Abstract
Polyoma virus middle T antigen (PyVmT) is a powerful viral oncogene; however, the mechanisms of PyVmT activation are poorly understood. The IGF-I receptor (IGF-IR) and the insulin receptor (IR) are known to be implicated in the development of many cancers. Furthermore, PyVmT-overexpressing mouse mammary carcinoma Met-1 cells are highly responsive to IGF-I and insulin. Herein, we demonstrate that PyVmT physically interacts with IGF-IR and IR in Met-1 cells. Insulin and IGF-I increase association of the IR and IGF-IR with PyVmT, enhance tyrosine phosphorylation of PyVmT and augment the recruitment of Src and PLCγ1 to PyVmT. This is accompanied by robust and sustained phosphorylation of Akt and ERK1/2, which are implicated in both PyVmT and IGF-IR/IR signalling. Both ligands significantly increase proliferation, survival, migration and invasion of Met-1 cells. Furthermore, orthotopic inoculation of Met-1 cells with shRNAmir-mediated knockdown of IR or IGF-IR fails to initiate tumour growth in recipient mice. In conclusion, our data indicate that the physical and functional interaction between PyVmT and cellular receptor tyrosine kinases, including IR and IGF-IR, is critical for PyVmT activation and tumour initiation. These results also provide a novel mechanism for oncogene activation in the host cell.
Keywords: oncogenes, polyoma virus middle T oncogene, insulin receptor, IGF-I receptor, crosstalk
Introduction
It is estimated that over 20% of human cancers are attributed to infectious pathogens including viruses. Polyoma viruses possess potent oncogenic activity in many species; however, their tumourigenic potential in humans is under debate. It is well established that two human polyoma viruses, BK and JC viruses, are primarily associated with haemorrhagic cystitis and progressive multifocal leukencephalopathy, respectively, in severely immunocompromised patients. Until recently, the role of polyoma viruses in human cancer, however, has remained elusive. In 2007–2008 three new human polyoma viruses were discovered: KI virus, WU virus and Merkel cell virus (MCV) (zur Hausen, 2008). Interestingly, the MCV genome and activity were identified in the vast majority of tumour samples obtained from patients with Merkel cell carcinoma, which is a highly metastatic and aggressive form of skin cancer (Feng et al., 2008; Kassem et al., 2008; Foulongne et al., 2008). These findings support a possible carcinogenic role of polyoma viruses in human cancers.
Identified by Ludwig Gross, murine polyoma virus (MuPyV) demonstrates a robust tumourigenic activity in rodents (Gross, 1951; Gross, 1953) and is closely related to the recently discovered MCV (Feng et al., 2008). The tumourigenic role of MuPyV is primarily mediated by polyoma virus middle T antigen, a powerful viral oncogene (Dilworth, 2002). Transgenic overexpression of PyVmT in mouse mammary epithelium results in aggressive breast cancer with a short latency period and 100% penetrance (Guy et al., 1992). These mammary tumours demonstrate morphological and functional similarity with human breast cancer. The process of PyVmT-induced mammary carcinogenesis involves the transition from benign hyperplastic lesions to malignant, poorly differentiated solid tumours with high metastatic potential. In addition, a gradual loss of estrogen and progesterone receptors, and upregulation of ErbB2/Neu and cyclin D1 occurs in both human breast cancer and PyVmT-induced mammary carcinoma (Lin et al., 2003). The association between oncogenic viruses and human breast cancer has not been established yet. Given the high degree of similarity between human breast cancer and PyVmT-induced mouse mammary tumours, it is conceivable that a novel polyoma virus that has not yet been identified could be implicated in the development of human breast cancer. The oncogenic activity of PyVmT is mediated by mimicking classical tyrosine kinase receptors. In fact, PyVmT-induced downstream signalling has been well studied. Once PyVmT is incorporated into the cell membrane, it undergoes tyrosine phosphorylation and recruits several signalling intermediates such as PI3K, Shc, PLCγ1 and Src. Shc and the p85 regulatory subunit of PI3K link PyVmT to the MAPK and PI3K/Akt pathways, which play an important role in oncogenic transformation (Dilworth, 2002).
However, the mechanisms of PyVmT activation in the host cell are less understood. In contrast to tyrosine kinase receptors, PyVmT lacks intrinsic kinase activity suggesting that cellular tyrosine kinases are essential for oncogene activation. Indeed, it was demonstrated that the epidermal growth factor receptor (EGFR), which has been implicated in several cancers, phosphorylates and activates PyVmT (Segawa and Ito, 1983). In addition to EGFR, the insulin-like growth factor I receptor (IGF-IR) and the insulin receptor (IR) also play an important role in tumourigenesis (Yakar et al., 2005; Clemmons, 2007; Belfiore and Frasca, 2008; Pollak, 2008). In the current study, we provide experimental evidence that the IGF-IR and the IR physically and functionally interact with PyVmT. IGF-I and insulin enhance the interaction of their cognate receptors with PyVmT and augment its activation in mouse mammary carcinoma Met-1 cells derived from PyVmT transgenic breast tumours. Furthermore, we also show that shRNA-mediated knockdown of both the IGF-R and the IR impairs the oncogenic potential of Met-1 cells and abrogates their ability to initiate tumour formation in vivo.
Results
Sustained PyVmT expression in Met-1 mouse mammary carcinoma cells
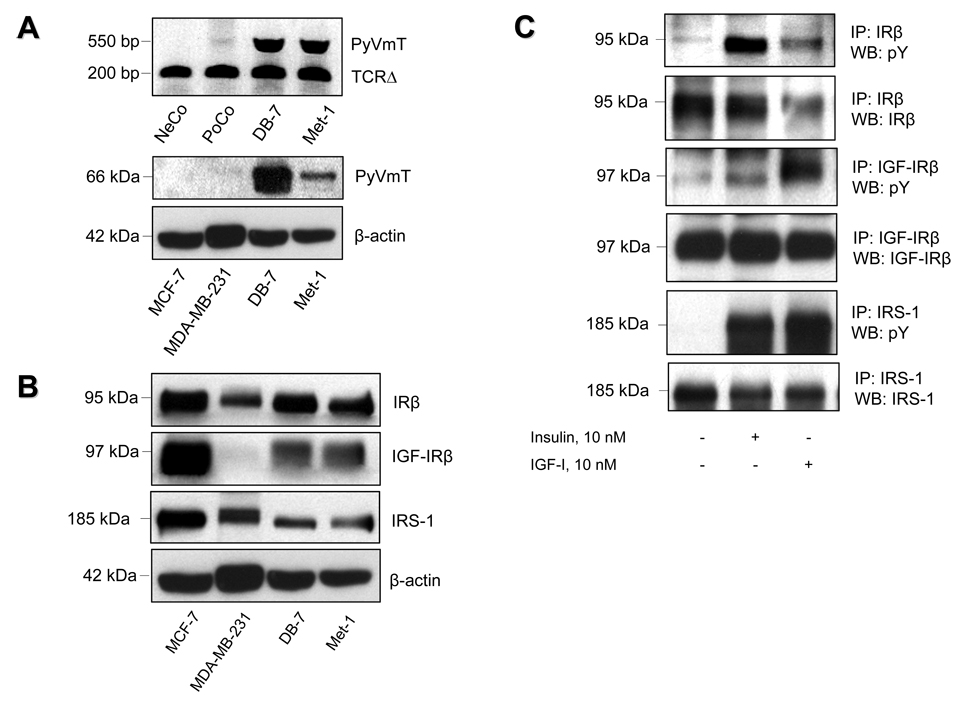
Met-1 and DB-7 cells were derived from two different lines of MMTV-PyVmT transgenic mice. To ensure that the PyVmT transgene was not lost during serial passages of these cells, PyVmT expression was tested by PCR and Western blot analysis. Both assays revealed a significant expression of the PyVmT transgene in Met-1 and DB-7 cells (Figure 1A).
