Abstract
Traditional approaches to treating chronic neuropathic pain largely focus on manipulations directly altering neuronal activity or neuron-to-neuron communication. Recently, however, it has become clear that glial cells (including microglia and astroglia) play a significant role in pain expression in a variety of neuropathic pain models. Multiple aspects of the inflammatory response of glial cells, commonly observed in neuropathic pain conditions, have been implicated in pain expression. Thus, glial cell inflammation has emerged as a potential therapeutic target in neuropathic pain. Our laboratory has been exploring the use of an anti-inflammatory cytokine, interleukin-10 (IL-10), to control glial inflammatory activation thereby controlling neuropathic pain. IL-10 protein delivery is limited by a short half-life and an inability to cross into the central nervous system from the periphery, making a centrally delivered gene therapy approach attractive. We have recently characterized a non-viral gene therapy approach using two injections of naked DNA to achieve long-term (>3 months) control of neuropathic pain in a peripheral nerve injury model. Timing and dose requirements leading to long-term pain control are discussed in this review, as is recent work using microparticle-encapsulated DNA to achieve long-term therapeutic efficacy with a single injection.
Keywords: IL-10, allodynia, glia, CNS
Glial activation is an important mediator of chronic pain in diverse pathologies
Chronic pain may arise from a wide variety of insults to the peripheral or central nervous system (CNS) that include infection, autoimmune disease and mechanical trauma.1–5 Symptoms associated with chronic pain include an enhanced sensitivity to painful stimuli (hyperalgesia) and the perception of normally non-painful stimuli as painful (allodynia). The intensity of symptom expression may change over time, as may the location of the pain. The complex nature of symptom expression along with the diversity of possible origins of chronic pain have contributed to the difficulty of developing effective therapeutic interventions.
A great deal of research in the past decade has highlighted the crucial role of microglial and astroglial cells (herein referred to as ‘glia’) in the inflammatory response to both injury and infection.6–9 These cells are commonly activated following a wide variety of infectious or mechanical insults, and constitute a vital signaling network that contribute to the pathology of injury. The term ‘tetrapartite synapse’ has been coined by DeLeo et al.10 to describe the intimate conversation that takes place between pre-synaptic neurons, post-synaptic neurons, microglia and astrocytes that ultimately defines the signaling that occurs within a neuronal network under normal circumstances, as well as during pathological conditions. The language used in this conversation among glia and neurons not only includes classic neuromodulators such as glutamate and nitric oxide, but also a large number of proteins initially identified for their role in immune signaling, namely cytokines and chemokines.11–16 Glial activation is often accompanied by both morphological changes as well as increased production of mediators termed proinflammatory cytokines (interleukin-1 (IL-1), tumor necrosis factor α, IL-6, IL-6).
Neuronal hyperexcitability is a potential downstream consequence of glial activation.17–19 This neuronal hyper-excitability is in turn thought to underlie the expression of classic symptoms of neuropathic pain, including allodynia and hyperalgesia.20–22 This line of research has identified glial activation as a cornerstone of injury-induced signaling within the CNS that is responsible for symptom expression, and therefore identifies this activation as an attractive potential therapeutic target.
Glial activation is subject to regulation through cytokine signaling
The cytokine inflammatory signaling network involves not only classic proinflammatory mediators such as IL-1 and tumor necrosis factorα, but also regulatory cytokines, including IL-10, that interfere with and counteract the effects of IL-1 and tumor necrosis factorα.23–25 IL-10 exerts its effects through a number of mechanisms including the downregulation of inflammatory second messengers such as nuclear factor κβ and the downregulation of surface receptors such as the IL-1 receptor, as well as the upregulation of some molecules such as suppressor of cytokine signaling and the activation of second messengers such as signal transducer and activator of transcription 3. The actions of IL-10 have been extensively reviewed elsewhere.26
Interleukin-10 is a particularly attractive therapeutic candidate in the control of glial activation for several reasons. First, IL-10 has the demonstrated capacity to suppress glial activation in a variety of contexts including infections, mechanical trauma and autoimmune inflammation.27 The use of IL-10 has been shown in vivo in animal models to control inflammation in a diversity of paradigms.26,28–30 In addition, in many CNS sites such as spinal cord, IL-10 receptors are present only on glia and not on neurons.31 The selective targeting of glial activation offers the possibility of reducing the inflammatory input driving neuronal hyperexcitability while leaving basal neuronal functioning intact. This is a major advantage over therapies that directly target neuronal activity and are often associated with severely limiting side effects.
