Abstract
In the beginning - Trk was an oncogene. Yet Neurotrophin-Trk signaling came to pre-eminence in the field of neurobiology. Now it is appreciated that Trks regulate important processes in non-neuronal cells and, in addition to their impact on tumors of neural origin, may contribute to the pathogenesis of carcinomas, myelomas, prostate and lymphoid tumors. While mutations and rearrangements of Trk are only sporadically seen in human cancers such as medullary thryoid carcinoma, a number of recent studies indicate that expression of TrkB contributes to tumor pathology. In neuroblastoma TrkA expression marks good prognosis which TrkB and Brain-derived neurotrophic factor (BDNF) expression marks poor prognosis. Activation of the BDNF/TrkB signal transduction pathway also stimulates tumor cell survival and angiogenesis and contributes to resistance to cytotoxic drugs and anoikis, enabling cells to acquire many of the characteristic features required for tumorigenesis. Small molecule inihibitors such as Cephalon's CEP-701 are in Phase I & II clinical trials, and a series of AstraZeneca Trk inhibitors are poised to enter the clinic. As monotherapy, inhibitors may only be effective in tumors with activating Trk mutations. Important clinical follow-up will be the assessment of Trk inhibitors in combination with standard chemo- or radiotherapy or other signal transduction pathway inhibitors.
Keywords: Trk, TrkB, Neuroblastoma, cancer, Prostate, Thryoid cancer Metastasis, Angiogenesis, Anoikis, Chemotherapy
Background
Trk (tropomyosin receptor kinase) was first functionally identified as a gene capable of transforming immortalized fibroblasts (1). Oncogenic Trk was composed of the 5′ region of the tropomyosin gene fused to an unknown tyrosine kinase (TK) domain similar to those frequently found in transmembrane receptors. The constitutive expression of Trk led to its activation and transforming capability. Mutations and rearrangements of Trk were identified in colon and thyroid cancers but at such a low frequency that oncologists turned their attention to oncogenes such as ras that were mutated at a higher frequency in human cancers. Research on Trks intensified among neurobiologists when it was recognized that the ligand-binding domain of proto-Trk was the long sought after receptor for Nerve Growth Factor (NGF) (2). Members of the Trk family are highly expressed in cells of neural origin. Activation of Trks (TrkA, TrkB & TrkC) by their preferred neurotrophins (NTs) (NGF to TrkA, Brain-derived neurotrophic Factor (BDNF) and NT4/5 to TrkB and NT3 to TrkC) mediates the survival and differentiation of neurons during development. Trk activation is also required throughout adulthood to sustain the growth and function of neuronal synapses (3). BDNF activation of TrkB is key for the enhancement of activity-stimulated excitation of neurons that is needed for memory development and maintenance. Moreover, the NT/Trk signaling pathway functions as an endogenous system that protects neurons after biochemical insults, transient ischemia or physical injury (4).
Trk signaling has been delineated in the TrkA-expressing rat pheochromocytoma tumor cell line PC12 and confirmed in primary cultures of normal sympathetic TrkA-expressing neurons. Upon NGF treatment, PC12 cells stop proliferating and extend neuritic processes, differentiating into sympathetic neurons almost indistinguishable from their normal counterparts (5). NGF-induced dimerization of TrkA or BDNF-induced dimerization of TrkB receptors leads to activation of the tyrosine kinase domain and reciprocal autophosphorylation of tyrosines (Y) in this catalytic domain as well as surrounding Y484 and Y785, which facilitates binding of SHC and PTB domain protein adaptors and PLCγ protein binding, respectively. This leads to activation of the major growth factor-regulated signaling pathways: the Ras/MAPK pathway, the PI3Kinase (PI3K)/ 3-phosphoinositide-dependent protein kinase-1 (PDK1)/Akt pathway and increases in Ca++ release and activation of the PLCγ pathway (Trk signaling reviewed in 3-6) Fig. 1.