Figure 1.
Expression of PyVmT, insulin and IGF-I receptors and their substrates in PyVmT-expressing mouse mammary carcinoma cells. (A) Upper panel: Total DNA obtained from DB-7 and Met-1 cells was subjected to PCR. Total DNA obtained from tails of wild type mice and PyVmT transgenic mice was used as negative control (NeCo) and positive control (PoCo), respectively. After PCR, amplified DNA was separated by 1% agarose gel electrophoresis, and subsequently subjected to ultraviolet shadowing. The presence of DNA in each sample was validated by PCR with primers specific for mouse T cell receptor delta (TCRΔ). Lower panel: Total protein (40 ug) extracted from whole cell lysates of human mammary carcinoma MCF-7 and MDA-MB-231 as well as from mouse mammary carcinoma DB-7 and Met-1 cells was size-fractionated by SDS-PAGE and immunoblotted with anti-PyVmT antibody. Equal loading of proteins was demonstrated by immunoblotting with an antibody directed against beta-actin. (B) Insulin receptor (IR), IGF-I receptor (IGF-IR) and insulin receptor substrate 1 (IRS-1) expression in DB-7 and Met-1 cells. Total protein (40 ug) extracted from whole cell lysates of human mammary carcinoma MCF-7 and MDA-MB-231 as well as from mouse mammary carcinoma DB-7 and Met-1 cells was size-fractionated by SDS-PAGE and immunoblotted with respective antibodies. Equal loading of proteins was demonstrated by immunoblotting with an antibody directed against beta-actin. (C) Insulin- and IGF-I-induced tyrosine phosphorylation of IR, IGF-IR and IRS-1 in Met-1 cells. After starvation in serum-free medium for 3 hours, Met-1 cells were treated with insulin or IGF-I (10 nM) for 5 minutes and immediately subjected to detergent lysis followed by immunoprecipitation (IP) of IRβ, IGF-IRβ or IRS-1. After SDS-PAGE and electroblotting, tyrosine phosphorylation of the precipitated proteins was evaluated by Western blot analysis (WB) with anti-phosphotyrosine antibody (anti-pY). Equal loading of proteins was ensured by re-probing the immunoblots with the antibodies used for immunoprecipitation.
Insulin receptor, IGF-I receptor and insulin receptor substrates 1 and 2 are robustly phosphorylated in Met-1 cells
Significant levels of insulin receptor (IR), IGF-I receptor (IGF-IR) and their major signalling intermediate, insulin receptor substrate 1 (IRS-1), were detected in Met-1 cells by Western blot analysis (Figure 1B). The activation of IR, IGF-IR and IRS-1 was evaluated by analysis of their tyrosine phosphorylation. For this purpose, lysates of Met-1 cells treated with insulin or IGF-I were subjected to immunoprecipitation of the β subunits of IR (IRβ) and IGF-1R (IGF-IRβ) as well as IRS-1 and immunoblotted with an anti-phosphotyrosine antibody. In Met-1 cells, the IRβ and the IGF-IRβ were robustly activated by their cognate ligands (Figure 1C). Moreover, IRS-1 phosphorylation was effectively induced by both insulin and IGF-I. Taken together, these data demonstrate that Met-1 cells express insulin and IGF-I receptors as well as their major adaptor protein, IRS-1, all of which are markedly phosphorylated in response to insulin or IGF-I.
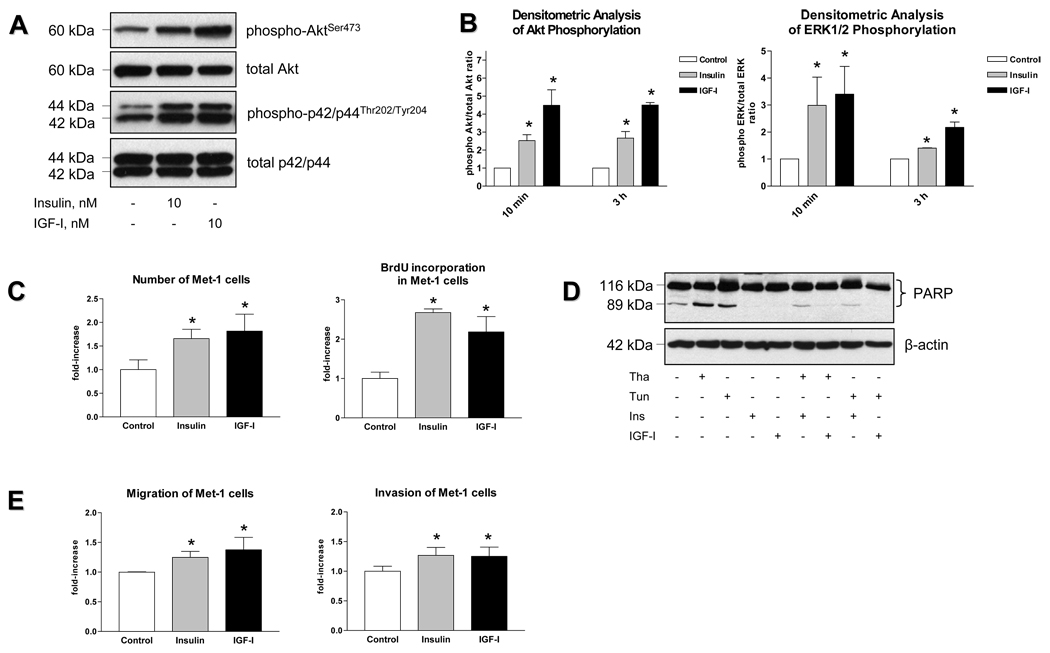
Sustained activation of PI3K and MAPK signalling pathways in Met-1cells
Activated PyVmT, IR and IGF-IR share common signalling pathways, the PI3K and the MAPK pathway. Therefore, when overexpressed, PyVmT may compete with IR and/or IGF-IR for binding to these signalling intermediates, thereby resulting in the attenuation of cellular response to insulin and/or IGF-I. Our data demonstrate that Met-1 cells, which overexpress PyVmT, are highly responsive to both insulin and IGF-I; treatment with these ligands results in sustained activation of the PI3K and the MAPK pathways as demonstrated by phosphorylation of AktS473 and ERK1/2T202/Y204 (Figure 2A, 2B). Treatment with insulin and IGF-I also increases the total number of Met-1 cells by enhancing mitogenesis (Figure 2C) and inhibiting apoptosis (Figure 2D). In addition, both ligands increase migration and invasion of Met-1 cells by 20–30% (Figure 2E) which is comparable to the effects of insulin and IGF-I in other breast cancer cell types (Bartucci et al., 2001). However, since the observed increase in migration and invasion rates can also be caused by proliferative effects of insulin and IGF-I, these results should be interpreted with caution.
