, Jahn R. Reinvestigation of the role of snapin in neurotransmitter release. J Biol Chem. 2004;279:26251–26256. doi: 10.1074/jbc.M404079200. [DOI] [PubMed] [Google Scholar]
- von Mering C, Krause R, Snel B, Cornell M, Oliver SG, Fields S, Bork P. Comparative assessment of large-scale data sets of protein-protein interactions. Nature. 2002;417:399–403. doi: 10.1038/nature750. [DOI] [PubMed] [Google Scholar]
- Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 and Rab32 control early post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Wolff S, Stöter M, Giamas G, Piesche M, Henne-Bruns D, Banting G, Knippschild U. Casein kinase 1 delta (CK1δ) interacts with the SNARE associated protein snapin. FEBS Lett. 2006;580:6477–6484. doi: 10.1016/j.febslet.2006.10.068. [DOI] [PubMed] [Google Scholar]
- Yin H, Laguna KA, Li G, Kuret J. Dysbindin structural homologue CK1BP is an isoform-selective binding partner of human casein kinase-1. Biochemistry. 2006;45:5297–5308. doi: 10.1021/bi052354e. [DOI] [PubMed] [Google Scholar]
- Yuan X, Shan Y, Zhao Z, Chen J, Cong Y. Interaction between snapin and G-CSF receptor. Cytokine. 2006;33:219–225. doi: 10.1016/j.cyto.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Zhang X-M, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–43034. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Table 1 Details of the scoring criteria used to rank candidate binding partners.
Suppl. Table 2 Information on the candidate binding partners of human BLOC-1 subunits.
Suppl. Table 3 Information on the candidate binding partners of Drosophila BLOC-1 subunits.