Abstract
Purpose
Radiotherapy has a central role in the treatment of non-small-cell lung cancer. Effectiveness of this modality, however, is often limited as resistance results from defects in cell death.
Experimental Design
We investigated whether simultaneous upregulation of apoptosis, via Bcl-2 inhibitor ABT-737, and autophagy, via mTOR inhibitor rapamycin, can be used to enhance radiosensitivity of H460 cells in vitro and growth delay in a xenograft model.
Results
In vitro studies confirmed that ABT-737 and rapamycin induce apoptosis and autophagy, respectively. ABT-737 induced cleaved caspase-3, a marker of apoptosis, and rapamycin correlated with an increase in punctate localization of GFP-LC3, characteristic of autophagy. The combination ABT-737/rapamycin markedly enhanced sensitivity of H460 cells to radiation (DER=2.47, p=0.002) in clonogenic assay. In addition, the combination ABT-737/rapamycin/radiation showed a dramatic tumor growth delay in a mouse xenograft model. In vivo immunohistochemistry staining showed that combination therapy yielded over a 100% increase in caspase-3 activity (apoptosis) and a 6-fold decrease in p62 protein level (indicative of autophagic flux) as compared to radiation alone control group. Moreover, cell proliferation (Ki67 staining) was reduced by 77% (p=0.001) and vascular density (vWF staining) by 67.5% (p=0.09) compared to radiation alone. Additional in vitro studies in human umbilical endothelial cells indicated that combined therapy also significantly decrease tubule formation.
Conclusion
These results suggest that concurrent induction of apoptosis and autophagy enhances radiation therapy both in vitro and in lung cancer xenograft models. Further investigations are warranted to assess the clinical potential of such strategy in lung cancer patients.
Keywords: Angiogenesis, apoptosis, autophagy, lung cancer, radiation
Introduction
In 2008, lung cancer remained the leading cause of cancer-related mortality in the United States, with an estimated 215,000 individuals diagnosed with lung cancer and a mortality exceeding 161,000 (1). Non-small-cell lung cancer (NSCLC) accounts for 75% of these cases and despite advances made in radio- and chemotherapy, the median overall survival is only 15 months, indicating a need for new strategies to improve outcome.
In recent years, apoptosis has become an attractive target for cancer therapy. Apoptosis is a genetically programmed cell death pathway, regulated by the complex interaction between two groups of Bcl-2 family proteins: anti-apoptotic proteins such as Bcl-2 itself, as well as Bcl-xL, Bcl-w and Mcl-1, and pro-apoptotic proteins, Bax, Bak, Bad and Bim (2). Defects in the apoptotic pathway correlate with cellular resistance to therapy and are frequently observed in NSCLC (3-5). Recently, ABT-737, a small-molecule BH3 domain mimetic which functions as a Bcl-2 inhibitor, has been shown to bind with high affinity (ki≤1nmol/L) to Bcl-2 and Bcl-xL, freeing Bax or Bak to trigger permeabilization of mitochondrial membrane and caspase-3 activation, and subsequently cell death (6). Furthermore, it has been demonstrated that ABT-737 potentiates anti-cancer treatments in lymphoma cell lines and SCLC xenograft models (7, 8).
Autophagy is a complex cellular process with a dual role. Under conditions of limited stress such as starvation, it promotes cell survival, degrading and recycling long lived proteins and cellular components (9). However, when the cell is exposed to prolonged or excessive conditions of stress, autophagy has been shown to result in death by self-digestion. The mammalian target of rapamycin (mTOR), which regulates cell growth, proliferation, angiogenesis and metabolism, is a major negative regulator of autophagy. In addition, the mTOR pathway has been shown to be constitutively activated in a variety of solid tumors (10), including an estimated 74% of resected NSCLC malignancies (11). Based on the frequent dysregulation of the mTOR signaling in cancer, inhibition has been proposed for cancer therapy (10). We and others have previously reported the inhibition of tumor proliferation by rapamycin and its analogues in several cancer xenograft models, including brain, breast, and NSCLC (12-14). Rapamycin is a natural macrolide antibiotic which cross-links with immunophilin FKBP-12, resulting in a complex that inhibits mTOR signaling and results in translation of RNA, cell cycle progression and importantly, induction of autophagy (15).
While both ABT-737 and rapamycin have suggested promise as cancer treatment options, neither drug has proven completely successful. Certain cell lines, including NSCLC and SCLC expressing high levels of Mcl-1 or low levels of Bcl-2, remain resistant to apoptosis even following treatment with ABT-737 (16-18). Similarly, rapamycin may not be able to sensitize all cell lines to radiotherapy (13). In this study, we tested the triple combination ABT-737/rapamycin and radiation to circumvent the defects of one single cell death pathway by simultaneously up-regulating both apoptosis and autophagy in lung cancer. Data on the efficacy of ABT-737 and rapamycin in combination with radiation in NSCLC cells and xenograft have direct implications for the clinical evaluation of Bcl-2 inhibitors in combination with mTOR inhibitors in patients with NSCLC.
Materials and Methods
Cell Culture and Chemical
H460 lung cancer cells were cultured in RPMI 1640 (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C and humidified 5% CO2. Human umbilical endothelial cells (HUVECs) were obtained from Clonetics (BioWhittaker). ABT-737 was provided by Abbott Laboratories (Abbott Park, Illinois) and rapamycin was purchased from Novartis Pharmaceutical (East Hanover, NJ).
Clonogenic assay
H460 cells were treated with DMSO, ABT-737 (500nM, for 2hrs), rapamycin (100nM, for 2hrs), or combined ABT-737 with rapamycin. Cells were irradiated with 0 to 6 Gy as described previously (14).
Immunoblotting
Cells (0.3×106) were treated with different combinations of radiation dose (0, 5, or 20 Gy) and drug, as described above. They were collected and washed with ice-cold PBS twice before the addition of lysis buffer. Equal amounts of protein were loaded into each well and the blots were incubated overnight with Caspase-3 (Cell signaling), LC-3 (Medical & Biological Laboratories Co. LTD), and Actin antibodies for 1hr at 4°C. Membranes were then incubated with goat anti-rabbit IgG secondary (1:5000, Santa Cruse Biotechnologies) for 45min at room temperature. Immunoblots were developed using the chemiluminescence detection system (PerkinElmer) according to the manufacturer's protocol and autoradiography.
Apoptosis Assay
H460 cells (5×105 cells) were plated into 10mm dishes for each data point. After overnight incubation at 37°C, cells were treated with ABT-737 (500nM for 2 hours) and irradiated with 5 Gy or 20 Gy. After 24h, cells were treated with 1ml of Accutase (Innovative cell technologies, Inc) for 4 min (keeping all floating cells) and counted for each sample. Cells were centrifuged and re-suspended in 1× Binding Buffer at a concentration of 1×106 cells/ml. 100μL of the solution (5× 105 cells) were transferred in a 5ml FACS tube, and combined with 1μL of Annexin-V FITC and 1μL of propidium iodide (PI). After incubation for 30min at room temperature in the dark, 300μL of 1× Binding Buffer was added to each tube. The rate of apoptosis was measured using the Annexin-V-fluorescein isothiocyanate apoptosis detection Kit I (Pharmingen) with flow cytometry.