Chronic pain, by definition, is a condition that is prolonged in time. Therefore, it is desirable that the therapy be equally prolonged in duration. As a proof of principle for the therapeutic use of IL-10 to suppress chronic pain, bolus IL-10 protein administration is able to block and reverse neuropathic pain in multiple animal models.32 However, the short-term efficacy of protein injections (several hours), which corresponds to the IL-10 half-life of protein in the intrathecal space, limits this approach.32 The short half-life of the protein makes a gene therapy approach an attractive alternative. Several attempts have been made to achieve long-term therapeutic efficacy by the use of viral vector overexpression of IL-10. These include adenoviral, adeno-associated viral and herpes simplex viral vectors through various routes of administration.32–35 Long-term efficacy has proven difficult to achieve and repeated administration of therapeutic viral vectors are often ineffective or potentially dangerous owing to the host immune response that they may provoke.36,37 This latter concern is especially noteworthy for CNS applications, given the negative repercussions of inflammation-induced neuronal loss.
Long-term therapeutic control of glial activation can be achieved through intrathecal non-viral IL-10 gene delivery
We have recently developed and characterized a non-viral gene therapy approach that makes use of two successive intrathecal injections of naked plasmid DNA coding for the anti-inflammatory cytokine IL-10F129S (Sloane et al.38 and Milligan et al.39). This cytokine contains a point mutation (serine substitution for phenylalanine at amino acid 129) from the wild-type IL-10 protein and has shown in vivo therapeutic efficacy in a variety of both peripheral and central inflammatory contexts. We have shown the ability of this novel procedure to control the allodynia associated with peripheral nerve injury, chemotherapy-induced neuropathy, and both the allodynia and paralysis associated with experimental autoimmune encephalomyelitis (a model of relapsing-remitting multiple sclerosis).30,39,40 Changes in the expression of glial activation and immune markers in both spinal cord and dorsal root ganglia associated with several of these inflammatory models are also prevented by pDNA-IL-10F129S therapy in these studies.
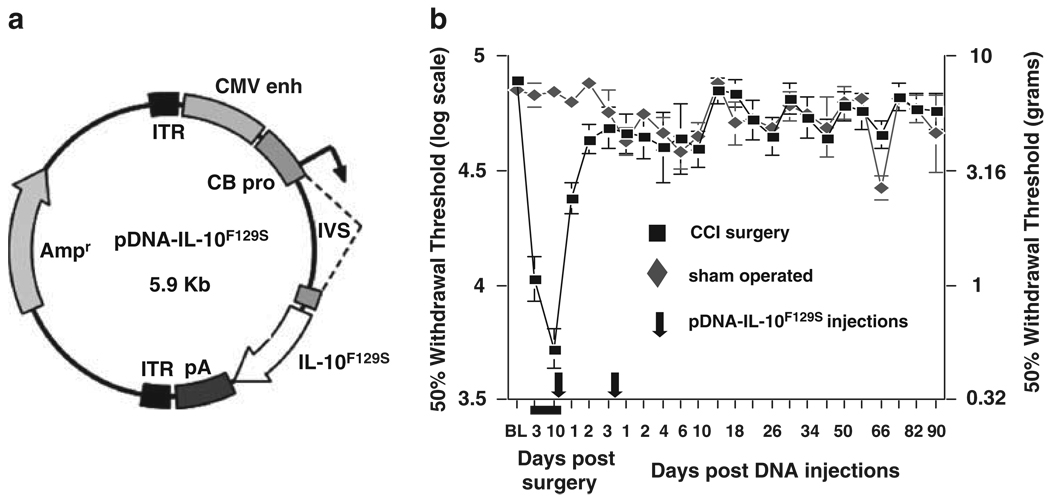
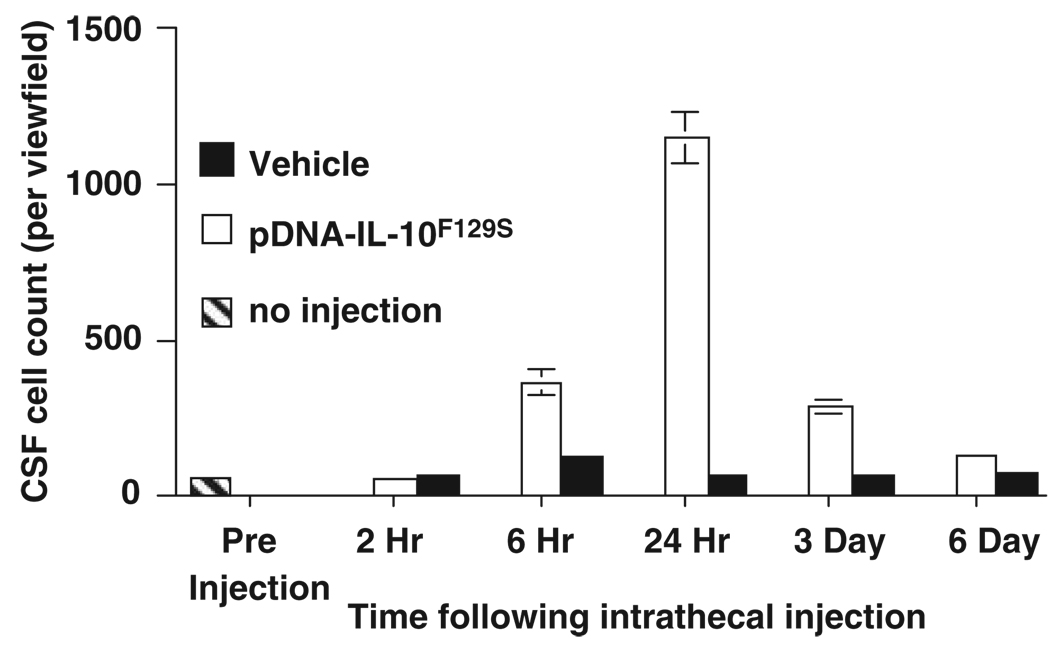
