Abstract
Alpha-lipoic acid (LA) has become a common ingredient in multivitamin formulas, anti-aging supplements, and even pet food. It is well-defined as a therapy for preventing diabetic polyneuropathies, and scavenges free radicals, chelates metals, and restores intracellular glutathione levels which otherwise decline with age. How do the biochemical properties of LA relate to its biological effects? Herein, we review the molecular mechanisms of LA discovered using cell and animal models, and the effects of LA on human subjects. Though LA has long been touted as an antioxidant, it has also been shown to improve glucose and ascorbate handling, increase eNOS activity, activate Phase II detoxification via the transcription factor Nrf2, and lower expression of MMP-9 and VCAM-1 through repression of NF-kappa-B. LA and its reduced form, dihydrolipoic acid, may use their chemical properties as a redox couple to alter protein conformations by forming mixed disulfides. Beneficial effects are achieved with low micromolar levels of LA, suggesting that some of its therapeutic potential extends beyond the strict definition of an antioxidant. Current trials are investigating whether these beneficial properties of LA make it an appropriate treatment not just for diabetes, but also for the prevention of vascular disease, hypertension, and inflammation.
Keywords: Alpha-lipoic acid, antioxidant, aging, glutathione, diabetes, dietary supplements
1. INTRODUCTION
Alpha-lipoic acid (LA), or 1,2-dithiolane-3-pentanoic acid, is a naturally occurring dithiol compound synthesized enzymatically in the mitochondrion from octanoic acid. LA is a necessary cofactor for mitochondrial α-ketoacid dehydrogenases, and thus serves a critical role in mitochondrial energy metabolism. In addition to synthesis, LA is also absorbed intact from dietary sources, and it transiently accumulates in many tissues. There is growing evidence that orally supplied LA may not be used as a metabolic cofactor but instead, elicits a unique set of biochemical activities with potential pharmacotherapeutic value against a host of pathophysiologic insults. LA has been described as a potent biological antioxidant, a detoxification agent, and a diabetes medicine; it has been used to improve age-associated cardiovascular, cognitive, and neuromuscular deficits, and has been implicated as a modulator of various inflammatory signaling pathways [1-8]. This impressive array of cellular and molecular functions has piqued considerable interest among the lay public and the research community for the use of LA both as a nutritive supplement and as a pharmacotherapy. In light of this growing interest, we will attempt to provide an update on the biochemical, toxicological, and pharmacological mechanisms of LA. As many excellent reviews already exist that outline the metabolic role of LA as a covalently bound enzyme cofactor, only a brief summary of this particular aspect of LA function will be presented herein. Instead, a focus mainly on the cellular actions of orally supplied, nonprotein-bound LA will be presented. Pertinent clinical benefits of LA will also be discussed in light of these molecular mechanisms.
2. DE NOVO SYNTHESIS OF LA AND ITS USE AS A COFACTOR
α-Lipoic acid, also known as 1,2-dithiolane-3-pentanoic acid or thioctic acid, has one chiral center and therefore exists in both R- and S-enantiomeric forms (Fig. 1). However, only R-LA is conjugated to conserved lysine residues in an amide linkage, thus making this isoform essential as a cofactor in biological systems [9]. Enzymes containing lipoamide are typically mitochondrial multi-enzyme complexes that catalyze the oxidative decarboxylation of α-keto acids (e.g. pyruvate dehydrogenase, 2-oxo-glutarate dehydrogenase, and transketolase) and glycine cleavage [10].
Figure 1.
The R and S enantiomers of lipoic acid.
Though de novo synthesis appears to supply all the necessary LA needed for its role in intermediary metabolism, LA can also be absorbed from the diet. While the direct roles of LA as a cofactor are well understood, less is known about the precise metabolic functions of orally supplied LA. The following sections will summarize the accumulating evidence suggesting that LA from the diet is both bioavailable, safe in moderate doses, and elicits a surprising array of metabolic and clinical effects.
3. UPTAKE, BIOAVAILABILITY, AND SAFETY OF ORALLY SUPPLIED LA
3.1. Dietary uptake
The potential biochemical and therapeutic actions of oral LA intake can only be appreciated with an understanding of its bioavailability, tissue accumulation and metabolic fate. It is now clear that cells maintain active systems to transport, utilize, and excrete nonprotein-bound LA. Typical dietary sources of LA are muscle meats, heart, kidney, and liver, and to a lesser degree, fruits and vegetables [11-13]. Though available from these normal nutritional sources, it is not likely that appreciable amounts of LA are consumed in the typical Western diet; rather, dietary supplements that typically range from 50 to 600 mg are the primary sources of LA, and most information as to its bioavailability comes from studies using supplements.
Takaishi et al. explored the mechanisms involved in LA uptake from dietary sources using a CACO-2 cell transwell model for transepithelial transport. Results showed that LA rapidly traversed the cell monolayer in a pH-dependent manner [14]. LA transport was also inhibitable by benzoic acid and medium-chain fatty acids, suggesting that the monocarboxylate transporter was the likely carrier responsible for intestinal absorption of LA. In addition to this transporter, other in vitro studies identified LA as a substrate for the Na+-dependent multivitamin transporter, which may not only contribute to its gastrointestinal uptake, but also may be involved in LA transport into tissues from the blood plasma [15, 16]. Thus, LA bioavailability may be dependent on multiple carrier proteins. Identification of such a multifaceted uptake and tissue distribution system suggests that various factors (e.g. substrate competition, transcriptional, translational and post-translational regulatory mechanisms) could influence overall LA absorption characteristics.
In concert with its multimodal means of transport, gastrointestinal absorption of LA appears to be quite variable. For example, Teichert et al. measured plasma LA in volunteers given 200 mg R,S-LA (the racemic mixture obtained by the manufacturing process) and observed that approximately 20-40% was absorbed [17]. The efficiency of LA uptake was also lowered by its administration in food, suggesting uptake competition with other nutrients for the carrier protein(s) involved. Carlson et al. followed appearance of R-LA in plasma following administration of its sodium salt to human subjects, and observed that both peak plasma appearance (Cmax) and total LA absorbed (area under the curve) were higher than that of the free acid [18]. Thus, overall LA bioavailability may fluctuate depending on whether it is ingested as a free acid or a salt, and with a meal or not. Table 1 lists selected studies of the pharmacokinetics of LA in humans.
Table 1.
Select lipoic acid (LA) studies in healthy volunteers.
| Reference | LA dose administered to human subjects | AUC of LA in plasma …† | Cmax of LA in plasma … | Subjects |
|---|---|---|---|---|
| Teichert et al. [17] | a) 200 mg racemic LA, oral tablet b) 600 mg racemic LA, oral tablets c) 200 mg racemic LA, intravenous |
a) 46.82 ±21.46 μg*min/ml b) 157.83 ±35.82 μg*min/ml c) 157.97 ±35.05 μg*min/ml |
a) 0.66 ±0.33 μg/ml b) 2.85 ±1.49 μg/ml c) 8.32 ±2.35 μg/ml |
12 (crossover) |
| Carlson et al. [18] | 600 mg Na-R-LA, oral solution | 441.6 ±160.2 μg*min/ml | 16.0 ±6.32 μg/ml | 12 |
| Breithaupt-Grogler et al. [19] |
600 mg racemic LA, oral tablet | R-LA: 2348.04 h*ng/ml S-LA: 1243.24 h*ng/ml |
R-LA: 1812.32 ng/ml S-LA: 978.20 ng/ml |
15 |
| Mignini et al. [20] | a) 600 mg Thioctacid, oral tablet b) 600 mg Tioctil N, oral tablet |
a) 3510.9 ±1088.6 ng*h/ml b) 3563.5 ±1374.1 ng*h/ml |
a) 1338.6 ±751.8 ng/ml b) 1215.8 ±560.5 ng/ml |
16 (crossover) |
| Bernkop-Schnurch et al. [21] |
766.8 mg LA bound to chitosan, once by oral sustained release tablets |
0-12h: 183.8 ±101.4 μg*min/ml 0-6h: 108.1 ±61.5 μg*min/ml 6-12h: 75.7 ±94.8 μg*min/ml |
0-12h: n/a 0-6h: 1354.5 ±807.1 ng/ml 6-12h: 497.5 ±1074 ng/ml |
8 |
| Amenta et al. [22] | a) 600 mg Thioctacid, oral tablet b) 600 mg Tiocronal, oral tablet c) 600 mg Biodynoral, oral tablet d) 600 mg Tiobec retard, oral tablet |
a) 3270.9 ±372.8 ng*h/g b) 2925.2 ±448.4 ng*h/g c) 3142.3 ±331.5 ng*h/g d) 3187.4 ±152.8 ng*h/g |
a) 1266.2 ±237.7 ng/g b) 2290.5 ±286.6 ng/g c) 2262.8 ±392.8 ng/g d) 1397.7 ±070.4 ng/g |
8 (crossover) |
| ‡ Teichert et al. [23] | 600 mg racemic LA, oral dose, q.d. for 4 days | Day 1: 891.0 nmol*min/ml Day 4: 995.5 nmol*min/ml |
Day 1: 19.8 nmol/ml Day 4: 21.1 nmol/ml |
8 |
| Teichert et al. [24] | 600 mg racemic LA, oral dose, q.d. for 4 days | Day 1: 923.5 ±216.91 nmol*min/ml Day 4: 998.91 ±145.8 nmol*min/ml |
Day 1: 36.19 ±10.35 nmol/ml Day 4: 31.45 ±8.2 nmol/ml |
9 |
Values are reported in the original units.
Areas under the curve (AUCs) are from last time measured, except the study [21] using sustained release LA tablets.
This study also included patients with kidney disease, not reported here.
Additionally, as most commercially available LA supplements are a mixture of both R and S enantiomers, questions as to preferential uptake of one isomer versus the other have arisen. In one study, volunteers were given 600 mg of R,S-LA, and plasma concentrations of R-LA were 40-50% higher than S-LA [19], the latter of which was apparently more rapidly cleared than R-LA. These results suggest that the R-enantiomer would be the most appropriate form to provide as oral supplements; however, S-LA in the racemic mixture may prevent the polymerization of R-LA and thereby enhance overall bioavailability. It has yet to be established whether R-LA, its salt form, or a racemic mixture would be best to use in future clinical studies.
3.2. Tissue distribution and metabolic fate
Rapid gastrointestinal uptake of LA and appearance in the plasma is followed by an equally rapid clearance, reflecting both transport into tissues as well as glomerular filtration and renal excretion [25]. LA primarily but transiently accumulates in the liver, heart, and skeletal muscle, but is found in other tissues as well. LA has been shown to cross the blood-brain barrier in a limited number of studies; i.v. doses of 25 mg/kg b.w. given to rats resulted in its accumulation in cerebral cortex within 60 minutes of administration [26], and i.p. doses of 100 mg/kg b.w. for 7-14 days in both young and old rats showed LA accumulation in various brain regions [27]. A more recent study, however, did not find significant levels of LA in the brain after oral gavage of 50 mg/kg b.w. in rats [28], particularly after correcting for residual blood volume. In light of the clinical benefits LA has on the brain, further research should attempt to measure not only LA, but its metabolites and its reduced form, dihydrolipoic acid (DHLA), any of which may exert therapeutic effects. Methods of administration should also be compared to determine whether this causes a difference in tissue distribution.
