Abstract
Hedgehog pathway activity has been demonstrated in malignant glioma. However, its role in tumor growth has not been determined. Here we demonstrate that pharmacological inhibition of the Hedgehog pathway in established orthotopic malignant glioma xenografts confers a survival advantage. Pathway inhibition is measured in transplanted human tumor cells and not in host mouse brain. Correspondingly, survival benefit is observed only in tumors with an operational Hedgehog pathway. These data indicate that Hedgehog signaling regulates the growth of select malignant gliomas. We also demonstrate that Hedgehog pathway component and gene target expression segregate to CD133+ tumor initiating cells. Treated mice eventually succumb to disease, thus targeting the Hedgehog pathway in CD133+ cells produces significant, but incomplete tumor regression. Therefore, our studies suggest that more complete tumor regression may require the inclusion of other therapeutic targets, including CD133− cells.
Keywords: Brain tumor, Glioma, Hedgehog, Xenotransplantation, Cyclopamine
Introduction
Malignant gliomas are characterized by invasive growth that is intractable to current therapies. One glioma cell type that confers resistance to radiation and chemotherapy has been identified by the expression of CD133 (Prominin-1) (Bao et al., 2006; Liu et al., 2006). Multipotent CD133+ cells have the capacity to initiate and passage disease in immunodeficient mice (Singh et al., 2004; Bao et al., 2006; Piccirillo et al., 2006), and thus represent at least one cellular component that can maintain aggressive glioma growth. Commonly referred to as tumor-initiating or cancer stem cells, the identification of CD133+ cells in malignant glioma has prompted the characterization of regulatory molecular pathways for therapeutic strategies.
Among signaling mechanisms that regulate stem cell self-renewal and commitment (Ahn and Joyner, 2005; Trowbridge et al., 2006), Hedgehog (Hh) pathway activation has been demonstrated in a subset of malignant gliomas (Bar et al., 2007; Clement et al., 2007; Ehtesham et al., 2007; Xu et al., 2008). However, the role of Hh signaling in glioma growth has not been determined as prior studies involved manipulations to inhibit the Hh pathway in cultured cell lines for in vitro assays and prior to xenotransplantation. Glioma cells are known to undergo phenotypic and genotypic transformation in culture (Lee et al., 2006). Therefore, we have initiated pathway inhibition in established glioma orthotopic xenografts from freshly resected patient specimens to test rigorously the requirement for Hedgehog signaling in tumor growth.
Hh signal transduction components and small molecule modulators have been well characterized (Rubin and de Sauvage, 2006; Rohatgi and Scott, 2007). Smoothened, a seven-pass transmembrane domain protein that is strictly required for signal transduction (Zhang et al., 2001), has been a common target of nonbiased small molecule screens (Chen et al., 2002b; Frank-Kamenetsky et al., 2002). For our experiments, pharmacological inhibition was mediated by cyclopamine, a Smoothened inhibitor (Chen et al., 2002a) that has been well-tolerated and effective for in vivo studies (Berman et al., 2002; Karhadkar et al., 2004).
Results
Cyclopamine confers a survival benefit in established glioma xenografts
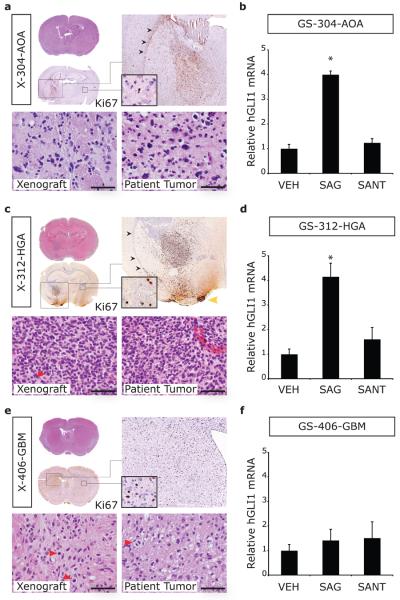
Malignant gliomas were modeled in NOD/SCID mice by orthotopic transplantation of CD133+ cells enriched from four primary tumor (PT) specimens on the day of resection (Table 1). Reflecting the heterogeneous phenotypes encompassed by WHO grades III and IV malignant glioma, the patient tumors included an anaplastic oligoastrocytoma (PT-304-AOA), a high-grade astrocytoma (PT-312-HGA), an anaplastic ganglioglioma (a rare malignant glioma phenotype; PT-302-AG) and a primary GBM (PT-406-GBM). From these specimens, xenotransplanted tumors formed with variable, but generally high, rates of engraftment (69-100%) and the median length of survival ranged from 64.5-145 days. Engraftment of transplanted cells could be visualized by hematoxylin and eosin (H&E) staining, in conjunction with immunohistochemistry for markers of cell proliferation (Ki67; Figure 1a, 1c and 1e; X-304-AOA, X-312-HGA, and X-406-GBM) or a pan-human nuclear epitope (anti-human nuclear; Supplementary Figure 1; X-302-AG) (Uchida et al., 2000). Recapitulation of malignant glioma pathological features was observed and included infiltrative growth, invasion of the corpus callosum to the contralateral hemisphere, and pleomorphic nuclei with mitotic figures (Figure 1a, 1c, and 1e).