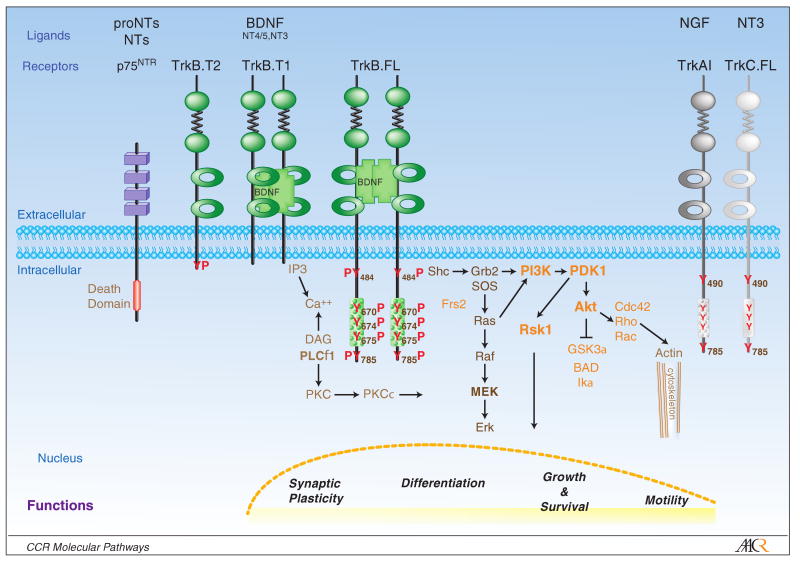
Figure 1.
Schematic representation of Trk receptor tyrosine kinases and major signal transduction pathways. The trk receptors have an extracellular ligand binding domain, a singular transmembrane region and an intracellular region of variable length. The round green filled circles represent the cysteine rich regions, which are separated by fibronectin type III repeats (jagged line). The open green circles represent the Ig-like domains that bind the dimeric BDNF monomers leading to dimerization of the TrkB receptors and activation of the intracellular TrkB-tyrosine kinase (TK) domain (rectangle filled with circles). Activation of the TrkB-TK leads to phosphorylation (P) of a number of tyrosines (Y) in the TK domain as well as the juxtamembrane domain. These PY residues serve as docking sites for cytoplamic proteins such as Shc, PLCγ whose recruitment in turn leads to activation of downstream mediators of the MAPK, PLCγ and PI-3 kinase pathways. Ultimately these signals are transduced to the nucleus to mediate transcriptional programs that regulate cellular functions such as synaptic plasticity, differentiation, growth and survival and motility. Other members of the Trk family, TrkA and TrkC are depicted to illustrate the structural conservation of this gene family. P75 is a pan neurotrophin (NT) receptor which is structurally in the “Death Receptor” family of membrane bound receptors.
Activation of ras mediates neurotrophin-induced survival and differentiation. Neurons containing constitutively active ras, either from introduction of mutant ras or from loss of a negative regulator of ras such as neurons from NF1-/- mice, survive without neurotrophins. Downstream of ras the PI-3 kinase pathway and to a lesser extent the raf/mek/erk pathway is required for survival (7). Constitutive activation of ERKs induces transcription factors including CREB, fos, jun and Egr-1, which regulate a number of genes involved in regulating neural differentiation and neurite outgrowth (8).
The phosphorylation of inositol-(3,4)-diphosphate to inositol-(3,4,5)-triphosphate by PI3K recruits PH-containing proteins PDK1 and AKT to the cell membrane, where PDK1 phosphorylation of AKT activates the AKT kinase. Akt subsequently phosphorylates and inactivates several proteins that inhibit a number of pro-apoptotic signaling pathways. Akt phosphorylation of GSK3β inactivates its kinase activity and high GSK3β kinase activity has been implicated in neurodegenerative diseases such as Alzheimer's disease and neurotoxic signaling. Akt phophorylates BAD, thus diminishing its binding to and inactivation of the anti-apoptotic protein, Bcl-XL (9). Akt phosphorylation of the Forkhead transcription factor FKHRL1 prevents its translocation to the nucleus, decreasing transcription of pro-apoptotic mediators, Bim and FASL (10). An alternative survival mechanism of increasing importance is PDK1 activation of RSK1/2. This Akt-independent pathway may require Erk activation and in some models leads to decreases in pro-apoptotic proteins such as Bim (11).