Trypan Blue assay
H460 cells (3×104 cells) were plated into 24 wells for each data point. They were incubated and radiated as described above. After 24h, 5μl Trypan Blue Solution (Invitrogen, Grand Island, NY) and 4μL PBS (Invitrogen, Grand Island, NY) were combined and added to 10μL resuspended cells. Following gentle mixing and incubation for 2min at room temperature, the total number of cells and the number of stained cells were calculated using a hemocytometer under a microscope to determine the percentage of dead (stained) cells.
Autophagy Assay
H460 cells (2×105 cells) were seeded in tissue culture in a 6-well plate overnight and then were transfected with 2μg of GFP-LC3 expression plasmid (a gift from Dr. Norboru Mizushima, Tokyo Medical and Dental University, Tokyo, Japan) using lipofectamine reagent (Invitrogen life technologies). After 12h, cells were treated with DMSO, ABT-737, rapamycin, or both, and received 5 Gy radiation as described above. Cells were then incubated for 48h at 37°C, after which GFP-LC3 fluorescence was observed under a confocal fluorescence microscope. Characteristic punctate GFP-LC3 signaling was considered a cell undergoing autophagy. The percent of punctate GFP cells per total GFP transfected cells was calculated and experiments were conducted in triplicate.
Tumor volume assessment
H460 cells were used in a xenograft model in female athymic nude mice (nu/nu, 5 to 6 weeks old [Harlan Sprague Dawley Inc., Indianapolis, IN]). A suspension of 1×106 cells in 100μL volume was injected subcutaneously into the right flank of mice using a 1-cc syringe with 27½-gauge needle. Tumors were grown for 6 to 8 days until average tumor volume reached 0.23 cm3. Treatment groups consisted of DMSO, ABT-737, rapamycin, combined ABT-737 with rapamycin, DMSO plus radiation, ABT-737 plus radiation, rapamycin plus radiation, and combined ABT-737 with rapamycin plus radiation Each treatment group contained 5 mice. DMSO and ABT-737 were administered at doses of 20mg/kg intraperitoneally, and rapamycin, 2mg/kg orally for 7 consecutive days. Mice in radiation groups were irradiated 1h after ABT-737 and rapamycin treatment with 2 Gy daily over 5 consecutive days. Tumors on the flanks of the mice were irradiated using an X-ray irradiator (Therapax, Agfa NDT, Inc., Lewis Town, PA). The non-tumor parts of the mice were shielded by lead blocks. Tumors were measured 2 or 3 times weekly in 3 perpendicular dimensions using a Vernier caliper. Tumor volumes were calculated using the modified ellipse volume formula (Volume = (Height × Width × Depth)/2). Growth delay was calculated as the number of days required to reach a tumor volume of 1.75cm3 for treatment groups relative to the control.
Histological sections, vWF, Ki-67, active caspase-3 and p62 staining
Mice were implanted with H460 lung cancer cells and treated as described above in the tumor volume studies. After 7 days of daily treatments, tumors from each mouse were resected and paraffin fixed. Slides from each treatment group were then stained for vWF using anti-vWF polyclonal antibody (Chemicon). Blood vessels were quantified by randomly selecting 400× fields and counting the number of blood vessels per field. This was done in triplicate and the average of the three counts was calculated. Ki67, active caspase-3 and p62 staining were performed in the Vanderbilt University Pathology Core laboratory using standard protocols. The number of positive cells per 400× fields were scored and graphed by averaging three repeated assessments.
Endothelial Cell Morphogenesis assay: Tubule Formation
Human umbilical vein endothelial cells (HUVECs) were used to examine tubule formation. HUVECs grown to ∼70% confluency were treated with DMSO, ABT-737 (500nM, for 2hrs), rapamycin (100nM, for 2h), or combined ABT-737 with rapamycin, with or without 5 Gy radiation. Cells were then trypsinized, counted, and seeded at 48000 per well on 24-well plates coated with 300μL of Matrigel (BD Biosciences). These cells were periodically observed by microscope as they differentiated into capillary-like tubule structures. One day later, cells were stained with hematoxylin and eosin (H&E) and photographs were taken via microscope. The average number of tubules was calculated from examination of three separate microscopic fields (100×) and representative photographs were taken.
Statistical analysis
Analysis of study results focused on testing the differences of the mean tumor volume among treatment groups and different time points. The data analysis was completed using the restricted/residual maximum likelihood-based mixed-effect model to adjust the intracorrelation effect for the mice that had multiple measurements. The model reported in the paper was selected on the basis of the Schwarz's Bayesian criterion. All tests of significance were 2-sided, and differences were considered statistically significant when p was less than 0.05. A statistical package was used for all analyses.
Results
ABT-737 increases radiation-induced apoptosis
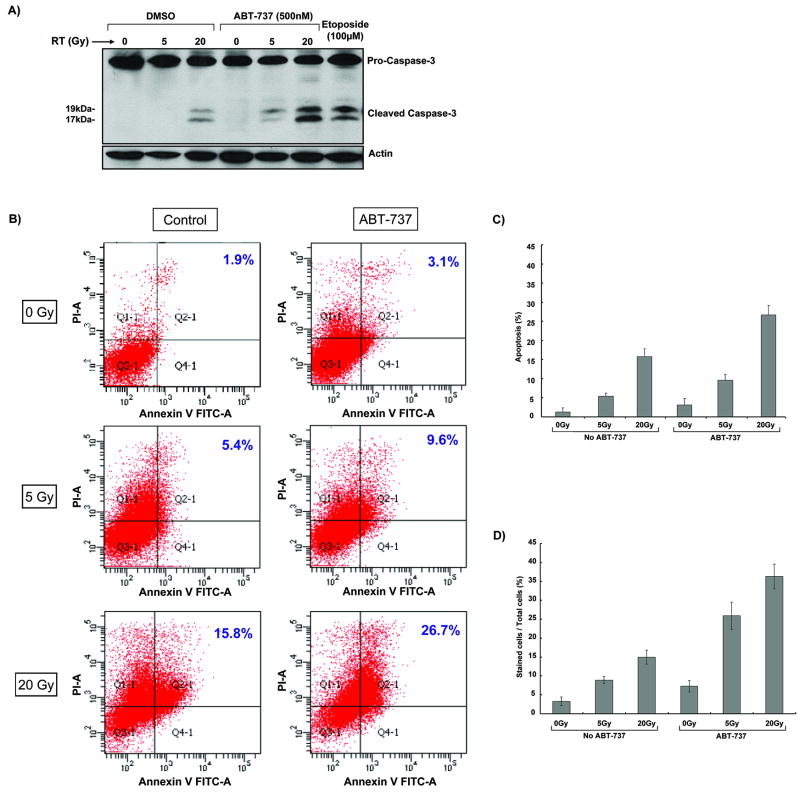
To determine whether ABT-737 enhances radiation-induced apoptosis in H460 cells, cleavage of caspase-3 was examined by Western blotting (Figure 1A). H460 cells were treated with DMSO or 500nM ABT-737 for two hours prior to receiving radiation (0, 5, or 20 Gy). In addition, Etoposide was used as a positive control. As shown in Figure 1A, cleaved caspase-3 was only detected at 20 Gy, indicating radioresistance in this lung cancer cell line. In H460 cells treated with ABT-737, cleaved caspase-3 is detected at 5 Gy, with significant increase at 20 Gy. These data suggest that ABT-737 promotes apoptosis in H460 radioresistant cells. Annexin-V flow cytometric analysis was used to confirm the above findings. As shown in Figure 1B and 1C, 5.4% and 15.8% of Annexin V positive cells were detected in H460 cells that received 5 Gy and 20 Gy, respectively. Treatment with ABT-737 resulted in a further increase in apoptosis (9.6% at 5 Gy and 26.7% at 20 Gy) compared to radiation alone. Finally, trypan blue staining showed that ABT-737 increased cell death amount from 8.9% to 25.9% at 5 Gy and from 15% to 36% at 20 Gy compared to radiation alone (p<0.001) (Figure 1D). ABT-737 alone yielded 7.3% positive dead cells compared to the control group. These results suggest a synergistic effect of ABT-737 in combination with ionizing radiation in H460 cells.
