Abstract
Metal oxide nanoparticles are often used as industrial catalysts and elevated levels of these particles have been clearly demonstrated at sites surrounding factories. To date, limited toxicity data on metal oxide nanoparticles are available. To understand the impact of these airborne pollutants on the respiratory system, airway epithelial (HEp-2) cells were exposed to increasing doses of silicon oxide (SiO2), ferric oxide (Fe2O3) and copper oxide (CuO) nanoparticles, the leading metal oxides found in ambient air surrounding factories. CuO induced the greatest amount of cytotoxicity in a dose dependent manner; while even high doses (400 µg/cm2) of SiO2 and Fe2O3 were non-toxic to HEp-2 cells. Although all metal oxide nanoparticles were able to generate ROS in HEp-2 cells, CuO was better able to overwhelm antioxidant defenses (e.g. catalase and glutathione reductase). A significant increase in the level of 8-isoprostanes and in the ratio of GSSG to total glutathione in cells exposed to CuO suggested that ROS generated by CuO induced oxidative stress in HEp-2 cells. Co-treatment of cells with CuO and the antioxidant resveratrol increased cell viability suggesting that oxidative stress may be the cause of the cytotoxic effect of CuO. These studies demonstrated that there is a high degree of variability in the cytotoxic effects of metal oxides, that this variability is not due to the solubility of the transition metal, and that this variability appears to involve sustained oxidative stress possibly due to redox cycling.
Keywords: Nanoparticles, HEp-2 cells, CuO, Oxidative stress, Resveratrol
Introduction
The massive increase in manufacturing and utilization of metal oxide nanoparticles has lead to major concerns regarding the potential health impact of these particles on the pulmonary system. Since these particles have a small aerodynamic diameter (<0.1 µm), they can escape air filters, contaminate ambient air, penetrate deep into the lungs, reach the alveolar region and evoke adverse pulmonary effects (Oberdorster et al., 2005).
Many epidemiological studies have demonstrated a correlation between the level of nanoparticles (PM0.1) in ambient air and a significant increase in pulmonary disease including exacerbation of bronchial asthma (Penttinen et al., 2001; Weichenthal et al., 2007). Since the chemical composition of airborne PM0.1 varies significantly according to the location and time of sample collection, the exact particle responsible for adverse pulmonary effects has remained elusive. Experimental studies have supported the epidemiological findings and have provided evidence suggesting a role for oxidative stress in these events (Yang et al., 2009). Oxidative stress generated in cells exposed to nanoparticles may stimulate inflammatory responses, oxidize lipids or even lead to cell death. Chemical analysis of different populations of PM0.1 has demonstrated the elevated presence of metal oxide nanoparticles at sites surrounding factories as compared to remote (i.e. “cleaner”) areas (Rogaczewska and Matczak, 1985). Despite the increase in the levels of these particles in ambient air, epidemiological studies rarely focus on the health impact associated with the exposure to these specific particles.
At present, metal oxide nanoparticles are used in manufacturing of hundreds of commercial products, and their industrial applications are expected to expand during the next decade. Silica which is composed of SiO2, is one of the most abundant oxides present in ambient air and comprises (up to 8%) of all total airborne nanoparticles (Balduzzi et al., 2004), typically in crystalline (quartz) or amorphous form. The amorphous form of silica is widely used in many industries and applications such as fillers in the rubber industry, anti-caking agents in powder materials such as paints and cosmetics (Merget et al., 2002). Copper oxide (CuO) nanoparticles are used in antimicrobial preparations, heat transfer fluids, semiconductors or intrauterine contraceptive devices (Aruoja et al., 2009). Ferric oxide (Fe2O3) nanoparticles are used as catalysts and in the manufacture of pigments (Montes-Hernandez et al., 2006).
Oxidative stress is often used to explain toxicity associated with particle exposure. Although the ability of crystalline silica to generate oxidative stress in pulmonary cells has been demonstrated (Fanizza et al., 2007), little is known about the ability of amorphous silica nanoparticles to induce oxidative stress. Amorphous silica nanoparticles demonstrate less ability to induce pulmonary inflammation (Warheit et al., 1995) and fibrosis (Reuzel et al., 1991) as compared to quartz particles of the same size. Furthermore, silicosis is associated with the exposure to quartz but not amorphous silica particles suggesting that these particles may have different toxicity profiles (Reuzel et al., 1991).
Both copper and iron ions are able to generate oxidative stress (Moriwaki et al., 2008). Although, oral administration of copper oxide nanoparticles induces hepatotoxicty and nephrotxicity in exposed rats (Lei et al., 2008), it is not known whether this toxicity is mediated by the generation of oxidative stress in the liver and the kidney tissues. Data demonstrating the toxic effect of ferric oxide nanoparticles remain controversial. While exposure to ferric oxide nanoparticles does not produce inflammation in vascular endothelial cells in vitro (Gojova et al., 2007), it significantly decreases cell viability in cancer cells (Choi et al., 2009). Although inhalation is the primary source of exposure to metal oxides in ambient air, data demonstrating the effect of metal oxide nanoparticles on the pulmonary system remain scarce. Therefore, comparative toxicological assessments need to be conducted to better understand the role of metal composition in the observed adverse pulmonary effects associated with the exposure to PM0.1. Because of their presence in airborne particulate matter, we choose to investigate the biological effects of three nanoparticles: amorphous silicon oxide (SiO2), ferric oxide (Fe2O3), and copper (II) oxide (CuO).
We hypothesized that different metal oxide particles will have different abilities to generate oxidative stress and alter cell viability based on the transition metal. The aim of this study was to compare the in vitro responses of respiratory epithelial cells following exposure to two types of commercially available metal oxide nanoparticles and amorphous SiO2 nanoparticles. In particular, we investigated the intrinsic ability of silicon oxide, ferric oxide and copper (II) oxide nanoparticles to decrease cell viability and generate oxidative stress in respiratory epithelial cells. Human laryngeal epithelial cells (HEp-2) were chosen, since they are used in many pulmonary toxicological assays (Rudolf et al., 2001; Kvolik et al., 2005) and represent target cells which are usually subjected to significant amounts of airborne particles.