This injection protocol requires two intrathecal injections of IL-10F129S DNA to be administered no less than 5 h, and no more than 3 days apart, to achieve long-term therapeutic efficacy. Injections separated by 2 h or given as a single combined dose bolus provide therapeutic benefits lasting only approximately 6 days. Maximal efficacy (>3 months) has been observed with an initial injection dose of 100 µg DNA and a second injection of 25 µg DNA separated by a 2–3 day interval (Figure 1). The timing requirements of this procedure appear to involve a sequence of events triggered by the initial DNA injection (the ‘priming’ injection). Within 6 h, a significant accumulation of innate immune cells (primarily cells strongly staining for markers of macrophages and undifferentiated monocytes) is observed in cerebrospinal fluid (CSF) at the intrathecal injection site (Figure 2). Whether these cells are recruited from the peripheral circulation or are intrinsic immune cells of the meninges that translocate to the CSF is as yet unknown. These cells appear to be of monocytic origin, with phenotypes consistent with those anticipated to be recruited following an immune challenge. It has been previously documented that innate immune cells, largely consisting of macrophages, infiltrate the CNS following local injection of various immunostimulatory substances including synthetic oligodeoxynucleotides. By 24 h following the priming DNA injection, the influx of cells into CSF peaks consists primarily of activated macrophages. This cell type is well known for its phagocytic capacity, and the increased presence of these cells would likely enhance the uptake and clearance of a subsequent DNA injection from CSF owing to the enriched local population. At 3 days following the priming DNA injection, local CSF still contains this enriched population of macrophages when the second, or ‘therapeutic’, DNA injection occurs. It is important to note that the innate immune response to the priming DNA injection occurs independent of the transgene contained in the plasmid. That is, 100 µg of a control plasmid that does not contain the IL-10 gene is perfectly capable of leading to long-term therapeutic efficacy, provided it is followed by a 25 µg therapeutic DNA injection that contains the gene of interest, IL-10F129S (Sloane et al.38). The reverse injection schedule, however, does not lead to a successful long-term therapy. An initial priming injection of 100 µg pDNA-IL-10F129S followed by 25 µg control plasmid leads to a therapy lasting only approximately 6 days.38
Figure 1.
(a) Design of the pDNA-IL-10F129S construct.39 Gene transcription is driven by a cytomegalovirus enhancer (CMV enh) and a chicken β actin promoter (CB pro).39 The expression cassette is flanked by two inverted terminal repeat (ITR) sequences and the backbone contains an ampicillin (AMP)-resistance gene. (b) Two injections of pDNA-IL-10F129S separated by 3 days leads to the long-term reversal of peripheral nerve injury-induced allodynia.
