Abstract
Previous studies have demonstrated that the Dictyostelium G alpha subunit G alpha 2 is essential for the cAMP-activation of adenylyl cyclase and guanylyl cyclase and that g alpha 2 null mutants do not aggregate. In this manuscript, we extend the analysis of the function of G alpha 2 in regulating downstream effectors by examining the in vivo developmental and physiological phenotypes of both wild-type and g alpha 2 null cells carrying a series of mutant G alpha 2 subunits expressed from the cloned G alpha 2 promoter. Our results show that wild-type cells expressing G alpha 2 subunits carrying mutations G40V and Q208L in the highly conserved GAGESG (residues 38-43) and GGQRS (residues 206-210) domains, which are expected to reduce the intrinsic GTPase activity, are blocked in multicellular development. Analysis of down-stream effector pathways essential for mediating aggregation indicates that cAMP-mediated activation of guanylyl cyclase and phosphatidylinositol-phospholipase C (PI-PLC) is almost completely inhibited and that there is a substantial reduction of cAMP-mediated activation of adenylyl cyclase. Moreover, neither mutant G alpha 2 subunit can complement g alpha 2 null mutants. Expression of G alpha 2(G43V) and G alpha 2(G207V) have little or no effect on the effector pathways and can partially complement g alpha 2 null cells. Our results suggest a model in which the dominant negative phenotypes resulting from the expression of G alpha 2(G40V) and G alpha 2(Q208L) are due to a constitutive adaptation of the effectors through a G alpha 2-mediated pathway. Analysis of PI-PLC in g alpha 2 null mutants and in cell lines expressing mutant G alpha 2 proteins also strongly suggests that G alpha 2 is the G alpha subunit that directly activates PI-PLC during aggregation. Moreover, overexpression of wild-type G alpha 2 results in the ability to precociously activate guanylyl cyclase by cAMP in vegetative cells, suggesting that G alpha 2 may be rate limiting in the developmental regulation of guanylyl cyclase activation. In agreement with previous results, the activation of adenylyl cyclase, while requiring G alpha 2 function in vivo, does not appear to be directly carried out by the G alpha 2 subunit. Our data are consistent with adenylyl cyclase being directly activated by either another G alpha subunit or by beta gamma subunits released on activation of the G protein containing G alpha 2.
Full text
PDF


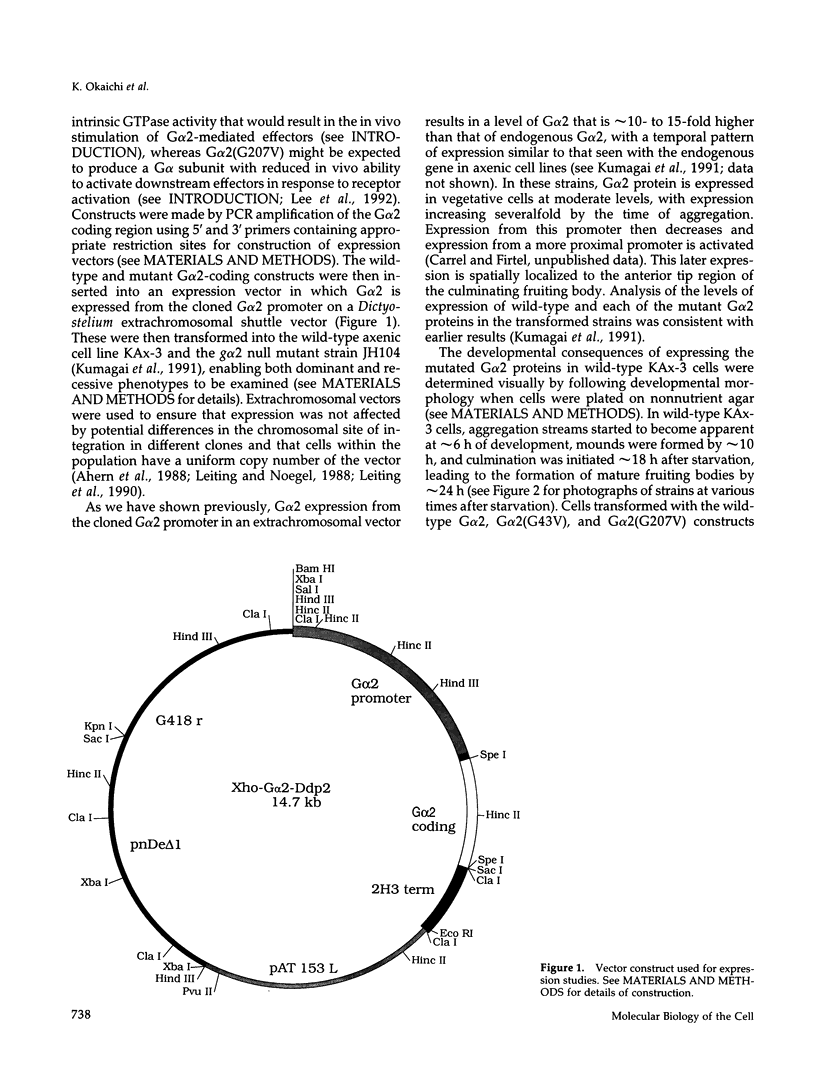

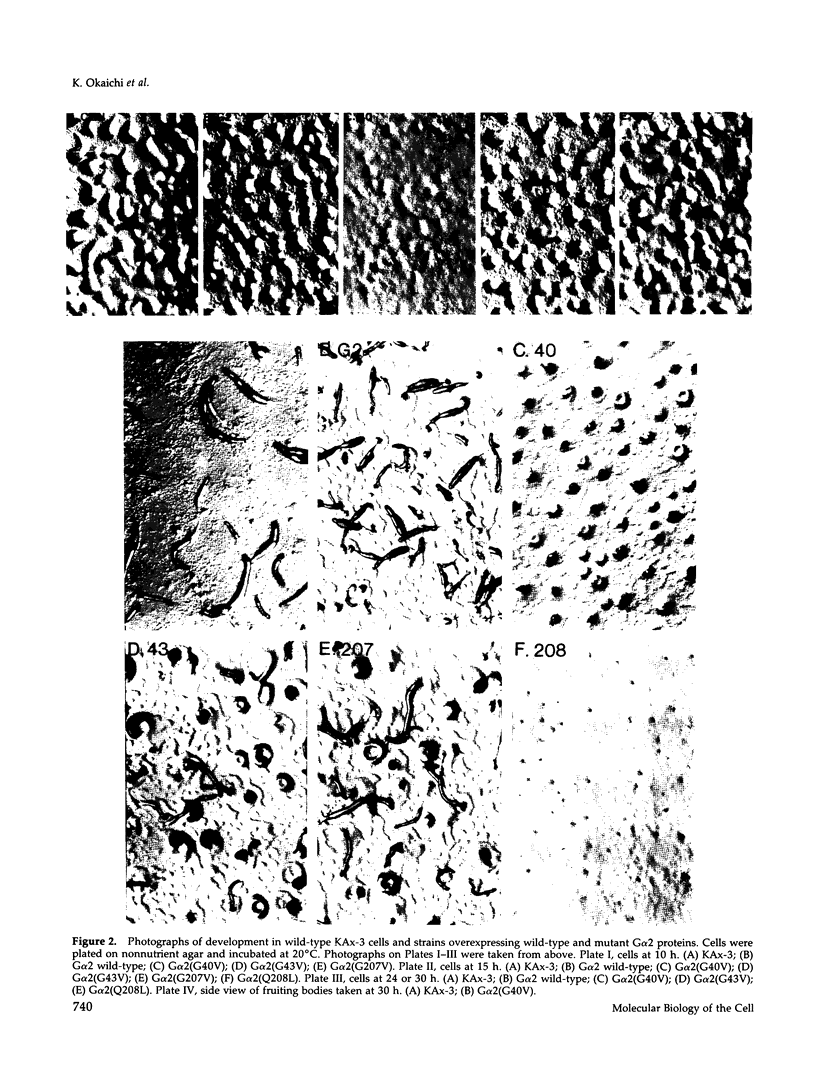