Materials and Methods
Reagents
2’,7’-dichlorofluorescein-diacetate (H2DCFDA), 5,5’-dithio-bis (2-nitrobenzoic acid) (DTNB), H2O2, oxidized glutathione (GSSG), reduced glutathione (GSH), β-nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt (NADPH), sulfosalisilic acid (SSA), superoxide disumutas (SOD) determination kit and glutathione assay kit were all obtained from Sigma (St Louis, MO). Copper (II) oxide (CuO) particles (30 nm, # 45407) and ferric oxide (Fe2O3) particles (20–40 nm, # 45007) were purchased from Alfa Aesar (Ward Hill, MA) and silicon oxide (SiO2) (80 nm, # 4830HT) was obtained from Nanostructured & Amorphous Materials, Inc (Los Alamos, NM). Resveratrol was purchased from Axxora (San Diego, CA) and Alamar Blue was obtained from Invitrogen (Carlsbad, USA). All organic solvents were of Fisher optima grade (Fisher Scientific, Hampton, NH).
Methods
Cell culture and treatment
Human laryngeal epithelial cells (HEp-2 cells) were purchased from ATCC (Manassas, VA) and were cultured in 75 cm2 flask at the density of 2 × 104 cell/cm2 in Dulbecco’s Modified Eagle’s Medium-Reduced Serum (DMEM-RS), supplemented with 2% heat inactivated fetal bovine serum (FBS) at 37°C in a humidified incubator containing 5% CO2. At 85 % confluence, cells were harvested using 0.25% trypsin and were sub-cultured into 75 cm2 flasks, 6-well plates or 96 well plates. Cells were allowed to recover for 2 days prior to treatment. Particles were suspended in cell culture medium by pulse sonication (30 s on, 30 s off) using 50% amplitude (Sonics and Materials Inc, CT, USA) for 4 min to avoid particle agglomeration, followed by vigorous vortexing for 1 min prior to administration to the cells. A serial dilution was established by mixing equal volumes of particle suspension and cell culture medium followed by vigorous vortexing. All experiments were performed using HEp-2 cells at passage 10–20 and were replicated with at least two independent cell passages.
Cell viability assay
The cytotoxic effect of particles on HEp-2 cells was determined by the Alamar blue assay as previously described (Baudouin et al., 2007). Briefly, HEp-2 cells were cultured in 96 well plates at the density of 2 × 104 cell/ cm2 and then, incubated with particles (4 to 400 µg/cm2), suspended in cell culture medium (200 µl/well) for 5 h to establish a dose response curve. Cell viability was estimated by measuring the emitted fluorescence of the reduced alamar blue using a plate reader (ex/em: 530/590) and was normalized to medium only treated cells (100% viability) and 0.1% saponin (0% viability). Unlike other cell viability assays, the presence of nanoparticles does not interfere with the Alamar blue assay (Simon-Deckers et al., 2008). A dose-response curve consisting of log doses of particles and percent cell viability associated with the exposure to each dose was plotted using the nonlinear fit (Third order polynomial; Graphpad Prism 5 software, La Jolla, CA). To investigate the influence of resveratrol, desferoxamine and D-penicillamine on the cytotoxic effect of copper oxide, cells were co-treated with 100 µM of resveratrol, 100 µM desferoxamine or 100 µM D-penicillamine prior to assessment of cell viability and the data were compared to medium containing 100 µM resveratrol, 100 µM desferoxamine or 100 µM D-penicillamine; respectively (100% viability) and 0.1% saponin (0% viability).
Measurement of cellular reactive oxygen species (ROS)
The production of reactive oxygen species in HEp-2 cells was measured by pre-loading the cells with 10 µM 2,7-dichlorofluorescin diacetate (H2DCFDA) at 37°C for 40 min in the dark in 6-well plate. The cells were then washed and incubated with particle suspension (80 µg/cm2; 125 µg/ml) for 30 min at 37°C. After the treatment, cells were washed, scraped, lysed, pulse sonicated for 15 s (1 s on, 1 s off) using 50% amplitude, and centrifuged at 12,000 × g for 15 min at 4°C. The intensity of DCF fluorescence in the cell lysate was measured using a plate reader (ex/em: 485/530) and was normalized to protein content measured by BCA protein assay (Thermo Fisher Scientific Inc., Waltham, MA).
Antioxidant enzyme activity
After treating the cells for 4 h with particle suspension (80 µg/cm2), cells were washed with PBS, scraped, lysed, sonicated for 15 s (1 s on, 1 s off) on ice and centrifuged at 12,000 × g for 15 min at 4°C. The supernatant (cell lysate) was removed and the protein concentration was measured by the BCA method. The activities of different antioxidant enzymes were then measured in the cell lysates.
The activity of SOD was measured using 15 µg protein of cell lysates. The cell lysates were incubated with xanthine oxidase enzyme and tetrazolium salt for 20 min at 37°C. The absorbance of the formazan salt resulting from the oxidation of tetrazolium salt was detected at 450 nm. The activity of SOD, expressed as percent inhibition of the formation of formazan, was then calculated.
The activity of catalase enzyme was measured as previously described (Aebi, 1984). An appropriate volume of cell lysate containing 50 µg protein was mixed with 1 ml of 50 mM potassium phosphate buffer (pH 7.0) containing 10 mM H2O2 in 1 ml quartz cuvette. The decrease in absorbance of H2O2 was followed at 240 nm for 4 min. Catalase activity was calculated from the slope of the H2O2 absorbance curve and normalized to protein concentration.
The activity of glutathione reductase (GR) was directly measured as previously described (Guthenberg et al., 1985) by mixing 50 µg protein of cell lysate with 1 ml of 0.1 M phosphate buffer supplied with 2 mM EDTA containing 20 mM NADPH and 20 mM GSSG. The decrease in NADPH absorbance was followed for 3 min at 340 nm. The activity of GR was calculated from the slope of NADPH absorbance curve and was normalized to protein content.
The activity of glutathione peroxidase (GPx) was measured using 50 µg protein of the cell lysates. The cell lysates were mixed with tert-butyl hydroperoxide (30 mM), reduced glutathione (2 mM), GR (0.5 unit/ml) and NADPH (0.25 mM) in 50 mM Tris HCl (pH 8) at 25°C. The decrease in NADPH absorbance was followed for 3 min at 340 nm. The activity of GPx was calculated from the slope of NADPH absorbance curve and was normalized to protein content.
Measurement of intracellular glutathione
HEp-2 cells grown to 85% confluence were exposed to nanoparticles (80 µg/cm2) for 4 h. After the treatment, the amount of total and oxidized glutathione was measured by the recycling method (Rahman et al., 2007). Particle-exposed cells were washed, scraped and lysed. Total glutathione was measured by reducing oxidized glutathione content using GR enzyme (3 units ml−1) and NADPH (0.8 mM). The assay is based on the chemical reaction between GSH and DTNB to form TNB. The change in TNB absorbance was measured at 412 nm using a plate reader. To measure the amount of GSSG, 100 µl of cell lysate was incubated with 2-vinyl pyridine which covalently reacts with GSH but not GSSG, and the cell lysates were then treated with GR, NADPH and DTNB. Data were compared to GSH and GSSG standard curves and were normalized to protein content. The ratio of GSSG to total glutathione was then calculated.
