Abstract
Purpose
Bevacizumab is an antibody against vascular endothelial growth factor (VEGF); sunitinib is an inhibitor of VEGF and related receptors. The safety and maximum tolerated dose (MTD) of sunitinib plus bevacizumab was assessed in this phase I trial.
Experimental Design
Patients with advanced solid tumors were treated on a 3+3 trial design. Patients received sunitinib daily (starting dose level 25 mg) for 4 weeks on followed by 2 weeks off and bevacizumab (starting dose level 5 mg/kg) on days 1, 15 and 29 of a 42-day cycle. Dose-limiting toxicities (DLTs) during the first 6-week cycle were used to determine the MTD.
Results
Thirty-eight patients were enrolled. Pts received a median of 3 cycles of treatment (range, 1–17+). There was one DLT (grade 4 hypertension) at 37.5 mg sunitinib and 5 mg/kg bevacizumab. Grade 3 or greater toxicity was observed in 87% of patients including hypertension (47%), fatigue (24%), thrombocytopenia (18%), proteinuria (13%), and hand-foot syndrome (13%). Dose modifications and delays were common at higher dose levels. No clinical or laboratory evidence of microangiopathic hemolytic anemia was observed. Seven patients had a confirmed RECIST-defined PR (18%; 95% confidence interval: 8–34%). Nineteen of the 32 patients with a post-baseline scan (59%) had at least some reduction in overall tumor burden (median 32%, range 3–73%).
Conclusions
The combination of sunitinib and bevacizumab in patients with advanced solid tumors is feasible, albeit with toxicity at higher dose levels and requiring dose modification with continued therapy. Anti-tumor activity was observed across multiple solid tumors.
Keywords: bevacizumab, sunitinib, phase I
Translational Relevance
This phase I study examined the safety and clinical effect of combination therapy against the VEGF pathway. Sunitinib, a VEGF receptor inhibitor, and bevacizumab, a VEGF ligand inhibitor, were combined in patients with a variety of advanced solid tumor malignancies. The combination of these drugs was able to be given at the highest dose levels tested, although with notable toxicities with prolonged therapy. Significant anti-tumor activity was observed, notably in tumors usually refractory with standard chemotherapy. Thus, combined VEGF pathway inhibition is feasible, and may be a strategy to overcome therapy resistance in select solid tumors.
Introduction
Vascular endothelial growth factor (VEGF) is a potent pro-angiogenic protein which is upregulated in many solid tumors.(1) Inhibition of VEGF, either via antibody-mediated VEGF binding or small molecule inhibition of the VEGF receptor (VEGF-R) family, has demonstrated clinically-relevant benefits across solid tumors.(2–6) Despite these advances, not all patients respond, complete responses are rare and eventual tumor resistance to VEGF-targeted therapy is nearly universal. Maximal inhibition of the VEGF pathway through a combination of VEGF-targeting agents with different mechanisms may allow for an enhanced anti-tumor effect.
Sunitinib (Sutent®, Pfizer Inc., New York, NY) is a small molecule tyrosine kinase inhibitor of a family of receptors including VEGF-R. Phase II and III trials have demonstrated significant anti-tumor activity of sunitinib as a single agent in metastatic renal cell carcinoma (RCC) and imatinib-refractory gastrointestinal stromal tumors (GIST) (5, 7–9) Common sunitinib toxicity includes fatigue, hand-foot syndrome, diarrhea, hypertension and hypothyroidism.(10)
An alternative approach to VEGF blockade involves bevacizumab (Avastin®, Genentech, South San Francisco, CA), a monoclonal antibody that binds and neutralizes circulating VEGF protein.(11) Bevacizumab has demonstrated anti-tumor activity both as monotherapy and in combination with interferon in metastatic RCC.(3, 12, 13) Bevacizumab has also shown significant clinical benefit in combination with chemotherapy in metastatic colorectal, lung and breast cancer.(4, 6, 14) Common bevacizumab toxicity includes hypertension and proteinuria.
Additive or synergistic VEGF blockade may be achieved through simultaneous targeting of the VEGF pathway. Bevacizumab leads to rapid clearance of circulating VEGF, but is not known to affect VEGF bound to receptor, as the epitope on VEGF recognized by bevacizumab is in the VEGF-R binding region. Further, other pro-angiogenic molecules may contribute to the angiogenic phenotype of solid tumors and require inhibition. Despite of the inhibition of the VEGF receptor, sunitinib leads to a compensatory plasma VEGF increase and may leave tumors exposed to VEGF effects during the 2 week off period of each cycle.(15) It is possible that a more maximal, constant and effective VEGF blockade will be achieved with therapeutics directed against both the VEGF ligand and receptor, resulting in an enhanced anti-tumor effect.
