SUMMARY
The cancer stem cell hypothesis suggests that, unlike most cancer cells within a tumor, cancer stem cells resist chemotherapeutic drugs and can regenerate the various cell types in the tumor, thereby causing relapse of the disease. Thus, drugs that selectively target cancer stem cells offer great promise for cancer treatment, particularly in combination with chemotherapy. Here, we show that low doses of metformin, a standard drug for diabetes, inhibits cellular transformation and selectively kills cancer stem cells in four genetically different types of breast cancer. The combination of metformin and a well-defined chemotherapeutic agent, doxorubicin, kills both cancer stem cells and non-stem cancer cells in culture. Furthermore, this combinatorial therapy reduces tumor mass and prevents relapse much more effectively than either drug alone in a xenograft mouse model. Mice appear to remain tumor-free for at least two months after combinatorial therapy with metformin and doxorubicin is ended. These results provide further evidence supporting the cancer stem cell hypothesis, and they provide a rationale and experimental basis for using the combination of metformin and chemotherapeutic drugs to improve treatment of patients with breast (and possibly other) cancers.
INTRODUCTION
Chemotherapeutic treatments for cancer can effectively reduce tumor mass, but the disease often relapses. To explain this phenomenon, the cancer stem cell hypothesis suggests that tumors contain a small number of tumor-forming, self-renewing, cancer stem cells within a population of non-tumor-forming cancer cells (1, 2). Unlike most cells within the tumor, cancer stem cells are resistant to well-defined chemotherapy, and after treatment, they can regenerate all the cell types in the tumor through their stem cell-like behavior. For this reason, drugs that selectively target cancer stem cells offer great promise for cancer treatment, although none are known at present.
Epidemiological studies indicate that diabetes is correlated with increased risk of breast and other cancers (3, 4), and we recently defined a transcriptional signature and drug-sensitivity profile of cellular transformation linking multiple types of cancer with diabetes and other metabolic diseases. Metformin is an extensively used and well-tolerated drug for treating individuals with type 2 diabetes, obesity, and polycystic ovarian syndrome. Diabetics treated with metformin have reduced cancer risk (5, 6), although it is unclear whether metformin affects cancer directly or indirectly by inhibiting the diabetic state. Metformin inhibits the growth of breast cancer cell lines, although it also affects non-transformed cells at the concentrations tested (7-9). In nude mice, metformin modestly inhibits tumor growth of xenografts of a triple-negative breast cancer cell line that lacks the estrogen, progesterone, and HER2 receptors (8). These observations suggest the possibility that metformin might be useful as an anti-cancer drug in non-diabetic contexts (10, 11).
Here, we show that metformin selectively kills cancer stem cells in four genetically different types of breast cancer. The combination of metformin and doxorubicin, a well-defined chemotherapeutic drug, kills both cancer stem cells and non-stem cancer cells in culture, and reduces tumor mass and prolongs remission much more effectively than either drug alone in a xenograft mouse model. These observations constitute independent support for the cancer stem cell hypothesis, and they provide a rationale for why the combination of metformin and chemotherapeutic drugs might improve treatment of patients with breast (and possibly other) cancers.
MATERIALS AND METHODS
Cell lines
MCF10A cells are mammary epithelial cells derived from fibrocystic breast tissue that was obtained from a mastectomy of a 36-years old woman with no family history of breast cancer and no evidence of disease (12). Genetic analysis did not reveal any amplification of HER2/neu oncogene or mutations in H-Ras oncogenes, and these cells do not express estrogen receptor. The experiments here use a derivative of MCF10A containing an integrated fusion of the v-Src oncoprotein with the ligand binding domain of estrogen receptor. MCF7 cells are mammary adenocarcinoma cells that express very high levels the estrogen receptor, are negative for HER2/neu, and do not have strong anchorage-independent properties (13). SKBR3 cells are mammary adenocarcinoma cells that overexpress the HER2/neu receptor, have anchorage-independent properties, and form tumors in xenografts (14). MDA-MB-468 cells are derived from a triple negative breast carcinoma that shows many of the recurrent basal-like molecular abnormalities including ER-PR-HER2-negative status, p53 deficiency, EGFR overexpression, PTEN loss and constitutive activation of the MEK/ERK pathway (15). MDA-MB-468 cells are very aggressive and form large tumors in xenograft experiments that resist treatment with tamoxifen or herceptin.