Detection of 8-isoprostane as a biomarker of lipid peroxidation
The supernatant of cells co-treated with CuO and resveratrol (100 µM), deferoxamine (100 µM) or D-penicillamine (100 µM) for 4 h was collected, centrifuged at 12,000 × g for 10 min to remove particles and cell debris and was used immediately to measure 8-isoprostane levels. 8-isoprostane, a biomarker of lipid peroxidation, was measured by a competitive enzyme-linked immunosorbent assay (ELISA) (Cayman Chemical, Ann Arbor, MI) using 50 µl of cell supernatant. The concentration of 8-isoprostane in samples were then calculated using an established standard curve.
Results
Cytotoxic effect of metal oxide nanoparticles on human laryngeal epithelial cells
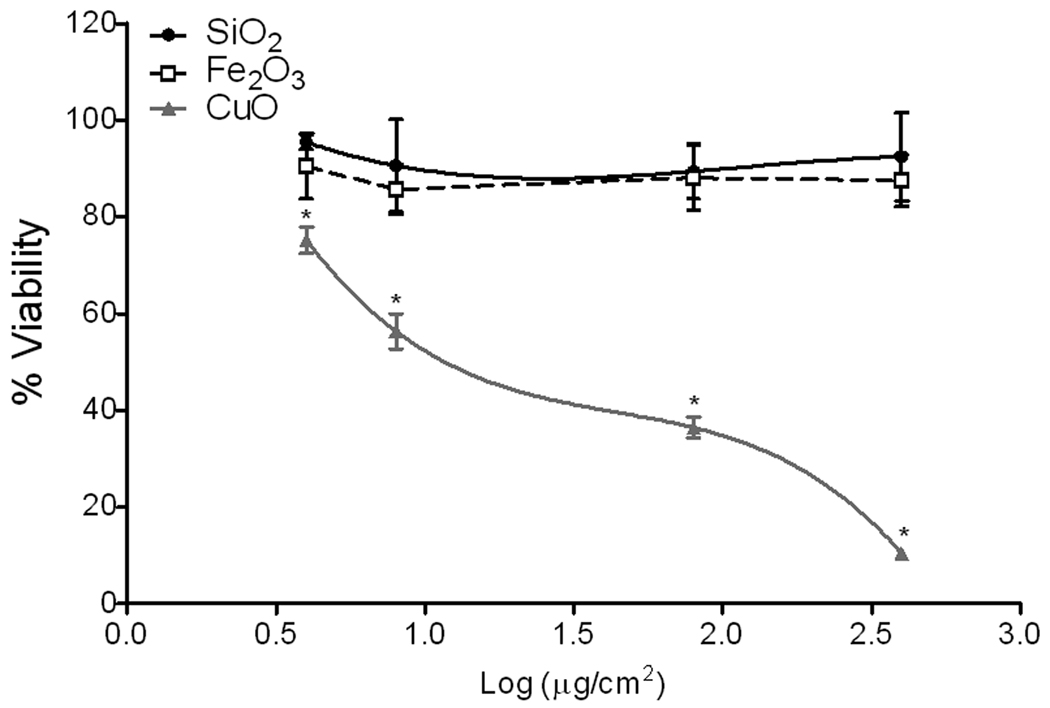
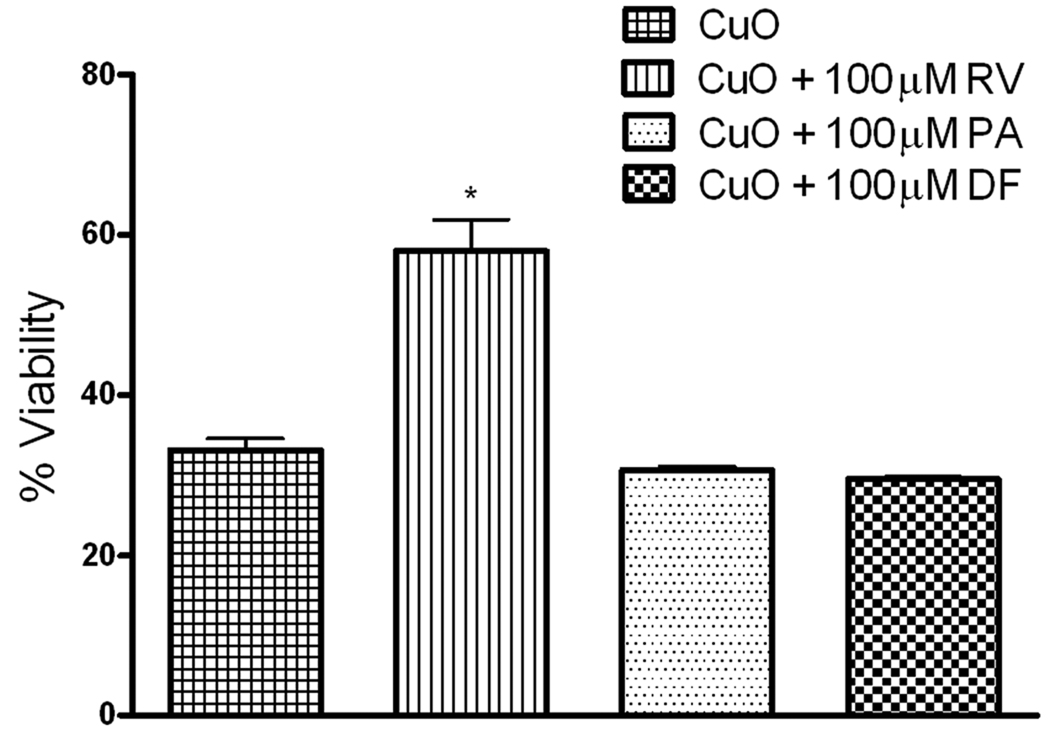
To assess the toxicity of metal oxide nanoparticles, HEp-2 cells were treated with these particles at different doses (4, 8, 80, and 400 µg/cm2) and viability after 5 h of exposure was determined. Cell viability decreased in a dose-dependent manner following exposure to CuO nanoparticles (Figure 1); however, SiO2 and Fe2O3 nanoparticles were non-toxic (less than 10% non-viable) at all exposure doses tested as compared to cells treated with medium. Cell viability also decreased in a time-dependent manner with maximal cell death occurring within 5 h post-treatment. No morphological changes were observed in the epithelial cells during the exposure to CuO or Fe2O3 nanoparticles. CuO significantly decreased cell viability by 60% at the exposure dose of 1.9 log µg /cm2 (80 µg/cm2). The cytotoxic effect of CuO nanoparticles was partially reversed when cells were co-treated with 100 µM resveratrol (42% non-viable) suggesting that oxidative stress at least in part mediated the cytotoxic effect of CuO (Figure 2). In contrast, the copper chelators desferoxamine and D-penicillamine did not alter the cytotoxic effect of CuO on HEp-2 cells, indicating that CuO-induced cytotoxicity is mediated by the particles and not any dissolved metal fraction (Figure 2).