METHODS
Patients
The study population consisted of patients ≥ 18 years old with a histologically-proven solid tumor malignancy not amenable to curative therapy. Patients were required to have a Karnofsky performance status of ≥ 60% and adequate bone marrow, hepatic and renal function (as defined by granulocytes ≥ 1,500/µL, hemoglobin ≥10.0 g/dL, platelet count ≥ 100,000/L, AST/ALT ≤ 2.5 × upper limit of normal (ULN), serum bilirubin ≤ 1.5 × ULN, urine protein creatinine (UPC) ratio as determined by urinalysis < 0.5 (for UPC ratio > 0.5, 24-hour urine protein must have been <1 gram) and serum creatinine ≤ 1.5 × ULN).
Patients with prior sunitinib or bevacizumab therapy were ineligible; there was no limit on the number of other prior systemic therapy regimens provided resolution of all acute toxic effects of prior (≥ 4 weeks) systemic therapy, radiotherapy or surgical toxicity to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0 grade ≤ 1. Patients with the following conditions were also excluded: squamous cell histology or any histology in close proximity to a major blood vessel, major surgical procedure, open biopsy or significant traumatic injury within 28 days, need for major surgical procedures during the study or biopsy within 7 days, evidence of bleeding diathesis or coagulopathy, history of or known brain metastases, spinal cord compression, carcinomatous meningitis, hypertension that could not be controlled by medications to < 140/90 mmHg, a history of abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess within the previous 28 days, serious, non-healing wound, ulcer, or bone fracture, known hypersensitivity of Chinese hamster ovary cell products or other recombinant human antibodies or known human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS)-related illness. Patients with any of the following within the 12 months prior to study were excluded: myocardial infarction, severe/unstable angina, severe peripheral vascular disease (claudication) or peripheral vasculature surgery, coronary/peripheral artery bypass graft, New York Heart Association (NYHA) grade II or greater congestive heart failure, cerebrovascular event, clinically significant bleeding, deep venous thrombosis or pulmonary embolism. Ongoing cardiac dysrhythmias of NCI CTCAE grade ≥ 2, atrial fibrillation of any grade, or prolongation of the QTc interval to >450 milliseconds (males) or >470 milliseconds (females), in addition to any history of ventricular arrhythmia, were excluded. Patients on full-dose anticoagulants were not eligible; however patients receiving low-dose anticoagulation therapy were eligible.
The protocol was approved by the Case Comprehensive Cancer Center Institutional Review Board (IRB), and all patients provided written informed consent.
Study Design
Bevacizumab and sunitinib were provided by the U.S. National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP). Bevacizumab was administered intravenously on day 1, 15 and 29 of each 42-day cycle. Sunitinib was administered orally on days 1–28 of a 42-day cycle. Patients underwent a history and physical exam with laboratory assessments (including complete blood count and chemistries at baseline, on day 1, 15 and 29 of cycle one and on days 1 and 29 of every subsequent cycle). Thyroid function tests (TFTs; including TSH, T3, T4 and free thyroxine index (FTI)) were performed at baseline and day 1 of every other cycle. Due to a safety signal of microangiopathic hemolytic anemia observed on a separate phase I trial of this combination conducted exclusively in metastatic renal cell carcinoma patients(16), additional laboratory monitoring was instituted as of January 2008 for the 10 patients on study at that time and 7 patients enrolled after this date to include the following: hematopathologist review of the peripheral blood smear, reticulocyte count, haptoglobin, and direct bilirubin drawn at the time of other laboratory analyses.
The initial dose level was arbitrarily chosen to be 50% of standard doses: 25 mg of sunitinib and 5 mg/kg of bevacizumab. Cohorts of 3–6 patients received therapy at each dose level based on the occurrence of dose-limiting toxicities (DLTs). Adverse events were graded according to the NCI CTCAE Version 3.0. DLTs were defined as any of the following that occurred within the first 42 days of treatment and were attributable to therapy: any grade 4 toxicity (except lymphopenia or increased uric acid), any grade 3 cardiac event (except hypertension) or grade 3 venous thrombosis, hypertension unable to be controlled to < 160/90 mmHg within 4 weeks by oral medications, any grade arterial thromboembolic event, any grade 3 non-cardiac toxicity that did not resolve to ≤ grade 1 within 6 weeks (except proteinuria, lymphopenia, hypophosphatemia, and asymptomatic hyperamylasemia / hyperlipasemia) or proteinuria > 3.5 gm/24 hours.
The protocol stipulated expansion of the MTD dose level to a total of at least 12 patients. After further discussion with CTEP during the course of the trial, dose level 2.5 (sunitinib 50 mg and bevacizumab 5 mg/kg) was added to accrue 12 patients to expand the safety experience with this regimen.
Patients experiencing a DLT had all therapy held until toxicity resolved to ≤ grade 1. For ≥ grade 3 toxicity outside the DLT evaluation period, sunitinib and/or bevacizumab were held per established guidelines for each agent, and reinstated upon resolution of toxicity to grade 1 or less. Dose reduction of sunitinib was permitted for subsequent cycles depending on the severity of toxicity. Bevacizumab was not dose-reduced, but subsequent doses could be held for toxicity. If toxicity required discontinuation of one drug (e.g. hand-foot syndrome due to sunitinib), the other drug could be continued as monotherapy at physician. There was no intra-patient dose-escalation.