Figure 2.
Time course of cell influx following pDNA-IL-10F129S injection. Between 6 h and 3 days following injection of DNA, but not vehicle, cell counts in cerebrospinal fluid (CSF) at the site of injection are significantly elevated. These cells primarily consist of immune cells of monocytic origin that positively stain for macrophage markers (ED1 and ED2, data not shown).
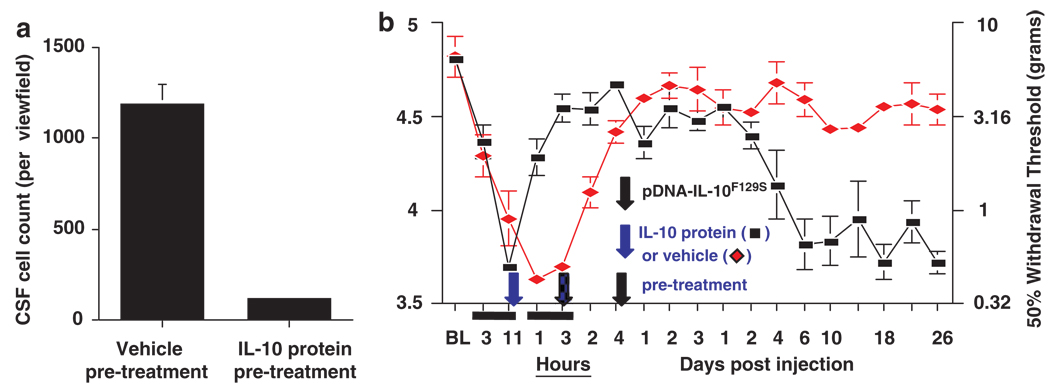
The similar time course of cell accumulation in CSF and the window of time in which a therapeutic DNA injection will lead to long-term efficacy appear to be more than coincidental. Cell influx following a 100 µg DNA injection can be blocked by intrathecal pretreatment with IL-10 protein and co-injection of IL-10 protein along with the DNA injection (Figure 3a). This suggests that an acute inflammatory response to the priming DNA injection is responsible for cell influx into CSF. Moreover, if cell influx is blocked through IL-10 protein pre- and co-treatment, therapeutic benefit is observed only for several days following a second pDNA-IL-10F129S injection as opposed to the normal duration of greater than 3 months (Figure 3b).
Figure 3.
Enriched cell population in cerebrospinal fluid (CSF) contributes to long-term therapeutic efficacy. (a) Accumulation of cells in CSF following DNA injection can be blocked by pre-treatment with interleukin (IL)-10 protein. (b) Pre-treatment with IL-10 protein also blocks the long-term reversal of allodynia that is normally seen in the two-injection paradigm.
The IL-10F129S transgene is not only required on the second injection for long-term therapeutic efficacy, but also there appears to be an ongoing requirement for anti-inflammatory signaling. Intrathecal administration of either the IL-10 neutralizing antibody (but not control IgG) or the inflammatory HIV coat protein, gp120, induces a rapid and sustained failure of the gene therapy.38 This finding suggests that the inclusion of an IL-10 gene or other regulatory cytokine genes along with any desired therapeutic gene of interest may be beneficial in providing long-term efficacy.
Non-viral IL-10 gene therapy may be applicable to a variety of neuropathic conditions
This two-injection paradigm, in which a priming DNA injection triggers the accumulation of phagocytic cells followed by a therapeutic DNA injection administered during the period of immune cell accumulation, has potential widespread application. This paradigm may serve as a general model in the development of other gene therapy applications and may provide a method to enhance gene uptake (viral or non-viral) by taking advantage of the inherent immunogenicity of gene therapy vectors.