Table 1. Clinical and pathological features of malignant gliomas.
Shown are the primary tumor identifier, pathology and WHO grade, age and gender of the patient, and prior therapy. P, primary tumor; R, recurrent tumor, NA, not applicable, TMZ, temozolamide.
| Identifier | Pathology | Primary/ Recurrent |
Age | Gender | Initial Pathology | Prior Therapy |
|---|---|---|---|---|---|---|
| PT-312-HGA | High grade astrocytoma WHO grade III |
P | 54 | M | NA | None |
| PT-304-AOA | Anaplastic oligoastrocytoma WHO grade III |
R | 44 | F | Infiltrating glioma with oligodendroglial features WHO grade II |
TMZ |
| PT-302-AG | Anaplastic gangliogioma WHO grade III |
R | 28 | M | Anaplastic ganglioglioma WHO grade III |
Gliadel, radiation and TMZ |
| PT-406-GBM | Glioblastoma multiforme WHO grade IV |
P | 83 | F | NA | None |
Figure 1. Characterization of tumor pathology in xenografts and Hh pathway responsiveness in gliomaspheres generated from malignant gliomas.
Malignant glioma specimens were dissociated with papain and divided into two portions. On the day of resection, one portion was enriched for CD133+ cells by immunomagnetic selection and transplanted directly into the striatum of NOD/SCID mice. The other portion was plated in NeuroCult media with bFGF and EGF for gliomasphere (GS) culture. (a, c and e) Tumor engraftment was revealed by H&E and Ki67 staining from an anaplastic oligoastrocytoma (X-304-AOA), a high grade astrocytoma (X-312-HGA), and a GBM (X-406-GBM). Xenotransplanted tumors recapitulated distinct features of high grade glioma, namely infiltrative growth with invasion of the corpus callosum (black arrowheads) to involve the contralateral hemisphere (insets) and of the leptomeninges (yellow arrowhead). Under high power magnification, similar cell density and morphology was observed for each xenograft and the corresponding patient tumor (scale bar = 50 μm; red arrowheads indicate mitotic figures). (b, d and e) Hh pathway response was assayed in GS cultured for 40 hours in the presence of either vehicle (VEH), a Smoothened agonist (SAG, 50 nM) or a Smoothened antagonist (SANT1, 200 nM). In triplicate cultures for each cell line and culture condition, hGLI1 levels were normalized to hGAPDH and expressed relative to vehicle-treated control GS. Relative hGLI1 mRNA levels were significantly elevated by SAG treatment in GS-304-AOA. In contrast, no response was elicited in GS-406-GBM. *, p ≤ 0.05.
As the Hh pathway is activated in subsets of malignant glioma specimens (Bar et al., 2007; Clement et al., 2007; Ehtesham et al., 2007; Xu et al., 2008), we first sought to determine the operational status of the Hh pathway in these malignant glioma specimens. Gliomaspheres (GS) were generated successfully from an unsorted portion for three of the four specimens (GS-304-AOA, GS-312-HGA, and GS-406-GBM) under culture conditions that are required for eliciting a Hh pathway response in glioma primary cell cultures (Ehtesham et al., 2007). Addition of a Smoothened agonist (SAG, 50 nM) (Frank-Kamenetsky et al., 2002) to GS-304-AOA and GS-312-HGA induced a 4-fold increase in hGLI1 mRNA expression (p < 0.05, Student's T-test; Figure 1b and 1d) suggesting an operational Hh pathway in these primary tumors. In contrast, GS-406-GBM cells did not respond to SAG treatment (Figure 1f) suggesting that this tumor lacks an operational Hh pathway. Consistent with previous Hh signaling assays in GS (Ehtesham et al., 2007), basal levels of pathway activity were not reduced by treatment with a Smoothened antagonist (SANT1, 200 nM; Figure 1b, 1d and 1f) (Chen et al., 2002b).