Figure 1.
Nanoparticles reduce human epithelial cell viability. Human epithelial (HEp-2) cells were incubated with increasing doses of SiO2, Fe2O3 and CuO nanoparticles prior to assessment of cell viability. Data were normalized to cell viability of cells treated with medium only (100% viable) or cells treated with 0.1% saponin (0% viable). CuO significantly reduced cell viability as compared to vehicle only treated cells. Results are expressed as mean ± SE of 3 replicates (n = 4). Two-way ANOVA *p< 0.05 vs. vehicle.
Figure 2.
Resveratrol alters the cytotoxic effect of CuO in epithelial cells. Cell viability of HEp-2 cells exposed to CuO (80 µg/cm2) with or without resveratrol (RV,100 µM), D-penicillamine (PA, 100 µM) or desferoxamine (DF, 100 µM) were assessed and were normalized to cell viability of medium-only, RV-only, PA-only and DF-only treated cells, respectively (100% viable) or 0.1% saponin treated cells (0% viable). Resveratrol but not desferoxamine or D-penicillamine alters the cytotoxic effect of CuO. Results are expressed as mean ± SE of 3 replicates, (n = 4). One-way ANOVA *p< 0.05 vs. CuO.
Metal oxide nanoparticles generate reactive oxygen species (ROS) in vitro
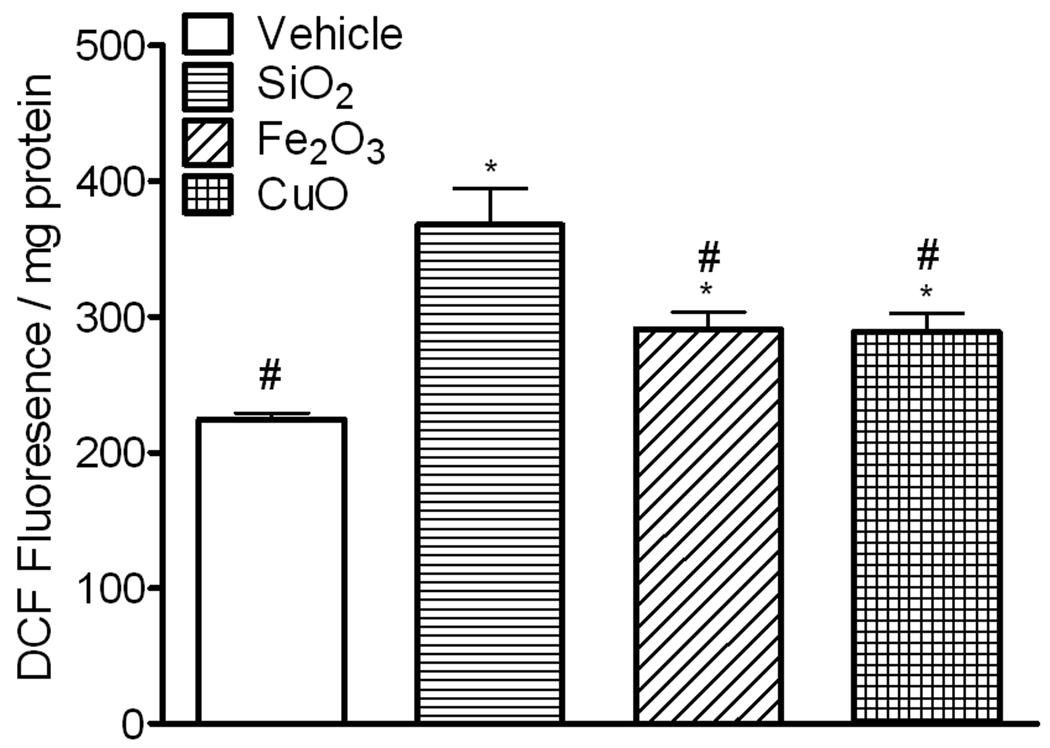
To compare the ability of metal oxide nanoparticles to induce oxidative stress in human epithelial cells, we first examined the ability of these particles to generate ROS in HEp-2 cells. Intracellular ROS was determined using 2,7 dichlorofluorescin diacetate (H2DCFDA). H2DCFDA diffuses into the cells and is hydrolyzed to H2DCF, which reacts with ROS to form DCF. HEp-2 cells were exposed to silica, Fe2O3, and CuO (80 µg/cm2) for 30 min; and DCF fluorescence was measured in cell lysates. Exposure of epithelial cells to SiO2, Fe2O3 and CuO nanoparticles resulted in significant increases in DCF fluorescence (167%, 132% and 131%, respectively), when compared to cells not exposed to these particles (Figure 3).
Figure 3.
ROS generated in HEp-2 cells exposed to nanoparticles. Cells were pre-loaded with H2DCFDA for 40 min and then treated with SiO2, Fe2O3, CuO (80 µg/cm2) or vehicle. The intensity of DCF fluorescence was measured in cell lysates, and normalized to protein concentration. All particles generated ROS in HEp-2 cells. Data are expressed as mean ± SE of 2 replicates, (n = 4). One-way ANOVA, *p< 0.05 vs. vehicle, # p< 0.05 vs. SiO2.