Figure 1. ABT-737 increases radiation-induced apoptosis in H460 cells.
(A) H460 cells were pretreated with 100 nM ABT-737 for 2 hours, followed by radiation (0, 5, or 20 Gy). Western blot analysis for active caspase-3 was performed after 24 hours. Actin immunoblot was used for normalization. (B) H460 cells were treated with ABT-737 (500nM for 2 hours) and immediately irradiated with 5 Gy or 20 Gy. After 24 h, cells were stained with Annexin V and propidium iodide, and analyzed by flow cytometry. This experiment was done in triplicate and representative diagrams of Annexin-V assays are shown. (C) Quantitative measurement of Annexin V flow cytometry analyses showing positive apoptotic cells in response to radiation/ABT-737. Columns, mean; bars, S.D. (D) H460 cells were treated with ABT-737 (500nM for 2 hours) and immediately irradiated with 5 Gy or 20 Gy. 24 hr later, they were stained with trypan blue. The total number of cells and the number of stained cells were determined using a hemocytometer under a microscope. This was repeated thrice. Columns, mean; bars, S.D.
Rapamycin increases radiation-induced autophagy
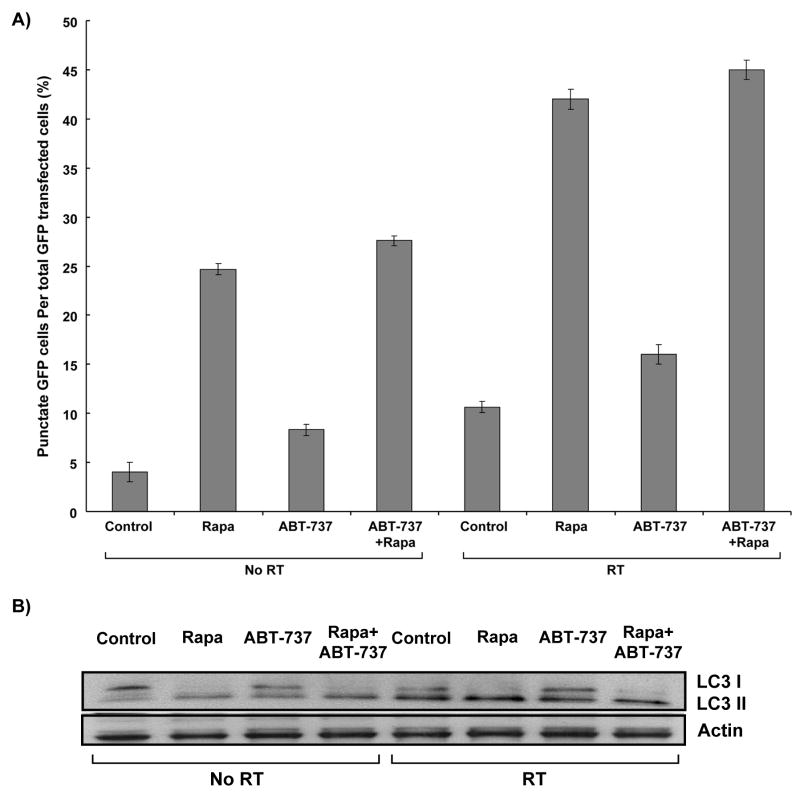
Published studies have indicated that Bcl-2 inhibition may up-regulate autophagy (19). To determine whether combination of Bcl-2 and mTOR inhibitor enhance radiation-induced autophagy, H460 cells were transfected with 2μg GFP-LC3 plasmid and treated with control, ABT-737 (500nM, 2 hr), rapamycin (100nM, 2 hrs), or both, followed by 5 Gy radiation. As shown in Figure 2A, less than 10% of cells receiving radiation alone were found to be undergoing autophagy (p=0.017), as opposed to 42% of irradiated cells treated with rapamycin (p=0.0002). ABT-737 increased autophagy from 4% to 8.3% (p=0.02) without radiation and from 10.7% to 16% (p=0.02) with radiation. When added to rapamycin treatment, however, ABT-737 induced only autophagy from 24.6% to 27.6% (p=0.003) without radiation and from 42% to 45% (p=0.0004) with radiation. These results were also confirmed by assessing the level of processed LC3 protein, in which the cells treated with rapamycin showed increased levels of LC3-II proteins following irradiation, in comparison to WT cells (Figure 2B).
Figure 2. Induction of autophagy by treatment with rapamycin in H460 cells.
(A) GFP-LC3 transfected cells were treated with 500 nM of ABT-737, 100 nM rapamycin, or both for two hours, with or without radiation (5 Gy). Cells were then examined by fluorescence microscopy after 48 hours. The percentage of cells with characteristic punctate GFP-LC3 fluorescence was calculated relative to all GFP-positive cells. This was repeated thrice. Columns, mean; bars, S.D. (B) LC-I and –II expression was determined by Western blot using lysates from H460 cells treated with 500 nM of ABT-737, 100 nM rapamycin, or both for two hours, with or without radiation (5 Gy). Actin was probed to demonstrate equal loading.