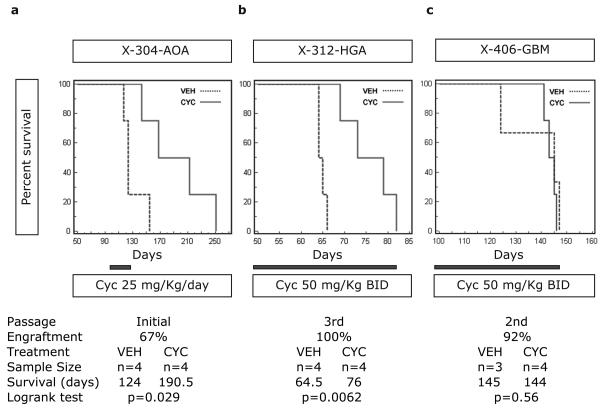
To determine the effect of in vivo Hh pathway inhibition on established tumors for which the operational status of the Hh pathway had been assayed in GS culture, X-304-AOA, X-312-HGA and X-406-GBM mice were treated with cyclopamine and assayed for survival. The survival studies were performed at initial transplantation (X-304-AOA and X-312-HGA) or first passage (X-406-GBM), depending upon adequate patient or xenograft material to generate sufficient numbers of mice. The mice were randomized to receive either cyclopamine or vehicle after the first animal of a cohort became symptomatic and was determined to have engraftment (Figure 1). Following published protocols, mice were treated by intraperitoneal injection with 25 mg/Kg/day of cyclopamine (Berman et al., 2002; Sanchez and Ruiz i Altaba, 2005). By this method, a significant survival benefit was observed only for X-304-AOA (p = 0.029, Logrank test; Figure 2a and data not shown). For the X-304-AOA survival study, treatment with either cyclopamine (25 mg/Kg/day; n=6) or vehicle (10% 2-Hydroxypropyl-ß-cyclodextrin; n=6) was initiated 90 days following transplantation, and was administered for 30 days. In the vehicle treatment group, 4 mice developed tumor and the median survival time was 124 days. In the cyclopamine treatment group 4 mice developed tumor and the median survival time was 190.5 days. The remaining mice (2 vehicle- and 2 cyclopamine-treated) were sacrificed 270 days after xenotransplantation and determined not to have engraftment. Thus, cyclopamine treatment conferred a median survival difference of 66.5 days (Figure 2a).
Figure 2. Hh pathway inhibition confers a survival advantage.
(a) 90 days after xenotransplantation, X-304-AOA mice were treated for 30 days with either cyclopamine (25 mg/Kg/day) or vehicle. The median survival for cyclopamine-treated mice was 66.5 days longer than for vehicle treated controls (p = 0.029, Logrank test). (b) Treatment was initiated in X-312-HGA mice 50 days after xenotransplantation and continued for the duration of the survival study. Cyclopamine-treated mice (50 mg/Kg twice daily) had a median survival of 11.5 days longer than vehicle-treated mice (p = 0.0062, Logrank test). (c) Continuous cyclopamine treatment of X-406-GBM mice (50 mg/Kg twice daily starting 100 days after xenotransplantation) does not enhance survival (p = 0.56, Logrank test). BID, twice daily.
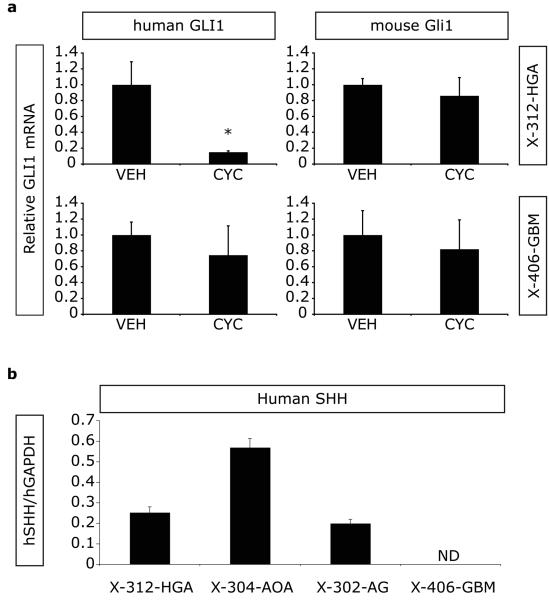
The lack of survival benefit in the X-312-HGA and X-406-GBM mice suggests that within these xenografts either an insufficient level or duration of pathway inhibition was achieved with daily dosing, or that the Hh pathway is not operational in these tumors. To test the first possibility, we determined the optimal dose and delivery schedule for cyclopamine. Because of limitations inherent to a direct orthotopic xenotransplantation model, namely the availability of sufficient CD133+ cells for passaging tumor in large numbers of animals and relatively slow growth kinetics, X-302-AG mice were used for dosage titration studies while X-312-HGA and X-406-GBM were undergoing serial passage. X-302-AG mice were injected with varying doses of cyclopamine (25-100 mg/Kg) and then sacrificed at varying times after injection (1.5-16 hours). Using species-specific primers, the expression levels of human GLI1 (hGLI1) and human GAPDH (hGAPDH) were measured in a portion of the injected hemisphere by quantitative real-time PCR (qRT-PCR). We measured suppression of hGLI1 expression in X-302-AG mice treated with cyclopamine, with maximal repression at 50 mg/Kg. Furthermore, hGLI1 inhibition was transient, with maximal inhibition measured at 5 hours after injection