Metal oxide nanoparticles alter the activity of antioxidant enzymes and the level of oxidized glutathione in cultured epithelial cells
When the production of ROS exceeds the ability of the cell to neutralize the effects of the radicals, accumulation of pro-oxidants occurs in the cell leading to a state of oxidative stress (Gilmour et al., 2006). As oxidative stress in the cell increases, different biological outcomes such as change in the activity of antioxidant enzymes and the depletion of glutathione occur (Li et al., 2003). To investigate the ability of metal oxide nanoparticles to alter the activity of cellular antioxidants, epithelial cells were exposed to each particle (80 µg/cm2) and the activity of antioxidant enzymes was assessed. There was no difference in the activity of SOD in any of the exposed cell populations (Table 1). Epithelial cells exposed to Fe2O3 or CuO showed significant inhibition (21% and 25%, respectively) of catalase. However, no significant differences were observed in SiO2 exposed cells as compared to control cells, which were not exposed to particles. SiO2 exposed cells exhibited a significant increase (15%) in the activity of GR, whereas CuO significantly inhibited (29%) the activity of GR in HEp-2 cells as compared to control cells not exposed to particles. The activity of GPx was significantly increased by CuO (150%); however, no significant differences were observed in SiO2 exposed cells as compared to control (Table 1). Fe2O3 exposed cells demonstrated no differences in the activity of GR and GPx as compared to control. The ratio of oxidized to total glutathione increased (150%) after exposure to CuO, but not after exposure to SiO2 or Fe2O3 (Figure 4). The oxidation of GSH indicates the inability of epithelial cells to scavenge ROS generated by CuO and the development of oxidative stress in these cells which may lead to oxidative damage.
Table 1.
Cellular antioxidant enzyme activities following exposure to nanoparticles (80 µg/cm2). The activity of superoxide dismutase (SOD), catalase, glutathione reductase, and glutathione peroxidase were measured and normalized to protein concentration. Data are expressed as mean ± SE of 2 replicates, (n =3). One-way ANOVA
| Enzyme activity | vehicle | SiO2 | Fe2O3 | CuO |
|---|---|---|---|---|
| total SOD (% inhibition rate) |
66.28 ± 2.765 | 77.13 ± 3.609 | 78.30 ± 3.293 | 74.61 ± 2.949 |
| catalase (unit/mg protein) |
5.494 ± 0.0733 | 5.107 ± 0.1148 | 4.359 ± 0.2050* | 4.105 ± 0.2176* |
| glutathione reductase (unit/mg protein) |
0.0145 ± 0.0002 | 0.0163 ± 0.0005* | 0.0140 ± 0.0003 | 0.0107 ± 0.0001* |
| glutathione peroxidase (unit/mg protein) |
0.1684 ± 0.0070 | 0.1867 ± 0.0165 | 0.2039 ± 0.0163 | 0.3958 ± 0.0093* |
p< 0.05 vs. vehicle.
Figure 4.
Ratio of oxidized to total glutathione in epithelial cells exposed to nanoparticles. The levels of GSSG and GSH were measured in cells exposed to SiO2, Fe2O3, CuO (80 µg/cm2) or vehicle and were normalized to protein concentration. CuO depletes GSH in exposed cells. Ratio is expressed as mean ± SE of 2 replicates, (n = 3). One-way ANOVA *p<0.05 vs. vehicle.
Copper oxide nanoparticles increase 8-isoprostane production in HEp-2 cells
Isoprostanes are produced by the random non-enzymatic oxidation of cellular phospholipids by oxygen radicals. SiO2 and Fe2O3 were excluded from this assay since they did not generate oxidative stress in HEp-2 cells as evidenced by normal ratio of GSSG to total GSH (similar to control cells exposed to medium) (Figure 4). In contrast, CuO induced oxidative stress, which can lead to oxidative damage and subsequently cell death. Analysis of culture supernatant from cells exposed to CuO nanoparticles indicated increased (1000%) levels of 8-isoprostane as compared to vehicle treated cells (Figure 5). Resveratrol, but not desferoxamine or D-penicillamine, significantly reduced (80%) the production of 8-isoprostane in CuO exposed cells.
Figure 5.
Resveratrol reduces the level of 8-isoprostane induced by CuO. The level of 8-isoprostane was measured in the supernatant of cells co-treated with CuO (80 µg/cm2) and desferoxamine (DF, 100 µM), D-penicillamine (PA, 100 µM) or Resveratrol (RV, 100 µM). Resveratrol but not desferoxamine or D-penicillamine reduces the level of 8-isoprostane induced by CuO. Data are presented as mean ± SE (n = 2). One-way ANOVA *p< 0.05 vs. vehicle, # p< 0.05 vs. CuO.
Discussion
The manufacture and use of metal oxide nanoparticles is continuously expanding due to their wide applications and unique physicochemical properties. Since they have a very small size (<0.1 µm in diameter), they readily contaminate the environment and may pose a risk to humans. Thus, it becomes increasingly important to investigate and identify their possible toxicological effects and to identify which particles pose the greatest harm to human health. Since inhalation is a significant route of exposure to metal oxide nanoparticles, we have studied the impact of SiO2, Fe2O3 and CuO nanoparticles on respiratory epithelial cells.
In our results there was significant variation in the ability of these particles to alter cell viability. CuO elicited a significant dose-dependent decrease in HEp-2 cell viability (Figure 1) as compared to cells treated with vehicle. In contrast, cell viability following exposure to SiO2 and Fe2O3 remained unchanged; these particles were non-toxic to HEp-2 cells. Since all three particle types are fairly uniform in size, the number of particles per mg weight and the surface area was comparable between all of them. Thus, the chemical composition of the particles appears directly responsible for the decreased cell viability of HEp-2 cells. This is consistent with documented data demonstrating enhanced cytotoxicity following ingestion of CuO nanoparticles (Aruoja et al., 2009). The mechanistic basis of this cytotoxicity; however was unknown.
Oxidative stress has been proposed as a common mechanism of cell damage induced by many types of nanoparticles (Stone et al., 2007). We hypothesized that the cytotoxicity induced by CuO exposure in our studies was mediated by the generation of oxidative stress in these cells. Indeed, co-treatment with the antioxidant resveratrol mitigated the cytotoxic effect of CuO, suggesting that oxidative stress was responsible, at least in part, for the decreased viability. All metal oxide nanoparticles were able to generate ROS in HEp-2 cells; however, CuO was better able to inhibit the activity of catalase and GR enzymes and increase the activity of GPx as compared to cells exposed only to medium. This finding suggests that not only was CuO able to generate ROS (e.g. H2O2) in the exposed epithelial cells but also that it was more efficient at blocking the antioxidant defenses of the cell as evidenced by an increase in the ratio of oxidized to total glutathione.