Combination treatment of ABT-737 and rapamycin increases radiosensitivity in H460 cells
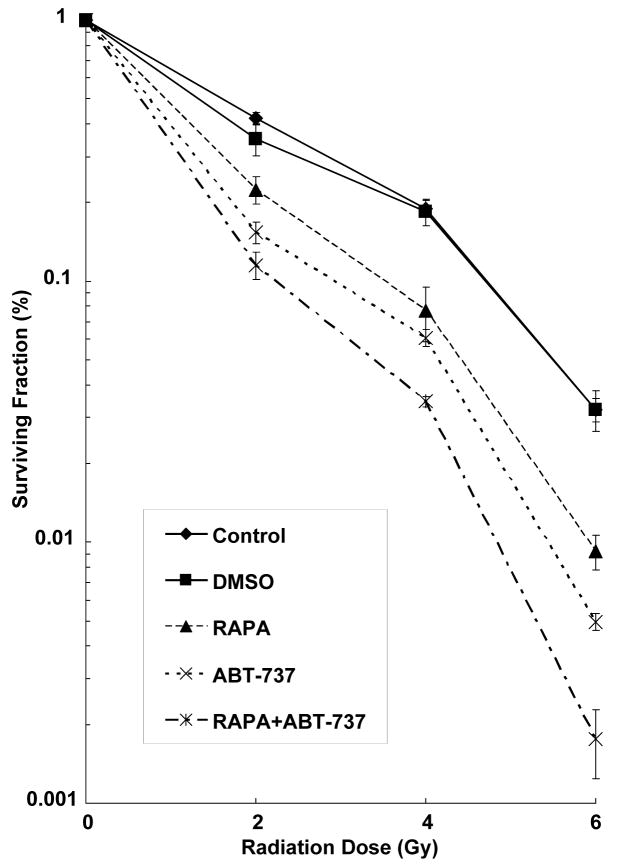
To determine if radiosensitization is maximized by simultaneous inhibition of Bcl-2 and mTOR pathways, we performed clonogenic assays in H460 cells treated with DMSO, ABT-737 (500 nM, 2 hrs), rapamycin (100 nM, 2 hrs), or both, and radiation (0-6 Gy). For all treatment groups, we calculated Dose Enhancement Ratios (DER), the ratio of radiation doses required to give an equivalent anti-tumor effect with radiosensitizer or without. Thus, in our study, a higher DER will imply that lower doses of radiation are required to achieve a similar cytotoxicity when radiation is combined to ABT-737 and/or rapamycin compared to radiation alone. As shown in Figure 3, the DER in H460 cancer cells treated with rapamycin or ABT-737 compared to control was 1.65 (p=0.006) and 2.16 (p=0.009), respectively. The combination treatment group of both rapamycin and ABT-737 yielded a DER of 2.47 (p=0002), compared to the DMSO group. These results suggest that either mTOR inhibition by rapamycin or Bcl-2 inhibition by ABT-737 increases radiation sensitivity and that dual inhibition of these pathways maximizes radiosensitivity in H460 lung cancer cells.
Figure 3. Combined ABT-737 and rapamycin treatment sensitizes H460 cells to radiation.
Clonogenic assay revealing radiosensitization of H460 cells treated with ABT-737 (500nM for 2hrs), rapamycin (100nM for 2hrs) or combination of both, and were irradiated with the indicated doses of radiation. After 8 d, surviving colonies were stained and the scored colonies were graphed. Shown are the mean +/- SD of three separate repeated experiments.
Combination treatment of ABT-737, rapamycin, and radiation results in extended tumor growth delay in lung xenograft model
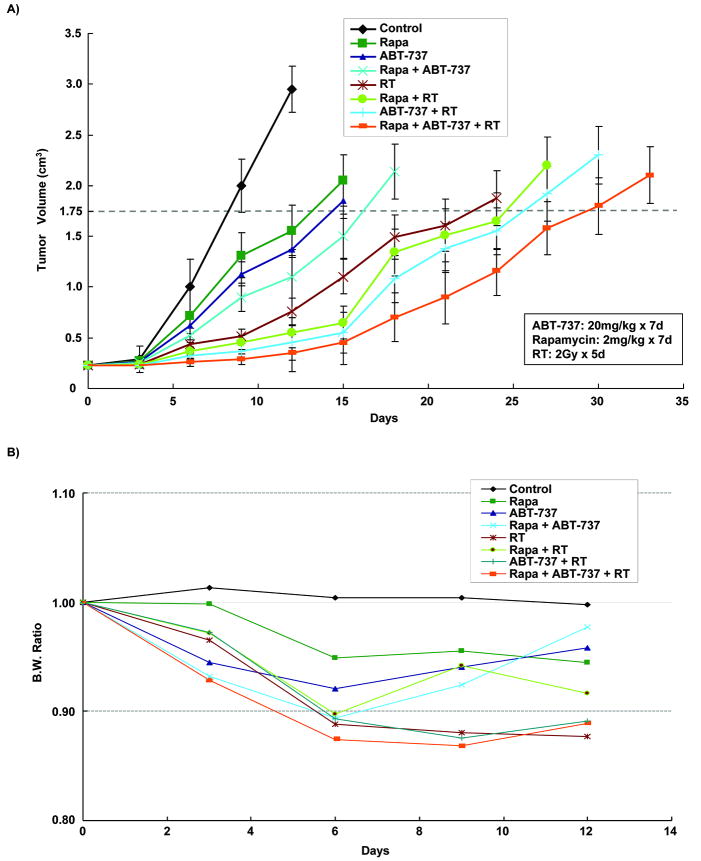
Having established the in vitro effects of combined Bcl-2 and mTOR inhibition on lung cancer radiosensitivity, mouse heterotopic xenograft models were used to confirm the biological effects of ABT-737, rapamycin, and radiation in vivo. The treatment groups consisted of DMSO (20mg/kg, ip), ABT-737 (20mg/kg, ip), rapamycin (2mg/kg, orally), or combination ABT-737 and rapamycin consecutively for 7 days, with or without 10 Gy radiation (2 Gy/day for 5 days). Growth delay was calculated as the number of days required to reach a tumor volume of 1.75 cm3 for treatment groups relative to control tumors. As shown in Figure 4A, a significant tumor growth delay was seen with combination therapy of ABT-737, rapamycin, and radiation compared to irradiation alone (22 vs 15 days, p=0.002), while ABT-737 or rapamycin alone did not significantly affect the tumor growth compared to control. Similarly, combination therapy of ABT-737/radiation and rapamycin/radiation resulted in a significant tumor growth delay, 3 (p=0.01) and 2 (p=0.001) days, respectively, as compared to irradiation alone. In addition, mouse body weights monitoring suggested that all treatments were relatively well tolerated (Figure 4B). Taken together, these results suggest that the combination therapy of ABT-737 and rapamycin increase lung cancer response to radiotherapy in vivo.
Figure 4. Extended tumor growth delay with combined ABT-737/rapamycin and radiotherapy at well-tolerated doses in xenografted H460 model.
H460 lung cancer cells were injected subcutaneously in athymic female mice and grown for 8-10 days until tumor volume averaged 0.23 cm3. Mice were treated daily for 7 days with vehicle control (DMSO), ABT-737 (20mg/kg/d), rapamycin (2mg/kg/d), or both in combination, and then were treated 1 hour after drug treatment with 2 Gy of radiation, daily over 5 consecutive days (detailed in Materials and Methods). (A) Tumors were measured regularly and volume was plotted vs. time. Growth delay was calculated as the number of days required to reach a tumor volume of 1.75 cm3 for treatment groups relative to control tumors. (B) Body weights were measured every 5 days and body weight ratio was calculated relative to baseline measurement.
