Abstract
Background
Variants in the gene for complement factor H (CFH) have been implicated as a major risk factor for the development of age-related macular degeneration (AMD). Little is known, however, about the factors regulating local expression and secretion of CFH by retinal pigment epithelial cells (RPE).
Methods
Cultured human early passage RPE cells, highly differentiated, polarized human RPE cultures, and bovine RPE explants were incubated in the presence or absence of recombinant human or bovine interferon-γ (IFN-γ; 25 ng/ml). CFH expression in cell lysates, and secretion into culture supernatants were examined by Western blot. CHF expression and localization was analyzed by confocal microscopy. Migration assay was performed in a modified Boyden chamber with early passage human RPE cells after stimulation with recombinant CFH protein (1–100 ng/ml).
Results
CFH was expressed in the cell lysates of RPE cells, and this expression was significantly upregulated by IFN-γ. Immunoreactivity for CFH was detected in RPE cells of bovine explants and highly differentiated human RPE monolayers, and the level of immunoreactivity increased after IFN-γ stimulation. Confocal microscopy revealed that CFH was predominantly localized in the apical cytoplasm of polarized human RPE. Western blot confirmed that IFN-γ increased CFH secretion into RPE supernatants. Dose dependent RPE cell chemotactic migration was induced by CFH.
Conclusion
IFN-γ promotes CFH expression in the apical compartment of RPE cells and increases secretion of CFH into RPE culture supernatants. Furthermore, CFH promotes chemotactic migration of RPE. This study suggests that interactions between CFH and IFN-γ have the potential to play a role in the pathogenesis of AMD.
Keywords: AMD, complement factor H, migration, retinal pigment epithelium, interferon-γ
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in the elderly in developed countries [1–5]. AMD is a complex disorder stemming from the interaction of aging with multiple genetic and environmental risk factors [6,7,8]. AMD, along with several other chronic disorders including Alzheimer disease, atherosclerosis and glomerulonephritis, is characterized by the presence of extracellular deposits and inflammation, which contribute to the pathogenesis and progression of the disease [3,9–14]. In AMD, these deposits appear as drusen, a hallmark of the early form of AMD [1]. Drusen contain various inflammatory components, suggesting that the innate immune response and the complement cascade may contribute to drusen formation [1,15–20]. Complement factor H (CFH) is the major inhibitor of the alternative complement pathway and is found within drusen [3,21]. Variants in the gene for CFH have been implicated as a major risk factor for the development of AMD [2,3,4,5,22,23,24]. Thus, a genetic variation in a regulator of the alternative complement pathway, when combined with a triggering event such as infection, oxidative stress or smoking, may underlie a major proportion of AMD in the human population [3,25].
Retinal pigment epithelial (RPE) cells represent a primary site of pathology in AMD and their function and phenotype may be modified by cytokines and inflammatory mediators. The cytokine interferon gamma (IFN-γ) has been shown to induce expression of CFH [26] in the human ARPE-19 cell line, and upregulate expression of major histocompatibility complex (MHC)-II in primary human RPE cells [27]. In contrast, a recent study by Chen et al. showed that CFH production in a cultured murine RPE cell line was not affected by IFN-γ [28]. Thus, the effects of IFN-γ on the expression of CFH in RPE cells may vary according to species, cell line or culture conditions. Therefore, in order to gain greater relevance to human disease, we determined the effects of IFN-γ on CFH expression in cultured human RPE, on highly differentiated and polarized human RPE cultures [29], and in primary bovine RPE explant cultures. We also hypothesized that CHF secretion into the extracellular microenvironment of the RPE may affect other RPE functions. Since cytokines have been shown to modulate RPE migration [30], and CFH has been reported to be chemotactic to monocytes [31], we evaluated the effects of CFH on RPE chemotactic migration.
MATERIALS AND METHODS
Human RPE cell cultures
The Institutional Review Board of the University of Southern California approved the use of cultured human RPE cells. RPE cells isolated from human fetal eyes (20 weeks gestation; Advanced Bioscience Resources, Inc., Alameda, CA) were cultured in Dulbecco's minimal Eagle's medium (DMEM; Fisher Scientific, Pittsburgh, PA) with 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (Sigma, St. Louis, MO), and 10% heat-inactivated fetal bovine serum (FBS; Irvine Scientific, Santa Ana, CA). All experiments were performed using first to fourth passage cells. Cells were confirmed to be RPE by their typical morphology, their immunoreactivity for cytokeratin (>95%) and the lack of immunoreactivity for macrophage or endothelial cell markers.
Western blot
Passage 2–4 human RPE cells (5×105) were cultured in Dulbecco’s minimal Eagle’s medium with 10% heat-inactivated fetal bovine serum (FBS) [30] on 6-well plates until they reached confluence. Twenty four hours prior to IFN-γ stimulation, the confluent cells were gently washed with serum-free medium and then the medium was replaced with Dulbecco’s minimal Eagle’s medium with no added serum; subsequently, recombinant human IFN-γ (25 ng/ml; Sigma, St,Louis,Mo) was applied and incubated for 24, 48 or 72 hours, after which the cells and supernatants were harvested. Parallel control non- IFN-γ –treated cultures were also evaluated after 24, 48 or 72 hours of incubation. Cell lysates were prepared using cell lysis buffer (Pierce Biotechnology, Rockford IL). The cell lysate (10 ug protein/lane) and supernatant (40 µg protein/lane) proteins were resolved on Tris hydrochloride 7.5% polyacrylamide gels (Ready Gel; Bio-Rad Laboratories, Hercules, CA) at 120 V. The proteins were transferred to a polyvinylidene fluoride blotting membrane (Millipore, Billerica, MA). These membranes were then probed with anti-CFH antisera (Quidel Corporation, San Diego, CA) 1:500), followed by horseradish peroxidase–conjugated anti–goat antibody (1:5000; Vector Laboratories) for 30 minutes at room temperature. Images were developed by adding chemiluminescence detection solution (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). As a loading control for cell lysates, membranes were also probed using antibodies against the housekeeping gene GAPDH. Band intensity was quantified using Image Pro Plus software. The integrated optical density, defined as the integrated total intensity of all pixels in each band was determined and corrected for background. The resulting absolute numerical values were expressed relative to GAPDH. Student-T test was then used to compare the integrated optical density between control and IFN stimulated RPE at each time point. The accepted level of significance was p<0.05.