and hGLI1 expression levels returning to baseline by 16 hours (data not shown). We therefore determined that injecting cyclopamine at 50 mg/Kg twice a day provides more complete and durable pathway inhibition than 25 mg/Kg/day. Thus to determine the operational status of the Hh pathway in X-312-HGA and X-406-GBM, mice were treated with either cyclopamine (50 mg/Kg/twice daily) or vehicle (twice daily). After one week of treatment, relative hGLI1 expression was determined by qRTPCR in vehicle (n=3 for each tumor) and cyclopamine (n=3 for each tumor) treated animals. Inhibition of hGLI1 was observed for X-312-HGA (85%, p = 0.036, Student's T-test) indicating an operational Hh pathway that can be modulated in vivo (Figure 3a). In contrast, relative hGLI1 levels were not inhibited by cyclopamine treatment in X-406-GBM (Figure 3a). The inability to modulate hGLI1 by small molecules in either in vitro (GS-406-GBM) or in vivo (X-406-GBM) assays (Figures 1f and 3a, respectively) suggests that the Hh pathway is not operational in this GBM.
Figure 3. Cyclopamine treatment inhibits the Hh pathway within xenotransplanted glioma cells.
(a) Mice bearing malignant glioma xenografts were treated for one week with either vehicle (twice daily; n=3 for each tumor type) or cyclopamine (50 mg/Kg twice daily; n=3 for each tumor type). Using species-specific primers, human GLI1 levels were normalized to hGAPDH and mouse Gli1 (mGLI1) levels were normalized to mGAPDH. Relative hGLI1 mRNA levels were significantly decreased in X-312-HGA cyclopamine-treated mice. No significant inhibition of hGLI1 was observed in X-406-GBM cyclopamine-treated mice. Relative mGLI1 mRNA levels were not altered by cyclopamine treatment in any of the malignant glioma xenografts. (b) Human SHH (hSHH) mRNA expression was detected in 6/6 X-312-HGA, 3/3 X-304-AOA, 6/6 X-302-AG, and 0/6 X-406-GBM xenografts (normalized to hGAPDH and expressed as average ± SEM). ND, not detected; *, p ≤ 0.05.
Hh pathway activity in malignant glioma is ligand-dependant (Bar et al., 2007; Clement et al., 2007; Ehtesham et al., 2007). Therefore, we assayed for human Shh (hSHH) mRNA expression in transplanted cells. Notably, hSHH expression can be measured in X-312-HGA, X-304-AOA and X-302-AG, but not in X-406-GBM (Figure 3b).
Collectively, the in vitro (GS-312-HGA, GS-304-AOA and GS-406-GBM), in vivo (X-312-HGA, X-304-AOA, X-302-AG and X-406-GBM) and hSHH expression (X-312-HGA, X-304-AOA, X-302-AG and X-406-GBM) studies identify Hh-responsive and non-responsive tumors. Furthermore, pathway activity may be directly related to active ligand production within the Hh-responsive tumors. Thus, given the evidence supporting an operational Hh pathway in X-312-HGA, we performed another survival study using more potent and durable cyclopamine dosing. By this method, continuous treatment with cyclopamine (50 mg/Kg twice daily) conferred a median survival difference of 11.5 days (p = 0.0062; Logrank test) in X-312-HGA mice (Figure 2b). Conversely, no difference in survival was measured in X-406-GBM mice receiving continuous treatment with a maximally inhibitory dose of cyclopamine (50 mg/Kg twice daily; Figure 2c).
The survival advantage from in vivo Hh inhibition in malignant gliomas with an operational pathway (X-304-AOA and X-312-HGA) indicates that Hh signaling regulates glioma growth. Notably, the degree of enhanced survival varies among Hh-responsive xenografts and the dosage of cyclopamine. This may relate to differences in growth kinetics of the two Hh-responsive xenografts. Survival was monitored in both treatment groups until death or endpoint criteria, and all of the mice in the cyclopamine treatment groups eventually died of disease (Figure 2).