Figure 2.
Biological response of Met-1 cells to insulin and IGF-I. (A) After serum starvation, mouse mammary carcinoma Met-1 cells were incubated in serum-free medium with insulin (10 nM) or IGF-I (10 nM) for 10 minutes or 3 hours. Control cultures were maintained in serum-free medium. Proteins (25 ug) extracted from whole cell lysates were size-fractionated by SDS-PAGE and immunoblotted with anti-phospho-p42/p44T202/Y204 and anti-phospho-AktS473 antibodies. Equal amounts of proteins were demonstrated by immunoblotting with antibodies directed against total p42/p44 and total Akt. The experiments were replicated three times, and autoradiographs representing the effect of insulin and IGF-I after 10 min treatment are presented. (B) The results of densitometric analysis of insulin and IGF-I-induced Akt and ERK1/2 phosphorylation are presented as a fold change compared to untreated cells. Statistically significant difference is indicated (*), P < 0.05 (Student’s t-test). (C) Effect of insulin and IGF-I on total cell number and DNA synthesis. After serum starvation, cells were incubated in serum-free medium with insulin (10 nM) or IGF-I (10 nM) for 24 hours. Total cell number was measured by direct counting using a hemocytometer, DNA synthesis was determined by BrdU incorporation assay. (D) Effect of insulin and IGF-I on apoptosis. Met-1 cells were incubated in the presence of thapsigargin (1000 nmol/L) or tunicamycin (5 ug/mL) and/or insulin (10 nmol/L) or IGF-I (10 nmol/L) for 6 hours. Proteins (25 ug) extracted from whole cell lysates were size-fractionated by SDS-PAGE and immunoblotted with anti-PARP antibodies. Apoptosis was evaluated by PARP cleavage. Equal loading of proteins was demonstrated by immunoblotting with an antibody directed against beta-actin. (E) Met-1 cells were incubated in serum-free medium with insulin (10 nM) or IGF-I (10 nM) for 20 and 36 hours to assess cell migration and invasion, respectively. Migration and invasion of Met-1 cells was analyzed by colorimetric cell migration and invasion assays. The results are presented as a fold change compared to untreated cells. Statistically significant difference is indicated (*), P < 0.05 (Student’s t-test).
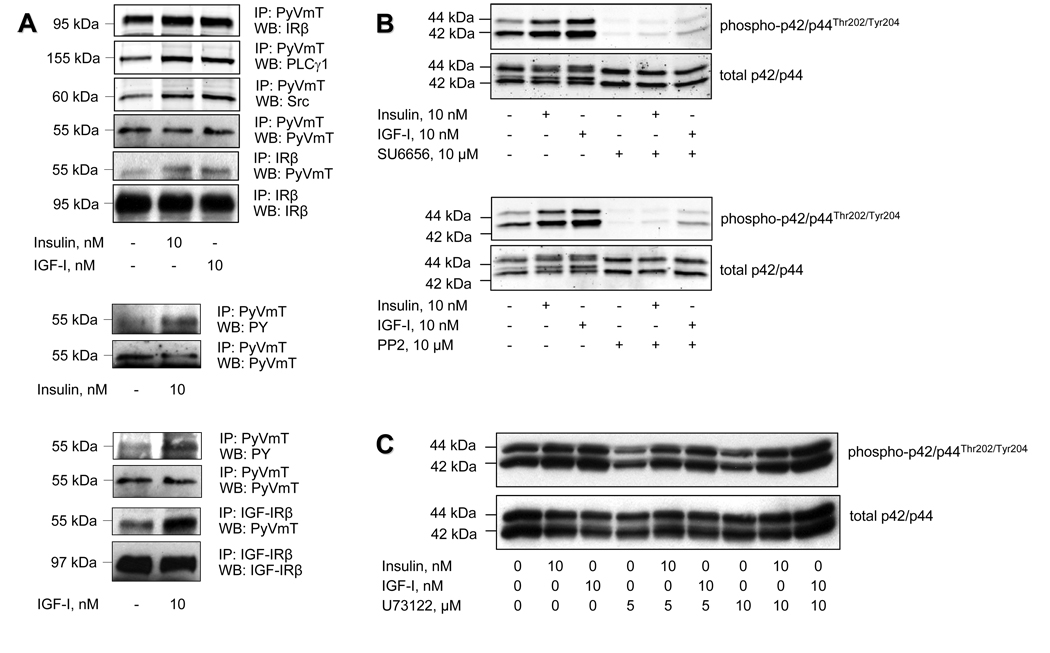
Bidirectional crosstalk between PyVmT and IR or IGF-IR
While PyVmT mimics the action of tyrosine kinase receptors, it lacks intrinsic tyrosine kinase activity and requires cellular tyrosine kinases for its activation. It was previously shown that the EGF receptor phosphorylates and thereby activates PyVmT (Segawa and Ito, 1983). Therefore, we hypothesized that IR and IGF-IR may play a similar role in Met-1 cells. Indeed, both receptors physically and functionally interact with PyVmT in Met-1 cells as demonstrated by co-precipitation studies (Figure 3A). Insulin and IGF-I enhance this interaction and increase PyVmT tyrosine phosphorylation. Moreover, recruitment of Src and PLCγ1, which are engaged by PyVmT is also augmented by insulin and IGF-I. However, Src is also important for insulin- and IGF-I-mediated signalling through the MAPK pathway, and blockade of Src with pharmacological inhibitors abrogates insulin- and IGF-I-induced ERK1/2 phosphorylation (Figure 3B), whereas PLC blockade does not affect MAPK signalling (Figure 3C). These results imply that in Met-1 cells there is mutual crosstalk between PyVmT and the IR and IGF-IR. The IR and IGF-IR are important for PyVmT activation and for recruitment of Src and PLCγ1 to promote tumourigenesis, whereas Src is required for insulin- and IGF-I-mediated activation of the MAPK pathway.