Cell culture
MCF-7, SKBR3, and MDA-MB-486 cells were grown in DMEM media (Invitrogen), 10% fetal bovine serum (Atlanta Biologicals), and penicillin/streptomycin (Invitrogen) at 37°C with 5% CO2. MCF10A ER-Src cells were cultured as described previously (16) and induced to transform with 1 μM 4OH-tamoxifen (TAM) dissolved (Sigma) in EtOH. Morphological changes, phenotypic transformation and foci formation occurred 24-36 h after TAM addition, and were monitored by phase-contrast microscopy. Metformin (Sigma) dissolved in water was typically added to 0.1 mM unless otherwise indicated.
Wound healing motility assay
Cells were seeded onto six-well dishes at 1×105/well. A single scratch wound was created using a p10 micropipette tip in to confluent cells. Cells were washed three times with PBS to remove cell debris, supplemented with assay medium, and monitored. Images were captured by phase-contrast microscopy at 0 and 12 h post wounding.
Colony formation assay
Triplicate samples of 5×104 cells from MCF10A ER-Src were mixed 4:1 (v/v) with 2.0% agarose in MCF-10A growth medium for a final concentration of 0.4% agarose. The cell mixture was plated on top of a solidified layer of 0.5% agarose in growth medium. Cells were fed every 6 to 7 days with growth medium containing 0.4% agarose. The number of colonies was counted after 15 days.
Mammosphere culture
Mammospheres were cultured in suspension (1000 cells/ml) in serum-free DMEM/F12 media, supplemented with B27 (1:50, Invitrogen), 0.4% BSA, 20 ng/ml EGF (Preprotech) and 4 μg/ml insulin (Sigma) as described previously (17). Mammosphere formation was tested by placing transformed cell populations in the presence of absence of metformin under these conditions, whereas mammosphere growth was examined by adding metformin to 6-day old mammospheres and counting the number of mammospheres 2 and 4 days after treatment.
Isolation and analysis of cancer stem cells
Flow cytometric cell sorting of transformed cell populations was performed on single cell suspensions. Cells were stained with CD44 antibody (FITC-conjugated) (555478, BD Biosciences) and with CD24 antibody (PE-conjugated) (555428, BD Biosciences). Cancer stem cells (CD44high/CD24low) and no-stem transformed cells (CD44low/CD24high) from MCF10A ER-Src (TAM-treated) and MCF7, SKBR3 and MDA-MD-486 cells were treated with 0.1 mM metformin and cell growth was assessed in different time points (12, 24, 48h). The experiments were performed in triplicate, and the data represent mean ± SD.
Tumor growth and relapse in xenografts
5×106 MCF10A ER-Src cells were injected into the right flank of 16 female nu/nu mice (Charles River Laboratories), all of which developed tumors in 10 days with size ~50mm3. The mice were randomly distributed into 4 groups that were untreated, or treated by intraperitoneal injections every 5 days (3 cycles) with 4 mg/kg doxorubicin, 100 μg/ml metformin, or the combination. Tumor volume (mean values and 95% confidence intervals) was measured at various times after the initial injection. All the mouse experiments were performed in accordance with Institutional Animal Care and Use Committee procedures and guidelines.
RESULTS AND DISCUSSION
To examine the anti-cancer properties of metformin, we first utilized an inducible transformation model consisting of non-transformed human mammary epithelial cells (MCF-10A) containing ER-Src, a fusion of the v-Src oncoprotein with the ligand-binding domain of estrogen receptor. When these cells are treated with tamoxifen, they become transformed within 24-36 hours. The transformed cell population contains 10% cancer stem cells, as defined by expression of the CD44 marker and the ability to form mammospheres, multicellular “micro-tumors” that are generated in non-adherent and non-differentiating conditions (18). In addition, we analyzed three other mammary adenocarcinoma cell lines derived from genetically and phenotypically different tumors that are treated with different drugs: ER-positive MCF7 (13); HER-positive SKBR3 (14); triple-negative MDA-MB-468 (15). These cell lines also contain a minority population of cancer stem cells capable of mammosphere formation. In all experiments, metformin was used at a concentration that does not affect the growth of non-transformed cells (0.1 or 0.3 mM; Fig. 1A). Previous experiments on cancer cell lines (7-9) used much higher concentrations of metformin (typically 10-30 mM), conditions that are also toxic for non-transformed cells.