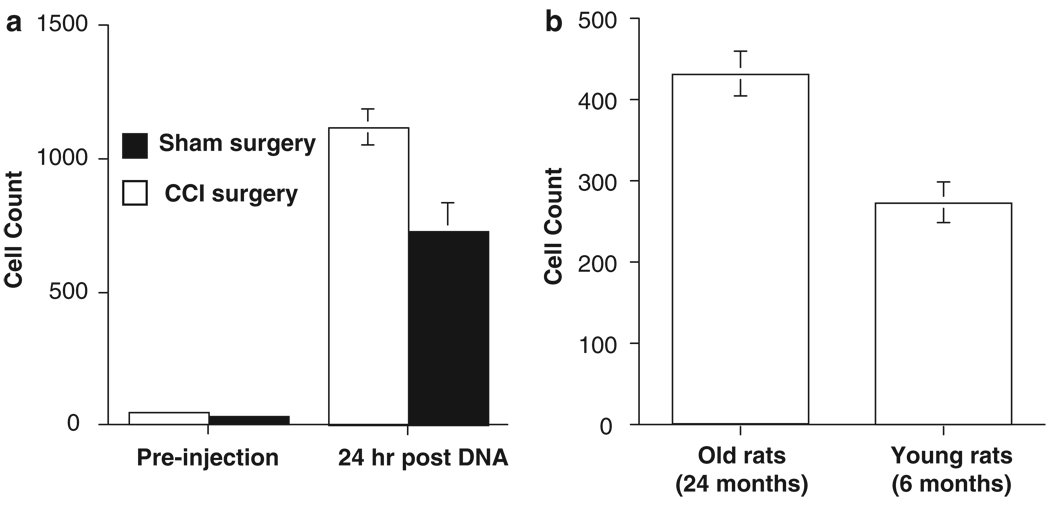
The proposed mechanism underlying the effectiveness of this two-injection paradigm is that an innate immune response to an initial injection potentiates a subsequent therapeutic injection through recruitment of phagocytic innate immune cells. The accumulation of phagocytic immune cells (primarily macrophages) is predicted to enhance uptake of plasmid DNA on a subsequent injection, thereby improving transfection efficiency of the local cell population. This idea further predicts that ongoing inflammation, or immune priming, at the site of gene therapy administration should enhance phagocytic cell recruitment following an initial injection, thereby optimizing a subsequent therapeutic injection. This prediction is supported by observations of increased cell recruitment following intrathecal DNA injection in the context of sciatic nerve injury-induced neuroinflammation compared with sham-operated controls (Figure 4a). Aging is a second context known to be associated with increased CNS immune reactivity, and in accordance with the above concepts, injection of naked DNA into aged rats (approximately 24 months) provokes a substantially greater accumulation of macrophages at 24 h post-injection as compared with younger rats (approximately 6 months) (Figure 4b).
Figure 4.
Pre-existing inflammation enhances cell accumulation in cerebrospinal fluid (CSF). (a) Lumbar CSF contains a significantly higher number of phagocytic immune cells both before and following injection of 100 µg pDNA in animals receiving chronic constriction injury (CCI; peripheral nerve injury model) of the sciatic nerve as compared with animals receiving sham surgery. (b) Rats that are aged also show a greater degree of cell accumulation in CSF following 100 µg pDNA injection as compared with younger rats.
An additional prediction that can be made is that long-term therapeutic efficacy requires the initial presence of sufficient numbers of phagocytic cells as well as sufficient copies of plasmid containing the therapeutic gene of interest. If it were possible to achieve both of these ends following a single intrathecal injection, this would offer a more attractive clinical approach. We have been pursuing this possibility with the use of pDNA-IL-10F129S encapsulated in microparticles consisting of a lactic and glycolic acid polymer. The primary benefit of microparticle encapsulation is the subsequent chronic release of DNA as the particles degrade over time. We have recently made the unexpected observation that microparticles injected intrathecally, which do not contain DNA, induce significant local accumulation of phagocytic immune cells.41 By injecting particles containing pDNA-IL-10F129S the requirements of sufficient phagocytic cell presence as well as sufficient exposure of these cells to therapeutic plasmid copies may both be fulfilled. We have recently observed therapeutic efficacy following lactic and glycolic acid polymer-encapsulated pDNA-IL-10F129S lasting approximately 3 months in the context of peripheral nerve injury.41
The principal goal driving this therapeutic approach is the control of inflammatory glial activation to control symptoms of painful neuropathy. This gene therapy has since been applied successfully to autoimmune inflammation in a model of multiple sclerosis, and has demonstrated the ability to control paralysis as well as pain in this context.30 Glial activation as a common end point arising from diverse sources of inflammation and underlying a variety of symptoms may provide a powerful therapeutic target for multiple neuroinflammatory diseases. This family of diseases may include such clinical challenges as Alzheimer’s disease, amyotrophic lateral sclerosis and diabetic neuropathy, all of which are associated with glial activation in CNS.42–46 The development of effective and chronic therapies that target CNS inflammation holds promise in the treatment of a range of diseases, including those leading to chronic pain.