Figure 3.
Physical and functional interaction between PyVmT and insulin/IGF-I receptors in mouse mammary carcinoma Met-1 cells. (A) After starvation in serum-free medium for 3 hours, Met-1 cells were treated with insulin or IGF-I for 5 minutes and immediately subjected to detergent lysis followed by immunoprecipitation (IP) of PyVmT, IRβ or IGF-IRβ After SDS-PAGE and electroblotting, PyVmT tyrosine phosphorylation was evaluated by Western blot analysis (WB) of immunoprecipitated PyVmT with anti-phosphotyrosine antibody (anti-pY). Coprecipitation of PyVmT with IRβ, IGF-IRβ, Src or PLCγ1 was determined by WB of immunoprecipitated PyVmT with anti-IRβ, anti-IGF-IRβ, anti-Src or anti-PLCγ1 antibodies. Coprecipitation of PyVmT with IRβ was confirmed by WB of immunoprecipitated IRβ with anti-PyVmT antibodies. Equal loading of proteins was ensured by reprobing the immunoblots with the antibodies used for immunoprecipitation. (B, C) Met-1 cells were pre-incubated in serum-free medium in the presence of Src inhibitors SU6656 (10 uM) or PP2 (10 uM) (B) and PLC inhibitor U73122 (5 and 10 uM) (C) for 3 hours and were further incubated with insulin (10 uM) or IGF-I (10 nM) for 10 minutes. Control cultures were maintained in serum-free medium. Proteins (25 ug) extracted from whole cell lysates were size-fractionated by SDS-PAGE and immunoblotted with anti-phospho-p42/p44T202/Y204 antibodies. Equal level of proteins was demonstrated by immunoblotting with antibodies directed against total p42/p44.
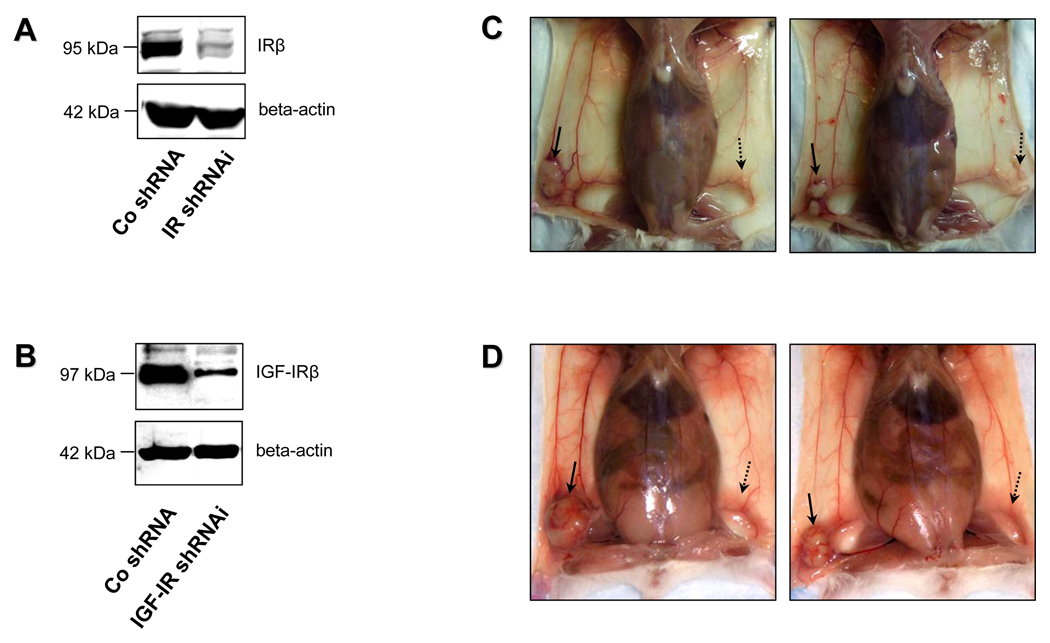
IGF-R and IR knockdown abrogates ability of Met-1 cells to initiate tumour formation in vivo
To further validate that IR and IGF-IR are essential for PyVmT-driven tumourigenesis in vivo, clones of Met-1 cells stably transfected with retroviral constructs encoding mouse IR and IGF-IR shRNAmir were employed. The efficiency of IR and IGF-IR knockdown in selected clones was confirmed by Western blot analysis (Figure 4A, 4B). Among 450 selected clones transfected with IR or IGF-IR shRNAmir, 16 were successfully propagated in culture; however, they demonstrated significantly impaired growth and higher rate of spontaneous apoptosis compared to clones transfected with control (scrambled) shRNA (data not shown). Implantation of Met-1 cells transfected with control shRNA (Figure 4C, Figure 4D, solid arrow) into the inguinal mammary fat pads of syngeneic FVB/N hosts (5–7 mice per group) resulted in tumour formation with 100% penetrance, whereas inoculation of cells with stable shRNAmir-mediated knockdown of IR or IGF-IR failed to initiate tumour growth (Figure 4C, 4D, dashed arrow). This experiment was reproduced twice, and identical results were also obtained with alternate clones of IR and IGF-IR knockdown cells. Taken together, these data further support our findings in vitro and strongly suggest that both IR and IGF-R play a critical role in PyVmT-induced tumour initiation in vivo.
Figure 4.
Effect of IR and IGF-IR knockdown on PyVmT-induced tumour growth in vivo. Efficiency of shRNAmir-mediated knockdown (KD) of the IR (A) and IGF-IR (B) was validated by Western blot analysis. Total protein (50 ug) extracted from whole cell lysates of mouse mammary carcinoma Met-1 cells stably transfected with retroviral constructs encoding scrambled shRNA, IR shRNAmir or IGF-IR shRNAmir was size-fractionated by SDS-PAGE and immunoblotted with IRP or IGF-IRP antibodies. Equal loading of proteins was demonstrated by immunoblotting with an antibody directed against beta-actin. (C, D) 500,000 Met-1 cells stably transfected with scrambled shRNA were injected into the right mammary fat pads of syngeneic FVB/N female mice (5–7 mice per group). The same number of cells transfected with IR shRNAmir or IGF-IR shRNAmir was implanted into the left mammary fat pad. 4 weeks after inoculation, the mice were sacrificed, and tumours arising from control (C, D, solid arrow), IR KD (C, dashed arrow) and IGF-IR KD (D, dashed arrow) cells were compared. Note that Met-1 cells with disrupted IR or IGF-IR expression failed to initiate tumour growth in the syngeneic recipients. Identical results were obtained with alternate clones of IR and IGF-IR knockdown cells.