Response and progression were assessed according to the RECIST criteria(17) and were determined by investigator assessment of radiographs performed at baseline and every 12 weeks.
Statistical Design and Data Analysis
The primary goal of this study was to determine the MTD of bevacizumab plus sunitinib using a standard 3+3 design. Two or more patients in a given cohort experiencing a DLT would exceed the MTD, while 0 or 1 patient with a DLT would define the MTD of this regimen. With this design, there was a ≥71% chance of escalating the combination if the underlying risk of DLT was ≤20% and a ≥91% chance of escalation if the underlying risk was ≤10%. In contrast, there was at most a 17% chance of escalation if the underlying DLT risk was ≥50% and ≤8% chance if the risk was ≥60%.
RESULTS
Patients
Between August 2006 and March 2008, 38 patients with advanced malignancies were enrolled on this study, with more than two thirds of patients having received at least one prior systemic treatment regimen (Table 1).
Table 1.
Patient Characteristics
| Characteristic | Number of Patients (n=38) |
|---|---|
| Gender | |
| Male | 22 (58%) |
| Female | 16 (42%) |
| Median Age, years (range) | 55 (25–83) |
| ECOG performance status | |
| 0 | 22 (58%) |
| 1 | 16 (42%) |
| Disease type | |
| Renal cell carcinoma | 6 (16%) |
| Adrenocortical carcinoma | 5 (13%) |
| Melanoma (1 uveal) | 4 (11%) |
| Non-small cell lung | 3 (8%) |
| Thyroid | 3 (8%) |
| Transitional cell carcinoma (bladder) | 3 (8%) |
| Prostate | 2 (5%) |
| Testis | 2 (5%) |
| Carcinoid (intestine) | 2 (5%) |
| Breast | 2 (5%) |
| Other* | 6 (16%) |
| Previous systemic therapy regimens | |
| 0 | 11 (29%) |
| 1 | 13 (34%) |
| 2 | 9 (24%) |
| 3 or more | 5 (13%) |
| Previous radiation therapy | 13 (34%) |
Treatment Administration
Pts received a median of 3 cycles of treatment (range, 1–17+) (Table 2). A total of 47% of patients received at least 4 cycles of therapy, including 13 of the 25 patients (52%) treated at the two highest dose levels. In general, escalated dose levels produced more toxicity and required more dose modifications. Thirty-five patients (92%) have discontinued treatment primarily for disease progression (19 patients; 50%) and either protocol-defined toxicity or patient withdrawal of consent due to adverse events not meeting criteria for study removal (16 patients; 42%).
Table 2.
Dose levels and drug delivery
| Dose Level |
Sunitinib dose |
Bevacizumab dose |
No. of patients enrolled |
Median (range) cycles received |
No. of pts with dose modification |
Sunitinib dose alterationa |
Bevacizumab doses held |
|---|---|---|---|---|---|---|---|
| Level 0 | 25 mg | 5 mg/kg | 3 | 2 (2–8) | 33% (1/3) | 42% (5/12) | (0%) 0/33 |
| Level + 1 | 37.5 mg | 5 mg/kg | 7 | 3 (1–10) | 29% (2/7) | 4% (1/28) | 4% (3/74) |
| Level + 2 | 37.5 mg | 10 mg/kg | 3 | 13 (2–17+) | 67% (2/3) | 38% (12/32) | 11% (10/94) |
| Level + 2.5 | 50 mg | 5 mg/kg | 13 | 2 (1–8) | 62% (8/13) | 42% (20/48) | 18% (24/130) |
| Level + 3 | 50 mg | 10 mg/kg | 12 | 4 (1–17+) | 75% (9/12) | 52% (39/75) | 13% (25/199) |
| Overall | - | - | 38 | 3 (1–17+) | 58% (22/38) | 39% (77/195) | 12% (62/530) |
Percent of cycles in which sunitinib was dose- reduced or doses missed for toxicity
Safety and MTD determination
There was one DLT (grade 4 headache and hypertension) at dose level 37.5 mg sunitinib and 5 mg/kg bevacizumab. This patient had a diagnosis of carcinoid syndrome and baseline hypertension requiring two anti-hypertensive medications, but did not have prior hypertensive episodes. Increasing systolic blood pressure was noted on day 3 of treatment and metoprolol dose was increased. Four days later, the patient reported severe headache with a 236/96 mmHg blood pressure and emesis. Brain imaging was negative and sunitinib was held. The patient was admitted the following day with change in mental status, blood pressure of 226/100 mmHg and was started on nitroprusside. Blood pressure normalized, symptoms resolved in approximately 2 days, and the patient was discharged on four anti-hypertensive medicines and removed from study. This cohort was expanded to 7 total patients (6 additional patients were enrolled as the second patient in this cohort came off study for disease progression prior to the end of the first cycle) without occurrence of another DLT. No other protocol-defined DLT was observed at any dose level. Thus, the protocol-defined MTD was 50 mg sunitinib 4 weeks on / 2 week off in combination with 10 mg/kg of bevacizumab every 2 weeks. As noted above, however, patients treated at the higher dose levels experienced more toxicity such as hypertension, fatigue and cytopenias, and often required dose reduction or delay with continued therapy (Table 2, 3).