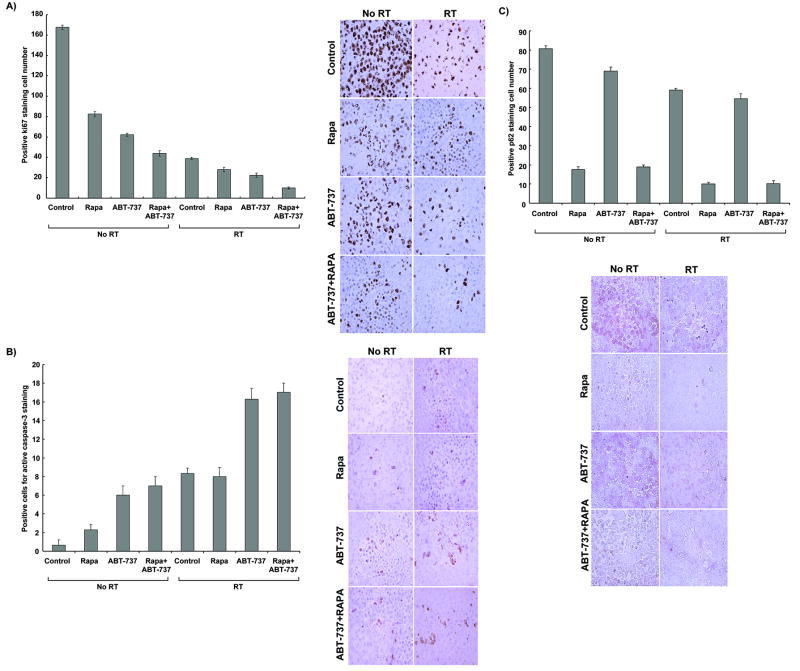
Combination treatment of ABT-737, rapamycin, and radiation reduces tumor proliferation index and induces both apoptosis and autophagy in irradiated H460 xenografts
To further characterize the effects of ABT-737 and rapamycin shown in the tumor growth delay model, we examined fixed H460 tumor sections in all treatment groups for proliferation (Ki67 staining, Figure 5C), apoptosis (active caspase-3 staining, Figure 5A), and autophagy (P62 staining, Figure 5B). The treatment groups were identical to those used for the tumor growth delay study. As shown in Figure 5C, Ki67 staining revealed a significant decrease in cell proliferation in the radiation combined to ABT-737 (22.3 ± 2 vs. 39 ± 1, p=0.01) or rapamycin (28 ± 2 vs. 39 ± 1, p=0.002) groups as compared to radiation alone, respectively. The greatest reduction in Ki67 proliferation index results from the combination of ABT-737, rapamycin, and radiation (10 ± 1 vs. 39 ± 1, p=0.001) compared to radiation alone. Apoptosis levels in fixed H460 tumor sections were assessed using active caspase-3 staining. As shown in Figure 5A, radiation plus ABT-737 increased apoptotic cells compared to radiation alone (16.3 ± 1 vs. 8 ± 1, p=0.001), while the addition of rapamycin to radiation had no increase in apoptosis compared to radiation alone. When rapamycin was combined with radiation and ABT-737, there was only a minor increase in apoptosis as compared to radiation plus ABT-737 alone. These results suggest that ABT-737 increases radiation-induced apoptosis and further reduce tumor cell proliferation after irradiation.
Figure 5. Combined ABT-737 and rapamycin therapy reduces cell proliferation and respectively increases apoptosis and autophagy in vivo.
Histologic sections were obtained from the tumors of the mice in each treatment group from the tumor volume study. The number of positive cells per 400× field was scored and graphed by averaging three repeated experiments. Representative histological photographs and quantitative graphs are shown for each type of staining. (A) Average Ki67 proliferative index of each treatment group was determined by the percent of Ki67-positive cells per microscopic field. This was repeated thrice. Columns, mean; bars, SD. (B) Active caspase-3 staining was performed on lung tumor sections, and apoptotic index was calculated by counting positive active caspase-3 stained cells per microscopic field. This was repeated thrice. Column, mean; bars, SD. (C) p62 antibody staining was also performed, and autophagy index was similarly calculated by counting positive p62 stained cells per microscopic field. This was repeated thrice. Column, mean; bars, SD.
To further explore the mechanisms of cell death resulting from the tri-combination treatment in vivo, we also examined fixed H460 tumor sections in all treatment groups for autophagy (P62 staining). P62 interacts and binds to LC3 and is removed in lysosomes by autophagy, which controls its turnover. Representative histological photographs of P62 staining on lung tumor sections are shown in Figure 5B. As shown in Figure 5B, rapamycin combined with radiation reduced P62 protein staining by 6-fold compared to radiation alone (59 ± 1.5 vs. 10 ± 1.5, p<0.001), while the ABT-737 plus radiation group exhibited an insignificant increase in autophagic level. There was no significant change in p62 staining with the addition of ABT-737 to rapamycin with radiation treatment, suggesting that mTOR inhibition is primarily responsible for autophagic cell death in vivo.
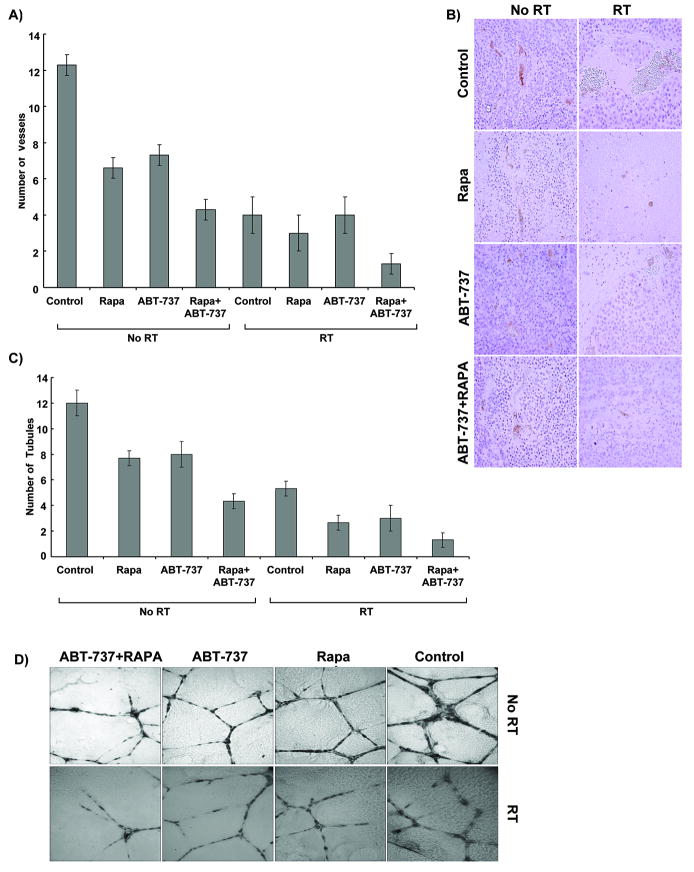
Combination treatment of ABT-737, rapamycin, and radiation reduces vascular density in irradiated H460 xenografts and sensitizes HUVECs to radiation
To determine the effects of Bcl-2/mTOR inhibition on tumor vasculature, vascular density study was performed using von Willebrand Factor (vWF) staining in each lung cancer xenograft treatment groups. The number of vessels per microscopic field was then quantified for each treatment group. As shown in Figure 6A, combination therapy with ABT-737 and rapamycin with radiation resulted in a 3-fold reduction relative to radiation therapy alone (4 ± 1.5 vs. 12.3 ± 0.5, p=0.01). To further investigate the effects of Bcl-2/mTOR inhibition on blood vessel formation, an endothelial cell morphogenesis assay was performed to examine the ability of treated HUVECs to produce capillary-like tubular structures. A representative image and the mean number of tubules from three separate (×400) fields are shown in Figures 6C and 6D. Treatment with rapamycin (2.6 ± 0.6 vs. 5.3 ± 0.6, p=0.05) or ABT-737 (3 ± 1 vs. 5.3 ± 0.6, p=0.07) combined with radiation reduced tubule formation as compared to radiation alone, respectively. The greatest reduction in tubule formation was seen following treatment with combination of rapamycin, ABT-737 and radiation (p=0.001). These results suggest that both ABT-737 and rapamycin have anti-angiogenic effects in addition to their radiosensitization effect.