Discussion
There is compelling evidence that viral infection plays an important role in the development and progression of many cancers (Morris et al., 1995). The causal role of viruses has been well documented for many human malignancies including Burkitt’s lymphoma, nasopharyngeal carcinoma, hepatocellular carcinoma, cervical cancer, T-cell leukemia and Kaposi’s sarcoma (Javier and Butel, 2008). Furthermore, the list of human oncogenic viruses continues to grow considerably due to advances in modern molecular technology. Polyoma viruses have been strong candidates as possible human tumourigenic viruses for a few decades. Genomic sequences of SV40, BK and JC polyoma viruses, which are tumourigenic in many species under experimental conditions, have been detected in many human tumours including prostate cancer, brain tumours, non-Hodgkin lymphoma, mesothelioma and osteosarcoma (White and Khalili, 2004). Their direct oncogenic role in humans, however, has never been proven. Interest in the tumourigenic potential of polyoma viruses has re-emerged due to the recent discovery of the Merkel cell virus (MCV), a novel human polyoma virus, which was recently identified in 80% of patients with Merkel cell carcinoma, an aggressive and metastatic form of skin cancer (Feng et al., 2008; Kassem et al., 2008; Foulongne et al., 2008). Interestingly, phylogenetic analysis revealed that MCV is closely related to the murine polyoma virus (MuPyV), which induces a variety of solid tumours in mice (Gross, 1951; Gross, 1953). It is well established that the tumourigenic potential of MuPyV is primarily caused by polyoma virus middle T antigen (PyVmT), which evolved millions of years ago (Soeda et al., 1980), and is one of the most powerful viral oncogenes.
The oncogenic activity of PyVmT is accomplished by mimicking the action of receptor tyrosine kinases, but it lacks intrinsic tyrosine kinase activity (Dilworth, 2002). PyVmT is incorporated into the cell membrane, and after tyrosine phosphorylation, it recruits several intracellular signalling intermediates such as PI3K, Shc, PLCγ1 and Src. While the mechanisms of its activation in the host cell are largely unknown, PyVmT seems to be dependent on cellular tyrosine kinases. It was previously shown that the EGF receptor (EGFR), which plays a key role in the development of many cancers (Hynes and Lane, 2005), phosphorylates and activates PyVmT (Segawa and Ito, 1983). Moreover, the presence of the EGFR is an absolute requirement for PyVmT-induced cell transformation (Ono et al., 1991).
In addition to EGF and the EGFR, insulin-like growth factor I (IGF-I), insulin and their receptors (IGF-IR and IR) have well established tumour-promoting effects (Pollak, 2008). High circulating IGF-I and insulin levels are associated with increased risk of many cancers, whereas low levels of these hormones are accompanied by delayed tumour development (Renehan et al., 2004; Yakar et al., 2005; Pisani, 2008). Cancer cells often demonstrate aberrant IGF-IR and/or IR expression, which results in elevated levels of the receptors in tumour tissue (Clemmons, 2007; Belfiore and Frasca, 2008). Moreover, it was previously demonstrated that several viral oncogenes (SV40 T antigen, papilloma virus E5 and E7 proteins, Kaposi’s sarcoma herpes virus oncogenes) are incapable of inducing tumour transformation in cells with disrupted IGF-IR (Baserga, 1999; Catrina et al., 2005). Taken together, these data strongly suggest that the IGF-IR is a key element in the process of cellular transformation. Numerous lines of evidence also suggest that the IR plays a similar role (Giorgino et al., 1991; Frittitta et al., 1995; Rose et al., 2007).
PyVmT is a potent activator of the PI3K/Akt and MAPK pathways (Dilworth, 2002), which are also utilized by IR and IGF-IR (LeRoith et al., 1995). Therefore, when overexpressed, it is feasible that PyVmT could compete with IR and/or IGF-IR for these signalling pathways and thus attenuate the cellular response to insulin and/or IGF-I. However, in the current study, we demonstrate that PyVmT-overexpressing Met-1 cells exert a marked responsiveness to both insulin and IGF-I. Therefore, we hypothesized that the IR and IGF-IR may be involved in PyVmT activation. Our results demonstrate that both receptors physically and functionally interact with PyVmT in Met-1 cells. Insulin and IGF-I augment the interaction of the IR and IGF-IR with PyVmT and induce phosphorylation of PyVmT on its tyrosine residues. These data are also supported by our findings in vivo. Implantation of Met-1 cells with abrogated IR or IGF-IR expression fails to induce tumour growth in syngeneic recipient mice. These results, therefore, provide compelling evidence that both receptors are critical for PyVmT activation and initiation of tumour growth and indicate that despite structural and functional similarities between the IR and the IGF-IR, these functions of the two receptors are non-redundant and cannot be mutually compensated. To our knowledge, this is the first report demonstrating a tumourigenic role of the IR in vivo. Moreover, we also show that the interaction of PyVmT with Src or PLCγ1 is increased in response to insulin and IGF-I. Both Src and PLCγ1 play a pivotal role in tumourigenesis (Guy et al., 1994; Shepard et al., 2007). It has been demonstrated previously that Src directly or indirectly associates with the IGF-IR and the IR (Lebrun et al., 1998; Arbet-Engels et al., 1999). Furthermore, in vascular smooth muscle cells, IGF-I activates Src kinase, which is essential for Shc activation and optimal MAPK activation (Lieskovska et al., 2006). In line with these data, our results show that Src, but not PLCγ1, is important for insulin-and IGF-I-mediated signalling through the MAPK pathway in the setting of PyVmT overexpression. These results imply that there is mutual crosstalk between PyVmT and the IR and IGF-IR in Met-1 cells. PyVmT requires the IR and IGF-IR for its activation and for further recruitment of Src and PLCγ1 to promote tumourigenesis, whereas Src is necessary for insulin-and IGF-I-mediated signal transduction through the MAPK pathway.
In conclusion, the present study provides insight into the mechanisms of PyVmT activation and identifies a unique role of the IGF-IR, IR and their ligands in this process (Figure 5). These findings thus complement the concept of the virocrine theory (DiMaio et al., 1998), which proposes the involvement of cellular signalling intermediates in virus-induced cell transformation. Our study further enhances this theory by demonstrating that products of viral oncogenes not only engage cellular downstream signalling intermediates but also utilize proximal signalling components (e.g. hormone and growth factor receptors such as IR and IGF-IR) for their activation in the host cell.