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern K. G., Howard P. K., Firtel R. A. Identification of regions essential for extrachromosomal replication and maintenance of an endogenous plasmid in Dictyostelium. Nucleic Acids Res. 1988 Jul 25;16(14B):6825–6837. doi: 10.1093/nar/16.14.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D., Comer J. F., Higinbotham K. G. A Ca2+-dependent signal transduction system participates in coupling expression of some cAMP-dependent prespore genes to the cell surface receptor. Dev Genet. 1988;9(4-5):359–369. doi: 10.1002/dvg.1020090417. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- Devreotes P. Dictyostelium discoideum: a model system for cell-cell interactions in development. Science. 1989 Sep 8;245(4922):1054–1058. doi: 10.1126/science.2672337. [DOI] [PubMed] [Google Scholar]
- Devreotes P., Fontana D., Klein P., Sherring J., Theibert A. Transmembrane signaling in Dictyostelium. Methods Cell Biol. 1987;28:299–331. doi: 10.1016/s0091-679x(08)61653-2. [DOI] [PubMed] [Google Scholar]
- Dinauer M. C., Steck T. L., Devreotes P. N. Cyclic 3',5'-AMP relay in Dictyostelium discoideum V. Adaptation of the cAMP signaling response during cAMP stimulation. J Cell Biol. 1980 Aug;86(2):554–561. doi: 10.1083/jcb.86.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Europe-Finner G. N., Ludérus M. E., Small N. V., Van Driel R., Reymond C. D., Firtel R. A., Newell P. C. Mutant ras gene induces elevated levels of inositol tris- and hexakisphosphates in Dictyostelium. J Cell Sci. 1988 Jan;89(Pt 1):13–20. doi: 10.1242/jcs.89.1.13. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Cyclic AMP stimulates accumulation of inositol trisphosphate in Dictyostelium. J Cell Sci. 1987 Mar;87(Pt 2):221–229. doi: 10.1242/jcs.87.2.221. [DOI] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. GTP analogues stimulate inositol trisphosphate formation transiently in Dictyostelium. J Cell Sci. 1987 May;87(Pt 4):513–518. doi: 10.1242/jcs.87.4.513. [DOI] [PubMed] [Google Scholar]
- Federman A. D., Conklin B. R., Schrader K. A., Reed R. R., Bourne H. R. Hormonal stimulation of adenylyl cyclase through Gi-protein beta gamma subunits. Nature. 1992 Mar 12;356(6365):159–161. doi: 10.1038/356159a0. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Chapman A. L. A role for cAMP-dependent protein kinase A in early Dictyostelium development. Genes Dev. 1990 Jan;4(1):18–28. doi: 10.1101/gad.4.1.18. [DOI] [PubMed] [Google Scholar]
- Firtel R. A. Signal transduction pathways controlling multicellular development in Dictyostelium. Trends Genet. 1991 Nov-Dec;7(11-12):381–388. doi: 10.1016/0168-9525(91)90260-w. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., van Haastert P. J., Kimmel A. R., Devreotes P. N. G protein linked signal transduction pathways in development: dictyostelium as an experimental system. Cell. 1989 Jul 28;58(2):235–239. doi: 10.1016/0092-8674(89)90837-4. [DOI] [PubMed] [Google Scholar]