Cyclopamine mediates Hedgehog pathway inhibition in transplanted human glioma cells and not in host neural parenchyma
Recent studies pertaining to epithelial-derived malignancies demonstrate that tumor-derived Shh ligand supports growth by generation of host stromal tissue (Yauch et al., 2008). Using species-specific primers, we find that cyclopamine treatment modulates hGLI1 and not mGli1 expression (Figure 3a). These results suggest that survival benefits observed in cyclopamine-treated X-304-AOA and X-312-HGA mice are not related to Hh pathway inhibition within host neural parenchyma, but rather in transplanted glioma cells.
PTCH and GLI1 expression segregate to CD133+ cells in malignant glioma xenografts
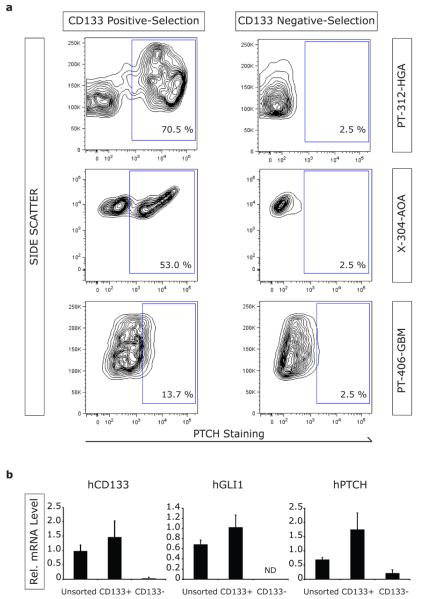
Within malignant gliomas, expression of the Hh receptor Patched (PTCH) has been detected in cells with stem or progenitor cell features (Ehtesham et al., 2007). To gain a better understanding of the glioma cellular compartments in which the Hh pathway might be operational, we evaluated PTCH expression by flow cytometry in the CD133 positive- and CD133 negative-selection cell populations from freshly resected tumors following immunomagnetic sorting. PTCH+ cells were highly enriched in the CD133 positive-selection fraction and depleted from the CD133 negative-selection fraction from PT-312-HGA, suggesting confinement of pathway responsiveness to CD133+ cells (Figure 4a). Likewise, PTCH expression segregated with CD133+ cell selection of X-304-AOA (Figure 4a). In contrast, there was no significant detection or segregation of PTCH expression in CD133-selected cell populations from PT-406-GBM (Figure 4a), consistent with the lack of in vitro or in vivo response to pathway modulation in this tumor.
Figure 4. PTCH expression segregates with CD133+ cell selection within malignant glioma.
(a) Expression of the Hh receptor PTCH was analyzed by flow cytometry in CD133 positive- and CD133 negative-selection cell populations. Analysis of PT-312-HGA revealed enrichment of PTCH+ cells with CD133 positive-selection, and their absence in the CD133 negative-selection cell populations. Similar results were obtained with analysis of xenotransplanted tissue from X-304-AOA. In contrast, PTCH expression did not segregate with CD133 positive- or negative-selection of the Hh non-responsive PT-406-GBM specimen. (b) Unsorted, CD133-enriched and CD133-depleted cells from X-302-AG were analyzed by qRT-PCR for expression of hCD133, hGLI1, hPTCH and hGAPDH. The relative expression levels of hCD133, hGLI1 and hPTCH were significantly elevated by CD133 positive selection. ND, not detected.
To corroborate and expand these findings, mRNA expression levels of the Hh pathway components and gene targets hPTCH and hGLI1 were measured in CD133 positive- and CD133 negative-selection cell populations following immunomagnetic sorting of X-302-AG. Compared to unsorted cells, elevated hGLI1 and hPTCH mRNA levels were detected in the CD133-enriched samples, and either not detected (hGLI1) or reduced (hPTCH) in the CD133-depleted samples (Figure 4b). Taken together, these data suggest pathway inhibition is mediated in CD133+ cells and that CD133− cells are not directly affected by cyclopamine treatment.
Discussion
These studies demonstrate that Hh pathway inhibition in established malignant glioma xenografts confers a significant survival advantage. Notably, survival studies performed in three xenotransplanted tumors reveal that the benefit of cyclopamine treatment is not uniform within the spectrum of malignant glioma. One key difference is the presence of an operational Hh pathway. Hh pathway activation can only be demonstrated in a subset of malignant gliomas in broad surveys of patient samples (Bar et al., 2007; Clement et al., 2007; Ehtesham et al., 2007; Xu et al., 2008). This trend was observed in our studies of more limited sample size, by the identification of Hh-responsive and non-responsive xenografts. Furthermore, and of importance for preclinical studies, the ability to modulate the Hh pathway in vitro has predictive value for in vivo modulation, as demonstrated by studies with GS-312-HGA and X-312-HGA. In support of this concept, hGLI1 levels could not be induced or suppressed in GS-406-GBM or X-406-GBM, respectively.