Preparation of polarized human RPE monolayer cultures
Highly differentiated, polarized, human RPE monolayer cultures were prepared as previously described [29]. Briefly, primary cultured, human fetal RPE cells were trypsinized and resuspended in DMEM supplemented with 100 U/mL penicillin-streptomycin, 2 mM L-glutamine, and 1% FBS. Cells (1.7×105 cells/cm2) were seeded onto individual fibronectin-coated polyester permeable supports (0.4 µm pore size, 12 mm diameter; Transwell; Corning Costar, Corning NY) in 0.5 ml of medium. The medium was changed twice a week. Cells were cultured for at least a month to form differentiated monolayers, with the apical domain corresponding to the retinal facing side of the RPE monolayer. Trans-epithelial resistance (TER) reached a plateau at the time of experimentation ranging between 200 and 300 Ω•cm2. Recombinant human IFN-γ (0, and 25 ng/ml, Sigma, St. Louis, MO) was added to the medium and incubated for 48 hours.
Bovine RPE-choroid explants
In order to determine CFH expression in situ, we evaluated CFH expression and its regulation by IFN-γ in bovine RPE-choroid explants. Fresh bovine eyes were obtained from a local abattoir and the eyes were cut circumferentially posterior to the limbus, and the vitreous and retina were removed. A 1-cm2 section of RPE with choroid was obtained and incubated in 12-well plates with DMEM containing 1% FBS for 24 hours, and then recombinant bovine IFN-γ (0, and 25 ng/ml; Sigma) was added for an additional 48 hours.
Immunocytochemistry
Immunocytochemistry was performed on bovine RPE/choroid explants, cryostat frozen sections (6 µm thick) of bovine RPE/choroid explants, and human polarized RPE monolayers which had been incubated with or without IFN-γ. Samples were fixed in 4% paraformaldehyde for 30 min , and then permeabilized using 0.2% Triton-X 100 at 37° C for 15 min. Blocking was achieved by addition of 1% rabbit serum for 20 min. The samples were incubated with primary anti-CFH antibody (Quidel Corporation; 1:1,000) for 1 hr at room temperature. After washing with PBS, secondary FITC-conjugated rabbit anti-goat antibody (1:400; Chemicon, Temecula, CA) was applied to the slides for 30 min at room temperature. As a negative control, sections were treated with secondary antibody alone. Sections were mounted on glass slides with mounting medium including propidium iodide (Vector Laboratories). Fluorescence was examined and photographed with a high resolution Zeiss laser-scanning microscope (LSM2, Carl Zeiss, Thornwood, NY) with a 40x oil-immersion lens.
Migration assay
Migration assay was performed in a modified 24 well plate Boyden chamber (Fibronectin coated, 1 µm thick polycarbonate inserts; 8 µm pore size; pore density of 1×105 pores/cm2 ; Becton Dickinson Labware, Franklin Lakes NJ) using passage 2–4 human fetal RPE cells (5 × 104 cells/ml in media containing 0.4% FBS). At the time of cell counting, the cells were evaluated microscopically to ensure a single cell suspension. Recombinant CFH (Quidel) was added to the lower chamber alone (chemotactic assay), or both lower and upper chambers (chemokinesis assay) at equal concentrations of 1, 10, 50, 100 ng/ml as the stimulant. After incubating for 5 hours, the inserts were washed 3 times with PBS, fixed with cold (4°C) methanol for 10 minutes, and counterstained with hematoxylin for 30 minutes. The number of migrated cells on the entire insert was counted using a phase-contrast microscope (×200). Three independent experiments were performed using triplicate inserts. Cross sectional analysis of the mean number of migrated cells in each group was performed to compare groups using the Poisson model for aggregate count data. The accepted level of significance was p<0.05.