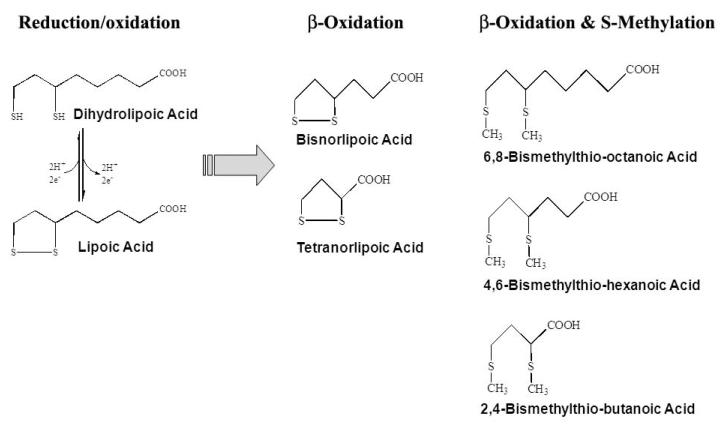
Following its uptake into tissues, LA is subject to extensive catabolism. Schupke et al. used liquid chromatography/tandem mass spectrometry to confirm that β-oxidation is the major metabolic fate of LA in vivo [29]. For rodents and dogs, no less than twelve major LA metabolites were observed that comprised sequential degradation of the carbon backbone and/or mono- and bis-methylation of the sulfhydryl groups. Regardless of the animal species studied, the most common metabolites of LA appear to be bisnorlipoate, tetranorlipoate, β-hydroxy-bisnorlipoate, or the bis-methylated mercapto derivatives of these compounds [25] (Fig. 2). In addition to its catabolism, in vitro studies indicate that LA is rapidly reduced to DHLA (Fig. 2), which is equally rapidly excreted from cells [30]. In all animal models studied, LA and its metabolites are readily excreted, primarily in the urine [33]. Thus LA, either from dietary sources or as a nutritional supplement, is readily absorbed, metabolized and excreted, resulting in negligible free LA being retained in tissues in the post-fed state.
Figure 2.
Lipoic acid and its reduced form, dihydrolipoic acid, with the 5 most common metabolites.
3.3. Safety and toxicity
The use of LA as a nutriceutical supplement has increased significantly, and therefore questions as to its safety and effectiveness have also arisen. While no upper limit for LA consumption in humans has been established, safe levels for acute oral LA intake have been defined in animals, with marked differences depending on the species (Table 2). For dogs, a LD50 of 400-500 mg LA/kg b.w. has been reported [32]; however, rats appear to be more tolerant of LA, as the acute LD50 for this species is >2000 mg/kg b.w. At 2000 mg/kg b.w., some rats “were reported to show signs of reduced well-being, including sedation, apathy, piloerection, hunched posture, and/or eye closure. There was no effect of treatment observable on body weight gain or on gross pathological examination [35].” In the case of chronically administered LA to male and female rats for four weeks by gavage, a “NOAEL” (no observed adverse effect level) was calculated to be 61.9 mg LA/kg b.w. per day based on “slight alterations in liver enzymes as well as histopathological effects on the liver and mammary gland” [35]. Additional work on long-term (24 month) oral LA supplementation to both male and female rats showed no adverse effects with regard to weight, histopathology and blood chemistry up to 60 mg/kg per day LA. However, at a higher chronic dose (180 mg/kg), body weight gain and food consumption were decreased [35, 36], though no gross pathology was evident. On that basis, a NOAEL of 60 mg/kg/day for long-term LA supplementation in rats was established.
Table 2.
Toxicity of lipoic acid (LA) in animals
For humans, a number of clinical trials using LA have been undertaken which also assessed adverse health effects in the participants (Table 3). The ALADIN (I, II, and III), SYDNEY (I and II), and ORPIL clinical trials used LA supplements up to 2400 mg/day with no reported adverse effects versus placebo. LA has also been administered intravenously in doses of 600 mg/day for three weeks with no evidence of serious side-effects [37]. Oral doses of 1800 mg LA (600 mg t.i.d.) for 6 months did not elicit significant adverse effects compared to placebo [39]. LA has been used in Germany for over 50 years as a therapy for diabetic neuropathy and retinopathy.
Table 3.
Select clinical trials using lipoic acid (LA).
| Clinical Trial | Ref. | LA Dose administered to human subjects | Subjects receiving LA |
Parameters measured† |
|---|---|---|---|---|
| Diabetes: ALADIN |
[37] | 100, 600, or 1200 mg, intravenous for 3 weeks | 328 | Neuropathic symptoms, HPAL, NDS |
| Diabetes: ALADIN II |
[38] | a) 600 mg, intravenous, for 5 days + 600 mg, orally for 2 years b) 1200 mg, intravenous for 5 days + 1200 mg, orally for 2 years |
a) 27 b) 18 |
NDS, electrophysiological attributes of the sural and tibial nerves |
| Diabetes: ALADIN III |
[39] | a) 600 mg, intravenous, for 3 weeks + 1800 mg (600 mg t.i.d.) for 6 months b) 600 mg, intravenous, for 3 weeks + placebo for 6 months |
a) 165 b) 173 |
TSS, NIS |
| Diabetes | [40] | a) 600 mg, oral, for 4 weeks b) 1200 mg (600 mg b.i.d.), orally for 4 weeks c) 1800 mg (600 mg t.i.d.), orally for 4 weeks |
a) 19 b) 18 c) 18 |
Insulin-stimulated glucose disposal |
| Diabetes: ORPIL |
[41] | 1800 mg (600 mg t.i.d.), orally for 3 weeks | 12 | TSS, HPAL, NDS |
| Diabetes: SYDNEY |
[42] | 600 mg, intravenous, 5 days a week for 14 treatments | 60 | NCS, TSS, NIS, quantitative sensation test, autonomic test |
| Diabetes: SYDNEY II |
[43] | a) 600 mg, orally for 5 weeks b) 1200 mg, orally for 5 weeks c) 1800 mg, orally for 5 weeks |
a) 45 b) 47 c) 46 |
TSS, NCS, NIS |
| Diabetes: DEKAN |
[44] | 800 mg (200 mg q.i.d.), orally for 4 months | 39 | Cardiac autonomic nerve function |
| Diabetes | [45] | 600 mg/day, orally for 3 months | 33 | Plasma lipid hydroperoxides, alpha-tocopherol, cholesterol |
| Multiple Sclerosis |
[46] | a) 1200 mg q.d. b) 1200 mg (600 mg b.i.d.) c) 2400 mg (1200 mg b.i.d.) |
a) 9 b) 7 b) 7 |
Serum LA, Matrix Metalloproteinase-9, and Intercellular Adhesion Molecule-1 |
| Metabolic Syndrome: ISLAND |
[47] | 300 mg, orally for 4 weeks | 15 | Endothelial function and proinflammatory markers |
HPAL = Hamburg Pain Adjective List, NDS = Neuropathy Disability Score, NIS = Neuropathy Impairment Score, NSC = Neuropathic Symptoms and Change Score, TSS = Total Symptom Score
However, despite the evidence attesting to its safety in moderate doses, precautions for the oral intake of LA have also been voiced. Cakatay et al. conducted a series of experiments in aged rats with intraperitoneal administration of racemic LA (100 mg/kg b.w./day for 2 weeks) and showed that this high chronic dose (the equivalent of 5 to 10 grams per day in humans) increased plasma lipid hydroperoxide levels and oxidative protein damage [48]. LA-mediated protein damage was noted in rat heart [49] and brain [50] but lipid hydroperoxide levels were beneficially decreased in both these organs. Apparently in keeping with its metal chelating abilities (see below), this group noted that LA lowered selenium levels in the serum, heart, brain, and muscle; manganese was lowered only in the heart, but increased in the brain and muscle [51]. Thus, while intake of moderate doses of LA have relatively few adverse side-effects, LA may mediate oxidative insult at higher doses or when administered intraperitoneally. More research is therefore warranted regarding both the safety and optimal dose of LA.
4. MECHANISMS OF ACTION
Despite the relatively transient and low cellular accumulation of LA following its oral intake, numerous studies have now shown that LA elicits an array of cellular actions, ranging from a potent antioxidant to a metal chelator to a mediator of cell signaling pathways. We will now discuss evidence for the biochemical interactions of LA with respect to particular cellular targets, which lead to this diverse mode of action.
4.1. LA/DHLA as an antioxidant
The chemical reactivity of LA is mainly conferred by its dithiolane ring. The oxidized (LA) and reduced (DHLA) forms create a potent redox couple that has a standard reduction potential of −0.32 V. This makes DHLA one of the most potent naturally occurring antioxidants [52]. In fact, there is evidence that both LA and DHLA are capable of scavenging a variety of reactive oxygen species (Table 4). Both LA and DHLA may scavenge hydroxyl radicals and hypochlorous acid, while LA also terminates singlet oxygen [2, 3, 55, 57, 58, 60]. Neither species is active against hydrogen peroxide [2, 60]. Furthermore, DHLA appears to regenerate other endogenous antioxidants (e.g. vitamins C and E) [33, 62] and has the salubrious property of neutralizing free radicals without itself becoming one in the process.
Table 4.
Antioxidant activities of lipoic acid (LA) and dihydrolipoic acid (DHLA)
| Oxidant | Scavenged by LA? Rate constant and reference |
Scavenged by DHLA? Rate constant and reference |
|---|---|---|
| Peroxynitrite | Yes, 1.4 × 103 M-1 s-1 [53] | Yes, 2.5 × 102 M-1 s-1 [53] |
| Nitric Oxide | No [54] | Yes, 3.19 M-1 s-1 [54] |
| Hydroxyl radical | Yes, 4.7 × 1010 M-1 s-1 [2] Yes [55] |
No [2] Yes [55] |
| Superoxide | No [2, 55] | No [2] Yes, 3.3 × 105 M-1 s-1 [55] Yes [56] |
| Singlet oxygen | Yes, 1.3 × 108 M-1 s-1 [57] Yes [3, 58] |
No [58] |
| Peroxyl radical | Yes, 1.8 × 108 M-1 s-1 [2] No [59] |
Yes, 2.3 × 107 M-1 s-1 [2] Yes [56, 59] |
| Hypochlorous acid | Yes [2, 60] | Yes [2, 60] |
| Hydrogen peroxide | No [2] | No [2, 60] |
4.2. LA as a metal chelator
In addition to being direct reactive oxygen species scavengers, both LA and DHLA chelate redox-active metals in vitro and in vivo. The oxidized and reduced forms bind a number of metal ions, but with different properties depending on the metal chelated. In vitro studies show that LA preferentially binds to Cu2+, Zn2+ and Pb2+, but cannot chelate Fe3+, while DHLA forms complexes with Cu2+, Zn2+, Pb2+, Hg2+ and Fe3+ [63]. We provided in vitro evidence that DHLA, but not LA, strongly inhibited Cu(II)(histidine)2-mediated ascorbate oxidation in a concentration-dependent manner [64]. These results were in agreement with a report showing that only DHLA prevented Cu(II)-mediated oxidation of LDL in vitro [6]. DHLA-mediated chelation of iron and copper in the brain had a positive effect in the pathobiology of Alzheimer’s Disease by lowering free radical damage [65]. Thus, a growing body of evidence suggests that DHLA chelates transition metals in a redox-inactive manner, and in turn mitigates metal-catalyzed free radical reactions in conditions where they accumulate.
Whether LA/DHLA effectively chelates and removes transition metals in vivo is still to be fully elucidated. In this regard, Goralska and co-workers showed that treating lens epithelial cells with LA significantly lowered the rate of iron uptake and the size of the intracellular labile iron pool [66]. Feeding R-LA to old rats for 2 weeks reversed the age-related increase in cerebral cortex iron [64]. Importantly, feeding LA did not affect either normal metal levels in young rats or lower iron status in old rats below that seen in young animals. Nor was LA or DHLA capable of removing iron from aconitase or copper from superoxide dismutase. These results imply, but do not yet prove, that LA supplementation may modulate the labile pool of redox active transition metals, without causing metal depletion.