Activated TrkB is an important mediator of synaptic plasticity via increases in intracellular Ca++ and activation of the PLCγ pathway. Since the growth cones of developing neurons and the synapses of mature neurons may be located far from their cell bodies (where the majority of protein synthesis occurs), local mRNA translation is important for the processes of axon guidance, synapse formation and plasticity. Synaptic formation and plasticity are essential for activity dependent learning (12). Neural activity stimulates expression and secretion of BDNF at synaptic membranes (4). TrkB located at the pre- and post-synaptic membranes is activated by BDNF, leading to increased PI3K/mTOR signaling and enhanced local translation of mRNAs such as Ca++ modulated proteins (13). Among these is CamKIIa, which phosphorylates the CREB transcription factor and enables its binding to cyclic AMP and Ca++ response elements in the promoters of target genes. Ca++ fluxes modulate the subsequent activation of CREB and transcription of target genes, one of which is BDNF. BDNF activation of TrkB also stimulates mobilization of neurotransmitter-containing synaptic vesicles via TrkB binding to an actin/Myo6/GIPC1 motor complex (14). Thus BDNF activation of TrkB amplifies neurotransmitter release, thereby stimulating and re-enforcing synaptic communication.
There are a number of Trk splice variants. For example, NTRK2 (TrkB) has 3 major variants; the p145kd catalytic tyrosine kinase receptor (TrkB.FL) and two others of p95kd (TrkB.T1 and TrkB.T2) that lack the tyrosine kinase domain yet have distinct intracellular regions (Fig. 1) (15,16). Under some conditions, TrkB.T1 or -T2 can function as dominant negatives, attenuating the effect of ligands when co-expressed with TrkB.FL. The ligand-binding domain of TrkB preferentially binds BNDF yet also to a lesser degree NT3 and NT4/5. Splice variants of TrkB and TrkA containing extra sequences in the juxtamembrane region enhance binding to non-preferred ligands.
Trk expression and activation status also can be influenced by a number of different receptor systems co-expressed by cells. Steroids receptors, G-protein coupled receptors (GPCR) and other receptor tyrosine kinases can also transactivate Trks and downstream PLCγ and PI3K paths in the absence of NTs (17,18). Intracellular accumulation of zinc (but not other divalent cations) via voltage-dependent cation channels activates TrkB (but not TrkA or TrkC) via inhibition of C-terminal Src kinase activating Src (also Yes and Fyn). Src kinase transactivates TrkB but results only in activation of the Erk1/2, CREB and PLCγ pathways (19). Activation of Trks via these alternative mechanisms can qualitatively alter the signaling threshold for downstream signaling intermediaries, resulting in distinct functional read-outs such as proliferation versus differentiation.
Finally, one cannot discuss the function of Trks without mentioning p75/NGFR. Structurally, p75 is in the Death Receptor Family yet binds all NTs and serves, in the presence of Trks, to inhibit non-preferred NT binding, thereby enhancing preferred NT activation of Trks (4,5,18,20). However, immature or unprocessed forms of NTs, pro-NTs, bind both p75 and sortilin (a member of the VPS10p-domain receptor family), and this complex transmits a “death” signal via the JNK kinase pathway (21). The VPS10p-domain receptors are involved in the process of intracellular organelle protein trafficking, which is important in highly polarized cells such as neurons. Another family member SORLA (sorting protein-related receptor with A-type repeats) prevents the inappropriate processing of amyloid precursor protein to amyloid-β, a neurotoxin which is found in the senile plaques of Alzheimer's disease patients (22).