Table 3.
Grade 2 or Greater Toxicity at Least Possibly Related to Therapy (all cycles)
| Sunitinib 25mg Bevacizumab 5mg/kg |
Sunitinib 37.5mg Bevacizumab 5mg/kg |
Sunitinib 37.5mg Bevacizumab 10mg/kg |
Sunitinib 50mg Bevacizumab 5mg/kg |
Sunitinib 50mg Bevacizumab 10mg/kg |
|
|---|---|---|---|---|---|
| Dose level | 0 | 1 | 2 | 2.5 | 3 |
| No. of patients | 3 | 7 | 3 | 13 | 12 |
| DLTs | None | 1 G4 hypertension |
None | None | None |
| Hypertension | -- | 1 G2, 4 G3, 1 G4 | 2 G3 | 2 G2, 4 G3 | 7 G3 |
|
Hand - Foot Syndrome |
1 G3 | 2 G2 | 1 G3 | 2 G3 | 1 G3 |
| Proteinuria | -- | 1 G2 | 1 G2, 1 G3 | 1 G2, 2 G3 | 4 G2, 2 G3 |
| Fatigue | 1 G2 | 2 G2, 2 G3, 1 G4 | 2 G2, 1 G3 | 6 G2, 2 G3,1 | 4 G2, 2 G3 |
| Thrombosis | -- | 1 G3 DVT | -- | 1 G3 DVT | -- |
| Hemorrhage | -- | 2 G2 | -- | 2 G2 | 1 G2, 1G4 |
| Nausea | 1 G2 | 3 G2 | 1 G3 | 1 G2, 2 G3 | 3 G2 |
| Diarrhea | -- | -- | 2 G2 | 2 G2, 1 G3 | 2 G2 |
| Mucositis | -- | 2 G2 | -- | 3 G2 | -- |
| Anorexia | 2 G2 | 2 G2, 1 G3 | -- | 1 G2 | 4 G2, 1G3 |
| GI Fistula | -- | -- | -- | 1 G2 | 1 G2 |
| Hypothyroidism | -- | 1 G2 | -- | 1 G2 | 3 G2 |
| Hematologic | |||||
| Anemia | 1 G2 | 1 G2 | -- | 1 G2 | 1 G2, 1 G4 |
| Neutropenia | 1 G2 | 2 G2 | 1 G2 | 2 G2, 2 G3,1 G4 | 2 G2, 2 G3 |
| Low platelets | 1 G2 | -- | 2 G2 | 2 G2, 3 G3 | 3 G3, 1 G4 |
| Lymphopenia | -- | 2 G3 | 2 G2 | 2 G2 | 1 G2, 1G3 |
| 3 G3 | 1 G2 | 1 G2 | |||
| Overall Worst | 2 G2 | 5 G3 | 8 G3 | 9 G3 | |
| Grade Toxicity | 1 G3 | 2 G4 | 3 G4 | 2 G4 | |
Other notable severe toxicity included anal fissure development in one patient (dose level 2.5, adrenocortical cancer), and anal fistula in two patients (1 at dose level 2.5, adrenocortical cancer; 1 at dose level 3, melanoma). The patient with anal fissure did not have known underlying bowel pathology, was diagnosed at end of cycles 2 and managed conservatively with dose modification. The second patient with anal fissue at end of cycle 3 subsequently underwent surgical repair. Both patients continued on therapy with slow resolution before coming off study for progressive disease. The third patient had tumor necrosis in a rectal wall tumor felt to be responsible for the fistula at end of cycle 4. The patient came off study and underwent successful surgical repair. No other cases of bowel perforation were observed.
Most patients (87%) experienced at least one toxicity that was grade 3 or higher (Table 3). The most commonly reported adverse event (all grades) was fatigue, which occurred in 84% of patients. Other treatment-related adverse events included thrombocytopenia (74%), nausea and vomiting (66%), elevated SGOT/SGPT (61%), hypertension (59%), leukopenia (58%), mucositis (55%) and anemia (55%).
No clinical or laboratory evidence of microangiopathic hemolytic anemia was observed. No schistocytes were observed on any peripheral blood smear, nor did any patient have evidence of hemolysis such as a decreased haptoglobin, elevated reticulocyte count or elevated indirect bilirubin.