4.3. Is LA a direct-acting antioxidant?
Although strong in vitro evidence supports the role of the LA/DHLA couple as a potent antioxidant, it remains questionable whether they can scavenge free radicals effectively in vivo. Because LA only transiently accumulates in vivo and is rapidly catabolized, it is difficult to envision how LA could augment endogenous antioxidant capacity on a sustained basis. The antioxidant properties of LA in vitro may be explained as follows: i) cell culture studies are often conducted with LA concentrations that are several fold higher than what has been seen in plasma or tissues after an oral dose (Table 1), and ii) LA and DHLA are not cleared from the culture media in a rate that replicates disposal in the body, where 98% of radiolabeled LA is excreted in the urine within 24 hours [29]. Thus, typical cell culture conditions likely overestimate the direct antioxidant capacity of LA via one-on-one interaction with free radicals. Alternatively, the ability of LA to indirectly induce or maintain endogenous antioxidant levels even in times of oxidative or toxicological stress may be more relevant than a direct-acting antioxidant role, and the data to support this will now be discussed.
4.4. LA as an inducer of endogenous antioxidants
There is growing evidence that LA may act indirectly to maintain cellular antioxidant status by either inducing the uptake or enhancing the synthesis of endogenous low molecular weight antioxidants or antioxidant enzymes. For instance, reports show that LA increases intracellular ascorbate levels. Even in rats, which synthesize ascorbate, LA feeding increases hepatic ascorbate levels, which otherwise decline with age [67]. Michels et al. extended this research to show that an age-related loss of sodium-dependent vitamin C transporter 1 (SLC23A1) was at least partly responsible for the decline in hepatic ascorbate [68]. Feeding LA to old rats may therefore induce ascorbate uptake from the exogenous milieu. Rat cardiomyocytes exhibit an age-related decline in ascorbate concentrations, but dietary R-LA restored ascorbate levels and lowered the rate of oxidant production to the level seen in young rats [69]. Moreover, Xu et al. observed that the reduction of dehydroascorbic acid to ascorbate in rat liver mitochondria was enhanced in the presence of LA [70]. These studies thus indicate that LA may improve endogenous ascorbate levels indirectly by inducing uptake from the blood plasma.
In concert with improving ascorbate status, LA markedly increases intracellular glutathione (GSH), an abundant natural thiol antioxidant and co-substrate for detoxification enzymes, in a variety of cell types and tissues [71, 72]. Packer and coworkers showed that LA treatment enhanced GSH levels in human cell lines and primary cells, including T cells, erythrocytes, lymphocytes, and glial and neuroblastoma cells [73]. These authors concluded that DHLA reduced cystine to cysteine, which is the limiting substrate for GSH synthesis. Additionally, LA may also increase cellular cysteine levels by enhancing cystine uptake from plasma followed by its reduction to cysteine. In this regard, we reported that LA reverses the age-related decline in myocardial GSH by increasing cysteine availability, thereby removing the constraints of this limiting substrate on GSH synthesis [74]. These results demonstrate that LA is an effective agent to restore both the age-associated decline in thiol redox ratio as well as increase GSH levels that otherwise decline with age. However, LA has also proven to be an effective regulator of signaling pathways. Some of our recent work has shown that LA induces de novo GSH synthesis transcriptionally [5], and this process will now be discussed.
4.5. Signal Transduction
Through changes in intracellular thiol redox status, the protein structures of signaling molecules may be changed, resulting in the alteration of transcription factor activities. Localized bursts of H2O2 upon ligand engagement of receptors has been shown to cause autophosphorylation and the induction of signaling cascades [75]. In a similar fashion, LA may oxidize sulfhydryl groups or form mixed disulfides on proteins. The multiplicative nature of signal transduction may explain some of LA’s beneficial effects, since LA accumulates at only micromolar levels, whereas the intracellular antioxidant GSH is present at millimolar levels. Indeed, clinical studies show that a 200-600 mg LA dose of orally supplied LA results in less than a 50 μM accumulation in the blood plasma (Table 1) with a Tmax of less than 90 min. [17]; hepatic levels of nonprotein-bound LA similarly peaked at ~60 μM in rats given 40 mg/kg b.w.i.p. (TMH, personal observation). This suggests that LA may not be present at high enough levels to directly recycle GSH, but that the positive clinical effects of LA may in fact be due to changes in the thiol redox status of signaling molecules.
4.5.1. LA-mediated induction of GSH through transcription factor Nrf2
In several cases, the stereochemistry of LA has been important for protection against ischemic damage, perhaps indicating that LA was not acting as a direct antioxidant, but instead was modulating GSH. For example, Kilic et al. [76] showed that in a cataract model, R-LA, unlike S-LA, was protective, and that it exerted its effect by maintaining GSH levels. In a rodent model of cerebral ischemia, Wolz et al. [77] reported that R- or S-LA (100 mg/kg b.w.) were as protective as DHLA (50 mg/kg b.w) two hours after subcutaneous administration. The investigators suggest that LA is reduced to DHLA to provide this effect. While both R- and S-LA are reduced to DHLA, the conversion of S-LA by lipoamide dehydrogenase is 28 times slower than the natural isoform [78]. Thus after 4 hours, DHLA formed from R-LA may have been cleared, because only S-LA was protective at this time point. Hagen et al. showed that R-LA was protective against t-BuOOH toxicity in hepatocytes from aged rats whereas S-LA provided no benefit [79].
Building on the above work from Hagen et al. showing that GSH levels decrease with age in rat hepatocytes [79], Suh et al. found that GSH was 35% lower on an age basis in whole liver tissue [5]. Activity of the rate-limiting enzyme in GSH synthesis, γ-glutamyl cysteine ligase (GCL), was 50% lower with age, which resulted from a significant loss in levels of its two protein subunits. As both subunits of GCL are products of genes that contain the Antioxidant Response Element (ARE), it was hypothesized that GSH synthetic capacity ultimately declined with age from deficits in ARE-mediated gene transcription. Indeed, it was observed that nuclear Nrf2 levels, the most important transcription factor regulating ARE-mediated genes, declined precipitously rat in livers on an age basis. However, old rats receiving R-LA (40 mg/kg b.w.i.p.) displayed significant increases in GCL activity and GSH concentration in the liver, up to the levels found in young control animals. As Nrf2 is critical for the Phase II detoxification response, it is likely that there is a mechanism in place to assure that Nrf2 responds to oxidative or electrophilic stress. In this case, LA may act as a pro-oxidant to cause a mild cellular insult that induces nuclear localization of Nrf2. Moini et al. [80] buttressed this concept when they observed that administration of R-LA in a cell culture model increased GSH only after 24 hours, a result that suggests an Nrf2-dependent mechanism rather than a direct antioxidant or GSH-recycling one.
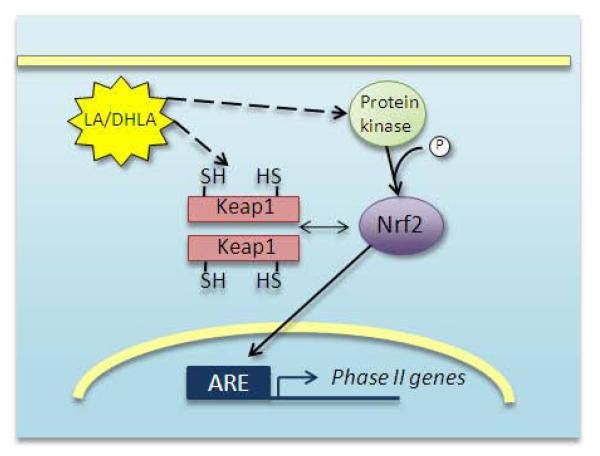
LA, acting as a pro-oxidant, may increase Nrf2-dependent transcriptional activity by forming lipoyl-cysteinyl mixed disulfides on Keap1 [81], the protein that sequesters Nrf2 and bridges it to ubiquitin ligases [82]. In this case, Nrf2 may not be released by Keap1, but rather, Nrf2 synthesized de novo would fail to bind Keap1 and would not be degraded [83-85] (Fig. 3). In support of this concept, recent research shows that the mutation of Keap1 critical cysteines 273 and 288 is not sufficient to release Nrf2 from Keap1, but prevents its degradation under normal conditions [84, 86]. Cysteine 151 also must be oxidized in order to halt Nrf2 degradation under conditions of stress [86] because this residue is necessary for Keap1’s interaction with the Cul3 ubiquitin ligase [87]. The phosphorylation of Nrf2 on Ser40, which allows it to dissociate from Keap1, represents an alternative trigger of Nrf2 nuclear translocation. It is known that certain protein kinases can phosphorylate Nrf2 [88-90], but whether this control mechanism is also impaired with age is not currently known. Other kinases have been investigated that could also phosphorylate Nrf2, but direct evidence of Nrf2’s release from Keap1 due to phosphorylation by kinases other than protein kinase C (PKC) is lacking, primarily because Ser40 is the only site on Nrf2 that has been definitively associated with the binding of Nrf2 to Keap1. It is currently not known whether LA may directly activate PKC in a redox-active manner, but it has been shown that PKCδ is activated in response to LA during Fas-mediated apoptosis [91].
Figure 3.
Proposed action of LA for induction of Phase II genes through Nrf2-mediated transcription.
LA may oxidize critical thiols on the Keap1 dimer to halt Nrf2 degradation, and to prevent Keap1 from binding newly synthesized Nrf2. LA may also activate protein kinase signaling pathways that cause phosphorylation of Nrf2 on Ser40. This is the event that allows it to dissociate from Keap1 [68, 69]. Nrf2 can then localize to the nucleus and bind to the ARE, promoting transcription of genes for the Phase II detoxification response.
4.5.2. Interaction of LA with kinases and phosphatases
In addition to PKCδ, LA activates Erk1/2 [92, 93], p38 MAPK [94], PI3 kinase [94], and Akt [94-97]. LA was also found to enhance expression of the insulin receptor substrate 1 (IRS1) protein in muscle of obese Zucker rats, and it also elicited association of IRS1 with the p85 regulatory subunit of PI3K [98]. In addition, LA decreases the activities of Protein Tyrosine Phosphatase 1B [99], Protein Phosphatase 2A [95], and the phosphatase and tensin homolog PTEN [95], all of which contain critical thiols that, when oxidized, repress their activities [100-103]. While LA may not target specific signaling molecules (the insulin receptor may be an exception, see Diesel et al. [104]), changes in the LA/DHLA redox couple may affect the redox status of critical cysteine residues on these proteins, causing conformational changes that activate or repress their activities. For instance, the suppression of PTEN and PP2A activities by LA contributes to the LA-induced increase in Akt phosphorylation observed in cultured primary hepatocytes treated with 50 μM LA, as well as in the liver tissue of rats gavaged with LA at 120 mg/kg b.w. [95].
Moreover, the well-established regulation of muscle glucose uptake by exercise/muscle contraction through protein kinases, including AMP-activated protein kinase (AMPK) [105, 106], is of interest because LA activates peripheral AMPK [107-109]. Thus, through AMPK, LA is thought to i) induce the phosphorylation of IRS1 Ser789 and activation of the IRS1/PI3K signaling [110, 111] and ii) stimulate GLUT4 translocation via inactivation of the Akt substrate of 160 kDa (AS160) independently of the IRS1/PI3K/Akt signaling cascade [112, 113].