RESULTS
CFH protein expression in cell lysates of RPE cells treated with IFN-γ
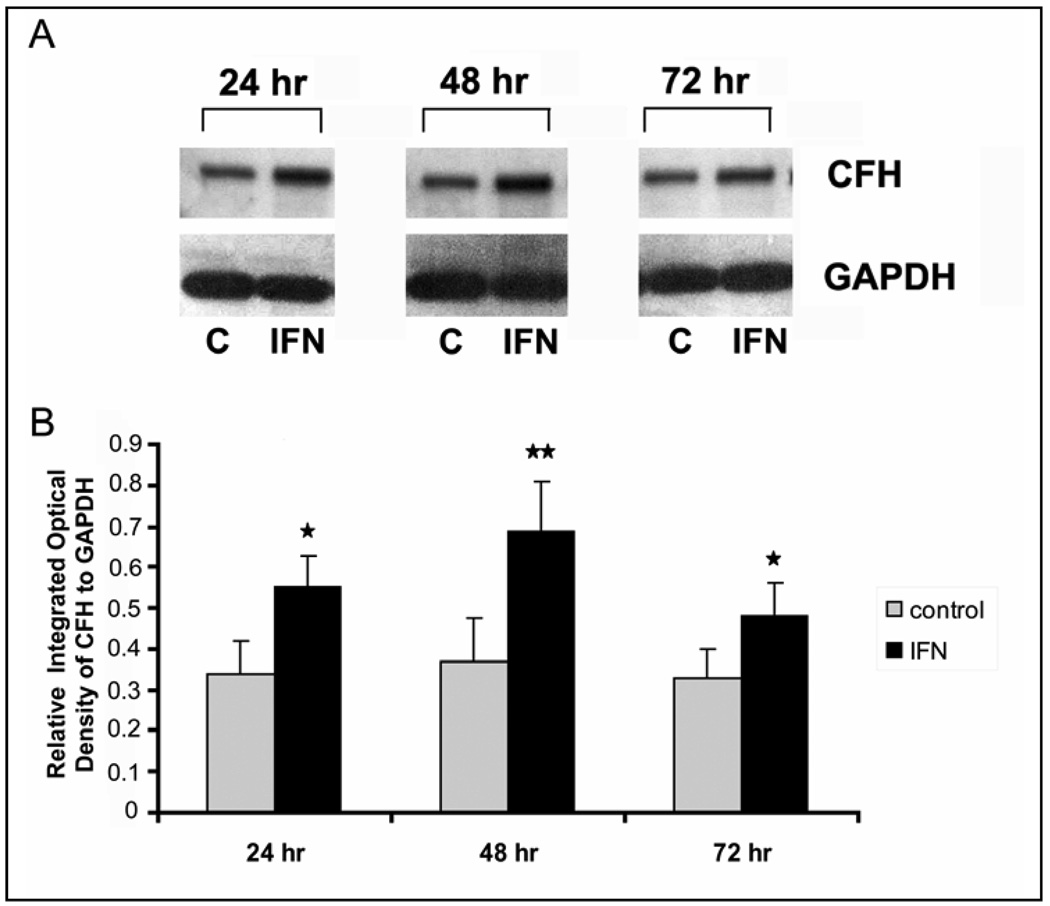
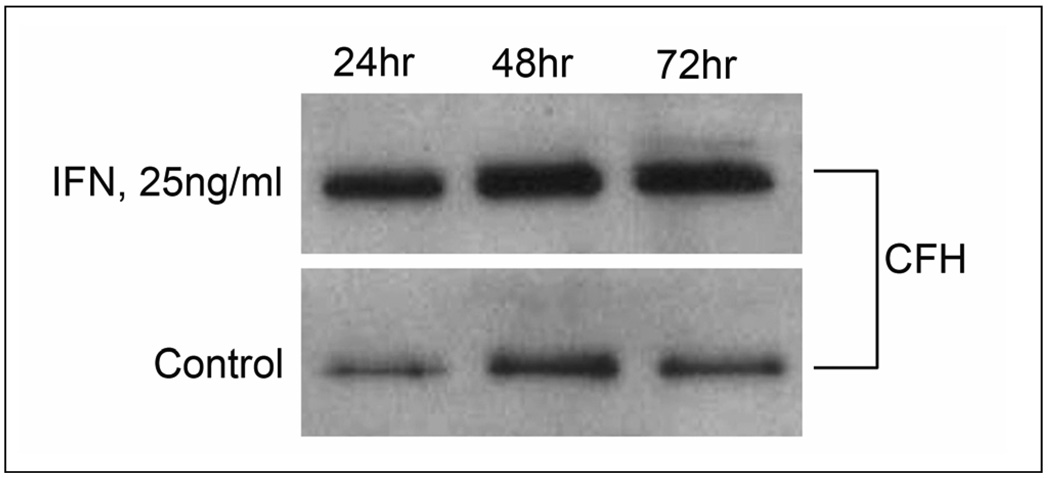
Western blots of RPE cell lysates revealed the presence of a major CFH immunoreactive band at 155 kDa CFH (Fig. 1a). CFH protein expression in RPE cell lysates was significantly upregulated by stimulation with IFN-γ at 24, 48, and 72 hours (Fig 1a). Semi-quantitative analysis of CFH protein expression by densitometry revealed that expression of CFH was significantly increased at all 3 time points with maximal expression at 48 hours after stimulation with IFN-γ (P<0.02) (Fig1 b).
Fig. 1.