Figure 6. ABT-737 and rapamycin reduce vascular density in irradiated H460 tumors in vivo and sensitize vascular endothelial cells to radiation in vitro.
(A) Histologic sections were obtained from the tumors of the mice in each treatment group from the in vivo tumor volume study, and stained for blood vessels using an antibody for vWF. Blood vessel density of each treatment group was determined by counting the number of blood vessels per 400× microscopic field. This was done in triplicate and the average of the three counts was calculated. Columns, average; bars, SD. (B) Representative histologic photographs following vWF staining. (C) Human umbilical vein endothelial cells (HUVECs) were treated with DMSO, ABT-737 (500μM for 2hrs), rapamycin (100nM for 2hrs) or combination therapy, and then irradiated with either 0 or 3 Gy. Six hours later, cells were trypsinized and replated on 24-well plates coated with Matrigel. After 24 h, cells were fixed and stained with H&E. The slides were examined by microscopy (×100), and stained tubules were then counted in three separate, randomly selected fields. Columns, mean; bars, S.D. (D) Representative histologic photographs of H&E stained HUVECs showing tubule formation.
Discussion
In the present report, we showed the effects of ABT-737, a Bcl-2 inhibitor, and rapamycin, an mTOR inhibitor, which resulted in the effective radiosensitization of lung cancer cells in vitro and in a lung cancer xenograft model. This study also suggests that the combination treatment of ABT-737 and rapamycin increases the effects of radiation on vasculature, which may partially explain the extended tumor growth delay. Interestingly, we found that both apoptosis and autophagy can simultaneously be induced and further enhance radiosensitivity of lung cancer.
It has been shown that ABT-737, a BH3 mimetic, binds to anti-apoptotic Bcl-2 proteins and disrupts the sequestering and neutralizing of proapoptotic proteins. ABT-737 and its oral analogue, ABT-263, have been demonstrated to promote apoptosis and cause in vitro regression of several hematologic malignancies and a variety of solid tumors, including small-cell lung cancer (20). The drug has demonstrated efficacy upon administration as both a monotherapy and in combination with cytotoxic therapies (20-22). Unfortunately, data were not consistent across all cell lines. In a study of a panel of NSCLC cell lines, however, ABT-737 (0-50μM for 48h) showed mixed results in several resistant cell lines with apoptosis levels remaining at ∼30% or lower (18). Consistently, our study similarly suggested that ABT-737 (500nM, 2h) was sufficient to further promote apoptosis in irradiated H460 cells, but remained relatively low (Figure 1). Even the use of higher dose of radiation (20 Gy) failed to result in cell death of more than 35% of cells (Figure 1C). Although some of the results are not synergistic, the clonogenic assays, however clearly demonstrated synergistic results with the tri-therapy compared to any other combinations (Figure 3). In addition, the trypan blue assay, which detects the total amount of cell death, also showed synergistic effects for the combination ABT-737/rapamycin/radiation over radiation alone (Figure 1D). We believe that this is probably due to the fact that some cells will undergo other types of cell death, such as necrosis, in addition to apoptosis or autophagy, which is also suggested by Figure 1B. Together, the results confirmed that like many NSCLC lines, H460 is relatively radio-resistant and ABT-737 alone remains limited for an adequate induction of cell death at reasonable doses of radiation.
As we know, defects in apoptosis are not limited to Bcl-2 or Bcl-xL proteins, as they occur at several cellular levels, which could potentially cause resistance to anti-cancer agents (23). Therefore, Bcl-2 inhibition by ABT-737 alone may not be effective enough in the induction of apoptosis on its own. Indeed, previous studies have suggested that, in order to effectively induce apoptosis, multiple points in the apoptotic pathway may need targeting, such as Mcl-1 inhibition or Bak induction (18). One can suggest that successful sensitization may require individually tailored molecular therapies targeting all the potential defects in the apoptotic pathway. As a result, the use of alternate mechanisms of cell death such as autophagy becomes an attractive strategy to overcome defects in apoptosis.
Consistent with previous findings (13, 14, 24, 25), we found that rapamycin alone significantly sensitizes H460 cells to radiation (Figure 3) and extends tumor growth delay in irradiated lung xenografts (Figure 4A), suggesting that it is possible to take advantage of autophagy to enhance radiation therapy in lung cancer. However, the enhancement of radiation by rapamycin may be limited by relative resistance to autophagy in cancer cells (13). Among the mechanisms known to restrict autophagy, a relevant example is the association of NSCLC with mutations in LKB1 tumor suppressor, which negatively regulates mTOR signaling and is involved in the stimulation of autophagy (26). In addition, there is suggestion of several resistance mechanisms to mTOR inhibitors that could potentially limit the clinical efficacy of these agents (27). Therefore, there is a rationale for combination therapy with mTOR inhibitors to induce autophagy and Bcl-2 inhibitors to induce apoptosis.
In addition, there is some evidence of cross-talk between those two pathways. Recent studies have shown that Bcl-2 interacts with autophagy, via Beclin-1, a haploinsufficient tumor suppressor that is essential for autophagy (28, 29). It has been shown that Beclin-1 mediates its interactions with Bcl-2 and Bcl-xL through a BH3 domain. This property allows the competitive inhibition of Beclin-1/Bcl-2 interaction by ABT-737, which results in stimulation of autophagy in HeLa cells (29). Thus, Bcl-2 acts as an anti-autophagy protein in addition to its anti-apoptotic function, suggesting a role for Bcl-2 in maintaining low apoptosis and autophagy levels for cell survival. In our study, the application of ABT-737 also resulted in an increase in autophagy, particularly in combination with radiation (Figure 2). In contrast, the concurrent use of ABT-737 and rapamycin yielded a lower increase in autophagy only compared to rapamycin alone. Similar findings were also observed after p62 staining in vivo (Figure 5B). These data suggest that ABT-737 does not influence rapamycin-induced autophagy in our lung cancer models, even though it may disrupt the interaction between Bcl-2 and Beclin-1 proteins.