4.5.3. Insulin pathway and glucose handling
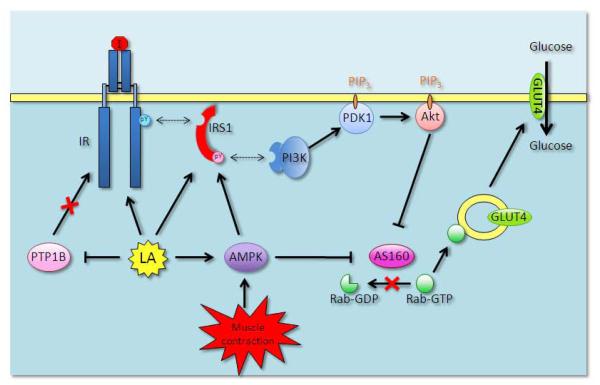
The interaction of LA and intracellular signaling is perceived to account for LA’s beneficial effects observed at 24 hours post-administration [5, 80], a time point that is much delayed from the plasma LA Tmax of ~1 hour [18]. This temporal difference is interesting in light of the rapid metabolism of LA and suggests a different mode of action versus other stimuli that LA mimics. For example, in cultured cells, insulin induced glucose uptake after 10 min. and a maximal effect after 30 min. [114], while LA required 1 hour to induce its maximal effect on glucose uptake, which could be achieved by insulin in half the time [115]. This delay is even evident when comparing the phosphorylation of Akt on Ser473 as induced by insulin versus LA [95]. Such a delay suggests that the effect of LA on glucose handling is not direct but necessitates the activation of additional mediator(s), and also supports the notion that LA or DHLA modulates the IR/PI3K/Akt pathway at different levels (Fig. 4).
Figure 4.
Role of lipoic acid in IR/PI3K/Akt-dependent activation of glucose uptake in skeletal muscle
Diesel et al. put forth the notion that LA may directly bind to and activate the tyrosine kinase domain of the insulin receptor (IR) b-subunit [84]. The authors based their claim on a computer modeling of the IR tyrosine kinase domain where LA would theoretically fit in a pocket located between Leu1133 and Phe1186. In contrast to a direct role of LA on IR, LA was proposed to oxidize critical cysteine thiols in protein tyrosine phosphatase B1 (PTPB1) thereby preventing the PTPB1-mediated inhibitory dephosphorylation of the IR tyrosine kinase domain [79]. Alternatively, LA was found to enhance the insulin receptor substrate 1 (IRS1) protein expression in muscle of obese Zucker rats and association of IRS1 with the p85 regulatory subunit of PI3K [78]. Moreover, the well-established regulation of muscle glucose uptake by exercise/muscle contraction through protein kinases, including AMP-activated protein kinase (AMPK) [85, 86] is of interest because LA activates peripheral AMPK [87, 89, 139]. Thus, through AMPK, LA is thought to i) induce the phosphorylation of IRS1 Ser789 and activation of the IRS1/PI3K signaling [90, 91] and ii) stimulate GLUT4 translocation via inactivation of the Akt substrate of 160 kDa (AS160) independently of the IRS1/PI3K/Akt signaling cascade [92, 93].
The effects of LA on IR/IRS1 will increase IRS1 association with PI3K and PI3K activity in the membrane environment. PtdIns-3,4,5-P3 (PIP3) production by PI3K recruits PtdIns-dependent kinase 1 (PDK1) to the membrane by Pleckstrin Homology domain:PIP3 interaction and stimulates PDK1-mediated phosphorylation of Akt Thr308. Following Ser473 phosphorylation, fully activated Akt regulates the trafficking of GLUT4 between storage vesicles and the plasma membrane through a mechanism involving the phosphorylation of AS160. In its active dephosphorylated form, AS160 inhibits GLUT4 vesicle trafficking to the plasma membrane by preventing Rab-GTP association with the vesicle. Akt-mediated phosphorylation of AS160 opposes the repressor role of AS160 and allows Rab-GTP binding to the GLUT4 vesicle. Domain structure analysis revealed that AS160 has a Rab-GAP (GTPase-activating protein) domain at the C-terminus and that the Rab-GAP activity promotes the hydrolysis of small G proteins Rab-GTP to Rab-GDP [140]. In its inactive GDP-loaded form, Rab is unable to associate with the GLUT4 vesicle nor elicit translocation to the plasma membrane. But Akt-mediated phosphorylation of AS160 inactivates the Rab-GAP activity of AS160 thus favoring the association of GTP-loaded active form of Rab with the GLUT4 vesicle.
In skeletal muscle, LA is proposed to recruit GLUT4 from its storage site in the Golgi to the sarcolemma, so that glucose uptake is stimulated by the local increase in transporter abundance. Evidence from cell culture experiments supports the involvement of the insulin-signaling cascade in LA-stimulated translocation of GLUT1 and GLUT4. Klip’s group [116] investigated the effects of R- and S-LA on glucose uptake in L6 myotubes and 3T3-L1 adipocytes. R-LA stimulated larger and more rapid glucose uptake than did S-LA or the racemic mixture, and when used in conjunction with insulin, enhanced its glucose uptake action. The cellular distribution of GLUT1 and GLUT4 glucose transporters responded to R-LA in a similar fashion as seen with insulin, and glucose uptake in response to all forms of LA was PI3K-dependent, as determined by use of the inhibitory compound, LY294002. Using the same cell culture models, Moini et al. [80] showed that R-LA stimulated glucose transport for up to 6 hours. While S-LA and the racemic mixture produced the same effect, DHLA did not. R-LA also resulted in tyrosine phosphorylation of the insulin receptor, and glucose uptake was PI3K dependent, as shown by using wortmannin.
In contrast to the above work done mainly in tissue culture, Henriksen et al. found that the action of LA on glucose uptake is not always PI3K-dependent [117]. This group incubated 2 mM R,S-LA with skeletal muscle isolated from either lean or obese Zucker rats (a model for insulin resistance) and showed that a significant portion (~75%) of LA-stimulated glucose uptake was independent of PI3K. The disparity between muscle cell lines and intact muscle preparations indicates that LA has the potential to impact various components of cellular signaling, depending on the chosen model and experimental conditions. Of note, these in vitro studies used LA concentrations that are much greater than peak plasma concentrations seen in healthy volunteers administered 200-600 mg [17, 18].
Tritschler’s group directly tested the effects of R-and S-LA by i.p. administration on glucose metabolism in the skeletal muscle of Zucker rats [118]. On an acute basis (100 mg/kg b.w. for 1 hour), only R-LA increased glucose uptake. Chronic 10-day administration of S-LA (50 mg/kg b.w.) did improve glucose uptake, but by less than half that of R-LA (30 mg/kg b.w.). This group also measured plasma insulin, finding it to be decreased by R-LA but increased by S-LA. Only R-LA was able to increase glycogen synthesis and glucose oxidation. In their model, GLUT4 levels were slightly reduced by chronic S-LA treatment, an undesirable effect. The evidence presented argues that R- and S-LA may not be equally useful in the treatment of diabetes.
Importantly, combining LA intake (30 mg/kg per day for 15 days) with endurance exercise training in an animal model of insulin resistance improved glucose transport activity and whole-body glucose tolerance further than either intervention alone [98]. A potential mechanism for this additive effect is the upregulation of GLUT4 protein expression in exercised muscle [119] combined with the enhanced translocation of GLUT4 to the plasma membrane induced by LA. Whether this combination of dietary supplement and exercise is beneficial to diabetic subjects remains to be investigated.
5. CLINICAL AND THERAPEUTIC EFFECTS OF LA
5.1. Diabetic polyneuropathies
The interaction of LA with regulatory components of the insulin signaling cascade has proved functionally beneficial to skeletal muscle glucose uptake, whole-body glucose tolerance, and helpful against insulin resistance in animal models [118, 120]. Improvements in glucose disposal were also observed in human patients with type 2 diabetes receiving LA either intravenously or orally [120-122]. Several clinical trials have been conducted to measure the efficacy of racemic LA in decreasing symptoms of diabetic polyneuropathies; these are the “alpha-lipoic acid in diabetic neuropathy” (ALADIN) trials and the “symptoms of diabetic polyneuropathy” (SYDNEY) trials. LA was given orally, intravenously, or i.v. with oral follow-up. A meta-analysis of four clinical trials using i.v. LA, including ALADIN, SYDNEY, and the first 3 weeks of ALADIN III, showed a significant improvement in diabetic polyneuropathies of the feet and lower limbs in patients infused with LA 600 mg/day, for three weeks [123]. Diabetic patients in the ALADIN II trial were administered LA i.v. at 600 or 1200 mg/day for 5 days, then oral LA for 2 years, resulting in improved indices of neuropathy [38]. Patients in the ALADIN III study received LA (600 mg/day i.v.) or placebo for three weeks, followed by oral LA (600 mg t.i.d.) or placebo for 6 months. The oral phase of this trial, however, was without clinically significant benefits [39]. One possible conclusion from these studies was that LA administered intravenously was more efficacious than oral LA, which may be due to either greater bioavailability or poor solubility of the medication in the stomach acid. However, some additional studies have found that oral LA is very effective. For example, the oral pilot (ORPIL) study showed a reduction in diabetic polyneuropathic symptoms after three weeks with 600 mg LA t.i.d. [41]. While the first SYDNEY trial used i.v. LA, [42], the SYDNEY II study used oral LA at 600, 1200, or 1800 mg q.d. for 5 weeks [43]; consequently, both studies showed significant improvements in neuropathic endpoints.
5.2. Effects of LA on the vascular system
Vascular endothelial cells, which line the blood vessel lumen, form the physical interface between the blood and the vessel wall, preventing platelet adhesion and regulating blood vessel patency. The elasticity of the vessel wall is regulated by nitric oxide (NO), a gas produced by endothelial nitric oxide synthase (eNOS). Loss of eNOS activity causes endothelial dysfunction due to NO limitation, and is characterized by reduced vasodilation, a proinflammatory milieu, and a prothrombic state. Oxidative stress has been implicated in endothelial dysfunction on the basis that antioxidants, such as ascorbate and LA, improve the redox state of the plasma and endothelium-dependent NO-mediated vasodilation [124, 125]. But the question remains as to how LA achieves this significant result. It is known, for instance, that the PI3K/Akt signaling pathway, cascading from the insulin receptor and stimulated by LA, plays an important role in eNOS activation [126, 127]. Treating human aortic endothelial cells with LA significantly increases NO synthesis [128], and LA improves the loss in eNOS phosphorylation seen in aorta from aged rats through Akt [97]. Furthermore, i.p. injection of LA into old rats restores vasorelaxation, characterized by an increased phosphorylation of both eNOS and Akt, as well as a decrease in neutral sphingomyelinase activity and a concomitant decrease in ceramide [129]. These studies using in vitro and animal models strengthen our understanding of the role of the insulin signaling pathway in vasomotor function, and underscore the health potential of LA therapy. Thus far, however, only the ISLAND clinical trial has examined LA as a potential remedy for endothelial dysfunction [47]. This trial was a randomized, double-blind, placebo-controlled study comparing LA to irbesartan, an angiotensin II receptor antagonist used mainly for the treatment of hypertension. Results showed that the oral administration of LA (300 mg/day for 4 weeks) and/or irbesartan (150 mg/day for 4 weeks) to 14-15 patients with metabolic syndrome improved endothelial-dependent flow-mediated vasodilation, which was measured by using the noninvasive brachial artery reactivity test. However, larger and more long-term studies are necessary in order to establish the efficacy of LA as a therapeutic for vascular endothelial dysfunction.