Time course of complement factor H (CFH) upregulation by recombinant human IFN-γ in human RPE cell homogenates as demonstrated by Western blot. High molecular weight CFH fragment (155 kDa) is seen in the Western blot (a). CFH protein expression is significantly upregulated by stimulation with IFN-γ (25ng/ml). Fig 1b shows the relative absolute “Integrated Optical Density” of each CFH band relative to the GAPDH band. All values were corrected for background. Values represent mean and standard deviation of three independent determinations. Asterisk denotes P < 0.05; Double Asterisks P< 0.02
Immunoreactivity for CFH in bovine RPE-choroid explants treated with bovine IFN-γ
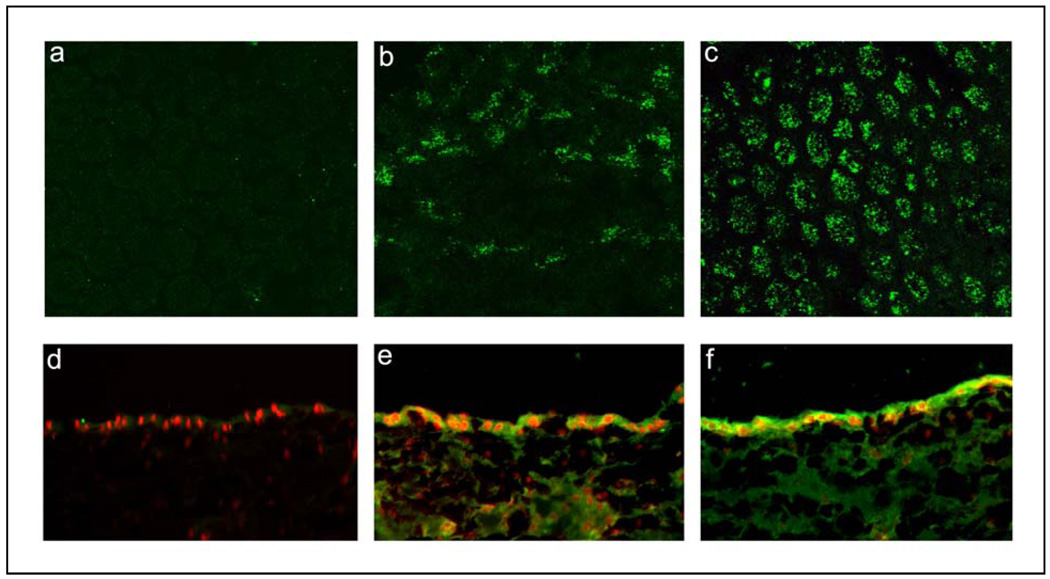
Cross reactivity of anti-CFH antibody with bovine CFH was confirmed by Western blot of bovine RPE cell homogenates; a major CFH immunoreactive band was identified at 155 kD similar to that seen in Figure 1 (results not shown). The expression of CFH in bovine RPE-choroid explants is shown in Fig 2. In untreated explants, scattered RPE cells showed weak to moderate cytoplasmic immunoreactivity for CFH (Fig. 2b). After treatment with IFN-γ (25 ng/ml) expression of CFH appeared to be much stronger and labeled almost every RPE cell in a punctuate pattern (Fig. 2c). No primary antibody controls revealed focal, weak, background staining (Fig. 2a). Cryostat sections of the RPE-choroid explants revealed that there was weak to moderate cytoplasmic staining for CFH in a subset of RPE cells; weak staining of cells within the choroid was also seen (Fig. 2e). Treatment with IFN-γ resulted in a prominent increase in CFH immunoreactivity in essentially all cells of the RPE layer (Fig. 2f). Very weak CFH immunoreactivity was present in the “no primary antibody” control (Fig. 2d)
Fig. 2.
Immunolocalization of CFH in RPE cells of bovine RPE/choroid explants (a–c) and cryostat sections of bovine explants (d–f) after 48 hrs incubation with 0 ng/ml or 25 ng/ml of bovine recombinant IFN-γ. Cytoplasmic immunoreactivity for CFH (green fluorescence) is detected in untreated RPE cells (b,e) and its level increases after treatment with IFN-γ (c,f). Weak staining is also seen in the choroid in both untreated and treated explants. Negative control specimens, in which primary antibody was omitted, show negligible background staining (a,d). Mounting media for cryostat sections (d–f) contains propidium iodide to label cell nuclei (red).