Acknowledgements
Financial support to the study was provided by NIH Grants DA018156, DA024044, DA015642, DA015656 and Avigen.
References
- 1.Macleod M, Stewart G, Zeidler M, Will R, Knight R. Sensory features of variant Creutzfeldt-Jakob disease. J Neurol. 2002;249:706–711. doi: 10.1007/s00415-002-0696-2. [DOI] [PubMed] [Google Scholar]
- 2.Osterberg A, Boivie J, Thuomas K. Central pain in multiple sclerosis—prevalence and clinical characteristics. Eur J Pain. 2005;9:531–542. doi: 10.1016/j.ejpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Kenner M, Menon U, Elliott D. Multiple Sclerosis as a painful disease. Int Rev Neurobiol. 2007;79:303–321. doi: 10.1016/S0074-7742(07)79013-X. [DOI] [PubMed] [Google Scholar]
- 4.Jensen M, Kuehn C, Amtmann D, Cardenas D. Symptom burden in persons with spinal cord injury. Arch Phys Med Rehab. 2007;88:638–645. doi: 10.1016/j.apmr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowbotham M, Fields H. The relationship of pain, allodynia, and thermal sensation in post-herpetic neuralgia. Brain. 1996;119:347–354. doi: 10.1093/brain/119.2.347. [DOI] [PubMed] [Google Scholar]
- 6.Milligan E, Twinning C, Chacur M, Biedenkapp J, O’Connor K, Poole S, et al. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watkins L, Milligan E, Maier S. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 8.Tsuda M, Inoue K, Salter M. Neuropathic pain and spinal microglia: a big problem from molecules in ‘small’ glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 9.McMahon S, Cafferty W, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.DeLeo J, Tawfik V, LaCroix-Fralish M. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Benveniste E. Cytokines: influence on glial cell gene expression and function. Neuroimmunoendocrinology. 1997;69:31–75. doi: 10.1159/000058653. [DOI] [PubMed] [Google Scholar]
- 12.Milligan E, Sloane E, Watkins L. Glia in pathological pain: a role for fractalkine. J Neuroimmunol. 2008;198:113–120. doi: 10.1016/j.jneuroim.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Araque A, Perea G. Glial modulation of synaptic transmission in culture. Glia. 2004;47:241–248. doi: 10.1002/glia.20026. [DOI] [PubMed] [Google Scholar]
- 14.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Chen W, Li S, Cai J, Li W, Xian X, et al. Fluorocitrate, an inhibitor of glial metabolism, inhibits the up-regulation of NOS expression, activity and NO production in the spinal cord induced by formalin test in rats. Neurochem Res. 2009;34:351–359. doi: 10.1007/s11064-008-9785-7. [DOI] [PubMed] [Google Scholar]
- 16.Holguin A, O’Connor K, Biedenkapp J, Campisi J, Wieseler-Frank J, Milligan E, et al. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-1 (nNOS) Pain. 2004;110:517–530. doi: 10.1016/j.pain.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens M, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Q, Li Y, Xin W, Zang Y, Ren W, Wei X, et al. ATP induces long-term potentiation of C-fiber-evoked field potentials in spinal dorsal horn: the roles of P2X(4) receptors and p38 MAPK in microglia. Glia. 2008 doi: 10.1002/glia.20786. [DOI] [PubMed] [Google Scholar]
- 19.Jabs R, Seifert G, Steinhauser C. Astrocytic function and its alteration in the epileptic brain. Epilepsia. 2008;49:3–12. doi: 10.1111/j.1528-1167.2008.01488.x. [DOI] [PubMed] [Google Scholar]
- 20.Xing G, Liu F, Qu X, Han J, Wan Y. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupunture in rats with neuropathic pain. Exp Neurol. 2007;208:323–332. doi: 10.1016/j.expneurol.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Gwak Y, Kang J, Leem J, Hulsebosch C. Spinal AMPA receptor inhibition attenuates mechanical allodynia and neuronal hyperexcitability following spinal cord injury in rats. J Neurosci Res. 2007;85:2352–2359. doi: 10.1002/jnr.21379. [DOI] [PubMed] [Google Scholar]
- 22.Waxman S, Hains B. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 2006;29:207–215. doi: 10.1016/j.tins.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Ledeboer A, Breve J, Poole S, Tilders F, Dam AV. Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. 2000;30:134–142. doi: 10.1002/(sici)1098-1136(200004)30:2<134::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Strle K, Zhou J, Shen W, Broussard S, Johnson R, Freund G, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- 25.Marques CP, Hu S, Sheng W, Cheeran MC-J, Cox D, Lokensgard JR. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia. 2004;47:358–366. doi: 10.1002/glia.20045. [DOI] [PubMed] [Google Scholar]