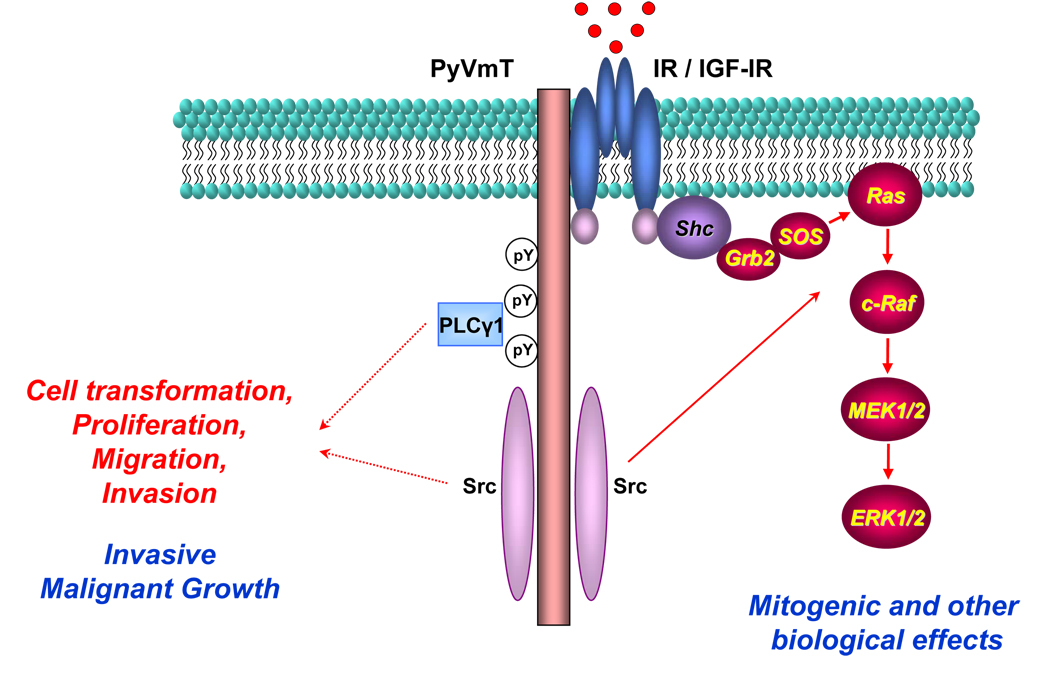
Figure 5.
The proposed model for the interaction between PyVmT and IR or IGF-IR. In the quiescent cell, PyVmT and IR or IGF-IR form a preexisting complex. Insulin and IGF-I activate their receptors, increase their association with PyVmT and stimulate its tyrosine phosphorylation. This enhances the recruitment of Src or PLCγ1, which are key oncogenic molecules in PyVmT-induced malignant transformation. In addition, Src is important for insulin-and IGF-I-mediated signalling through the MAPK pathway. These data imply that PyVmT physically and functionally interacts with IR and IGF-IR. PyVmT requires IR and IGF-IR for its full activation and for the recruitment of Src and PLCγ1 to promote tumourigenesis, whereas PyVmT-activated Src is necessary for insulin- and IGF-I-mediated signal transduction through the MAPK pathway.
Materials and Methods
Materials
Chemicals and materials were obtained from the following sources: PP2, U73122 (Alexis ® Biochemicals, San Diego, CA, USA); Trans-Blot nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), recombinant human insulin (Eli Lilly and Company, Indianapolis, IN, USA); pre-stained protein ladder RPN800, protein G sepharose 4 fast flow (GE Healthcare Piscataway, Piscataway, NJ, USA); recombinant human IGF-I (Genentech, San Francisco, CA, USA); bovine serum albumin (BSA), fetal bovine serum (FBS), L-glutamine, penicillin/streptomycin, trypsin, Novex® Tris-Glycine Gels, Benchmark™ (Invitrogen, Carlsbad, CA, USA); X-ray films BioMax MR films (Kodak, Rochester, NY, USA); Dulbecco’s modified Eagle medium (DMEM), improved MEM (IMEM) (Mediatech, Herndon, VA, USA); Arrest-IN™ transfection reagent (Open Biosystems, Huntsville, AL, USA); BCA protein assay, bovine albumin, SuperSignal® West Pico chemiluminescent substrate (Pierce, Indianapolis, IN, USA); Complete® protease inhibitor cocktail (Roche, Indianapolis, IN, USA); sodium fluoride, sodium orthovanadate, tetrasodium pyrophosphate, SU6656 (Sigma-Aldrich, Saint Louis, MO, USA). All other common reagents were purchased from Fisher Scientific (Pittsburg, PA, USA) or VWR (Westchester, PA, USA).
Antibodies
The following antibodies and sera were purchased from the following sources: rabbit polyclonal antibody raised against phospho-Akt (Ser 473, #9271), total Akt (#9271), phospho-p44/42 MAP kinase (Thr202/Tyr204, #9101), total p44/42 MAP kinase (#9102), PARP (#9542) rabbit monoclonal antibodies raised against Src (#2109) (Cell Signalling Technology. Danvers, MA, USA); rabbit polyclonal antibody against IRβ (sc-711), IGF-IRβ (sc-713), PLCγ1 (sc-81) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); mouse monoclonal antibody raised against polyoma virus middle T antigen (PyVmT, DP10L) (Calbiochem, San Diego, CA, USA); rabbit polyclonal antibody raised against phosphotyrosine (#06–427) and IRS-1 (#06–248) (Upstate,Lake Placid, NY, USA); mouse monoclonal antibody raised against β-actin (clone AC-15,#A5441) (Sigma-Aldrich, Saint Louis, MO, USA); secondary peroxidase-conjugated antibodies (GE Healthcare Piscataway, Piscataway, NJ, USA).
shRNAmir constructs
Frozen glycerol stocks of E.coli (PirPlus™ DH10βpir116) transformed with pSHAG-MAGIC 2 retroviral vectors were obtained from Open Biosystems. These E.coli clones contained shRNAmir against mouse IGF-IR (clones V2MM_23388 and V2MM_188397), mouse IR (clones V2MM_72722, V2MM_75164 and V2MM_79169) and non-silencing shRNA control (scrambled shRNA). Transformed E. coli clones were propagated in LB broth supplemented with chloramphenicol and kanamycin. Plasmid preparation was done by Qiagen Plasmid Maxi Kit (Qiagen, Valencia, CA, USA). Quality of pSHAG-MAGIC 2 vectors was validated by analytical restriction digestion with HindIII/XbaI.
Primers
Screening of mouse mammary carcinoma DB-7 and Met-1 cells for PyVmT was performed according to the Jackson Laboratories genotyping protocol (Tg(MMTV-PyVT)634Mul, Standard PCR, Version 1). The following primers were employed for this purpose: 5´-GGA AGC AAG TAC TTC ACA AGG G-3´ and 5´-GGA AAG TCA CTA GGA GCA GGG-3´ were used to amplify a 556 bp fragment of the PyVmT transgene, whereas 5´-CAA ATG TTG CTT GTC TGG TG-3´ and 5´-GTC AGT CGA GTG CAC AGT TT-3´ were applied to amplify a 200 bp fragment of T cell receptor delta (TCRΔ) gene from the wild type allele (internal control).