Immunoreactivity for CFH in polarized human RPE cultures treated with IFN-γ
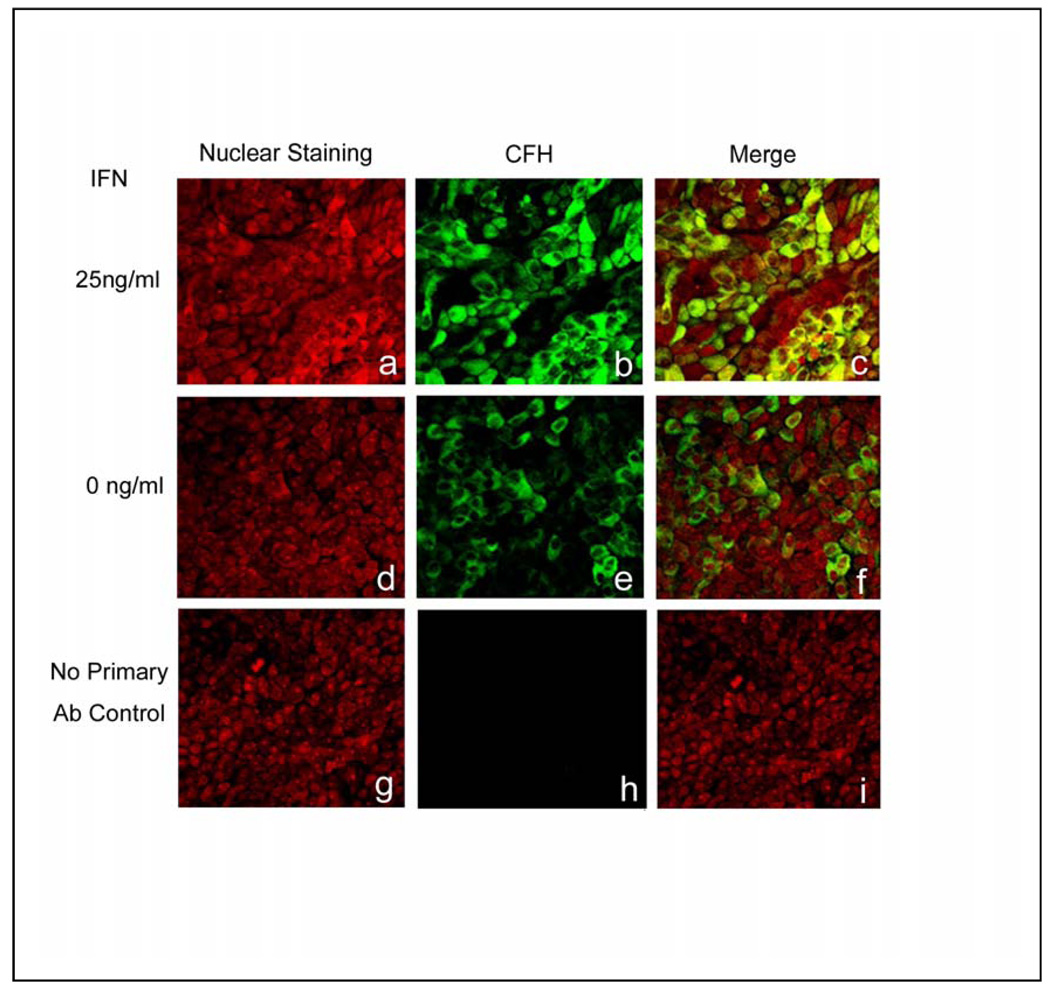
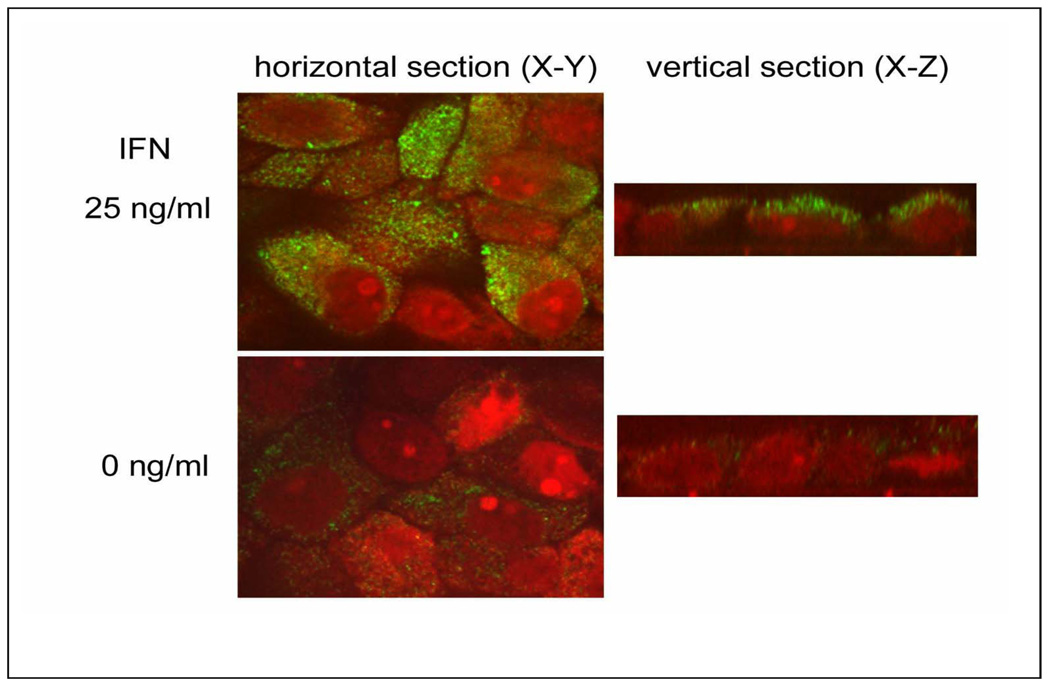
Evaluation of CFH protein expression in polarized, human RPE cell monolayers, revealed diffuse, cytoplasmic expression in most of the RPE cells in the untreated cultures (Fig. 3; d–f,). After treatment with 25 ng/ml IFN-γ (Fig. 3; a–c), the intensity of expression became more prominent. No primary antibody control sections showed insignificant background staining (Fig. 3; g–i). Higher magnification confocal images of the polarized, human RPE cell monolayers (Fig. 4) confirmed that CFH staining was cytoplasmic and punctate and that the intensity of the immunoreactivity was greater in the IFN-γ –treated cells. Confocal vertical sections (X–Z) revealed that expression of CFH was predominantly localized in the apical compartment of both untreated and treated cells (Fig. 4; right panel).
Fig. 3.
Immunolocalization of CFH (b, c, e, f, h, i; green) in human polarized monolayer RPE cultures after 0 ng/ml (e–f), and 25 ng/ml (b–c) of recombinant human interferon (IFN)-γ. Cytoplasmic immunoreactivity for CFH is detected in RPE cells (e–f) and its level increases after treatment with IFN-γ (b,c). Propidium iodide staining provides nuclear counterstain (red). Negative control specimens, in which primary antibody was omitted, show negligible immunoreactivity for CFH (h–i).
Fig. 4.
Immunolocalization of CFH (green) in differentiated human RPE monolayer cultures after 0 ng/ml (lower panel), and 25 ng/ml (upper panel) of recombinant human interferon (IFN)-γ. Left and right panels show confocal horizontal (X–Y), and vertical sections (X–Z), respectively. Immunoreactivity for CFH is weak and punctate in RPE cells with no IFN-γ addition (left lower panel). The CFH immunoreactivity is strongly and diffusely detected in the cytoplasm of RPE cells treated with 25 ng/ml IFN-γ (left upper panel).Vertical section of confocal microscopy (X–Z) reveals immunoreactivity for CFH is located in the apical cytoplasmic compartment of RPE cells (right panel).