- 26.Moore K, Malefyt R, Coffman R, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 27.Plunkett J, Yu C, Easton J, Bethea J, Yezierski R. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol. 2001;168:144–154. doi: 10.1006/exnr.2000.7604. [DOI] [PubMed] [Google Scholar]
- 28.Schif-Zuck S, Wildbaum G, Karin N. Coadministration of plasmid DNA constructs encoding an encephalitogenic determinant and IL-10 elicits regulatory T cell-mediated protective immunity in the central nervous system. J Immunol. 2006;177:8241–8247. doi: 10.4049/jimmunol.177.11.8241. [DOI] [PubMed] [Google Scholar]
- 29.Milligan E, Soderquist R, Malone S, Mahoney J, Hughes T, Langer S, et al. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biology. 2006;2:293–308. doi: 10.1017/S1740925X07000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier S, et al. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis. Brain Behav Immun. 2009;23:92–100. doi: 10.1016/j.bbi.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledeboer A, Wierinckx A, Bol J, Floris S, de Lavalette R, de Vries H, et al. Regional and temporal expression patterns of interleukin-10, interleukin-10 receptor and adhesion molecules in the rat spinal cord during chronic relapsing EAE. J Neuroimmunol. 2003;136:94–103. doi: 10.1016/s0165-5728(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 32.Milligan E, Langer S, Sloane E, He L, Wieseler-Frank J, O’Connor K, et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatroy cytokine, interleukin-10. Eur J Neurosci. 2005;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- 33.Storek B, Reinhardt M, Wang C, Janssen W, Harder N, Banck M, et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. PNAS. 2008;105:1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z, Peng X, Hao S, Fink D, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor α in spinal cord microglia. Gene Therapy. 2008;15:183–190. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milligan E, Sloane E, Langer S, Cruz P, Chacur M, Spataro L, et al. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain. 2005;1:9. doi: 10.1186/1744-8069-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Driesse M, Esandi M, Kros J, Avezaat C, Vecht C, Zurcher C, et al. Intra-CSF administered recombinant adenovirus causes an immune response-mediated toxicity. Gene Therapy. 2000;7:1401–1409. doi: 10.1038/sj.gt.3301250. [DOI] [PubMed] [Google Scholar]
- 37.Zaiss A, Muruve D. Immune responses to adeno-associated virus vectors. Curr Gene Ther. 2005;5:323–331. doi: 10.2174/1566523054065039. [DOI] [PubMed] [Google Scholar]
- 38.Sloane E, Langer S, Jekich B, Mahoney J, Hughes T, Seibert W, et al. Immunological priming potentiates non-viral anti-inflammatory gene therapy treatment of neuropathic pain. In Revision. 2008 doi: 10.1038/gt.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milligan E, Sloane E, Langer S, Hughes T, Jekich B, Frank M, et al. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126:294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Ledboer A, Jekich B, Sloane E, Mahoney J, Langer S, Milligan E, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soderquist R, Sloane E, Harrison J, Loram L, Lewis M, Chavez R, et al. Microparticle mediated therapeutic pDNA delivery facilitates long term reversal of neuropathic pain following a single intrathecal administration. In Preparation. 2008 [Google Scholar]
- 42.Schwab C, McGeer P. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimers Dis. 2008;13:359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- 43.Boillee S, Velde C, Cleveland D. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Zeng H-Y, Green W, Tso M. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 45.Tsuda M, Ueno H, Kataoka A, Tozaki-Saitoh H, Inoue K. Activation of dorsal horn microglia contributes to diabetes-induced tactile allodynia via extracellular signal-related protein kinase signaling. Glia. 2008;56:378–386. doi: 10.1002/glia.20623. [DOI] [PubMed] [Google Scholar]
- 46.Daulhac L, Mallet C, Couteix C, Etienne M, Duroux E, Privat A, et al. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-Methyl-D-aspartate-dependent mechanisms. Mol Pharmacol. 2006;70:1246–1254. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]