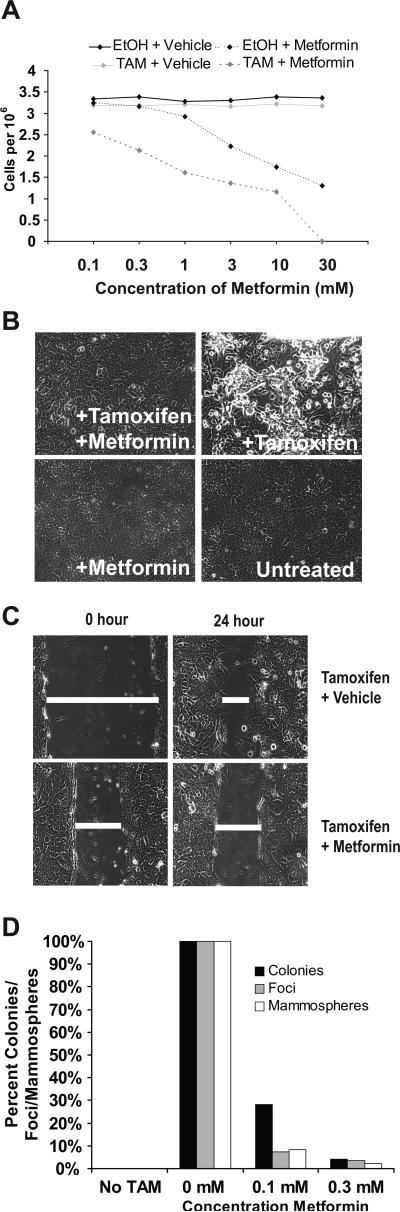
Figure 1.
Metformin prevents transformation of MCF10A-ER-Src cells. A, Number of cells grown in the presence or absence of 1 μM 4-hydroxy tamoxifen (TAM) with the indicated concentrations of metformin for 24 hours. B, Phase-contrast images of cells grown in the presence or absence of 0.1 mM metformin and/or TAM for 36 hours. C, Wound-healing/invasion response assay of cells grown in the presence or absence of 0.1 mM metformin and/or TAM. D, Relative number of foci, colonies in soft agar, and mammospheres in untreated or TAM-treated cells in the presence of the indicated concentration of metformin.
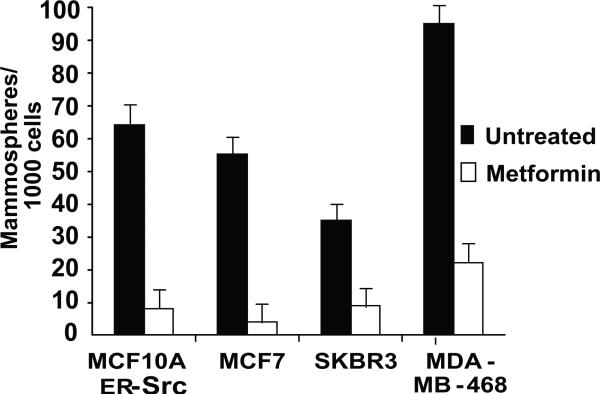
In the inducible MCF-10A model, metformin strongly inhibits morphological transformation (Fig. 1B), invasive growth in wound-healing assays (Fig. 1C), focus formation, formation of colonies in soft agar, and generation of mammospheres (Fig. 1D). Furthermore, metformin treatment of mammospheres derived from all four breast cancer cell lines causes a dramatic reduction in the number of mammospheres within 48 hours (Fig. 2) as a consequence of cell death. As mammospheres are composed primarily of cancer stem cells (18), this latter observation suggests that metformin may kill cancer stem cells.
Figure 2.
Metformin inhibits growth of mammospheres. 6-day old mammospheres from the indicated cell lines were or were not treated with 0.1 mM metformin for 48 hr, and the number of mammospheres counted.