5.3. LA as a hypotensive agent
Hypertension is a risk factor for stroke, heart attack and arterial aneurysm, and a leading cause of chronic kidney failure. Even moderate elevation in arterial blood pressure correlates with shortened life expectancy. The rationale for the therapeutic use of LA against hypertension stemmed from its ability to increase tissue GSH levels and prevent deleterious sulfhydryl group modification in Ca2+ channels. Feeding LA to hypertensive rats normalized systolic blood pressure and cytosolic free Ca2+, and attenuated adverse renal vascular changes [130-134]. The role of LA in regenerating reduced GSH was further put forth by El Midaoui and de Champlain [135, 136] who associated the restoration of glutathione peroxidase activity seen in LA-fed rats with the normalization of aortic superoxide production and blood pressure. It was also suggested that dietary LA inhibits renal and vascular overproduction of endothelin-1, a vasoconstrictor secreted by the endothelium [137]. Because NO is the main vasodilator in conduit arteries and the recent finding that LA improves endothelial NO synthesis [129], pharmacologists have a new rationale to investigate the role of LA and high blood pressure. Clinically, LA administration (in combination with acetyl-L-carnitine) showed some promise as an antihypertensive therapy by decreasing systolic pressure in high blood pressure patients and subjects with the metabolic syndrome [138]. In contrast, the administration of LA (300 mg/day for 4 weeks) to patients with the metabolic syndrome had no significant effect on blood pressure compared to placebo group [47].
5.4. LA as an anti-inflammatory agent
Inflammation results from the innate biological response of vascular tissues to harmful agents, such as pathogens or irritants. It is an attempt by the organism to remove the injurious stimuli, protect the surrounding tissue, and initiate the healing process. However, unabated chronic inflammation also contributes to a host of diseases, such as atherosclerosis, asthma, and rheumatoid arthritis. Elevated levels of oxidative stress play an important role in chronic inflammation. Oxidative stress-associated inflammation is thought to provoke early vascular events in atherogenesis, including the upregulation of vascular adhesion molecules and matrix metalloproteinase activity. These events require the activation of NF-kappaB, a transcription factor that induces expression of many genes involved in inflammation and endothelial cell migration. Given the oxidative nature of inflammation, therapeutic strategies aimed at mitigating oxidant production and oxidative damage have been investigated for decades in various models of inflammation.
In keeping with this strategy, LA has been studied for its antioxidant properties in cytokine-induced inflammation; it is also widely known as an inhibitor of NF-kappaB [32]. Results show that LA lowers expression of vascular cell adhesion molecule-1 (VCAM-1) and endothelial adhesion of human monocytes [139], and inhibits NF-kappaB-dependent expression of metalloproteinase-9 in vitro [140]. Similarly, LA (25-100 μg/ml = 122-486 μM) prevents the upregulation of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) in spinal cords and in TNF-alpha stimulated cultured brain endothelial cells [141]. Collagen-induced arthritis was attenuated by LA (10-100 mg/kg i.p.) in DBA/1 mice by reduction of inflammatory cytokines like TNF-alpha, and partial inhibition of NF-kappaB binding to DNA [142]. In this study, LA also inhibited osteoclast formation, suggesting that LA may be useful in the prevention of bone erosion and joint destruction in rheumatoid arthritis. In another study, pretreatment of collagen sheets with LA (2 mg) prior to implantation decreased TNF-alpha-induced bone resorption in ICR mice [142]. In experimental autoimmune encephalomyelitis (an animal model of multiple sclerosis) LA-treated mice showed marked improvement in central nervous system infiltrating T-cells and macrophages, decreased demyelination and spinal cord expression of adhesion molecules (ICAM-1 and VCAM-1) [141, 144]. The downregulation of surface CD4 seen in LA-treated blood mononuclear cells was proposed to account, at least in part, for the modulation of inflammatory cell infiltration into the central nervous system [145]. This is because co-receptor CD4 amplifies the signal generated at the T-cell receptor by recruiting lymphocyte protein kinase Lck, which in turn triggers a cascade of events leading to T-cell activation. Interestingly, DHLA did not downregulate CD4 from the surface of human peripheral blood mononuclear cells [145]. As an alternative or in addition to CD4 downregulation, the immunomodulatory properties of LA may involve the upregulation of cAMP in T-cells and natural killer cells [146]. Cell migration and neovascularization were also inhibited by LA (86 μg/day in drinking water) in c57/black mice injected with Kaposi’s sarcoma in a matrigel sponge, as well as in nude mice injected with KS cells [147]. In a mouse model of bronchial asthma, dietary LA significantly attenuated airway hyper-responsiveness, lowered the eosinophil count among bronchoalveolar lavage cells, and significantly improved pathologic lesion scores of the lungs [148]. LA inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells via a mechanism seemingly distinct from antioxidants, such as ascorbate or reduced GSH, but consistent with the workings of a metal chelator [149]. Recently, the inhibition of endotoxin-induced acute inflammation by LA was associated with the stimulation of the PI3K/Akt pathway [96].
To date, the anti-inflammatory properties of LA have rarely been investigated in humans. The ISLAND trial showed a 15% significant decrease in serum interleukin-6 levels following 4 weeks of supplementation with LA (300 mg/day) [47]. This finding may prove important to human health because interleukin-6 is a recognized marker of inflammation in coronary atherosclerotic plaques, and also regulates the expression of other inflammatory cytokines, such as interleukin-1 and TNF-alpha [150]. However, the body of evidence is currently too limited to be conclusive.
6. SUMMARY AND FUTURE DIRECTIONS
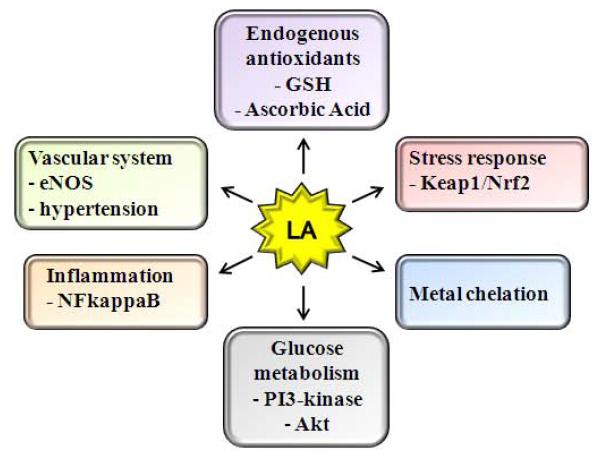
As described in this review, the biological role(s) of diet-derived LA are quite diverse (Fig. 5). In fact, to our knowledge there are few compounds as multifaceted as LA as a bioactive agent. It is an inducer of cellular signaling pathways, an insulin mimetic, a hypotriglyceridemic agent, a vasorelaxant/anti-hypertensive compound, a metal chelator, and an adjuvant for neuro-cognitive function. Thus, it will be important to define the precise cause-and-effect relationship between LA and its cellular targets of immediate action. An area that warrants further study is whether LA directly regulates hormonal signals, which in turn initiate downstream biochemical actions on target organs. In this regard, LA stimulates an AMPK-dependent anorectic effect in rodents [107] and improves learning and short-term memory in aged rodents [4, 31, 151, 152]. Thus diet-derived LA may owe some of its diverse physiological actions on stimulating neuro-hormonal function and thereby indirectly influencing multiple cell signaling pathways in peripheral tissues.
Figure 5.
Proposed biological actions of lipoic acid
In concert with its potential for centralized action, it is also apparent that orally supplied LA affects multiple signaling and transcriptional paradigms at the cellular level. Again, the question that immediately arises is whether LA has multiple or only a few actual cellular targets, in the latter case mediating its strong antioxidant and metabolic effects indirectly. For example, LA acts as an insulin mimetic in that it improves glucose handling; however, the timing to which LA stimulates glucose metabolism is significantly delayed from that of insulin itself. Thus, it is still to be determined whether LA truly targets the insulin-signaling pathway via thiol/disulfide interactions on the insulin receptor or its direct protein substrates or alternatively, indirectly influences this pathway by affecting phosphatases.
As this review was being compiled, we were struck by the lack of consistency and sheer magnitude of dose used to examine LA action, particularly for in vitro models. Low micromolar to millimolar concentrations of LA have been employed for cultured cells where LA has variously been shown to induce apoptosis, directly act as an antioxidant, induce H2O2 release, and stimulate stress response mechanisms to name a few. Considering the transient cellular accumulation of LA following an oral dose, which does not exceed low micromolar levels, it is entirely possible that some of the cellular effects of LA when given at supraphysiological concentrations may be not be clinically or physiologically relevant. This may be particularly important in characterizing the cellular role of LA or DHLA as effective direct-acting cellular antioxidants. One must ask how a dithiol agent that only transiently accumulates at very low levels in vivo could act as a potent antioxidant on a stoichiometric basis. Significantly more research is necessary to understand physiological uptake, accumulation and metabolism of LA and its metabolites in order to set up appropriate cell-based models to examine LA action. Only then will the most biologically relevant cellular actions of LA be elucidated.
Although some important aspects of LA’s mechanism of action in vivo are yet to be uncovered, it is apparent that oral LA supplements are clinically effective in mitigating complications of diabetes and potentially, other vascular diseases. Because current evidence is based mainly on data from rodent studies, additional placebo-controlled trials are advisable to determine the potential for LA to maintain or improve neurological disorders (e.g. Alzheimer’s disease, multiple sclerosis), limit progression of cardiovascular disease, mitigate chronic inflammatory conditions, as well as improve or maintain antioxidant/detoxification defenses that otherwise decline with age. Prior to any large-scale clinical work using LA, an initial focus should be placed on optimizing conditions as to effective dose, as well as identification of the appropriate enantiomeric isoform.