Animals
Wild-type mice on FVB/N background were housed and maintained in an animal facility at the Mount Sinai School of Medicine. Animals were kept on a 12-hour light/dark cycle with access to a standard laboratory chow diet and fresh water ad libitum.
Cell cultures and treatment
Mouse mammary carcinoma Met-1 and DB-7 cells derived from MMTV-PyVmT transgenic animals on FVB/N background were provided by S. Hurstings and K.W. Hunter, NCI, Bethesda, MD; human mammary carcinoma MCF-7 and MDA-MB-231 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Met-1, DB-7, and MDA-MB-231 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in 5% CO2 atmosphere and 95% humidity. MCF-7 cells were cultured in Improved modified Eagle’s medium (IMEM) supplemented with 10% foetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37 °C in 5% CO2 atmosphere and 95% humidity. Cells were allowed to grow until 80% confluence, and were then detached by trypsin and subcultured for several passages. Before the addition of stimuli, cell cultures were washed in phosphate-buffered saline (PBS), maintained in serum-free DMEM supplemented with 0.1% BSA for 3 hours, and subsequently incubated in the presence or absence of insulin (10 nM), IGF-I (10 nM), or a Src inhibitor (SU6656, 10 µM or PP2, 10 µM). After treatment, the cells were washed with ice cold PBS and further subjected to protein extraction.
shRNAmir-mediated knockdown of insulin and IGF-I receptors
Gene silencing was achieved by transfecting Met-1 cells with pSHAG-MAGIC 2 bacterial vectors encoding shRNAmir targeting mouse IR or IGF-IR mRNA (Open Biosystems). Transfection was performed according to instructions of the manufacturer (RNAintro™ shRNA Transfection Kit, Open Biosystems). Transfected cells were selected in DMEM supplemented with 0.5 µg/ml puromycin (Mediatech). IR and IGF-IR knockdown in individual clones of puromycin-resistant cells was validated by Western blot analysis.
Syngeneic Met-1 orthotopic tumour model
Met-1 cells stably transfected with control (scrambled), IR or IGF-IR shRNAmir were allowed to grow until confluence, detached by non-enzymatic cell dissociation solution, and 500,000 cells were resuspended in 100 µl of sterile PBS and injected into the left (cell transfected with scrambled shRNA) or right (cells transfected with IR or IGF-IR shRNAmir) inguinal mammary fat pads of 8 week-old wild-type FVB/N mice. To monitor orthograft growth, mammary fat pads were examined by finger palpation once a week. 4 weeks after cell inoculation mice were euthanized, and the size of Met-1 orthograft tumours was evaluated. Portions of the tumours were either snap-frozen in liquid nitrogen or stored in formalin for further studies.
Protein extraction and Western blot (WB) analysis
Cells were lysed in buffer (pH 7.4) containing 50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1.25% CHAPS, 1 mM sodium orthovanadate, 2 mM sodium fluoride, 10 mM sodium pyrophosphate, 8 mM β-glycerophosphate and Complete® Protease Inhibitor Cocktail. Concentrations of cellular proteins were determined by BCA protein assay using bovine albumin as a protein standard. 10–50 µg of cell lysates were resuspended in 3x loading buffer supplemented with DTT, denatured by boiling at 96°C, subjected to SDS-PAGE (8–10% acrylamide), and transferred to nitrocellulose membrane. The membrane was sequentially probed with primary and secondary antibodies, incubated with SuperSignal® West Pico chemiluminescent substrate, and exposed to X-ray films. Equal loading of proteins was confirmed by immunoblotting with antibodies to β-actin.
Immunoprecipitation (IP)
Cells were lysed and protein concentrations were determined as above. 5 µg of primary antibody was added to each sample (1000 µg of cell lysate) and allowed to incubate at 4°C overnight with end-over-end rotation. 200 µL of 30% Protein G Sepharose was added to each sample and incubated at 4°C for 4 hours to precipitate the desired immune complexes. The slurry was subjected to centrifugation at 13,000 rpm for 1 minute, the supernatant was removed, and the sepharose beads were collected. The pelleted sepharose was washed three times with 500 µl of ice-cold 50 mM Tris buffer (pH 7.4), and the proteins were resuspended in 50 µl of 3x loading buffer supplemented with DTT and boiled at 96°C for 5 minutes. Finally, dissociated sepharose beads were pelleted by centrifugation at 13,000 rpm for 30 seconds. Immunoprecipitates were then size-fractionated by SDS-PAGE and transferred to nitrocellulose membrane. First, the membrane was subjected to immunoblotting with anti-phosphotyrosine antibodies or the appropriate antibody to detect the presence of co-immunoprecipitates. Equal amount of precipitated protein was ensured by reblotting with the antibody used for protein precipitation.Prior to co-precipitation studies, the specificity of PyVmT immunoprecipitation protocol was validated. We failed to detect the 55 kDa band corresponding to PyVmT when PyVmT-negative protein extracts (mouse kidney lysates) or control isotype-specific antibody (mouse IgG1) were used.
Proliferation, migration and invasion assays
Met-1 cells were plated on 60 mm diameter dishes (150,000 cells per dish). The cells were starved in serum-reduced DMEM supplemented with 0.3% FBS for 1 hour and then stimulated with insulin or IGF-I for 24 hours. Proliferation in response to insulin (10 nM) or IGF-I (10 nM) was quantified by direct cell counting using a hemocytometer. DNA synthesis in Met-1 cells was measured by 5-bromo-2’-deoxyuridine incorporation assay (Roche). The effect of insulin and IGF-I on migration and invasion of Met-1 cells was analyzed by colorimetric cell migration and invasion assays according to the manufacturer’s instructions (Chemicon, Temecula, CA). Cells were cultured on 24 well plates (300,000 cells per well) in serum-reduced DMEM supplemented with 0.3% FBS in the presence or absence of insulin (10 nM) or IGF-I (10 nM) for 20 or 36 hours.
Densitometric analysis
Densitometric analysis was performed using MacBAS V2.52software.
Statistical analysis
Results are expressed as the mean ± SEM. Statistical analyses were calculated using the student’s t-test in Microsoft Excel.
Acknowledgements
We thank S.D. Hurstings (Department of Nutritional Sciences, University of Texas, Austin, TX, and Department of Carcinogenesis, University of Texas - M.D. Anderson Cancer Center, Smithville, TX, USA) and N.P.Nunez (Department of Nutritional Sciences, University of Texas, Austin, TX, USA) for donating Met-1 cells. This work was funded by National Institutes of Health/National Cancer Institute grant 1RO1CA128799-O1A1. Danielle Lann was supported by National Institutes of Health grant T32 DK007792. Yvonne Fierz was funded by Swiss National Science Foundation grant PBBSB-120851 and Novartis Foundation grant.