CFH secretion into culture supernatants induced by IFN-γ
Since we showed that RPE cells show regulated expression of CFH in their cytoplasmic compartment, we next wanted to determine whether CFH was secreted, consistent with a previous finding that human keratinocytes released CFH into the culture medium [32]. To determine whether CFH was secreted into the serum-free RPE cell culture medium, CFH expression was examined in the cell-free supernatants of RPE cells with or without IFN-γ treatment using Western blot analysis. High molecular weight CFH was detected in the supernatants in control samples and was increased in amount after treatment with IFN-γ for 24, 48 and 72 hours when compared with the time-matched non- IFN-γ treated controls (Fig.5). In order to determine whether the level of CFH secreted from RPE cultures was relevant to subsequent migration experiments, we established a Western blot standard curve using the pixel density of immunoblotted serum free culture media spiked with recombinant CFH (5, 10, 20, 30, 50, and 100 ng/ml). When 48 hour, IFN-γ-treated cultures (3 independent samples) were run along side the spiked standards, an average concentration of 22 ±6 ng/ml was found (results not shown).
Fig. 5.
Secretion of CFH by human RPE cultures measured by Western blot. Accumulation of complement factor H (CFH) in the supernatants of human RPE cultures (serum free medium) treated with 25 ng/ml of IFN-γ was measured after 24, 48 and 72 hours. Western blot for CFH was performed using 40 µg of total protein in each lane. After treatment with IFN-γ, increased secretion of CFH is found in the 24, 48 and 72 hour supernatants when compared to time-matched non- IFN-γ treated control supernatants.
Modulation of RPE cell migration by recombinant CFH protein
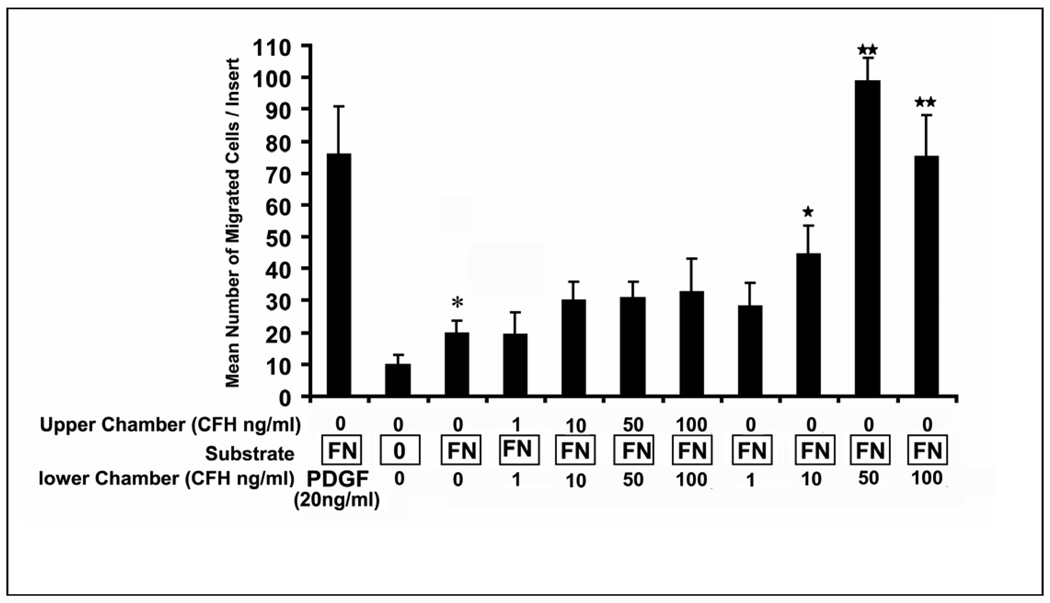
Our results showing that RPE cells secreted CFH protein into the culture supernatant, prompted us to determine whether CFH could affect RPE function. This is relevant, since Lommatzsch et al [33] found that CFH was highly expressed extracellularly in basal laminar deposits from human CNV membranes associated with AMD. The finding that RPE cells migrate into the stroma in response to growth factors found in CNV membranes [34–36] suggested that we evaluate RPE cell migration induced by exposure to CFH protein. Since RPE migration typically occurs in the context of a provisional extracellular matrix, and fibronectin has been localized in human CNV membranes [37] and in diffuse drusen [38], we first evaluated whether a migratory response to fibronectin was present. Baseline migration doubled in the presence of fibronectin and therefore all subsequent migration experiments were performed in the presence of fibronectin (Fig 6). When equal concentrations of CFH were placed in the upper and lower compartments, there was a modest but statistically insignificant increase in migration indicating that RPE lack a significant increase in random migration with CFH. However, a much stronger, dose-dependent chemotactic response was obtained when CFH was added to the lower compartment alone. A maximal chemotactic effect was found at 50 ng/ml (p<0.01; Fig. 6). Interestingly, CFH-induced RPE chemotactic migration was as strong as that stimulated by the known RPE chemotactic factor platelet derived growth factor (PDGF)-BB. In separate experiments, RPE migration was completely blocked by pre-incubating CFH with anti-CFH antibody (data not shown).
Fig. 6.