ACKNOWLEDGEMENTS
We are indebted to Dr. Dove Keith, Dr. Alexander Michels, and Judy A. Butler for careful review and helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem. 2004;11:1135–46. doi: 10.2174/0929867043365387. [DOI] [PubMed] [Google Scholar]
- [2].Scott BC, Aruoma OI, Evans PJ, O’Neill C, Van der Vliet A, Cross CE, Tritschler H, Halliwell B. Lipoic and dihydrolipoic acids as antioxidants. A critical evaluation. Free Radic Res. 1994;20:119–33. doi: 10.3109/10715769409147509. [DOI] [PubMed] [Google Scholar]
- [3].Devasagayam TP, Subramanian M, Pradhan DS, Sies H. Prevention of singlet oxygen-induced DNA damage by lipoate. Chem Biol Interact. 1993;86:79–92. doi: 10.1016/0009-2797(93)90113-d. [DOI] [PubMed] [Google Scholar]
- [4].Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–61. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–6. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lodge JK, Traber MG, Packer L. Thiol chelation of Cu2+ by dihydrolipoic acid prevents human low density lipoprotein peroxidation. Free Radic Biol Med. 1998;25:287–97. doi: 10.1016/s0891-5849(98)00048-3. [DOI] [PubMed] [Google Scholar]
- [7].Anuradha B, Varalakshmi P. Protective role of DL-alpha-lipoic acid against mercury-induced neural lipid peroxidation. Pharmacol Res. 1999;39:67–80. doi: 10.1006/phrs.1998.0408. [DOI] [PubMed] [Google Scholar]
- [8].Han D, Sen CK, Roy S, Kobayashi MS, Tritschler HJ, Packer L. Protection against glutamate-induced cytotoxicity in C6 glial cells by thiol antioxidants. Am J Physiol. 1997;273:R1771–8. doi: 10.1152/ajpregu.1997.273.5.R1771. [DOI] [PubMed] [Google Scholar]
- [9].Reed L. Multienzyme complexes. Accts Chem Res. 1974;7:40–46. [Google Scholar]
- [10].Boom T.J. Vanden, Reed KE, Cronan JE., Jr. Lipoic acid metabolism in Escherichia coli: isolation of null mutants defective in lipoic acid biosynthesis, molecular cloning and characterization of the E. coli lip locus, and identification of the lipoylated protein of the glycine cleavage system. J Bacteriol. 1991;173:6411–20. doi: 10.1128/jb.173.20.6411-6420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Akiba S, Matsugo S, Packer L, Konishi T. Assay of protein-bound lipoic acid in tissues by a new enzymatic method. Anal Biochem. 1998;258:299–304. doi: 10.1006/abio.1998.2615. [DOI] [PubMed] [Google Scholar]
- [12].Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition. 2001;17:888–95. doi: 10.1016/s0899-9007(01)00658-x. [DOI] [PubMed] [Google Scholar]
- [13].Wollin SD, Jones PJ. Alpha-lipoic acid and cardiovascular disease. J Nutr. 2003;133:3327–30. doi: 10.1093/jn/133.11.3327. [DOI] [PubMed] [Google Scholar]
- [14].Takaishi N, Yoshida K, Satsu H, Shimizu M. Transepithelial transport of alpha-lipoic acid across human intestinal Caco-2 cell monolayers. J Agric Food Chem. 2007;55:5253–9. doi: 10.1021/jf063624i. [DOI] [PubMed] [Google Scholar]
- [15].Balamurugan K, Vaziri ND, Said HM. Biotin uptake by human proximal tubular epithelial cells: cellular and molecular aspects. Am J Physiol Renal Physiol. 2005;288:F823–31. doi: 10.1152/ajprenal.00375.2004. [DOI] [PubMed] [Google Scholar]
- [16].Prasad PD, Wang H, Kekuda R, Fujita T, Fei YJ, Devoe LD, Leibach FH, Ganapathy V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J Biol Chem. 1998;273:7501–6. doi: 10.1074/jbc.273.13.7501. [DOI] [PubMed] [Google Scholar]
- [17].Teichert J, Kern J, Tritschler HJ, Ulrich H, Preiss R. Investigations on the pharmacokinetics of alpha-lipoic acid in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:625–8. [PubMed] [Google Scholar]
- [18].Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007;12:343–51. [PubMed] [Google Scholar]
- [19].Breithaupt-Grogler K, Niebch G, Schneider E, Erb K, Hermann R, Blume HH, Schug BS, Belz GG. Dose-proportionality of oral thioctic acid--coincidence of assessments via pooled plasma and individual data. Eur J Pharm Sci. 1999;8:57–65. doi: 10.1016/s0928-0987(98)00061-x. [DOI] [PubMed] [Google Scholar]
- [20].Mignini F, Streccioni V, Tomassoni D, Traini E, Amenta F. Comparative crossover, randomized, open-label bioequivalence study on the bioequivalence of two formulations of thioctic acid in healthy volunteers. Clin Exp Hypertens. 2007;29:575–86. doi: 10.1080/10641960701744111. [DOI] [PubMed] [Google Scholar]
- [21].Bernkop-Schnurch A, Reich-Rohrwig E, Marschutz M, Schuhbauer H, Kratzel M. Development of a sustained release dosage form for alpha-lipoic acid. II. Evaluation in human volunteers. Drug Dev Ind Pharm. 2004;30:35–42. doi: 10.1081/ddc-120027509. [DOI] [PubMed] [Google Scholar]
- [22].Amenta F, Traini E, Tomassoni D, Mignini F. Pharmacokinetics of different formulations of tioctic (alpha-lipoic) acid in healthy volunteers. Clin Exp Hypertens. 2008;30:767–75. doi: 10.1080/10641960802563568. [DOI] [PubMed] [Google Scholar]
- [23].Teichert J, Tuemmers T, Achenbach H, Preiss C, Hermann R, Ruus P, Preiss R. Pharmacokinetics of alpha-lipoic acid in subjects with severe kidney damage and end-stage renal disease. J Clin Pharmacol. 2005;45:313–28. doi: 10.1177/0091270004270792. [DOI] [PubMed] [Google Scholar]
- [24].Teichert J, Hermann R, Ruus P, Preiss R. Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers. J Clin Pharmacol. 2003;43:1257–67. doi: 10.1177/0091270003258654. [DOI] [PubMed] [Google Scholar]
- [25].Harrison EH, McCormick DB. The metabolism of dl-(1,6-14C)lipoic acid in the rat. Arch Biochem Biophys. 1974;160:514–22. doi: 10.1016/0003-9861(74)90428-7. [DOI] [PubMed] [Google Scholar]
- [26].Panigrahi M, Sadguna Y, Shivakumar BR, Kolluri SV, Roy S, Packer L, Ravindranath V. alpha-Lipoic acid protects against reperfusion injury following cerebral ischemia in rats. Brain Res. 1996;717:184–8. doi: 10.1016/0006-8993(96)00009-1. [DOI] [PubMed] [Google Scholar]
- [27].Arivazhagan P, Shila S, Kumaran S, Panneerselvam C. Effect of DL-alpha-lipoic acid on the status of lipid peroxidation and antioxidant enzymes in various brain regions of aged rats. Exp Gerontol. 2002;37:803–11. doi: 10.1016/s0531-5565(02)00015-3. [DOI] [PubMed] [Google Scholar]
- [28].Chng HT, New LS, Neo AH, Goh CW, Browne ER, Chan EC. Distribution study of orally administered lipoic acid in rat brain tissues. Brain Res. 2009;1251:80–6. doi: 10.1016/j.brainres.2008.11.025. [DOI] [PubMed] [Google Scholar]
- [29].Schupke H, Hempel R, Peter G, Hermann R, Wessel K, Engel J, Kronbach T. New metabolic pathways of alpha-lipoic acid. Drug Metab Dispos. 2001;29:855–62. [PubMed] [Google Scholar]
- [30].Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- [31].Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–83. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- [32].Packer L, Witt EH, Tritschler HJ. alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–50. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- [33].Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29:315–31. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- [34].Hill AS, Werner JA, Rogers QR, O’Neill SL, Christopher MM. Lipoic acid is 10 times more toxic in cats than reported in humans, dogs or rats. J Anim Physiol Anim Nutr (Berl) 2004;88:150–6. doi: 10.1111/j.1439-0396.2003.00472.x. [DOI] [PubMed] [Google Scholar]
- [35].Cremer DR, Rabeler R, Roberts A, Lynch B. Safety evaluation of alpha-lipoic acid (ALA) Regul Toxicol Pharmacol. 2006;46:29–41. doi: 10.1016/j.yrtph.2006.06.004. [DOI] [PubMed] [Google Scholar]
- [36].Cremer DR, Rabeler R, Roberts A, Lynch B. Long-term safety of alpha-lipoic acid (ALA) consumption: A 2-year study. Regul Toxicol Pharmacol. 2006;46:193–201. doi: 10.1016/j.yrtph.2006.06.003. [DOI] [PubMed] [Google Scholar]
- [37].Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schutte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study) Diabetologia. 1995;38:1425–33. doi: 10.1007/BF00400603. [DOI] [PubMed] [Google Scholar]
- [38].Reljanovic M, Reichel G, Rett K, Lobisch M, Schuette K, Moller W, Tritschler HJ, Mehnert H. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy. Free Radic Res. 1999;31:171–9. doi: 10.1080/10715769900300721. [DOI] [PubMed] [Google Scholar]
- [39].Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schutte K, Kerum G, Malessa R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999;22:1296–301. doi: 10.2337/diacare.22.8.1296. [DOI] [PubMed] [Google Scholar]
- [40].Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W, Augustin HJ, Dietze GJ, Rett K. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Radic Biol Med. 1999;27:309–14. doi: 10.1016/s0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]
- [41].Ruhnau KJ, Meissner HP, Finn JR, Reljanovic M, Lobisch M, Schutte K, Nehrdich D, Tritschler HJ, Mehnert H, Ziegler D. Effects of 3-week oral treatment with the antioxidant thioctic acid (alpha-lipoic acid) in symptomatic diabetic polyneuropathy. Diabet Med. 1999;16:1040–3. doi: 10.1046/j.1464-5491.1999.00190.x. [DOI] [PubMed] [Google Scholar]
- [42].Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, Litchy WJ, Low PA, Nehrdich D, Novosadova M, O’Brien PC, Reljanovic M, Samigullin R, Schuette K, Strokov I, Tritschler HJ, Wessel K, Yakhno N, Ziegler D. The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: the SYDNEY trial. Diabetes Care. 2003;26:770–6. doi: 10.2337/diacare.26.3.770. [DOI] [PubMed] [Google Scholar]
- [43].Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29:2365–70. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- [44].Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H, Reichel G. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Care. 1997;20:369–73. doi: 10.2337/diacare.20.3.369. [DOI] [PubMed] [Google Scholar]
- [45].Borcea V, Nourooz-Zadeh J, Wolff SP, Klevesath M, Hofmann M, Urich H, Wahl P, Ziegler R, Tritschler H, Halliwell B, Nawroth PP. alpha-Lipoic acid decreases oxidative stress even in diabetic patients with poor glycemic control and albuminuria. Free Radic Biol Med. 1999;26:1495–500. doi: 10.1016/s0891-5849(99)00011-8. [DOI] [PubMed] [Google Scholar]
- [46].Yadav V, Marracci G, Lovera J, Woodward W, Bogardus K, Marquardt W, Shinto L, Morris C, Bourdette D. Lipoic acid in multiple sclerosis: a pilot study. Mult Scler. 2005;11:159–65. doi: 10.1191/1352458505ms1143oa. [DOI] [PubMed] [Google Scholar]
- [47].Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, Khan BV. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–8. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- [48].Cakatay U, Kayali R. Plasma protein oxidation in aging rats after alpha-lipoic acid administration. Biogerontology. 2005;6:87–93. doi: 10.1007/s10522-005-3462-x. [DOI] [PubMed] [Google Scholar]
- [49].Cakatay U, Kayali R, Sivas A, Tekeli F. Prooxidant activities of alpha-lipoic acid on oxidative protein damage in the aging rat heart muscle. Arch Gerontol Geriatr. 2005;40:231–40. doi: 10.1016/j.archger.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [50].Kayali R, Cakatay U, Akcay T, Altug T. Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem Funct. 2006;24:79–85. doi: 10.1002/cbf.1190. [DOI] [PubMed] [Google Scholar]
- [51].Cakatay U, Kayali R, Kiziler AR, Aydemir B. Postmitotic tissue selenium and manganese levels in alpha-lipoic acid-supplemented aged rats. Chem Biol Interact. 2008;171:306–11. doi: 10.1016/j.cbi.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [52].Searls RL, Sanadi DR. alpha-Ketoglutaric dehydrogenase. 8. Isolation and some properties of a flavoprotein compnent. J Biol Chem. 1960;235:2485–91. [PubMed] [Google Scholar]