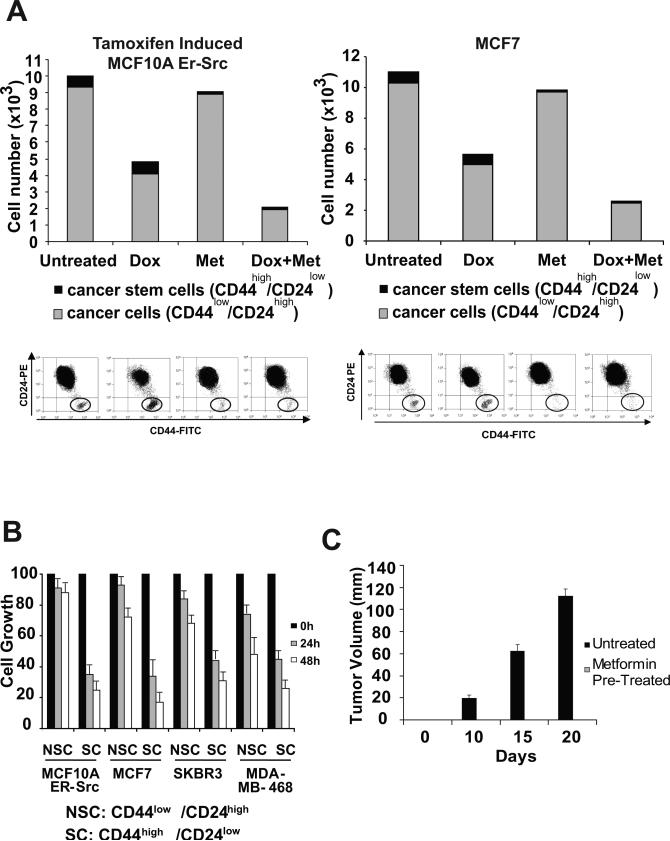
Strikingly, metformin preferentially kills cancer stem cells (CD44high/CD24low) within a population of transformed MCF-10A or MCF-7 cells (Fig. 3A). Similarly, when all four cancer cell lines are sorted, cancer stem cells are quite susceptible to metformin, whereas the standard cancer cell population remains essentially unaffected (Fig. 3B). Furthermore, treatment of MCF-10A cancer stem cells with metformin for just 1 hour blocks the ability of these cells to form tumors in nude mice, even though the drug is not present for the month after injection (Fig. 3C). The ability of metformin to selectively kill cancer stem cells is in marked contrast to doxorubicin, a chemotherapeutic agent that kills cancer cells, but not cancer stem cells. As expected from their distinct properties, metformin works together with doxorubicin to reduce both non-stem cancer cells and cancer stem cells in the mixed transformed population (Fig. 3A).
Figure 3.
Metformin selectively kills cancer stem cells and functions synergistically with doxorubicin. A, Number of cancer stem cells (CD44high/CD24low; black) and cancer cells (CD44low/CD24high; grey) in the transformed (36 h TAM treatment) MCF-10A population that was treated with doxorubicin, 0.1 mM metformin, or both (n = 3). B, Cancer stem cells (SC) and non-stem cancer cells (NSC) obtained by sorting were treated with 0.1 mM metformin for 0, 24, and 48 hours. C, Tumor volume in nude mice at the indicated number of days after injection of MCF10A-ER-Src cancer stem cells that were or were not treated with 0.1 mM metformin for 1 hr prior to injection.
In accord with the above results in cell lines, the synergy between metformin and doxorubicin is observed upon treatment of tumors that arise 10 days after injection of MCF-10A-ER-Src cells into nude mice. After 15 days of treatment (3 cycles every 5 days), this drug combination virtually eliminates tumors, whereas doxorubicin alone causes only a 2-fold decrease in tumor volume and metformin alone has little effect (Fig. 4A). Doxorubin-treated mice show a further reduction in tumor volume after an additional 10 days (day 35). The minimal effect of metformin alone is in contrast to more significant effects seen in an independent report (8), but there are many differences in experimental protocol between these studies.
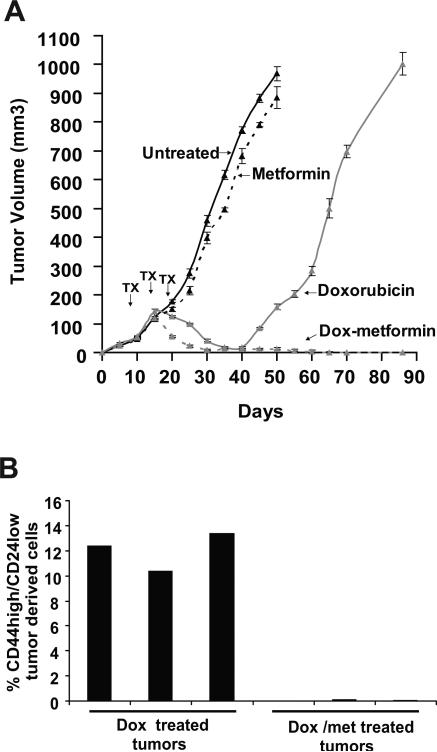
Figure 4.
Metformin and doxorubicin act in combination to reduce tumor mass and prolong remission in nude mice. A, Tumor volume (mean values and 95% confidence intervals) of mice injected with transformed MCF10A-ER-Src cells (time 0 indicates the time of injection) that were untreated, or treated by intraperitoneal injections every 5 days (3 cycles; arrows indicate the day or injections) with 4 mg/kg doxorubicin (Dox), 100 μg/ml metformin (Met), or both. B, Number of cancer stem cells (CD44high/CD24low) in cells obtained from tumors treated with Dox or the combination of Dox + Met after 3 cycles of treatment (day 25).