- Gerisch G. Cyclic AMP and other signals controlling cell development and differentiation in Dictyostelium. Annu Rev Biochem. 1987;56:853–879. doi: 10.1146/annurev.bi.56.070187.004225. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Ginsburg G., Kimmel A. R. Inositol trisphosphate and diacylglycerol can differentially modulate gene expression in Dictyostelium. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9332–9336. doi: 10.1073/pnas.86.23.9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M. P., Gilman A. G. Synthesis in Escherichia coli of GTPase-deficient mutants of Gs alpha. J Biol Chem. 1989 Sep 15;264(26):15475–15482. [PubMed] [Google Scholar]
- Gundersen R. E., Devreotes P. N. In vivo receptor-mediated phosphorylation of a G protein in Dictyostelium. Science. 1990 May 4;248(4955):591–593. doi: 10.1126/science.2110382. [DOI] [PubMed] [Google Scholar]
- Haberstroh L., Firtel R. A. A spatial gradient of expression of a cAMP-regulated prespore cell-type-specific gene in Dictyostelium. Genes Dev. 1990 Apr;4(4):596–612. doi: 10.1101/gad.4.4.596. [DOI] [PubMed] [Google Scholar]
- Howard P. K., Ahern K. G., Firtel R. A. Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res. 1988 Mar 25;16(6):2613–2623. doi: 10.1093/nar/16.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens P. M., Van Haastert P. J. Molecular basis of transmembrane signal transduction in Dictyostelium discoideum. Microbiol Rev. 1987 Dec;51(4):396–418. doi: 10.1128/mr.51.4.396-418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. L., Dhanasekaran N. The G-protein family and their interaction with receptors. Endocr Rev. 1989 Aug;10(3):317–331. doi: 10.1210/edrv-10-3-317. [DOI] [PubMed] [Google Scholar]
- Kesbeke F., Snaar-Jagalska B. E., Van Haastert P. J. Signal transduction in Dictyostelium fgd A mutants with a defective interaction between surface cAMP receptors and a GTP-binding regulatory protein. J Cell Biol. 1988 Aug;107(2):521–528. doi: 10.1083/jcb.107.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R. Different molecular mechanisms for cAMP regulation of gene expression during Dictyostelium development. Dev Biol. 1987 Jul;122(1):163–171. doi: 10.1016/0012-1606(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. cAMP signal transduction pathways regulating development of Dictyostelium discoideum. Curr Opin Genet Dev. 1991 Oct;1(3):383–390. doi: 10.1016/s0959-437x(05)80304-1. [DOI] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., Devreotes P. N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988 Sep 16;241(4872):1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Hadwiger J. A., Pupillo M., Firtel R. A. Molecular genetic analysis of two G alpha protein subunits in Dictyostelium. J Biol Chem. 1991 Jan 15;266(2):1220–1228. [PubMed] [Google Scholar]