Efficacy
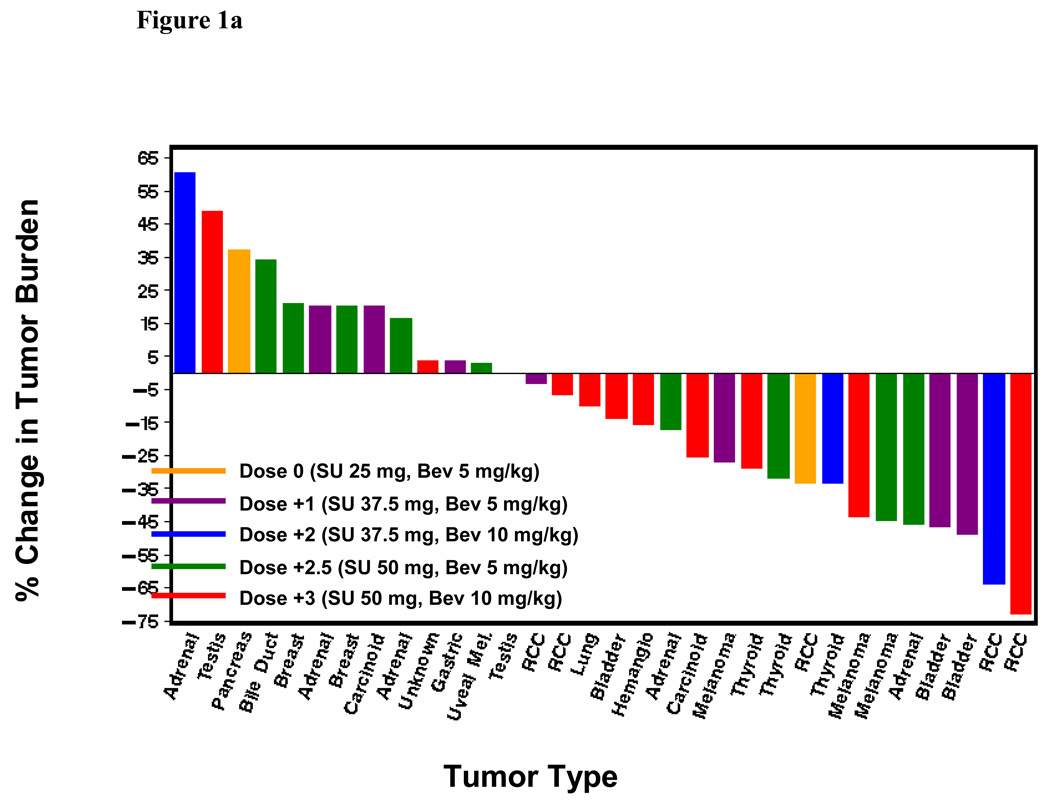
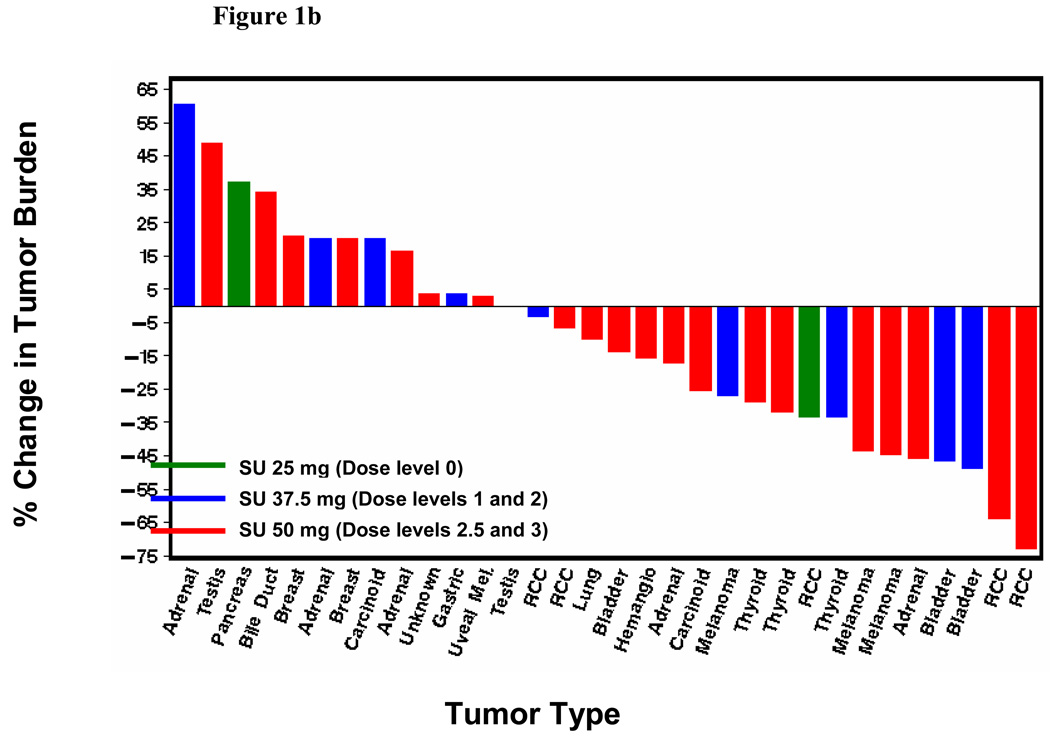
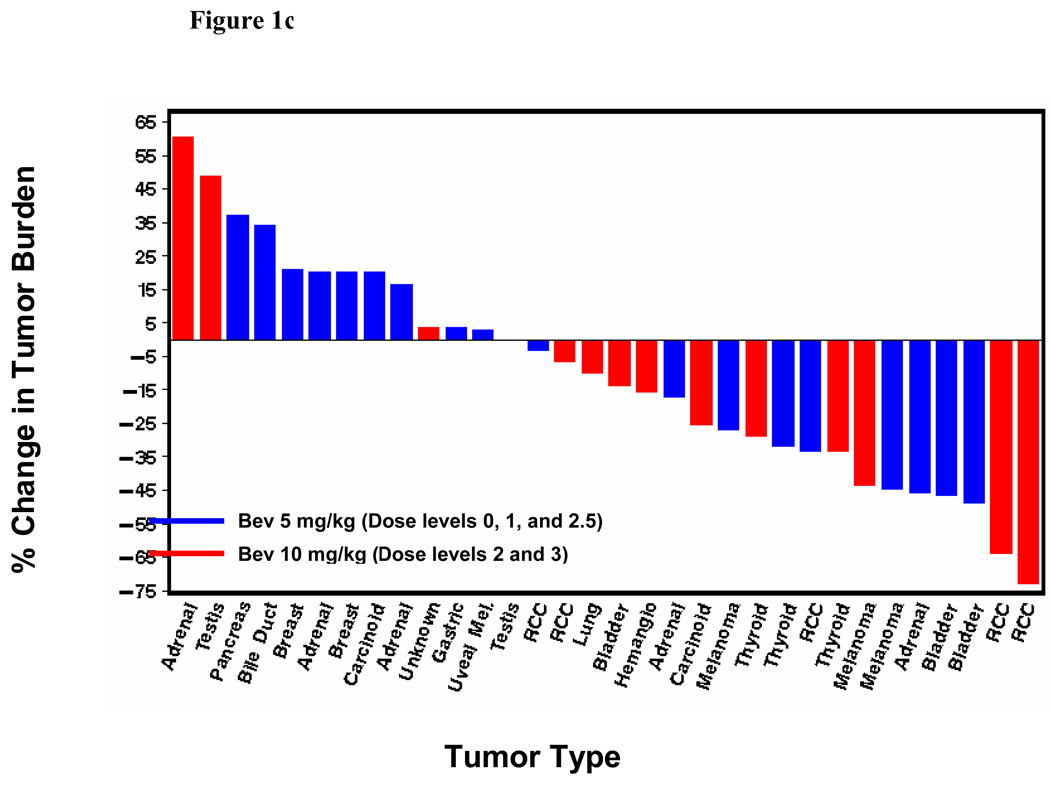
Seven of the 38 patients treated had a confirmed RECIST-defined partial response (PR) (18%; 95% confidence interval: 8–34%). Two additional patients achieved an unconfirmed RECIST-defined PR. The confirmed responses occurred across all dose levels except dose level 0. Anti-tumor effect was observed in RCC and notably in advanced solid tumors typically refractory to traditional chemotherapy including cisplatin-resistant transitional cell carcinoma of the bladder, melanoma, thyroid cancer and adrenocortical carcinoma. The responses have lasted 2.9+–13.7+ months (3 patients with PD and 4 with an ongoing PR). Figure 1 depicts the maximum tumor shrinakge after the start of therapy for all patients with at least one post-baseline radiograph (n=32), according to dose level (Figure 1a), sunitinib dose (Figure 1b) and bevacizumab dose (Figure 1c). Radiographic changes consistent with tumor necrosis without reduction in tumor size, as has been observed with VEGF-targeted agents in RCC previously, were also observed in the present study (Figure 2). Overall, the median change in tumor burden was an 11.8% decrease (range, 73% decrease to 61% increase). Nineteen of the 32 patients with a post-baseline scan (59%) had at least some reduction in overall tumor burden (median 32%, range 3–73%).
Figure 1.