- [53].Trujillo M, Radi R. Peroxynitrite reaction with the reduced and the oxidized forms of lipoic acid: new insights into the reaction of peroxynitrite with thiols. Arch Biochem Biophys. 2002;397:91–8. doi: 10.1006/abbi.2001.2619. [DOI] [PubMed] [Google Scholar]
- [54].Vriesman MF, Haenen GR, Westerveld GJ, Paquay JB, Voss HP, Bast A. A method for measuring nitric oxide radical scavenging activity. Scavenging properties of sulfur-containing compounds. Pharm World Sci. 1997;19:283–6. doi: 10.1023/a:1008601327920. [DOI] [PubMed] [Google Scholar]
- [55].Suzuki YJ, Tsuchiya M, Packer L. Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Radic Res Commun. 1991;15:255–63. doi: 10.3109/10715769109105221. [DOI] [PubMed] [Google Scholar]
- [56].Suzuki YJ, Tsuchiya M, Packer L. Antioxidant activities of dihydrolipoic acid and its structural homologues. Free Radic Res Commun. 1993;18:115–22. doi: 10.3109/10715769309147348. [DOI] [PubMed] [Google Scholar]
- [57].Kaiser S, di Mascio P, Sies H. Lipoat und Singulettsauerstoff. In: Borbe HO, Ulrich H, editors. Thioctseure. pmi Verlag GmbH; Frankfurt: 1989. pp. 69–76. [Google Scholar]
- [58].Devasagayam TP, Di Mascio P, Kaiser S, Sies H. Singlet oxygen induced single-strand breaks in plasmid pBR322 DNA: the enhancing effect of thiols. Biochim Biophys Acta. 1991;1088:409–12. doi: 10.1016/0167-4781(91)90133-7. [DOI] [PubMed] [Google Scholar]
- [59].Kagan VE, Shvedova A, Serbinova E, Khan S, Swanson C, Powell R, Packer L. Dihydrolipoic acid--a universal antioxidant both in the membrane and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem Pharmacol. 1992;44:1637–49. doi: 10.1016/0006-2952(92)90482-x. [DOI] [PubMed] [Google Scholar]
- [60].Haenen GR, Bast A. Scavenging of hypochlorous acid by lipoic acid. Biochem Pharmacol. 1991;42:2244–6. doi: 10.1016/0006-2952(91)90363-a. [DOI] [PubMed] [Google Scholar]
- [61].Yan LJ, Traber MG, Kobuchi H, Matsugo S, Tritschler HJ, Packer L. Efficacy of hypochlorous acid scavengers in the prevention of protein carbonyl formation. Arch Biochem Biophys. 1996;327:330–4. doi: 10.1006/abbi.1996.0130. [DOI] [PubMed] [Google Scholar]
- [62].Bast A, Haenen GR. Lipoic acid: a multifunctional antioxidant. Biofactors. 2003;17:207–13. doi: 10.1002/biof.5520170120. [DOI] [PubMed] [Google Scholar]
- [63].Ou P, Tritschler HJ, Wolff SP. Thioctic (lipoic) acid: a therapeutic metal-chelating antioxidant? Biochem Pharmacol. 1995;50:123–6. doi: 10.1016/0006-2952(95)00116-h. [DOI] [PubMed] [Google Scholar]
- [64].Suh JH, Moreau R, Heath SH, Hagen TM. Dietary supplementation with (R)-alpha-lipoic acid reverses the age-related accumulation of iron and depletion of antioxidants in the rat cerebral cortex. Redox Rep. 2005;10:52–60. doi: 10.1179/135100005X21624. [DOI] [PubMed] [Google Scholar]
- [65].Bush AI. Metal complexing agents as therapies for Alzheimer’s disease. Neurobiol Aging. 2002;23:1031–8. doi: 10.1016/s0197-4580(02)00120-3. [DOI] [PubMed] [Google Scholar]
- [66].Goralska M, Dackor R, Holley B, McGahan MC. Alpha lipoic acid changes iron uptake and storage in lens epithelial cells. Exp Eye Res. 2003;76:241–8. doi: 10.1016/s0014-4835(02)00307-x. [DOI] [PubMed] [Google Scholar]
- [67].Lykkesfeldt J, Hagen TM, Vinarsky V, Ames BN. Age-associated decline in ascorbic acid concentration, recycling, and biosynthesis in rat hepatocytes--reversal with (R)-alpha-lipoic acid supplementation. Faseb J. 1998;12:1183–9. doi: 10.1096/fasebj.12.12.1183. [DOI] [PubMed] [Google Scholar]
- [68].Michels AJ, Joisher N, Hagen TM. Age-related decline of sodium-dependent ascorbic acid transport in isolated rat hepatocytes. Arch Biochem Biophys. 2003;410:112–20. doi: 10.1016/s0003-9861(02)00678-1. [DOI] [PubMed] [Google Scholar]
- [69].Suh JH, Shigeno ET, Morrow JD, Cox B, Rocha AE, Frei B, Hagen TM. Oxidative stress in the aging rat heart is reversed by dietary supplementation with (R)-(alpha)-lipoic acid. Faseb J. 2001;15:700–6. doi: 10.1096/fj.00-0176com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Xu DP, Wells WW. alpha-Lipoic acid dependent regeneration of ascorbic acid from dehydroascorbic acid in rat liver mitochondria. J Bioenerg Biomembr. 1996;28:77–85. [PubMed] [Google Scholar]
- [71].Bast A, Haenen GR. Interplay between lipoic acid and glutathione in the protection against microsomal lipid peroxidation. Biochim Biophys Acta. 1988;963:558–61. doi: 10.1016/0005-2760(88)90326-8. [DOI] [PubMed] [Google Scholar]
- [72].Busse E, Zimmer G, Schopohl B, Kornhuber B. Influence of alpha-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneimittelforschung. 1992;42:829–31. [PubMed] [Google Scholar]
- [73].Han D, Handelman G, Marcocci L, Sen CK, Roy S, Kobuchi H, Tritschler HJ, Flohe L, Packer L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6:321–38. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- [74].Suh JH, Wang H, Liu RM, Liu J, Hagen TM. (R)-alpha-lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for GSH synthesis. Arch Biochem Biophys. 2004;423:126–35. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–72. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- [76].Kilic F, Handelman GJ, Traber K, Tsang K, Packer L, Trevithick JR. Modelling cortical cataractogenesis XX. In vitro effect of alpha-lipoic acid on glutathione concentrations in lens in model diabetic cataractogenesis. Biochem Mol Biol Int. 1998;46:585–95. doi: 10.1080/15216549800204112. [DOI] [PubMed] [Google Scholar]
- [77].Wolz P, Krieglstein J. Neuroprotective effects of alpha-lipoic acid and its enantiomers demonstrated in rodent models of focal cerebral ischemia. Neuropharmacology. 1996;35:369–75. doi: 10.1016/0028-3908(95)00172-7. [DOI] [PubMed] [Google Scholar]
- [78].Biewenga GP, Dorstijn MA, Verhagen JV, Haenen GR, Bast A. Reduction of lipoic acid by lipoamide dehydrogenase. Biochem Pharmacol. 1996;51:233–8. doi: 10.1016/0006-2952(95)02124-8. [DOI] [PubMed] [Google Scholar]
- [79].Hagen TM, Vinarsky V, Wehr CM, Ames BN. (R)-alpha-lipoic acid reverses the age-associated increase in susceptibility of hepatocytes to tert-butylhydroperoxide both in vitro and in vivo. Antioxid Redox Signal. 2000;2:473–83. doi: 10.1089/15230860050192251. [DOI] [PubMed] [Google Scholar]
- [80].Moini H, Tirosh O, Park YC, Cho KJ, Packer L. R-alpha-lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes. Arch Biochem Biophys. 2002;397:384–91. doi: 10.1006/abbi.2001.2680. [DOI] [PubMed] [Google Scholar]
- [81].Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–13. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–53. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, Yamamoto M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–9. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Eggler AL, Liu G, Pezzuto JM, van Breemen RB, Mesecar AD. Modifying specific cysteines of the electrophile-sensing human Keap1 protein is insufficient to disrupt binding to the Nrf2 domain Neh2. Proc Natl Acad Sci U S A. 2005;102:10070–5. doi: 10.1073/pnas.0502402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hong F, Sekhar KR, Freeman ML, Liebler DC. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J Biol Chem. 2005;280:31768–75. doi: 10.1074/jbc.M503346200. [DOI] [PubMed] [Google Scholar]
- [86].Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, Motohashi H, Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol. 2008;28:2758–70. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rachakonda G, Xiong Y, Sekhar KR, Stamer SL, Liebler DC, Freeman ML. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chem Res Toxicol. 2008;21:705–10. doi: 10.1021/tx700302s. [DOI] [PubMed] [Google Scholar]
- [88].Huang HC, Nguyen T, Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–74. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- [89].Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675–82. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- [90].Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci U S A. 2000;97:12475–80. doi: 10.1073/pnas.220418997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sen CK, Sashwati R, Packer L. Fas mediated apoptosis of human Jurkat T-cells: intracellular events and potentiation by redox-active alpha-lipoic acid. Cell Death Differ. 1999;6:481–91. doi: 10.1038/sj.cdd.4400514. [DOI] [PubMed] [Google Scholar]
- [92].Suzuki YJ, Shi SS, Day RM, Blumberg JB. Differential regulation of MAP kinase signaling by pro- and antioxidant biothiols. Ann N Y Acad Sci. 2000;899:159–67. doi: 10.1111/j.1749-6632.2000.tb06184.x. [DOI] [PubMed] [Google Scholar]
- [93].Shi SS, Day RM, Halpner AD, Blumberg JB, Suzuki YJ. Homocysteine and alpha-lipoic acid regulate p44/42 MAP kinase phosphorylation in NIH/3T3 cells. Antioxid Redox Signal. 1999;1:123–8. doi: 10.1089/ars.1999.1.1-123. [DOI] [PubMed] [Google Scholar]
- [94].Konrad D, Somwar R, Sweeney G, Yaworsky K, Hayashi M, Ramlal T, Klip A. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes. 2001;50:1464–71. doi: 10.2337/diabetes.50.6.1464. [DOI] [PubMed] [Google Scholar]
- [95].Shay K. Petersen, Hagen TM. Age-associated impairment of Akt phosphorylation in primary rat hepatocytes is remediated by alpha-lipoic acid through PI3 kinase, PTEN, and PP2A. Biogerontology. 2009;10:443–456. doi: 10.1007/s10522-008-9187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–82. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Smith AR, Hagen TM. Vascular endothelial dysfunction in aging: loss of Akt-dependent endothelial nitric oxide synthase phosphorylation and partial restoration by (R)-alpha-lipoic acid. Biochem Soc Trans. 2003;31:1447–9. doi: 10.1042/bst0311447. [DOI] [PubMed] [Google Scholar]
- [98].Saengsirisuwan V, Perez FR, Sloniger JA, Maier T, Henriksen EJ. Interactions of exercise training and alpha-lipoic acid on insulin signaling in skeletal muscle of obese Zucker rats. Am J Physiol Endocrinol Metab. 2004;287:E529–36. doi: 10.1152/ajpendo.00013.2004. [DOI] [PubMed] [Google Scholar]
- [99].Cho KJ, Moini H, Shon HK, Chung AS, Packer L. Alpha-lipoic acid decreases thiol reactivity of the insulin receptor and protein tyrosine phosphatase 1B in 3T3-L1 adipocytes. Biochem Pharmacol. 2003;66:849–58. doi: 10.1016/s0006-2952(03)00395-2. [DOI] [PubMed] [Google Scholar]
- [100].Foley TD, Petro LA, Stredny CM, Coppa TM. Oxidative inhibition of protein phosphatase 2A activity: role of catalytic subunit disulfides. Neurochem Res. 2007;32:1957–64. doi: 10.1007/s11064-007-9394-x. [DOI] [PubMed] [Google Scholar]
- [101].Boivin B, Zhang S, Arbiser JL, Zhang ZY, Tonks NK. A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc Natl Acad Sci U S A. 2008;105:9959–64. doi: 10.1073/pnas.0804336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ross SH, Lindsay Y, Safrany ST, Lorenzo O, Villa F, Toth R, Clague MJ, Downes CP, Leslie NR. Differential redox regulation within the PTP superfamily. Cell Signal. 2007;19:1521–30. doi: 10.1016/j.cellsig.2007.01.026. [DOI] [PubMed] [Google Scholar]
- [103].Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–42. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- [104].Diesel B, Kulhanek-Heinze S, Holtje M, Brandt B, Holtje HD, Vollmar AM, Kiemer AK. Alpha-lipoic acid as a directly binding activator of the insulin receptor: protection from hepatocyte apoptosis. Biochemistry. 2007;46:2146–55. doi: 10.1021/bi602547m. [DOI] [PubMed] [Google Scholar]