Reference List
- Arbet-Engels C, Tartare-Deckert S, Eckhart W. C-terminal Src kinase associates with ligand-stimulated insulin-like growth factor-I receptor. J. Biol. Chem. 1999;274:5422–5428. doi: 10.1074/jbc.274.9.5422. [DOI] [PubMed] [Google Scholar]
- Bartucci M, Morelli C, Mauro L, Ando S, Surmacz E. Differential insulin-like growth factor I receptor signaling and function in estrogen receptor (ER)-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells. Cancer Res. 2001;61:6747–6754. [PubMed] [Google Scholar]
- Baserga R. The IGF-I receptor in cancer research. Exp. Cell Res. 1999;253:1–6. doi: 10.1006/excr.1999.4667. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F. IGF and insulin receptor signaling in breast cancer. J. Mammary. Gland. Biol. Neoplasia. 2008;13:381–406. doi: 10.1007/s10911-008-9099-z. [DOI] [PubMed] [Google Scholar]
- Catrina SB, Lewitt M, Massambu C, Dricu A, Grunler J, Axelson M, et al. Insulin-like growth factor-I receptor activity is essential for Kaposi's sarcoma growth and survival. Br. J. Cancer. 2005;92:1467–1474. doi: 10.1038/sj.bjc.6602408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat. Rev. Drug Discov. 2007;6:821–833. doi: 10.1038/nrd2359. [DOI] [PubMed] [Google Scholar]
- Dilworth SM. Polyoma virus middle T antigen and its role in identifying cancer-related molecules. Nat. Rev. Cancer. 2002;2:951–956. doi: 10.1038/nrc946. [DOI] [PubMed] [Google Scholar]
- DiMaio D, Lai CC, Klein O. Virocrine transformation: the intersection between viral transforming proteins and cellular signal transduction pathways. Annu. Rev. Microbiol. 1998;52:397–421. doi: 10.1146/annurev.micro.52.1.397. [DOI] [PubMed] [Google Scholar]
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulongne V, Kluger N, Dereure O, Brieu N, Guillot B, Segondy M. Merkel cell polyomavirus and Merkel cell carcinoma, France. Emerg. Infect. Dis. 2008;14:1491–1493. doi: 10.3201/eid1409.080651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frittitta L, Vigneri R, Stampfer MR, Goldfine ID. Insulin receptor overexpression in 184B5 human mammary epithelial cells induces a ligand-dependent transformed phenotype. J. Cell Biochem. 1995;57:666–669. doi: 10.1002/jcb.240570411. [DOI] [PubMed] [Google Scholar]
- Giorgino F, Belfiore A, Milazzo G, Costantino A, Maddux B, Whittaker J, et al. Overexpression of insulin receptors in fibroblast and ovary cells induces a ligand-mediated transformed phenotype. Mol. Endocrinol. 1991;5:452–459. doi: 10.1210/mend-5-3-452. [DOI] [PubMed] [Google Scholar]
- Gross L. "Spontaneous" leukemia developing in C3H mice following inoculation in infancy, with AK-leukemic extracts, or AK-embrvos. Proc. Soc. Exp. Biol. Med. 1951;76:27–32. [PubMed] [Google Scholar]
- Gross L. A filterable agent, recovered from Ak leukemic extracts, causing salivary gland carcinomas in C3H mice. Proc. Soc. Exp. Biol. Med. 1953;83:414–421. doi: 10.3181/00379727-83-20376. [DOI] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy CT, Muthuswamy SK, Cardiff RD, Soriano P, Muller WJ. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 1994;8:23–32. doi: 10.1101/gad.8.1.23. [DOI] [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Javier RT, Butel JS. The history of tumor virology. Cancer Res. 2008;68:7693–7706. doi: 10.1158/0008-5472.CAN-08-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68:5009–5013. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- Lebrun P, Mothe-Satney I, Delahaye L, Van OE, Baron V. Insulin receptor substrate-1 as a signaling molecule for focal adhesion kinase pp125(FAK) and pp60(src) J. Biol. Chem. 1998;273:32244–32253. doi: 10.1074/jbc.273.48.32244. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr. Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- Lieskovska J, Ling Y, Badley-Clarke J, Clemmons DR. The role of Src kinase in insulin-like growth factor-dependent mitogenic signaling in vascular smooth muscle cells. J. Biol. Chem. 2006;281:25041–25053. doi: 10.1074/jbc.M602866200. [DOI] [PubMed] [Google Scholar]
- Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JD, Eddleston AL, Crook T. Viral infection and cancer. Lancet. 1995;346:754–758. doi: 10.1016/s0140-6736(95)91510-9. [DOI] [PubMed] [Google Scholar]
- Ono M, Kuwano M, Kung HF. Malignant transformation of mouse BALB/3T3 cells by polyoma middle T antigen requires epidermal growth factor receptor expression. Cell Growth Differ. 1991;2:317–322. [PubMed] [Google Scholar]
- Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch. Physiol Biochem. 2008;114:63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- Rose PP, Carroll JM, Carroll PA, DeFilippis VR, Lagunoff M, Moses AV, et al. The insulin receptor is essential for virus-induced tumorigenesis of Kaposi’s sarcoma. Oncogene. 2007;26:1995–2005. doi: 10.1038/sj.onc.1210006. [DOI] [PubMed] [Google Scholar]
- Segawa K, Ito Y. Enhancement of polyoma virus middle T antigen tyrosine phosphorylation by epidermal growth factor. Nature. 1983;304:742–744. doi: 10.1038/304742a0. [DOI] [PubMed] [Google Scholar]
- Shepard CR, Kassis J, Whaley DL, Kim HG, Wells A. PLC gamma contributes to metastasis of in situ-occurring mammary and prostate tumors. Oncogene. 2007;26:3020–3026. doi: 10.1038/sj.onc.1210115. [DOI] [PubMed] [Google Scholar]
- Soeda E, Maruyama T, Arrand JR, Griffin BE. Host-dependent evolution of three papova viruses. Nature. 1980;285:165–167. doi: 10.1038/285165a0. [DOI] [PubMed] [Google Scholar]
- White MK, Khalili K. Polyomaviruses and human cancer: molecular mechanisms underlying patterns of tumorigenesis. Virology. 2004;324:1–16. doi: 10.1016/j.virol.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Yakar S, LeRoith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: Lessons from animal models. Cytokine Growth Factor Rev. 2005;16:407–420. doi: 10.1016/j.cytogfr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Novel human polyomaviruses--re-emergence of a well known virus family as possible human carcinogens. Int. J. Cancer. 2008;123:247–250. doi: 10.1002/ijc.23620. [DOI] [PubMed] [Google Scholar]