- Kumagai A., Pupillo M., Gundersen R., Miake-Lye R., Devreotes P. N., Firtel R. A. Regulation and function of G alpha protein subunits in Dictyostelium. Cell. 1989 Apr 21;57(2):265–275. doi: 10.1016/0092-8674(89)90964-1. [DOI] [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Lee E., Taussig R., Gilman A. G. The G226A mutant of Gs alpha highlights the requirement for dissociation of G protein subunits. J Biol Chem. 1992 Jan 15;267(2):1212–1218. [PubMed] [Google Scholar]
- Leiting B., Lindner I. J., Noegel A. A. The extrachromosomal replication of Dictyostelium plasmid Ddp2 requires a cis-acting element and a plasmid-encoded trans-acting factor. Mol Cell Biol. 1990 Jul;10(7):3727–3736. doi: 10.1128/mcb.10.7.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiting B., Noegel A. Construction of an extrachromosomally replicating transformation vector for Dictyostelium discoideum. Plasmid. 1988 Nov;20(3):241–248. doi: 10.1016/0147-619x(88)90030-3. [DOI] [PubMed] [Google Scholar]
- Lochrie M. A., Simon M. I. G protein multiplicity in eukaryotic signal transduction systems. Biochemistry. 1988 Jul 12;27(14):4957–4965. doi: 10.1021/bi00414a001. [DOI] [PubMed] [Google Scholar]
- Mann S. K., Firtel R. A. Cyclic AMP regulation of early gene expression in Dictyostelium discoideum: mediation via the cell surface cyclic AMP receptor. Mol Cell Biol. 1987 Jan;7(1):458–469. doi: 10.1128/mcb.7.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. K., Firtel R. A. Two-phase regulatory pathway controls cAMP receptor-mediated expression of early genes in Dictyostelium. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1924–1928. doi: 10.1073/pnas.86.6.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S. K., Pinko C., Firtel R. A. cAMP regulation of early gene expression in signal transduction mutants of Dictyostelium. Dev Biol. 1988 Nov;130(1):294–303. doi: 10.1016/0012-1606(88)90435-6. [DOI] [PubMed] [Google Scholar]
- Masters S. B., Miller R. T., Chi M. H., Chang F. H., Beiderman B., Lopez N. G., Bourne H. R. Mutations in the GTP-binding site of GS alpha alter stimulation of adenylyl cyclase. J Biol Chem. 1989 Sep 15;264(26):15467–15474. [PubMed] [Google Scholar]
- Milne J. L., Coukell M. B. A Ca2+ transport system associated with the plasma membrane of Dictyostelium discoideum is activated by different chemoattractant receptors. J Cell Biol. 1991 Jan;112(1):103–110. doi: 10.1083/jcb.112.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupillo M., Kumagai A., Pitt G. S., Firtel R. A., Devreotes P. N. Multiple alpha subunits of guanine nucleotide-binding proteins in Dictyostelium. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4892–4896. doi: 10.1073/pnas.86.13.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Small N. V., Europe-Finner G. N., Newell P. C. Adaptation to chemotactic cyclic AMP signals in Dictyostelium involves the G-protein. J Cell Sci. 1987 Nov;88(Pt 4):537–545. doi: 10.1242/jcs.88.4.537. [DOI] [PubMed] [Google Scholar]