Alternatively, mTOR has been correlated to apoptosis, which can be promoted by rapamycin and its analogues dependent on the cell type (30). Although it has been shown that rapamycin-induced apoptosis is minimal on its own, it has potential to enhance the effects of DNA damaging agents, such as cisplatin (31). Interestingly, it has been suggested that expression of Bcl-2 was associated with “resistance” to rapamycin and analogues (32, 33), and that sensitivity to rapamycin was restored by Bcl-2 antisense (32). Another study demonstrated that rapamycin (100nM) in conjunction with ABT-263 (39 nM or 156 nM) resulted in increased apoptosis in lymphoma cell lines (34). Consistently, similar studies in neuroblastoma, lymphoid, and hepatocellular carcinoma showed that inhibition of mTOR induces or sensitizes cells to apoptosis (35-38). In this study, we only observed a small effect of rapamycin when administered alone for induction of apoptosis in vivo, while combination with ABT-737, radiation, or both did not significantly promote apoptosis (Figure 5A). Differences in findings may be due to the intrinsic nature of the hematological cell lines as opposed to the solid NSCLC xenograft tumor or to the differences in concentration of the Bcl-2 inhibitor (39nmol/L or 156nmol/L vs. 500nmol/L). Taken together, our results suggest that a potential clinical benefit from the combination of ABT-737 and rapamycin will probably be secondary to the individual targeting of each pathway rather than a cross-talk between them.
In contrast, we previously illustrated a cross-talk at a different level, between inhibition of apoptosis and up-regulation of autophagy (14). More precisely, H460 radiosensitivity was increased when rapamycin was administered in presence of Z-DEVD, a caspase-3 inhibitor (14). Thus, it appears that autophagy may serve as a backup death mechanism when apoptosis is unavailable. In the present study, instead of channeling radiation-induced cell death through autophagy only, we wanted to take advantage of the two death pathways simultaneously to maximize cell death. By doing this, we found that the targeting of both pathways is better than the induction of one pathway alone, but one drug did not significantly induce a synergistic effect on the alternate pathway. This also illustrates the complex role of autophagy and suggests that more studies are needed to further determine the mechanisms of autophagy.
To investigate autophagy in vivo, we stained histological sections for p62 antibody (Figure 5B). In vivo detection of autophagy has long been a challenge and p62 detection may offer an important solution. p62, or, sequestosome 1 (SQSTM1), is a common component of protein aggregates, responsible for linkage of polyubiquitinated proteins to autophagic machinery (39, 40). Both p62-and LC3-positive bodies are degraded in autolysosomes and inhibition of autophagy leads to an increase in p62 protein levels (41). Detection of p62 in vivo has previously been proposed as a way to monitor autophagic flux, though to our knowledge, previous use of this protocol has not been published (42). Currently, there is no other means of detecting autophagy in vivo and we believe that obtained data are a good representation of autophagy levels in examined histological sections. Indeed, in vivo results suggested that rapamycin and not ABT-737 resulted in autophagy induction, both with and without radiation (Figure 5B). This is consistent with our in vitro experiments, which showed similarly that ABT-737 does not result in a significant increase in autophagosome formation as opposed to rapamycin (Figure 2), and suggests that the p62 in vivo staining may be used in future investigations.
Since, tumor neo-vascularization is a poor prognostic factor in NSCLC patients (43), we also investigated the effects of Bcl-2 and mTOR inhibition on vascular density and angiogenesis. Ionizing radiation is known to exhibit contrasting effects on vascularization, resulting in an increase in pro-angiogenic factors such as the vascular endothelial growth factor (VEGF), as well as antivascular effects (44-46). We report here that the triple combination ABT-737/rapamycin/radiation reduced the vessels density (vWF staining) compared to radiation alone (p=0.01) (Figure 6A). We confirmed the vascular effects observed in vivo, by determining the ability of treated endothelial cells to produce capillary-like tubular structures in vitro (Figure 6B). Although the bi-combination was not previously explored, anti-angiogenic effects of Bcl-2 (47) and mTOR (48) inhibitors were individually reported. Additionally, it has been shown that rapamycin exerts anti-angiogenic effects possibly by reducing VEGF production and also by blockage of VEGF-induced endothelial cell signaling (49). We also recently reported that rapamycin and analogues decreased clonogenic survival of HUVECs and sensitized them to apoptosis (50). Further studies are needed to fully elucidate the complex mechanisms by which ABT-737 and rapamycin mediate their anti-vascular effects.
In conclusion, this preclinical study supports the therapeutic potential for the combination treatment of ABT-737, a Bcl-2 inhibitor and rapamycin, an mTOR inhibitor, to sensitize lung cancer to radiotherapy. The rational therapeutic targeting of Bcl-2 and mTOR pathway simultaneously is a promising strategy to overcome possible resistance in NSCLC to standard radiotherapy. Clinical trials are warranted to determine if this novel strategy might benefit patients with NSCLC.
Footnotes
Statement of Translational Relevance: Treatment of non-small-cell lung cancer (NSCLC) is characterized by intensive chemo-radiation regimens which, in the instance of locally-advanced disease, succeed only to prolong patient survival by a few months. Treatment failure is partially attributed to the relative resistance of NSCLC to apoptosis. We have previously demonstrated that an alternate cell death type, autophagy, has the potential to enhance radiation. In this study, we simultaneously induce apoptosis, using ABT-737, and autophagy, via rapamycin to maximize radiation-induced cell death. As a result, the tri-modality treatment leads to radiosensitization of H460 lung cancer cells and extended tumor growth delay in an in vivo mouse xenograft model. Immunohistochemistry analyses (active caspase-3 and p62 stainings) confirm an increase in both processes as well as decreased vascular density (vWF staining). We conclude that the simultaneous induction of apoptosis and autophagy in combination with radiation warrants further investigation and may one day offer a viable option for treatment of resistant tumors.