- [105].Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol. 2005;99:330–7. doi: 10.1152/japplphysiol.00175.2005. [DOI] [PubMed] [Google Scholar]
- [106].Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab. 2007;32:557–66. doi: 10.1139/H07-026. [DOI] [PubMed] [Google Scholar]
- [107].Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–33. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- [108].Lee WJ, Lee IK, Kim HS, Kim YM, Koh EH, Won JC, Han SM, Kim MS, Jo I, Oh GT, Park IS, Youn JH, Park SW, Lee KU, Park JY. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:2488–94. doi: 10.1161/01.ATV.0000190667.33224.4c. [DOI] [PubMed] [Google Scholar]
- [109].Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005;332:885–91. doi: 10.1016/j.bbrc.2005.05.035. [DOI] [PubMed] [Google Scholar]
- [110].Paz K, Hemi R, LeRoith D, Karasik A, Elhanany E, Kanety H, Zick Y. A molecular basis for insulin resistance. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. J Biol Chem. 1997;272:29911–8. doi: 10.1074/jbc.272.47.29911. [DOI] [PubMed] [Google Scholar]
- [111].Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem. 2001;276:46912–6. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- [112].Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–8. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- [113].Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by {alpha}-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol. 2007 doi: 10.1152/ajpcell.00115.2007. [DOI] [PubMed] [Google Scholar]
- [114].Tsakiridis T, McDowell HE, Walker T, Downes CP, Hundal HS, Vranic M, Klip A. Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose transport, amino acid transport, and glucose transporters in L6 skeletal muscle cells. Endocrinology. 1995;136:4315–22. doi: 10.1210/endo.136.10.7664650. [DOI] [PubMed] [Google Scholar]
- [115].Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A. Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha-lipoic acid in 3T3-L1 adipocytes. Diabetologia. 2000;43:294–303. doi: 10.1007/s001250050047. [DOI] [PubMed] [Google Scholar]
- [116].Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H, Klip A. Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes. 1996;45:1798–804. doi: 10.2337/diab.45.12.1798. [DOI] [PubMed] [Google Scholar]
- [117].Henriksen EJ, Jacob S, Streeper RS, Fogt DL, Hokama JY, Tritschler HJ. Stimulation by alpha-lipoic acid of glucose transport activity in skeletal muscle of lean and obese Zucker rats. Life Sci. 1997;61:805–12. doi: 10.1016/s0024-3205(97)00562-6. [DOI] [PubMed] [Google Scholar]
- [118].Streeper RS, Henriksen EJ, Jacob S, Hokama JY, Fogt DL, Tritschler HJ. Differential effects of lipoic acid stereoisomers on glucose metabolism in insulin-resistant skeletal muscle. Am J Physiol. 1997;273:E185–91. doi: 10.1152/ajpendo.1997.273.1.E185. [DOI] [PubMed] [Google Scholar]
- [119].Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol. 1993;264:E855–62. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- [120].Jacob S, Streeper RS, Fogt DL, Hokama JY, Tritschler HJ, Dietze GJ, Henriksen EJ. The antioxidant alpha-lipoic acid enhances insulin-stimulated glucose metabolism in insulin-resistant rat skeletal muscle. Diabetes. 1996;45:1024–9. doi: 10.2337/diab.45.8.1024. [DOI] [PubMed] [Google Scholar]
- [121].Jacob S, Henriksen EJ, Schiemann AL, Simon I, Clancy DE, Tritschler HJ, Jung WI, Augustin HJ, Dietze GJ. Enhancement of glucose disposal in patients with type 2 diabetes by alpha-lipoic acid. Arzneimittelforschung. 1995;45:872–4. [PubMed] [Google Scholar]
- [122].Konrad T, Vicini P, Kusterer K, Hoflich A, Assadkhani A, Bohles HJ, Sewell A, Tritschler HJ, Cobelli C, Usadel KH. alpha-Lipoic acid treatment decreases serum lactate and pyruvate concentrations and improves glucose effectiveness in lean and obese patients with type 2 diabetes. Diabetes Care. 1999;22:280–7. doi: 10.2337/diacare.22.2.280. [DOI] [PubMed] [Google Scholar]
- [123].Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004;21:114–21. doi: 10.1111/j.1464-5491.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- [124].Heitzer T, Finckh B, Albers S, Krohn K, Kohlschutter A, Meinertz T. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: relation to parameters of oxidative stress. Free Radic Biol Med. 2001;31:53–61. doi: 10.1016/s0891-5849(01)00551-2. [DOI] [PubMed] [Google Scholar]
- [125].Sena CM, Nunes E, Louro T, Proenca T, Fernandes R, Boarder MR, Seica RM. Effects of alpha-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br J Pharmacol. 2007 doi: 10.1038/sj.bjp.0707474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol. 2002;16:1931–42. doi: 10.1210/me.2002-0074. [DOI] [PubMed] [Google Scholar]
- [127].Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, Sbraccia P, Spagnoli LG, Sesti G, Lauro R. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–72. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- [128].Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the aging rat heart: evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann N Y Acad Sci. 2002;959:491–507. doi: 10.1111/j.1749-6632.2002.tb02119.x. [DOI] [PubMed] [Google Scholar]
- [129].Shay K. Petersen, Moreau RF, Smith EJ, Hagen TM. Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life. 2008;60:362–7. doi: 10.1002/iub.40. [DOI] [PubMed] [Google Scholar]
- [130].Vasdev S, Gill V, Longerich L, Parai S, Gadag V. Salt-induced hypertension in WKY rats: prevention by alpha-lipoic acid supplementation. Mol Cell Biochem. 2003;254:319–26. doi: 10.1023/a:1027354005498. [DOI] [PubMed] [Google Scholar]
- [131].Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary lipoic acid supplementation prevents fructose-induced hypertension in rats. Nutr Metab Cardiovasc Dis. 2000;10:339–46. [PubMed] [Google Scholar]
- [132].Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary alpha-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. J Hypertens. 2000;18:567–73. doi: 10.1097/00004872-200018050-00009. [DOI] [PubMed] [Google Scholar]
- [133].Vasdev S, Gill V, Parai S, Gadag V. Dietary lipoic acid supplementation attenuates hypertension in Dahl salt sensitive rats. Mol Cell Biochem. 2005;275:135–41. doi: 10.1007/s11010-005-1095-7. [DOI] [PubMed] [Google Scholar]
- [134].Louhelainen M, Merasto S, Finckenberg P, Lapatto R, Cheng ZJ, Mervaala EM. Lipoic acid supplementation prevents cyclosporine-induced hypertension and nephrotoxicity in spontaneously hypertensive rats. J Hypertens. 2006;24:947–56. doi: 10.1097/01.hjh.0000222766.37971.9f. [DOI] [PubMed] [Google Scholar]
- [135].El Midaoui A, de Champlain J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension. 2002;39:303–7. doi: 10.1161/hy0202.104345. [DOI] [PubMed] [Google Scholar]
- [136].Midaoui AE, Elimadi A, Wu L, Haddad PS, de Champlain J. Lipoic acid prevents hypertension, hyperglycemia, and the increase in heart mitochondrial superoxide production. Am J Hypertens. 2003;16:173–9. doi: 10.1016/s0895-7061(02)03253-3. [DOI] [PubMed] [Google Scholar]
- [137].Takaoka M, Kobayashi Y, Yuba M, Ohkita M, Matsumura Y. Effects of alpha-lipoic acid on deoxycorticosterone acetate-salt-induced hypertension in rats. Eur J Pharmacol. 2001;424:121–9. doi: 10.1016/s0014-2999(01)01120-7. [DOI] [PubMed] [Google Scholar]
- [138].McMackin CJ, Widlansky ME, Hamburg NM, Huang AL, Weller S, Holbrook M, Gokce N, Hagen TM, Keaney JF, Jr., Vita JA. Effect of combined treatment with alpha-Lipoic acid and acetyl-L-carnitine on vascular function and blood pressure in patients with coronary artery disease. J Clin Hypertens (Greenwich) 2007;9:249–55. doi: 10.1111/j.1524-6175.2007.06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kunt T, Forst T, Wilhelm A, Tritschler H, Pfuetzner A, Harzer O, Engelbach M, Zschaebitz A, Stofft E, Beyer J. Alpha-lipoic acid reduces expression of vascular cell adhesion molecule-1 and endothelial adhesion of human monocytes after stimulation with advanced glycation end products. Clin Sci (Lond) 1999;96:75–82. [PubMed] [Google Scholar]
- [140].Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK, Park JY, Lee KU, Kim JG, Lee IK. Alpha-lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-kappaB transcriptional activity. Exp Mol Med. 2007;39:106–13. doi: 10.1038/emm.2007.12. [DOI] [PubMed] [Google Scholar]
- [141].Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;175:87–96. doi: 10.1016/j.jneuroim.2006.03.007. [DOI] [PubMed] [Google Scholar]
- [142].Lee EY, Lee CK, Lee KU, Park JY, Cho KJ, Cho YS, Lee HR, Moon SH, Moon HB, Yoo B. Alpha-lipoic acid suppresses the development of collagen-induced arthritis and protects against bone destruction in mice. Rheumatol Int. 2007;27:225–33. doi: 10.1007/s00296-006-0193-5. [DOI] [PubMed] [Google Scholar]
- [143].Xu C, Yuan X, Pan Z, Shen G, Kim JH, Yu S, Khor TO, Li W, Ma J, Kong AN. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;5:1918–26. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- [144].Morini M, Roccatagliata L, Dell’Eva R, Pedemonte E, Furlan R, Minghelli S, Giunti D, Pfeffer U, Marchese M, Noonan D, Mancardi G, Albini A, Uccelli A. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:146–53. doi: 10.1016/j.jneuroim.2003.11.021. [DOI] [PubMed] [Google Scholar]
- [145].Marracci GH, Marquardt WE, Strehlow A, McKeon GP, Gross J, Buck DC, Kozell LB, Bourdette DN. Lipoic acid downmodulates CD4 from human T lymphocytes by dissociation of p56(Lck) Biochem Biophys Res Commun. 2006;344:963–71. doi: 10.1016/j.bbrc.2006.03.172. [DOI] [PubMed] [Google Scholar]
- [146].Schillace RV, Pisenti N, Pattamanuch N, Galligan S, Marracci GH, Bourdette DN, Carr DW. Lipoic acid stimulates cAMP production in T lymphocytes and NK cells. Biochem Biophys Res Commun. 2007;354:259–64. doi: 10.1016/j.bbrc.2006.12.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Larghero P, Vene R, Minghelli S, Travaini G, Morini M, Ferrari N, Pfeffer U, Noonan DM, Albini A, Benelli R. Biological assays and genomic analysis reveal lipoic acid modulation of endothelial cell behavior and gene expression. Carcinogenesis. 2007;28:1008–20. doi: 10.1093/carcin/bgl233. [DOI] [PubMed] [Google Scholar]
- [148].Cho YS, Lee J, Lee TH, Lee EY, Lee KU, Park JY, Moon HB. alpha-Lipoic acid inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Allergy Clin Immunol. 2004;114:429–35. doi: 10.1016/j.jaci.2004.04.004. [DOI] [PubMed] [Google Scholar]
- [149].Zhang WJ, Frei B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. Faseb J. 2001;15:2423–32. doi: 10.1096/fj.01-0260com. [DOI] [PubMed] [Google Scholar]
- [150].Ikeda U, Ito T, Shimada K. Interleukin-6 and acute coronary syndrome. Clin Cardiol. 2001;24:701–4. doi: 10.1002/clc.4960241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Wang SJ, Chen HH. Presynaptic mechanisms underlying the alpha-lipoic acid facilitation of glutamate exocytosis in rat cerebral cortex nerve terminals. Neurochem Int. 2007;50:51–60. doi: 10.1016/j.neuint.2006.06.011. [DOI] [PubMed] [Google Scholar]
- [152].Stoll S, Hartmann H, Cohen SA, Muller WE. The potent free radical scavenger alpha-lipoic acid improves memory in aged mice: putative relationship to NMDA receptor deficits. Pharmacol Biochem Behav. 1993;46:799–805. doi: 10.1016/0091-3057(93)90204-7. [DOI] [PubMed] [Google Scholar]