- Sun T. J., Devreotes P. N. Gene targeting of the aggregation stage cAMP receptor cAR1 in Dictyostelium. Genes Dev. 1991 Apr;5(4):572–582. doi: 10.1101/gad.5.4.572. [DOI] [PubMed] [Google Scholar]
- Sun T. J., Van Haastert P. J., Devreotes P. N. Surface cAMP receptors mediate multiple responses during development in Dictyostelium: evidenced by antisense mutagenesis. J Cell Biol. 1990 May;110(5):1549–1554. doi: 10.1083/jcb.110.5.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science. 1991 Dec 6;254(5037):1500–1503. doi: 10.1126/science.1962211. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Krupinski J., Gilman A. G. Expression and characterization of calmodulin-activated (type I) adenylylcyclase. J Biol Chem. 1991 May 5;266(13):8595–8603. [PubMed] [Google Scholar]
- Van Haastert P. J. Binding of cAMP and adenosine derivatives to Dictyostelium discoideum cells. Relationships of binding, chemotactic, and antagonistic activities. J Biol Chem. 1983 Aug 25;258(16):9643–9648. [PubMed] [Google Scholar]
- Van Haastert P. J. Determination of inositol 1,4,5-trisphosphate levels in Dictyostelium by isotope dilution assay. Anal Biochem. 1989 Feb 15;177(1):115–119. doi: 10.1016/0003-2697(89)90024-9. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J. Differential effects of temperature on cAMP-induced excitation, adaptation, and deadaptation of adenylate and guanylate cyclase in Dictyostelium discoideum. J Cell Biol. 1987 Nov;105(5):2301–2306. doi: 10.1083/jcb.105.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J. Sensory adaptation of Dictyostelium discoideum cells to chemotactic signals. J Cell Biol. 1983 Jun;96(6):1559–1565. doi: 10.1083/jcb.96.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan R. A., Devreotes P. N. Ligand-induced phosphorylation of the cAMP receptor from Dictyostelium discoideum. J Biol Chem. 1988 Oct 5;263(28):14538–14543. [PubMed] [Google Scholar]
- Woon C. W., Heasley L., Osawa S., Johnson G. L. Mutation of glycine 49 to valine in the alpha subunit of GS results in the constitutive elevation of cyclic AMP synthesis. Biochemistry. 1989 May 30;28(11):4547–4551. doi: 10.1021/bi00437a006. [DOI] [PubMed] [Google Scholar]
- Wu L. J., Devreotes P. N. Dictyostelium transiently expresses eight distinct G-protein alpha-subunits during its developmental program. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1141–1147. doi: 10.1016/0006-291x(91)91690-e. [DOI] [PubMed] [Google Scholar]