Usually cells respond to oxidative burden by fortifying their antioxidant defense mechanisms in order to protect themselves from any oxidative damage. However, if the defense mechanisms fail to neutralize the oxidative burden protein oxidation (Ramirez-Prieto et al., 2006), lipid peroxidation (Gutteridge, 1995), DNA damage, mitochondrial perturbation and apoptosis occur (Li et al., 2003). One of the eicosanoids produced by the oxidation of phospholipids is 8-isoprostane, a stable, water-soluble compound. Therefore, 8-isoprostane has been proposed as an ideal marker of oxidative stress and lipid peroxidation (Morrow et al., 1995; Morrow and Roberts, 1997). The levels of 8-isoprostane in the supernatant of cells exposed to CuO was significantly elevated demonstrating that CuO induced oxidative damage in HEp-2 cells. Resveratrol completely protected HEp-2 cells from the oxidative damage associated with CuO exposure as evidence by the massive decrease in the level of 8-isoprostanes in the supernatant of these cells. However, it exhibited only partial protection from the cytotoxicity associated with CuO exposure in these cells. This suggests that oxidative stress was only partially responsible for the reduced viability induced by exposure to CuO and suggests that other mechanism(s) may be responsible for further reductions in cell viability.
Although SiO2 and Fe2O3 were able to generate ROS in HEp-2 cells, the antioxidant defense system remained intact and no cytotoxicity was observed. Cell exposed to amorphous SiO2 nanoparticles showed an increase in the activity of GR as compared to control. No differences in the activity of GPx, catalase, SOD or the ratio of oxidized to total glutathione were observed, suggesting that ROS generated in HEp-2 cells were scavenged by the antioxidant defense system and SiO2 failed to generate oxidative stress. This was associated with the inability of SiO2 particle to promote cell death even at the highest dose tested. Our data are consistent with previously published data demonstrating that amorphous silica nanoparticles do not significantly decrease the viability of respiratory epithelial cells and do not deplete GSH molecules (Cha and Myung, 2007). However, the exact reason why ROS was successfully scavenged in SiO2 and Fe2O3, but not CuO, exposed cells is not clear. It is possible that different particles generated different radicals with different oxidative potency in HEp-2 cells.
Fe2O3 inhibited the activity of catalase enzyme in exposed cells, whereas, no differences in the activity of GR, GPx, SOD or the ratio of oxidized to total GSH as compared to cells exposed to vehicle were observed. Our data are consistent with recently published data demonstrating that Fe2O3 nanoparticles were not able to generate oxidative stress characterized by normal levels of oxidized DNA as compared to control in human alveolar epithelial cells (A549), even when these cells were incubated with Fe2O3 particles at the concentration of (40 µg/cm2) for 18 h (Karlsson et al., 2008). Additionally, Fe2O3 nanoparticles (53 µg/cm2) were not able to decrease the viability of bronchial epithelial cells (BEAS-2B) cells after 24 h of exposure (Veranth et al., 2007).
In our studies, CuO nanoparticles generated cytotoxicity at even the lowest doses. The cytotoxic effect of Cu2+ ion has been extensively studied. It has been proposed that Cu2+ decreases cell viability by binding to DNA resulting in DNA damage and cell death (Aruoma et al., 1991). In addition, Cu2+ ions are able to induce apoptosis in neuronal cells by directly altering the expression of apoptotic genes (Chan et al., 2008). Finally, it has been demonstrated that Cu ions can be released from the surface of CuO nanoparticles when they are suspended in Dulbecco’s Modified Eagle’s Medium and that the released component is responsible for some of the toxic effects (Midander et al., 2009). Therefore, we tested whether Cu ions released from the particles to the cell media contribute in the cytotoxicity of CuO nanoparticles; however, both Cu-chelators utilized, desferoxamine and D-penicillamine, failed to mitigate the cytotoxic effect of CuO on HEp-2 cells. In addition, no differences in the level of 8-isoprostane was observed in the supernatant of cells treated with Cu-chelators and CuO nanoparticles as compared to cells exposed to CuO only. Finally, desferoxamine and D-penicillamine are cell permeable and are capable of binding intracellular and extracellular free Cu ions should they become available. Our data suggest that even if Cu2+ ions were released in the cell or in the cell culture media, they do not significantly contribute to the cytotoxic effect or the oxidative damage associated with the exposure to CuO nanoparticles. This result is consistent with previous findings demonstrating that the dissolved portion of Cu from nanoparticles is insufficient to produce mortality in zebrafish exposed to CuO particles (Griffitt et al., 2007).
Interestingly, different metal oxide nanoparticles have been shown to penetrate the cell membrane of respiratory epithelial cells with different efficacies (Park et al., 2007). Once the particles are engulfed inside the cells, they are delivered to the lysosomes. Since the interior of the lysosmes is acidic (pH 4.5), many metal oxides, including CuO and Fe2O3 can be dissolved and released as Cu2+ and Fe3+, respectively (Guo et al., 2009). It remains possible that of all the particles studied here, CuO is the most efficient at penetrating the cell membrane and once inside the cell Cu2+ may be released and initiate the production of intracellular ROS or directly damage intracellular proteins prompting cell death. However, both Cu chelators, which can easily penetrate the cell membrane, did not mitigate the oxidative damage or cytotoxicty produced by CuO nanoparticles. This suggests that the biological effect seen in CuO exposed cells is least likely to be mediated through the release of Cu2+ ions either outside or inside the cells.
In our model, CuO but not Fe2O3 generated oxidative stress and induced cytotoxicity in HEp-2 cells. It has been proposed that transition metals such as Cu and Fe may contribute to the production of intracellular ROS via Fenton-type reaction (Stohs and Bagchi, 1995). Fe3+ and Cu2+ theoretically can be reduced by superoxide anion to Fe2+ and Cu+ respectively, which then react with H2O2 to produce (OH.) (Stohs and Bagchi, 1995). However, the reduction capability of Fe3+ to Fe2+ in the absence of a reductant, which is crucial for initiation of the Fenton reaction, is not easily achievable under physiological conditions and occurs at an even slower rate when it is bound to chelators (Petrat et al., 2003). Indeed, it was demonstrated that Fe2O3 nanoparticles are able to penetrate respiratory epithelial cell membrane and release Fe3+ in the acidic lysosomes, however, these Fe3+ ions were not reduced to Fe2+ under normal physiological conditions and hence did not cause oxidative stress (Guo et al., 2009). Collectively, it appears that the oxidative stress and the decrease in cell viability associated with exposure to CuO are generated by the particle itself rather than any released Cu2+ ions making the Fenton reaction an unlikely cause of oxidative stress in CuO exposed cells. On the other hand, Cu2+ rapidly reacts with superoxide making it difficult to scavenge and possibly allowing it to participate in redox cycling leading to sustained oxidative stress.