(a) Best tumor burden change (vs. baseline) in all patients (n=32) with measurable disease and at least one post-baseline radiograph according dose level. Six patients did not undergo post-baseline radiographs due to early termination of therapy from clinical progressive disease, toxicity or withdrawal of consent; (b) Best tumor burden change according to sunitinib dosing; (c) Best tumor burden change according to bevacizumab dosing
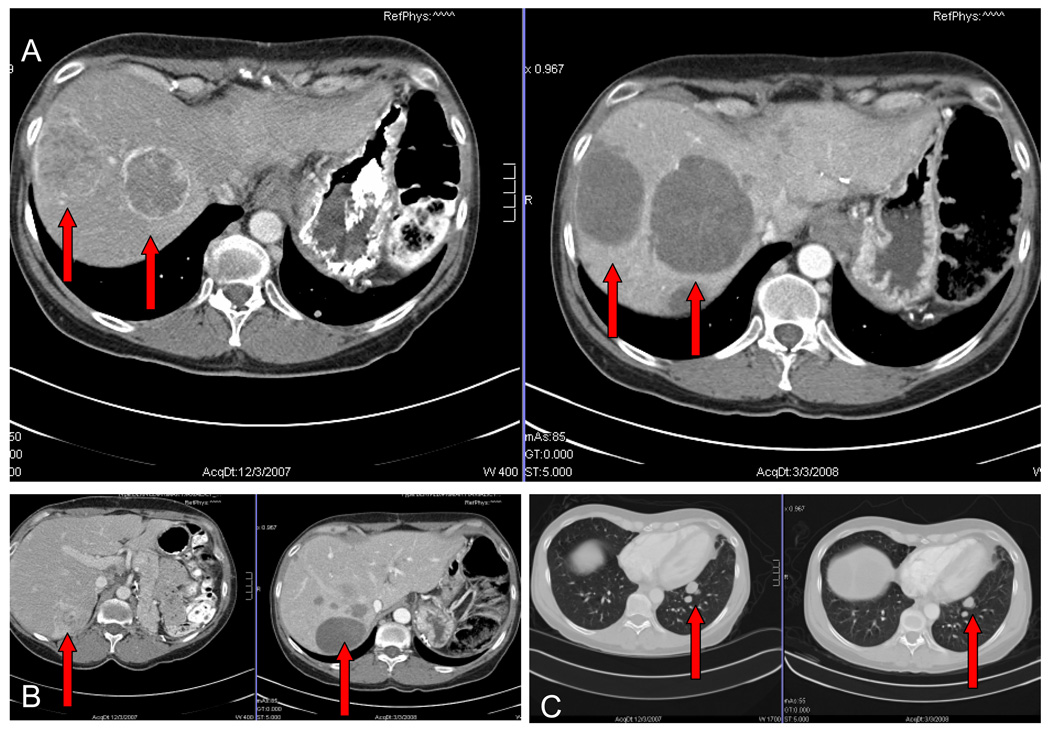
Figure 2.
CT scans pre- (A, B and C left panel) and after 2 cycles (A, B and C right panel) of sunitinib 50 mg and bevacizumab 5 mg/kg in a 47 year old female with metastatic adrenocortical cancer. Post-therapy hepatic metastases are larger but with radiographic changes consistent with tumor necrosis.
DISCUSSION
VEGF engagement of its cognate receptor represents the most potent pathway promoting tumor-associated angiogenesis. As such, blockade of various elements of this pathway has emerged as a therapeutic force in solid tumor oncology. Bevacizumab and sunitinib, VEGF ligand and receptor inhibitors respectively, are among the most clinically developed agents that inhibit this pathway, and each has shown robust clinical activity. Based on the hypothesis that these agents could be safely combined, a phase I study was undertaken. Only 1 protocol-defined DLT was observed, and the agents were tolerated up to the highest dose level tested, with several patients tolerating therapy over multiple cycles. Importantly, however, dose reductions and interruptions of both agents were often required with continued therapy, especially at higher dose levels. These observations highlight the severe limitation of MTD determination based on one cycle of therapy, given the need for dose modification with continued therapy that impacts the overall tolerability of a given regimen. In contrast to a previous phase I trial of this combination (16), no evidence of a microangiopathic hemolytic anemia was observed in any patients at any dose level.
The MTD defined in this study was sunitinib 50mg and bevacizumab 10mg/kg. The optimal dose and schedule of this combination, however, remains to be defined. It is possible that bevacizumab, which binds and neutralizes VEGF at doses as low as 0.3 mg/kg and with a half-life of up to 21 days(18)could be given at reduced doses or less frequently. Similarly, several patients in the current study achieved an objective response at doses of sunitinib lower than 50 mg. It is highly likely that individual tumor types as well as patient-related factors will impact the optimal dose and schedule of this combination. It must also be recognized that tumor types with significant single agent effect to each of these drugs such as RCC may more optimally be treated with sequential monotherapy. Thus, further phase I and phase II testing will be required in each tumor type to fully define the optimal dose /schedule and assess the safety and efficacy profile of this combination.