To determine the basis for why the combination of metformin and doxorubicin is more effective than doxorubicin alone, we examined the population of cells recovered from tumors after 3 cycles of treatment (day 25). In accord with our results in cell lines, cancer stem cells are virtually absent from mice treated with the drug combination, whereas they are easily detected in tumors from mice treated with doxorubicin alone (Fig. 4B). Thus, the therapeutic advantage of metformin in the context of conventional chemotherapy is linked to its ability to kill cancer stem cells.
The cancer stem cell hypothesis for the progression of human disease is based on the differential tumor-forming properties and responses to well-defined chemotherapy of cancer stem cells and non-stem cancer cells. A prediction of this model, heretofore untested, is that drugs that selectively inhibit cancer stem cells should function synergistically with chemotherapeutic drugs to delay relapse. Strikingly, mice treated with the combination of metformin and doxorubicin remain in remission for at least 60 days after treatment is ended (Fig. 4A). In contrast, tumor growth resumes 20 days after mice are treated with doxorubicin alone, and the rate of tumor growth after relapse is comparable to that observed in the initial disease (i.e. in the absence of treatment). Thus, combinatorial therapy has a dramatic effect on prolonging remission, and indeed may even represent a cure of these xenograft-generated tumors. In addition to their potential medical significance, these observations provide independent and further support for the cancer stem cell hypothesis.
To our knowledge, the ability of metformin to selectively kill cancer stem cells and to function synergistically with doxorubicin to block both cancer stem cells and non-stem transformed cells is unique. In the case of breast cancer, herceptin and tamoxifen are useful drugs for cancer types that, respectively, express the HER2 and estrogen receptors, but some forms of breast cancer lack these receptors resist these treatments. For all of these types of breast cancer, metformin selectively inhibits cancer stem cell growth, and hence is likely to function synergistically with chemotherapeutic drugs. In addition, as metformin inhibits transformation of MCF10A-ER-Src cells, it might have a potential use in preventing the development of cancer, as opposed to treating cancer that has already occurred. Indeed, the ability of metformin to inhibit cellular transformation might underlie the epidemiological observation that diabetics treated with metformin have a lower incidence of cancer (5, 6). As a cancer preventative, metformin would be required on a long-term basis, and in this regard, the concentration of metformin needed for the anti-cancer effects observed here is considerably below that used for the treatment of diabetes. Lastly, the selectivity of metformin and doxorubicin for distinct types of cells in the tumor can explain the striking combinatorial effects on reducing tumor mass and prolonging remission in nude mice, and it provides the rationale for combining metformin with chemotherapy as a new treatment for breast (and possibly other) cancers.
ACKNOWLEDGMENTS
This work was supported by a postdoctoral fellowship to H.A.H. from the American Cancer Society and research grants to P.N.T (CA 57436) and K.S. (CA 107486) from the National Institutes of Health.
REFERENCES
- 1.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–6. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cells traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. International journal of cancer. 2007;121:856–62. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- 4.Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr. 2007;86:867–71. doi: 10.1093/ajcn/86.3.867S. [DOI] [PubMed] [Google Scholar]
- 5.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ (Clinical research ed. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–15. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–40. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 9.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer research. 2006;66:10269–73. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 10.Cazzaniga M, Bonanni B, Guerrieri-Gonzaga A, Decensi A. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol Biomarkers Prev. 2009;18:701–5. doi: 10.1158/1055-9965.EPI-08-0871. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin PJ, Ligibel JA, Stambolic V. Metformin in breast cancer: Time for action. J Clin Oncol. 2009;27:3271–3. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- 12.Soule HD, Maloney TM, Wolman SR, et al. Isolation and characterization of a spontaneously immortallized human breast epithelial cell line, MCF10. Cancer research. 1990;50:6075–86. [PubMed] [Google Scholar]
- 13.Brooks SC, Locke ER, Soule HD. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973;248:6251–3. [PubMed] [Google Scholar]
- 14.Bergman I, Barmada MA, Griffin JA, Slamon DJ. Treatment of meningeal breast cancer xenografts in the rat using an anti-p185/HER2 antibody. Clin Cancer Res. 2001;7:2050–6. [PubMed] [Google Scholar]
- 15.Oliveras-Ferraros C, Vazquez-Martin A, Lopez-Bonet E, et al. Growth and molecular interactions of the anti-EGFR antibody cetuximab and the DNA crosslinking agent cisplatin in gefitinib-resistant MDA-MB-468 cells: new prospects in the treatment of triple-negative/basal-like breast cancer. Int J Oncol. 2008;33:1165–76. [PubMed] [Google Scholar]
- 16.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30(3):256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 17.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimshaw MJ, Cooper L, Papazisis K, et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]