Complement factor H (CFH) induces RPE cell migration in a Boyden chamber assay. .Inserts coated with fibronectin (FN) show increased migration in the absence of any factors added to upper or lower compartment (P<0.05). Chemokinesis was evaluated by added equal concentrations of CFH in the upper and lower compartments; no significant increase was seen at 10, 50 or 100 ng/ml CFH. Chemotaxis was evaluated by adding CFH to the lower compartment alone. Dose dependent chemotactic migration was found with a maximal effect at 50 ng/ml. (p<0.01). PDGF-BB (20 ng/ml) is used as a positive control. Values represent mean number of migrated cells/insert and standard deviation from three independent experiments. *; P<0.05; ** P<0.01
Discussion
The importance of CFH in pathogenesis of AMD has been well established and the localization of CFH in drusen suggests local synthesis of CFH by RPE [3,18]. Previous studies in the human ARPE-19 cell line suggested that CFH was not constitutively expressed in RPE [26], while immunostaining and RT-PCR studies in mice showed constitutive expression in RPE [39]. Our results indicate that non-immortalized, passage 2–4 human RPE cell cultures constitutively express CFH as demonstrated by Western blot analysis of cell lysates, and immunofluorescence images of cultured bovine RPE explants and highly differentiated, polarized human cultured RPE monolayers. RPE cells are known to respond to stimulation by pro-inflammatory cytokines such as IFN-γ by up-regulating expression of cytokine receptors and MHC class II molecules [27,40]. These results suggest that IFN-γ may participate in and magnify local inflammatory responses in the outer retina; however, IFN-γ may also up-regulate expression of genes that dampen inflammatory responses in the delicate environment of the retina. In the current study, we found that IFN-γ stimulation results in up-regulation of CFH expression. This result differs from that published by Chen et al. [28]. They reported that in murine RPE, IFN-γ had no effect on CFH expression while TNF-α and IL-6 downregulated CFH expression. These conflicting results on cytokine regulation of CFH expression may be due to the difference between species, as varying results are obtained even among different cell lines [21,41]. Similar to our results, Wu et al. demonstrated that CFH was upregulated by IFN-γ in RPE, however, their study, performed using the ARPE-19 cell line, did not show constitutive expression of CFH [26]. In summary, our work clearly supports the contention that IFN-γ up-regulates CFH expression in RPE and that in vitro studies evaluating regulated gene expression in immortalized cell lines should be re-evaluated using differentiated, human primary cell cultures, in situ evaluation or explant cultures.
This study is the first to demonstrate polarized expression of CFH in RPE. Using highly differentiated human RPE monolayer cultures we showed that CFH was clearly localized to the apical compartment. We also demonstrate that CFH is actually secreted from RPE cells where it accumulates in the culture supernatants and that IFN-γ promotes this secretion. Importantly, we show that CFH accumulates in RPE culture supernatants at a level that promotes RPE chemotaxis in Boyden chamber assays suggesting that the level is physiologically relevant. Apical secretion of CFH would promote a CFH gradient that is highest in the subretinal space, where it may be acting to protect photoreceptors from effects of inappropriate complement activation. We also demonstrate here, for the first time, that CFH promotes the chemotactic migration of RPE cells consistent with its effects as a chemotactic agent for monocytes [31]. Therefore, apical secretion of CFH from RPE may have the potential to promote migration of RPE into the stroma of choroidal neovascular lesions.
In conclusion, we demonstrate that CFH is constitutively expressed in human RPE cell cultures and bovine RPE explants and that CFH expression is localized to the apical compartment of polarized RPE cells. The expression and secretion of CFH was significantly increased by the stimulation with IFN-γ. Furthermore, the secretion of CFH by RPE may have functional significance beyond that of modulating complement activation by stimulation of RPE migration. This study suggests that interactions between CFH and inflammatory cytokines may play an important role in the pathogenesis of AMD.
Acknowledgements
This work was supported by the Arnold and Mabel Beckman Foundation and by National Institutes for Health grant EY01545 and Core grant EY03040.
The authors would like to thank Chris Spee and Ernesto Barron for technical assistance, Dr. Tom Ogden for reviewing the paper and Susan Clarke for editorial assistance.
References
- 1.Bok D. Evidence for an inflammatory process in age-related macular degeneration gains new support. Proc Natl Acad Sci U S A. 2005;102:7053–7054. doi: 10.1073/pnas.0502819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 3.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnusson KP, Duan S, Sigurdsson H, Petursson H, Yang Z, Zhao Y, Bernstein PS, Ge J, Jonasson F, Stefansson E, Helgadottir G, Zabriskie NA, Jonsson T, Bjornsson A, Thorlacius T, Jonsson PV, Thorleifsson G, Kong A, Stefansson H, Zhang K, Stefansson K, Gulcher JR. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med. 2006;3:e5. doi: 10.1371/journal.pmed.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 7.Daiger SP. Genetics. Was the Human Genome Project worth the effort? Science. 2005;308:362–364. doi: 10.1126/science.1111655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiggs JL. Complement factor H and macular degeneration: the genome yields an important clue. Arch Ophthalmol. 2006;124:577–578. doi: 10.1001/archopht.124.4.577. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torzewski J, Bowyer DE, Waltenberger J, Fitzsimmons C. Processes in atherogenesis: complement activation. Atherosclerosis. 1997;132:131–138. doi: 10.1016/s0021-9150(97)00100-7. [DOI] [PubMed] [Google Scholar]