It has been proposed that the surface activity of metal oxide nanoparticles influence the biological effect of these particles. The manufacturing processes employed make the particles hydrophilic or lipophilic, catalytically active or passive and alter the electronic properties of the particle surface. It is possible that the surface of CuO has the ability to generate ROS such as H2O2 in cell medium which can then diffuse across the cell membrane of HEp-2 cells (Schubert and Wilmer, 1991). Alternatively, the extracellularly generated ROS may oxidize cell membrane lipids to produce 8-isoprostane which in turn can diffuse into the cell and initiate the production of intracellular or intramitochondrial ROS (Landar et al., 2006). Interestingly, it was demonstrated that CuO nanoparticles were able to generate H2O2 in aqueous medium using O2 in the presence of light (Bandara et al., 2005). Additionally, CuO nanoparticles may directly oxidize membrane lipids and generate 8-isoprostane. Finally, CuO may penetrate the cell membrane and trigger an intracellular signaling network leading to the development of oxidative stress and apoptosis as previously documented with other metal oxide nanoparticles (Park et al., 2007). Understanding the relationship between the cellular response and the oxidative stress endpoints will be extremely useful in understanding the exact mechanism by which nanoparticles generate ROS in respiratory epithelial cells and should be further investigated.
Our data are to some extent consistent with a recently published report demonstrating the greater ability of CuO vs. Fe2O3 nanoparticles and CuO vs. Cu ions to induce cytotoxicity in a respiratory cell line (Karlsson et al., 2008). In contrast to Karlsson’s work, we observed the generation of significant levels of ROS in HEp-2 cells treated with CuO using the same assay (i.e. DCF fluorescence). The reasons for this are unclear and could simply be due to the use of different cell lines: A549 (human epithelial cells derived from a lung carcinoma) vs HEp-2 (human epithelial cells derived from an epidermoid carcinoma of the larynx) or different doses: 20 and 40 µg/cm2 vs. 80 µg/cm2 (the derived LD50) of nanoparticles or different production mechanisms (Sigma vs. Alfa Aesar). Karlsson’s data also clearly demonstrates that Cu ions at concentrations up to 20 µg/cm2 have little effect on cell viability. To rule out the possibility that Cu ions were being released from CuO (either extracelllarly or intracellular) and were themselves responsible for the adverse effects observed upon CuO exposure, we repeated many of our studies in the presence of two cell permeable Cu chelators, desferoxamine and D-penicillamine. Although our approach to address this same issue was different, we both arrived at a similar conclusion - Cu ions were not responsible for the observed adverse effects of CuO. Finally, our data extended Karlsson’s work by demonstrating the following: 1) cell viability could be improved and oxidative damage reduced by alleviating the oxidative burden through the use of the antioxidant resveratrol, and 2) CuO diminished the activity of a variety of antioxidant enzymes.
In summary, our study demonstrated that there is significant variation among different metal oxide nanoparticles regarding their ability to generate oxidative stress and promote cell death. In fact, CuO nanoparticles were the only particles capable of inducing cell death. They were also the most potent at inducing oxidative stress. These effects were not due to the solubility of the transition metal and appear to involve sustained oxidative stress possibly due to redox cycling. This demonstrates that the chemical composition and possibly the reductive capacity of the particles have a great influence on the biological response of exposed cells. Exposure to CuO rather than Fe2o3 or SiO2 nanoparticles may endanger human health and produce pulmonary diseases and/ or exacerbations of pre-existing respiratory diseases.
Acknowledgement
This work was supported by a grant from the National Institute of Environmental Health Science Center (NIEHS) to SAC (R01ES015050). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH or NIEHS.
Footnotes
Declaration of interest:
The authors report no conflicts of interest.
References
- Aebi H. Catalase in vitro. Methods in Enzymology. 1984:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Aruoja V, Dubourguier HC, Kasemets K, Kahru A. Toxicity of nanoparticles of CuO, ZnO and TiO(2) to microalgae Pseudokirchneriella subcapitata. Science of the Total Environemnt. 2009;407:1461–1468. doi: 10.1016/j.scitotenv.2008.10.053. [DOI] [PubMed] [Google Scholar]
- Aruoma OI, Halliwell B, Gajewski E, Dizdaroglu M. Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochemical Journal ( Pt 3) 1991;273:601–604. doi: 10.1042/bj2730601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduzzi M, Diociaiuti M, De Berardis B, Paradisi S, Paoletti L. In vitro effects on macrophages induced by noncytotoxic doses of silica particles possibly relevant to ambient exposure. Environmental Research. 2004;96:62–71. doi: 10.1016/j.envres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Bandara J, Guasaquillo I, Bowen P, Soare L, Jardim WF, Kiwi J. Photocatalytic storing of O2 as H2O2 mediated by high surface area CuO. Evidence for a reductive-oxidative interfacial mechanism. Langmuir. 2005;21:8554–8559. doi: 10.1021/la0504661. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Riancho L, Warnet JM, Brignole F. In Vitro Studies of Antiglaucomatous Prostaglandin Analogues: Travoprost with and without Benzalkonium Chloride and Preserved Latanoprost. Investigative ophthalmology & visual science. 2007;48:4123–4128. doi: 10.1167/iovs.07-0266. [DOI] [PubMed] [Google Scholar]
- Cha KE, Myung H. Cytotoxic effects of nanoparticles assessed in vitro and in vivo. Journal of Microbiology and Biotechnology. 2007:1573–1578. [PubMed] [Google Scholar]
- Chan HW, Liu T, Verdile G, Bishop G, Haasl RJ, Smith MA, Perry G, Martins RN, Atwood CS. Copper Induces Apoptosis of Neuroblastoma Cells Via Post-translational Regulation of the Expression of Bcl-2-family Proteins and the tx Mouse is a Better Model of Hepatic than Brain Cu Toxicity. International Journal of Clinical and Experimental Medicine. 2008;1:76–88. [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Oh JM, Choy JH. Toxicological effects of inorganic nanoparticles on human lung cancer A549 cells. Journal of Inorganic Biochemistry. 2009 doi: 10.1016/j.jinorgbio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Fanizza C, Ursini CL, Paba E, Ciervo A, DiFrancesco A, Maiello R, De Simone P, Cavallo D. Cytotoxicity and DNA-damage in human lung epithelial cells exposed to respirable alpha-quartz. Toxicology In Vitro. 2007;21:586–594. doi: 10.1016/j.tiv.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environmental health perspectives. 2006;114:627–633. doi: 10.1289/ehp.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environmental health perspectives. 2007;115:403–409. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D, Barber DS. Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio) Environmental Science and Technology. 2007;41:8178–8186. doi: 10.1021/es071235e. [DOI] [PubMed] [Google Scholar]