References
- 1.American Cancer Society. Cancer Facts and Figures 2008. Atlanta: American Cancer Society; 2008. [Google Scholar]
- 2.Green DR. At the gates of death. Cancer Cell. 2006;9:328–30. doi: 10.1016/j.ccr.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Haura EB, Cress WD, Chellappan S, Zheng Z, Bepler G. Antiapoptotic signaling pathways in non-small-cell lung cancer: biology and therapeutic strategies. Clin Lung Cancer. 2004;6:113–22. doi: 10.3816/CLC.2004.n.025. [DOI] [PubMed] [Google Scholar]
- 4.Borner MM, Brousset P, Pfanner-Meyer B, et al. Expression of apoptosis regulatory proteins of the Bcl-2 family and p53 in primary resected non-small-cell lung cancer. Br J Cancer. 1999;79:952–8. doi: 10.1038/sj.bjc.6690152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pezzella F, Turley H, Kuzu I, et al. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993;329:690–4. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- 6.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–21. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 7.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 8.Cory S, Adams JM. Killing cancer cells by flipping the Bcl-2/Bax switch. Cancer Cell. 2005;8:5–6. doi: 10.1016/j.ccr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 10.Marinov M, Fischer B, Arcaro A. Targeting mTOR signaling in lung cancer. Crit Rev Oncol Hematol. 2007;63:172–82. doi: 10.1016/j.critrevonc.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Balsara BR, Pei J, Mitsuuchi Y, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–9. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 12.Kasukabe T, Okabe-Kado J, Kato N, Sassa T, Honma Y. Effects of combined treatment with rapamycin and cotylenin A, a novel differentiation-inducing agent, on human breast carcinoma MCF-7 cells and xenografts. Breast Cancer Res. 2005;7:R1097–110. doi: 10.1186/bcr1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weppler SA, Krause M, Zyromska A, Lambin P, Baumann M, Wouters BG. Response of U87 glioma xenografts treated with concurrent rapamycin and fractionated radiotherapy: possible role for thrombosis. Radiother Oncol. 2007;82:96–104. doi: 10.1016/j.radonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Kim KW, Hwang M, Moretti L, Jaboin JJ, Cha YI, Lu B. Autophagy upregulation by inhibitors of caspase-3 and mTOR enhances radiotherapy in a mouse model of lung cancer. Autophagy. 2008;4:659–68. doi: 10.4161/auto.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82:381–8. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- 16.Tahir SK, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 17.Hann CL, Daniel VC, Sugar EA, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008;68:2321–8. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesarg E, Hoffarth S, Wiewrodt R, et al. Targeting BCL-2 family proteins to overcome drug resistance in non-small cell lung cancer. Int J Cancer. 2007;121:2387–94. doi: 10.1002/ijc.22977. [DOI] [PubMed] [Google Scholar]
- 19.Maiuri MC, Criollo A, Tasdemir E, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–6. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 20.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 21.Shoemaker AR, Mitten MJ, Adickes J, et al. Activity of the Bcl-2 family inhibitor ABT-263 in a panel of small cell lung cancer xenograft models. Clin Cancer Res. 2008;14:3268–77. doi: 10.1158/1078-0432.CCR-07-4622. [DOI] [PubMed] [Google Scholar]
- 22.Witham J, Valenti MR, De-Haven-Brandon AK, et al. The Bcl-2/Bcl-XL family inhibitor ABT-737 sensitizes ovarian cancer cells to carboplatin. Clin Cancer Res. 2007;13:7191–8. doi: 10.1158/1078-0432.CCR-07-0362. [DOI] [PubMed] [Google Scholar]
- 23.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–85. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 24.Paglin S, Lee NY, Nakar C, et al. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005;65:11061–70. doi: 10.1158/0008-5472.CAN-05-1083. [DOI] [PubMed] [Google Scholar]
- 25.Cao C, Subhawong T, Albert JM, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–7. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 26.Ji H, Ramsey MR, Hayes DN, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–10. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 27.Kurmasheva RT, Huang S, Houghton PJ. Predicted mechanisms of resistance to mTOR inhibitors. Br J Cancer. 2006;95:955–60. doi: 10.1038/sj.bjc.6603353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pattingre S, Tassa A, Qu X, et al. Bcl-2 Antiapoptotic Proteins Inhibit Beclin 1-Dependent Autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Maiuri MC, Le Toumelin G, Criollo A, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–39. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castedo M, Ferri KF, Blanco J, et al. Human immunodeficiency virus 1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/FKBP12-rapamycin-associated protein-mediated p53 phosphorylation. J Exp Med. 2001;194:1097–110. doi: 10.1084/jem.194.8.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–59. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 32.Aguirre D, Boya P, Bellet D, et al. Bcl-2 and CCND1/CDK4 expression levels predict the cellular effects of mTOR inhibitors in human ovarian carcinoma. Apoptosis. 2004;9:797–805. doi: 10.1023/B:APPT.0000045781.46314.e2. [DOI] [PubMed] [Google Scholar]
- 33.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 34.Ackler S, Xiao Y, Mitten MJ, et al. ABT-263 and rapamycin act cooperatively to kill lymphoma cells in vitro and in vivo. Mol Cancer Ther. 2008;7:3265–74. doi: 10.1158/1535-7163.MCT-08-0268. [DOI] [PubMed] [Google Scholar]
- 35.Gu L, Gao J, Li Q, et al. Rapamycin reverses NPM-ALK-induced glucocorticoid resistance in lymphoid tumor cells by inhibiting mTOR signaling pathway, enhancing G1 cell cycle arrest and apoptosis. Leukemia. 2008;22:2091–6. doi: 10.1038/leu.2008.204. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JF, Liu JJ, Lu MQ, et al. Rapamycin inhibits cell growth by induction of apoptosis on hepatocellular carcinoma cells in vitro. Transpl Immunol. 2007;17:162–8. doi: 10.1016/j.trim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Bu X, Le C, Jia F, et al. Synergistic effect of mTOR inhibitor rapamycin and fluorouracil in inducing apoptosis and cell senescence in hepatocarcinoma cells. Cancer Biol Ther. 2008;7:392–6. doi: 10.4161/cbt.7.3.5366. [DOI] [PubMed] [Google Scholar]
- 38.Marimpietri D, Brignole C, Nico B, et al. Combined therapeutic effects of vinblastine and rapamycin on human neuroblastoma growth, apoptosis, and angiogenesis. Clin Cancer Res. 2007;13:3977–88. doi: 10.1158/1078-0432.CCR-06-2757. [DOI] [PubMed] [Google Scholar]
- 39.Bjorkoy G, Lamark T, Brech A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–14. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pankiv S, Clausen TH, Lamark T, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 41.Ichimura Y, Kumanomidou T, Sou YS, et al. Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem. 2008;283:22847–57. doi: 10.1074/jbc.M802182200. [DOI] [PubMed] [Google Scholar]
- 42.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–5. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 43.Fontanini G, Lucchi M, Vignati S, et al. Angiogenesis as a prognostic indicator of survival in non-small-cell lung carcinoma: a prospective study. J Natl Cancer Inst. 1997;89:881–6. doi: 10.1093/jnci/89.12.881. [DOI] [PubMed] [Google Scholar]
- 44.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–8. [PubMed] [Google Scholar]
- 45.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 46.Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9:1957–71. [PubMed] [Google Scholar]
- 47.Zeitlin BD, Joo E, Dong Z, et al. Antiangiogenic effect of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res. 2006;66:8698–706. doi: 10.1158/0008-5472.CAN-05-3691. [DOI] [PubMed] [Google Scholar]
- 48.Manegold PC, Paringer C, Kulka U, et al. Antiangiogenic therapy with mammalian target of rapamycin inhibitor RAD001 (Everolimus) increases radiosensitivity in solid cancer. Clin Cancer Res. 2008;14:892–900. doi: 10.1158/1078-0432.CCR-07-0955. [DOI] [PubMed] [Google Scholar]
- 49.Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 50.Shinohara ET, Cao C, Niermann K, et al. Enhanced radiation damage of tumor vasculature by mTOR inhibitors. Oncogene. 2005;24:5414–22. doi: 10.1038/sj.onc.1208715. [DOI] [PubMed] [Google Scholar]