This study is notable for lack of thrombotic microangiopathy (TMA) which has been reported in metastatic RCC patients receiving this combination on a separate phase I study.(16) Intensive monitoring for evidence of TMA was instituted in the present trial when this safety signal emerged. No laboratory or clinical evidence of TMA was observed in any patent, including the six RCC patients (median 8 cycles of therapy (range, 2–13)), all of whom had undergone nephrectomy, and patients treated at the highest dose levels up to 20 months of treatment. The reason for this apparent discrepancy is not clear. The understanding of TMA in relation to VEGF blockade is emerging. VEGF gene deletion in a murine model lead to glomerular fibrin deposits and intracapillary thrombi.(19) Schistocytes were also observed in the peripheral blood in a subset of patients described in this report, but platelet counts were normal. Notably, hypertension occurred after glomerular damage occurred, and thus the inciting event was VEGF depletion in the glomerulus. Further, human patients receiving single agent bevacizumab or sunitinib have shown findings of renal TMA on biopsy, and sometimes, but not always, demonstrated evidence of TMA in the peripheral blood.(19–21) It can be hypothesized that more complete VEGF blockade with combination therapy may lead to more severe renal TMA, and further to more widespread endothelial damage creating a thrombogenic endothelium. The presence of a single kidney may accelerate this process and in part explain TMA observed in the separate study which enrolled only RCC patients who had undergone prior nephrectomy.(16) TMA was also not reported in two phase I trials of bevacizumab plus sorafenib, also a VEGF receptor inhibitor, albeit with lower than full doses of each agent due to other toxicity. (22, 23) Further elucidation of the biology of TMA in relation to VEGF blockade and specific predisposing factors including underlying disease is necessary.
This study has several limitations. No pharmacokinetic or pharmacodynamic parameters were measured given distinct drug clearance mechanisms of an antibody and a tyrosine kinase inhibitor. As such, determination of the optimal biologic dose for VEGF pathway inhibition, potentially at lower than maximal doses to reduce acute and chronic toxicity, is not possible. Although a phase I study of the combination of bevacizumab plus sorafenib did not show altered pharmacokinetics compared to single agent therapy, data specific to the present combination is not available.(22, 23)
In conclusion, the combination of sunitinib and bevacizumab is feasible, with notable toxicities with prolonged therapy and higher doses. Significant anti-tumor activity was observed across several solid tumors. Further clinical investigation of this combination in select solid tumors (especially in tumors with limited response to single agent VEGF-targeted therapy) is warranted.
Acknowledgments
Research support: This study was supported in part by the Cancer Therapy Evaluation Program (U01CA062502) through the Case Comprehensive Cancer Center and Pfizer, Inc.
Footnotes
Presented in part at:
1. Cooney MM, Garcia JA, Rini BI et al.: Sunitinib and bevacizumab in advanced solid tumors: A phase I trial. American Society of Clinical Oncology Annual Meeting; Chicago, IL, abstract 3530, 2008
2. Garcia JA, Mekhail T, Rini BI et al.: Sunitinib and bevacizumab in advanced solid tumors: Preliminary results of a phase I trial. Genitourinary Cancer Symposium, abstract 352, 2008
References
- 1.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002 Nov 1;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007 Jan 11;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007 Dec 22;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz HI, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007 Jan 11;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006 Dec 14;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Dror Michaelson M, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006 Dec 5;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. Jama. 2006 Jun 7;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006 Oct 14;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Tamaskar I, Shaheen P, Salas R, Garcia J, Wood L, et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. Journal of the National Cancer Institute. 2007 Jan 3;99:81–83. doi: 10.1093/jnci/djk008. [DOI] [PubMed] [Google Scholar]
- 11.Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 12.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003 Jul 31;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008 Nov 20;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 15.Deprimo SE, Bello CL, Smeraglia J, Baum CM, Spinella D, Rini BI, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. Journal of translational medicine. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman DR, Baum MS, Ginsberg MS, Hassoun H, Flombaum CD, Velasco S, et al. Phase I Trial of Bevacizumab Plus Escalated Doses of Sunitinib in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2009 Mar 20;27:1432–1439. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Gordon MS, Margolin K, Talpaz M, Sledge GW, Jr, Holmgren E, Benjamin R, et al. Phase I safety and pharmacokinetic study of recombinant human anti- vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 19.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008 Mar 13;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bollee G, Patey N, Cazajous G, Robert C, Goujon JM, Fakhouri F, et al. Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant. 2009 Feb;24:682–685. doi: 10.1093/ndt/gfn657. [DOI] [PubMed] [Google Scholar]
- 21.Frangie C, Lefaucheur C, Medioni J, Jacquot C, Hill GS, Nochy D. Renal thrombotic microangiopathy caused by anti-VEGF-antibody treatment for metastatic renal-cell carcinoma. Lancet Oncol. 2007 Feb;8:177–178. doi: 10.1016/S1470-2045(07)70037-2. [DOI] [PubMed] [Google Scholar]
- 22.Azad NS, Posadas EM, Kwitkowski VE, Steinberg SM, Jain L, Annunziata CM, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008 Aug 1;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sosman JA, Flaherty KT, Atkins MA, et al. Updated results of phase I trial of sorafenib (S) and bevacizumab (B) in patients with metastatic renal cell cancer (mRCC) J Clin Oncol. 2008 May 20;26 suppl abstr 5011. [Google Scholar]