- 11.Schwertz R, Rother U, Anders D, Gretz N, Scharer K, Kirschfink M. Complement analysis in children with idiopathic membranoproliferative glomerulonephritis: a long-term follow-up. Pediatr Allergy Immunol. 2001;12:166–172. doi: 10.1034/j.1399-3038.2001.012003166.x. [DOI] [PubMed] [Google Scholar]
- 12.Mullins RF, Aptsiauri N, Hageman GS. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye. 2001;15:390–395. doi: 10.1038/eye.2001.142. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien C, Duvall-Young J, Brown M, Short C, Bone M. Electrophysiology of type II mesangiocapillary glomerulonephritis with associated fundus abnormalities. Br J Ophthalmol. 1993;77:778–780. doi: 10.1136/bjo.77.12.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colville D, Guymer R, Sinclair RA, Savige J. Visual impairment caused by retinal abnormalities in mesangiocapillary (membranoproliferative) glomerulonephritis type II (“dense deposit disease”) Am J Kidney Dis. 2003;42:E2–E5. doi: 10.1016/s0272-6386(03)00665-6. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–431. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 16.Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- 18.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–896. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 19.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. Faseb J. 2000;14:835–846. [PubMed] [Google Scholar]
- 21.Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Mol Immunol. 2004;41:355–367. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 23.Zareparsi S, Branham KE, Li M, Shah S, Klein RJ, Ott J, Hoh J, Abecasis GR, Swaroop A. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005;77:149–153. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes AE, Orr N, Patterson C, Esfandiary H, Hogg R, McConnell V, Silvestri G, Chakravarthy U. Neovascular age-related macular degeneration risk based on CFH, LOC387713?HTRA1, and smoking. PLoS Med. 2007;4:e355. doi: 10.1371/journal.pmed.0040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–152/. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, Lauer TW, Sick A, Hackett SF, Campochiaro PA. Oxidative stress modulates complement factor H expression in retinal pigmented epithelial cells by acetylation of FOXO3. J Biol Chem. 2007;282:22414–22425. doi: 10.1074/jbc.M702321200. [DOI] [PubMed] [Google Scholar]
- 27.Gabrielian K, Osusky R, Sippy BD, Ryan SJ, Hinton DR. Effect of TGF-beta on interferon-gamma-induced HLA-DR expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:4253–4259. [PubMed] [Google Scholar]
- 28.Chen M, Forrester JV, Xu H. Synthesis of complement factor H by retinal pigment epithelial cells is down-regulated by oxidized photoreceptor outer segments. Exp Eye Res. 2007;84:635–645. doi: 10.1016/j.exer.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Sreekumar PG, Zhou J, Sohn J, Spee C, Ryan SJ, Maurer BJ, Kannan R, Hinton DR. N-(4-hydroxyphenyl) retinamide augments laser-induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci. 2008;49:1210–1220. doi: 10.1167/iovs.07-0667. [DOI] [PubMed] [Google Scholar]
- 30.Jin M, He S, Worpel V, Ryan SJ, Hinton DR. Promotion of adhesion and migration of RPE cells to provisional extracellular matrices by TNF-alpha. Invest Ophthalmol Vis Sci. 2000;41:4324–4332. [PubMed] [Google Scholar]
- 31.Nabil K, Rihn B, Jaurand MC, Vignaud JM, Ripoche J, Martinet Y, Martinet N. Identification of human complement factor H as a chemotactic protein for monocytes. Biochem J. 1997;326(Pt 2):377–383. doi: 10.1042/bj3260377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timar KK, Pasch MC, van den Bosch NH, Jarva H, Junnikkala S, Meri S, Bos JD, Asghar SS. Human keratinocytes produce the complement inhibitor factor H: synthesis is regulated by interferon-gamma. Mol Immunol. 2006;43:317–325. doi: 10.1016/j.molimm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Lommatzsch A, Hermans P, Weber B, Pauleikhoff D. Complement factor H variant Y402H and basal laminar deposits in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245:1713–1716. doi: 10.1007/s00417-007-0649-7. [DOI] [PubMed] [Google Scholar]
- 34.He S, Jin ML, Worpel V, Hinton DR. A role for connective tissue growth factor in the pathogenesis of choroidal neovascularization. Arch Ophthalmol. 2003;121:1283–1288. doi: 10.1001/archopht.121.9.1283. [DOI] [PubMed] [Google Scholar]
- 35.Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157:135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–868. [PubMed] [Google Scholar]
- 37.Grossniklaus ME, Martinez JA, Brown VB, Lambert HM, Sternberg P, Jr, Capone A, Jr, Aaberg TM, Lopez PF. Immunohistochemical and histochemical properties of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am J Ophthalmol. 1992;114:464–472. doi: 10.1016/s0002-9394(14)71859-8. [DOI] [PubMed] [Google Scholar]
- 38.Newsome DA, Hewitt AT, Huh W, Robey PG, Hassell JR. Detection of specific extracellular matrix molecules in drusen, Bruch's membrane, and ciliary body. AM J Ophthalmol. 1987;104:373–381. doi: 10.1016/0002-9394(87)90227-3. [DOI] [PubMed] [Google Scholar]
- 39.Mandal MN, Ayyagari R. Complement factor H: spatial and temporal expression and localization in the eye. Invest Ophthalmol Vis Sci. 2006;47:4091–4097. doi: 10.1167/iovs.05-1655. [DOI] [PubMed] [Google Scholar]
- 40.Hollborn M, Kohen L, Wiedemann P, Enzmann V. The influence of pro-inflammatory cytokines on human retinal pigment epithelium cell receptors. Graefes Arch Clin Exp Ophthalmol. 2001;239:294–301. doi: 10.1007/s004170100263. [DOI] [PubMed] [Google Scholar]
- 41.Friese MA, Hellwage J, Jokiranta TS, Meri S, Peter HH, Eibel H, Zipfel PF. FHL-1/reconectin and factor H: two human complement regulators which are encoded by the same gene are differently expressed and regulated. Mol Immunol. 1999;36:809–818. doi: 10.1016/s0161-5890(99)00101-7. [DOI] [PubMed] [Google Scholar]