- Guo B, Zebda R, Drake SJ, Sayes CM. Synergistic effect of co-exposure to carbon black and Fe2O3 nanoparticles on oxidative stress in cultured lung epithelial cells. Particle and Fibre Toxicology. 2009;6:4. doi: 10.1186/1743-8977-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthenberg C, Alin P, Mannervik B. Glutathione transferase from rat testis. Methods in Enzymology. 1985;113:507–510. doi: 10.1016/s0076-6879(85)13067-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clinical Chemistry. 1995;41:1819–1828. [PubMed] [Google Scholar]
- Karlsson HL, Cronholm P, Gustafsson J, Moller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chemical Research in Toxicology. 2008;21:1726–1732. doi: 10.1021/tx800064j. [DOI] [PubMed] [Google Scholar]
- Kvolik S, Glavas-Obrovac L, Bares V, Karner I. Effects of inhalation anesthetics halothane, sevoflurane, and isoflurane on human cell lines. Life Science. 2005;77:2369–2383. doi: 10.1016/j.lfs.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Landar A, Zmijewski JW, Dickinson DA, LeGoffe C, Johnson MS, Milne GL, Zanoni G, Vidari G, Morrow JD, Darley-Usmar VM. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2006;290:H1777–H1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- Lei R, Wu C, Yang B, Ma H, Shi C, Wang Q, Yuan Y, Liao M. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: a rapid in vivo screening method for nanotoxicity. Toxicology and Applied Pharmacology. 2008;232:292–301. doi: 10.1016/j.taap.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clinical Immunology. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Merget R, Bauer T, Kupper HU, Philippou S, Bauer HD, Breitstadt R, Bruening T. Health hazards due to the inhalation of amorphous silica. Archives of Toxicology. 2002;75:625–634. doi: 10.1007/s002040100266. [DOI] [PubMed] [Google Scholar]
- Midander K, Cronholm P, Karlsson HL, Elihn K, Moller L, Leygraf C, Wallinder IO. Surface characteristics, copper release, and toxicity of nano- and micrometer-sized copper and copper(II) oxide particles: a cross-disciplinary study. Small. 2009;115:389–399. doi: 10.1002/smll.200801220. [DOI] [PubMed] [Google Scholar]
- Montes-Hernandez G, Pironon J, Villieras F. Synthesis of a red iron oxide/montmorillonite pigment in a CO2-rich brine solution. Journal of Colloid and Interface Science. 2006;303:472–476. doi: 10.1016/j.jcis.2006.07.064. [DOI] [PubMed] [Google Scholar]
- Moriwaki H, Osborne MR, Phillips DH. Effects of mixing metal ions on oxidative DNA damage mediated by a Fenton-type reduction. Toxicology in Vitro. 2008;22:36–44. doi: 10.1016/j.tiv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. The New England Journal of Medicine. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Progress in Lipid Research. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental health perspectives. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee YK, Jung M, Kim KH, Chung N, Ahn EK, Lim Y, Lee KH. Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhalation Toxicology. 2007;19 Suppl 1.:59–65. doi: 10.1080/08958370701493282. [DOI] [PubMed] [Google Scholar]
- Penttinen P, Timonen KL, Tiittanen P, Mirme A, Ruuskanen J, Pekkanen J. Ultrafine particles in urban air and respiratory health among adult asthmatics. European Respiratory Journal. 2001;117:428–435. doi: 10.1183/09031936.01.17304280. [DOI] [PubMed] [Google Scholar]
- Petrat F, Paluch S, Dogruoz E, Dorfler P, Kirsch M, Korth HG, Sustmann R, de Groot H. Reduction of Fe(III) ions complexed to physiological ligands by lipoyl dehydrogenase and other flavoenzymes in vitro: implications for an enzymatic reduction of Fe(III) ions of the labile iron pool. Journal of Biological Chemistry. 2003;278:46403–46413. doi: 10.1074/jbc.M305291200. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nature Protocols. 2007;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Ramirez-Prieto MT, Garcia-Rio F, Villamor J. [Role of oxidative stress in respiratory diseases and its monitoring] Medicina Clinica. 2006;127:386–396. doi: 10.1157/13092440. [DOI] [PubMed] [Google Scholar]
- Reuzel PG, Bruijntjes JP, Feron VJ, Woutersen RA. Subchronic inhalation toxicity of amorphous silicas and quartz dust in rats. Food and Chemical Toxicology. 1991;29:341–354. doi: 10.1016/0278-6915(91)90205-l. [DOI] [PubMed] [Google Scholar]
- Rogaczewska T, Matczak W. [Evaluation of occupational exposure to cadmium based on air analysis of the work area. I. Cadmium oxide level in the air of work areas in a cadmium and nickel cumulator factory] Medycyna Pracy. 1985;36:273–279. [PubMed] [Google Scholar]
- Rudolf E, Peychl J, Cervinka M. Toxic effects of chromium acetate hydroxide on cells cultivated in vitro. Alternatives to Laboratory Animals. 2001;29:163–177. doi: 10.1177/026119290102900209. [DOI] [PubMed] [Google Scholar]
- Schubert J, Wilmer JW. Does hydrogen peroxide exist “free” in biological systems? Free Radic Biol Med. 1991;11:545–555. doi: 10.1016/0891-5849(91)90135-p. [DOI] [PubMed] [Google Scholar]
- Simon-Deckers A, Gouget B, Mayne-L’hermite M, Herlin-Boime N, Reynaud C, Carriere M. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology. 2008;253:137–146. doi: 10.1016/j.tox.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biology & Medicine. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Stone V, Johnston H, Clift MJ. Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions. IEEE Transactions on Nanobioscience. 2007;6:331–340. doi: 10.1109/tnb.2007.909005. [DOI] [PubMed] [Google Scholar]
- Veranth JM, Kaser EG, Veranth MM, Koch M, Yost GS. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Particle and Fibre Toxicology. 2007;4:2. doi: 10.1186/1743-8977-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB, McHugh TA, Hartsky MA. Differential pulmonary responses in rats inhaling crystalline, colloidal or amorphous silica dusts. Scandinavian Journal of Work Environment & Health. 1995;21 Suppl 2.:19–21. [PubMed] [Google Scholar]
- Weichenthal S, Dufresne A, Infante-Rivard C. Indoor ultrafine particles and childhood asthma: exploring a potential public health concern. Indoor Air. 2007;17:81–91. doi: 10.1111/j.1600-0668.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu C, Yang D, Zhang H, Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. Journal of Applied Toxicology. 2009;29:69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]