Another example of nonuniformity in cyclopamine response was observed between the two Hh-responsive xenografts. A longer survival benefit was measured for X-304-AOA mice with a lower dose and duration of cyclopamine treatment than for X-312-HGA. Many factors may have influenced this difference including prior treatment in the patient, tumor engraftment rate and growth kinetics, or cell selection pressures of tumor passage. With regard to tumor growth kinetics, in this study cyclopamine treatment was initiated after the first animal of a cohort developed symptoms. Thus for the more slow-growing X-304-AOA, treatment was initiated earlier in the course of disease (day 90 with a median survival of 124 days in control animals). In contrast, X-312-HGA displayed faster growth kinetics as all mice bearing the initial xenotransplant died or met criteria for sacrifice within a span of 7 days following initiation of treatment with cyclopamine at 25 mg/Kg/day. These observations suggest a greater survival benefit may be obtained with initiation of pathway inhibition earlier in the course of tumor growth. Future studies to address these differences in cyclopamine response would benefit from the use of small-animal imaging to establish tumor engraftment and thus enable earlier treatment initiation.
Prior studies pertaining to Hh signaling and ligand-independent growth of medulloblastoma have utilized systemic doses of cyclopamine as low as 10 mg/Kg every other day to achieve meaningful pathway inhibition in the cerebellum (Sanchez and Ruiz i Altaba, 2005). Our results indicate that much higher doses of cyclopamine are required for inhibiting the growth of gliomas with ligand-dependent Hh pathway activation. This difference in sensitivity is consistent with the hypothesis of “oncogene-addiction” (Weinstein and Joe, 2008), such that lower doses of cyclopamine are sufficient for medulloblastoma in which tumor initiation and growth are associated with constitutive activation of the Hh pathway by loss of PTCH function {Berman, 2002 #34}.
With regard to Hh-dependant tumor growth, signaling within either tumor cells or host tissue has been demonstrated, depending upon the tumor type (Berman et al., 2002; Yauch et al., 2008). Our studies indicate that in the case of malignant glioma, cyclopamine inhibits the Hh pathway in xenotransplanted cells and not in host cerebrum. In this context, our observation that a survival advantage can be demonstrated only in mice xenotransplanted with Hh-responsive gliomas argues strongly that this benefit is a result of cyclopamine-mediated Hh pathway inhibition and not due to off-target effects.
We have previously demonstrated a requirement for the addition of exogenous Hh ligand to induce a pathway response in primary GS cultures (Ehtesham et al., 2007). Furthermore, the Hh pathway in GS appears to be at a basal level as the addition of SANT1 does not reduce relative hGLI1 levels. Thus the finding from our in vivo model that transplanted cells generate hSHH transcript, indicates that Shh production is not maintained under these GS culture conditions. Notably, hSHH transcript is detected only in Hh-responsive xenografts and suggests that tumor cells may be a source of ligand. Whether this underlies autocrine or paracrine signaling among glioma cells has not been addressed in these studies.
Within solid and hematologic cancers, the frequency and phenotypes of tumorigenic cells in transplantation models remain to be fully characterized (Kelly et al., 2007; Quintana et al., 2008). However, to date CD133 is the best characterized surface marker for prospective isolation of tumor-initiating cells from malignant glioma. Despite several caveats, the concept of tumor-initiating cells provides a framework for conceptualizing the cellular heterogeneity of tumors, with a hierarchical arrangement of multipotent tumor cells giving rise to transient amplifying progenitor and ultimately postmitotic cells (Clarke et al., 2006; Barker et al., 2009; Zhu et al., 2009). The segregation of Hh pathway components and gene targets, PTCH and GLI1, to CD133+ cells suggests that CD133− cell behavior is not subject to Hh pathway regulation. Thus, CD133− transient amplifying cells (Ligon et al., 2007) might contribute to tumor growth in spite of cyclopamine treatment. Additionally, the CD133− compartment may contain other Hh-unresponsive tumor-initiating cells, as CD133− cells isolated directly from malignant gliomas have been shown to engraft in nude rats (Ogden et al., 2008). Therefore, greater tumor regression might be achieved by combining Hh pathway inhibition with therapies directed at other glioma cell types such as CD133− transient amplifying cells or other tumor-propagating cells.
Common approaches for modeling human cancer in mice include germ-line genetic modification or xenotranplantation (Suggitt and Bibby, 2005; Fomchenko and Holland, 2006; Gutmann et al., 2006; Shu et al., 2008). In these studies, a direct orthotopic glioma xenotransplantation model was utilized to reduce the potential for cell transformation in culture (Lee et al., 2006). Inherent limitations of this model include availability of adequate patient material and initial engraftment rate, the number of CD133+ cells available for initial transplantation and subsequent passage and relatively slow growth kinetics. Importantly though, it has afforded the opportunity to: (i) define the presence or absence of an operational Hh pathway in an array of malignant glioma specimens; (ii) determine the optimal inhibitory dose and delivery schedule for cyclopamine; (iii) identify the glioma cell type in which Hh signaling occurs; and (iv) demonstrate enhanced survival by pharmacological inhibition of Hh signaling. These findings indicate that Hh signaling regulates the growth of select malignant gliomas, and the utility of targeting this pathway in a preclinical model.
Methods
Tissue procurement
Excess brain tumor specimens were obtained for research purposes from patients in accordance with a protocol approved by Vanderbilt Medical Center Institutional Review Board. Primary brain tumors were phenotyped and graded as described (Kleihues et al., 1995; Kleihues et al., 2002).
Immunohistochemical analysis of tumors
Immunodetection was performed for Ki67 (1:50; Dako) on paraffin-embedded sections and for anti-human nuclear epitope (1:50; Chemicon) on frozen sections as described (Ehtesham et al., 2007).
CD133 immunomagnetic cell selection
Tumor samples were minced mechanically, dissociated with papain (Worthington Biochemical Corporation), and passed through a 40 μm filter. Cells were labeled with a CD133/1 (15 ug/ml; Miltenyi Biotec) antibody crosslinked to magnetic nanoparticles and subjected to immunomagnetic cell separation using the EasySep Magnetic Selection Kit (Stem Cell Technologies). Purity of the CD133-enriched population was assessed by flow cytometry after labeling enriched cells with CD133/2-APC (Miltenyi Biotec) antibody. CD133-enriched and CD133-depleted populations were then incubated with PTCH antibody (1:200; Santa Cruz Biotechnologies, G19 Lot # J2505), followed by AlexaFluor-488 secondary antibody labeling (1:1000; Invitrogen) and analyzed by flow cytometry. Isotype-matched controls were included in the analysis to determine background signal levels. Gates thresholds were set relative to the CD133 negative-selection samples. A portion of the CD133-enriched and CD133-depleted fractions were also used for gene expression measurements using quantitative qRT-PCR.
Orthotopic xenotransplantation
104-105 CD133-enriched cells were transplanted into the striatum of NOD/SCID mice according to a protocol approved by the Vanderbilt Medical Center Institutional Animal Care and Use Committee. Mice were anesthetized with ketamine and xylazine, and securely placed on a stereotactic frame. Using aseptic surgical procedures, an incision was made in the scalp and a small burr-hole was drilled 2.5 mm lateral to the bregma. CD133-enriched cells were implanted 2.5 mm into the right striatum using a Hamilton syringe. Mice were maintained until development of neurological symptoms or signs of distress (piloerection and/or hunched posture).
Gliomasphere cell culture and Hh signaling assays
Tumor samples were dissociated (Papain, Worthington Biochemical Corporation). Cells were plated in non-treated polystyrene flasks (BD-Falcon) in NeuroCult medium with supplements (NeuroCult® NS-A Proliferation Kit; Stem Cell Technologies), 2 μg/mL heparin (Sigma), 20 ng/mL EGF (ProSpec TechnoGene), 10 ng/mL bFGF (ProSpec TechnoGene), and 1X Penicillin-Streptomycin (Invitrogen). GS were transferred to multiwell plates and cultured in triplicate for 40 hours either alone, with 50 nM SAG, or 200nM SANT1. GLI1 and GAPDH levels were measured by qRT-PCR as described below.
RNA extraction, cDNA synthesis, and qRT-PCR
Total RNA was extracted from brain tissue and GS cells with the PureLink™ RNA Mini Kit (Invitrogen). Genomic DNA was removed (RNase-Free DNase Set; QIAGEN) and purified RNA quantified (RiboGreen RNA Quantitation Kit; Invitrogen). Single-stranded cDNA was synthesized with oligo(dT) and random hexamer primers (iScript cDNA Synthesis Kit; Bio-Rad). For negative controls, reverse transcriptase was omitted from the synthesis reaction (-RT). qRT-PCR was performed in triplicate for each sample and on the corresponding -RT control with TaqMan® Fast Universal PCR Master Mix (Applied Biosystems; ABI), cDNA template, and ABI's TaqMan® Gene Expression Assay for hPTCH (Hs00970979_m1), hGLI1 (Hs00171790_m1), hGAPDH (Hs99999905_m1), hCD133 (Hs01009261_m1), hSHH (Hs00179843_m1), mGli1 (Mm00494654_m1), or mGAPDH (Mm99999915_g1). For standard curves, qRT-PCR was performed on serial dilutions of a cDNA mixture (Ehtesham et al., 2007). For each amplicon, quantities were determined according to the standard curve method (User Bulletin #2, PE Applied Biosystems).
Supplementary Material
(a and b) Tumor engraftment is revealed in serial sections by H&E and anti-human nuclear (hNu) staining. (c and d) High magnification H&E images of the xenograft and patient tumor samples show similar cell density and morphology (scale bar = 50 μm).
Acknowledgments
We are grateful to Infinity Pharmaceuticals for supplying cyclopamine. Histological services were performed in part by the Vanderbilt Medical Center (VMC) Human Tissue Acquisition and Pathology Shared Resource (supported by the Vanderbilt Ingram Cancer Center, P30 CA68485). Flow cytometry experiments were performed in the VMC Flow Cytometry Shared Resource (supported by the Vanderbilt Ingram Cancer Center, P30 CA68485, and the Vanderbilt Digestive Disease Research Center, DK058404). This work was supported by grants to M.K.C. from the NINDS (K02 NS053614), the Burroughs Wellcome Fund and the Doris Duke Charitable Foundation (for human tissues) and VMC development funds (for animal studies).
Footnotes
Disclosure/Conflict of Interest The authors declare no conflict of interest.
References
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–7. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–33. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, Chen JK, Cooper MK, Taipale J, Olson JM, Beachy PA. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–61. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002a;16:2743–8. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci U S A. 2002b;99:14071–6. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, Thompson RC, Cooper MK. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–61. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–97. doi: 10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, Rubin LL, Porter JA. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J Biol. 2002;1:10. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH, Maher EA, Van Dyke T. Mouse Models of Human Cancers Consortium Workshop on Nervous System Tumors. Cancer Res. 2006;66:10–3. doi: 10.1158/0008-5472.CAN-05-3180. [DOI] [PubMed] [Google Scholar]
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–25. doi: 10.1093/jnen/61.3.215. discussion 226-9. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Soylemezoglu F, Schauble B, Scheithauer BW, Burger PC. Histopathology, classification, and grading of gliomas. Glia. 1995;15:211–21. doi: 10.1002/glia.440150303. [DOI] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, Bachoo RM, Kane M, Louis DN, Depinho RA, Anderson DJ, Stiles CD, Rowitch DH. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–17. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden AT, Waziri AE, Lochhead RA, Fusco D, Lopez K, Ellis JA, Kang J, Assanah M, McKhann GM, Sisti MB, McCormick PC, Canoll P, Bruce JN. Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery. 2008;62:505–14. doi: 10.1227/01.neu.0000316019.28421.95. discussion 514-5. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–5. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Scott MP. Patching the gaps in Hedgehog signalling. Nat Cell Biol. 2007;9:1005–9. doi: 10.1038/ncb435. [DOI] [PubMed] [Google Scholar]
- Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–33. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Ruiz i Altaba A. In vivo inhibition of endogenous brain tumors through systemic interference of Hedgehog signaling in mice. Mech Dev. 2005;122:223–30. doi: 10.1016/j.mod.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Shu Q, Wong KK, Su JM, Adesina AM, Yu LT, Tsang YT, Antalffy BC, Baxter P, Perlaky L, Yang J, Dauser RC, Chintagumpala M, Blaney SM, Lau CC, Li XN. Direct orthotopic transplantation of fresh surgical specimen preserves CD133+ tumor cells in clinically relevant mouse models of medulloblastoma and glioma. Stem Cells. 2008;26:1414–24. doi: 10.1634/stemcells.2007-1009. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Suggitt M, Bibby MC. 50 years of preclinical anticancer drug screening: empirical to target-driven approaches. Clin Cancer Res. 2005;11:971–81. [PubMed] [Google Scholar]
- Trowbridge JJ, Scott MP, Bhatia M. Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc Natl Acad Sci U S A. 2006;103:14134–9. doi: 10.1073/pnas.0604568103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 2000;97:14720–5. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–80. doi: 10.1158/0008-5472.CAN-07-3293. discussion 3080. [DOI] [PubMed] [Google Scholar]
- Xu Q, Yuan X, Liu G, Black KL, Yu JS. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells. 2008;26:3018–26. doi: 10.1634/stemcells.2008-0459. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–92. [PubMed] [Google Scholar]
- Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–7. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a and b) Tumor engraftment is revealed in serial sections by H&E and anti-human nuclear (hNu) staining. (c and d) High magnification H&E images of the xenograft and patient tumor samples show similar cell density and morphology (scale bar = 50 μm).