NTs are synthesized by different organs throughout the body and are essential for appropriate innervations of target organs. Trks are important during the normal development of kidney, prostate (23), B-lymphocytes (24), eosinophils (25) and bone marrow derived-endothelial precursor cells (26) and impact heart (27), muscle (28) and ovarian (29) differentiation. Moreover, embryonic stem cells express TrkB and TrkC, and their survival and clonability is largely dependent on the expression of their cognate ligands in the surrounding milieu (5).
Activation of the Trk signal transduction pathways is influenced by the concentration and type of NTs expressed in the local environment and by the cellular co-expression of Trk splice variants, p75 receptor, accessory receptors and the activation of G-coupled protein receptors. The ultimate signal transduced after NT activation of Trk signaling is the consequence of the integration of a number of distinct receptor signaling as well as biologic processing pathways.
Trks in Cancer
Trks may play either a good or bad role in oncogenesis. The first clinical association of Trks and cancer came with the findings of activating mutations caused by chromosomal rearrangements or mutations in NTRK1 (TrkA) in papillary and medullary thyroid carcinoma, respectively (30). However, even in these tumor types the frequency of genetic alterations in Trk genes is low, and such alterations have not been consistently identified in other tumors. Over the years a number of studies have detailed Trk expression in different tumor types, correlating expression with prognosis or tumor stage. However, there is no clear pattern of association of a particular Trk isoform with prognosis. In neuroblastoma (NB), patients whose tumors have elevated levels of TrkA (31) or TrkC (32) expression have a better prognosis, while those whose tumors express elevated levels of TrkB and BDNF have a poor prognosis (33,34). Elevated TrkC expression also is associated with a better prognosis in medulloblastoma (35). In medullary thyroid cancer, TrkB is expressed in hyperplastic C-cells while TrkC is more highly expressed in the late stages of disease (36).
Different splice variants of the same isoform may also have variable patterns of expression. For example, high expression of TrkB.FL in Wilm's tumor is a poor prognostic marker while high expression of the TrkB.T1 or T2 forms is associated with a better prognosis (37). Recently, a splice variant of TrkA (TrkAIII) was identified and found to be more highly expressed in tumors of neuroblastoma patients with a poor prognosis (38). These studies indicate that different Trk isoforms function as prognostic markers in different tumor types as well as within a single tumor type. The differential Trk or Trk isoform expression in tumor cells may reflect regulatory programs operant during development and/or different environmental conditions (such as hypoxia).
Clinical-Translational Advances
In cases where Trks are not translocated or mutated, questions remain; do they function as developmental markers or tumor markers, or do they have a pathophysiologic role in the biology of tumor cells? Since NT activation of Trk mediates survival signals and stimulates neuritogenesis and migration in normal neurons (39), these same processes could be exploited by tumor cells to survive cytotoxic insults or metastasize. TrkB-expressing neuroblastoma tumor cells treated with BDNF are less sensitive to cytotoxic drugs (40,41), survive under conditions of limiting growth factors (33,42,43), and display enhanced invasiveness (42) and increased VEGF production (44). Neuroblastoma cells that survive repeated exposures to cytotoxic agents express increasing levels of BDNF, suggesting that the NT-Trk signal transduction pathway contributes to a multi-drug resistant phenotype (42). The recent finding that hypoxia induces increases in TrkB (45) may contribute to a phenomenon in the tumor microenvironment whereby the autocrine activation of the BDNF/TrkB signal transduction pathway enables survival of residual tumor cells after repeated rounds of chemotherapy. In fact, even though many aggressive, poor prognosis neuroblastoma tumors are initially sensitive to cytotoxic drugs, the majority relapse.
Elevated expression of TrkA in neuroblastoma tumor specimens is associated with a good prognosis. Consistent with this observation, high TrkA expression in neuroblastoma cell lines is associated with decreased invasiveness (42), tumor cell growth (46) and low expression of angiogenic growth factors (47). TrkA and TrkB are differentially regulated in neuroblastoma tumor cells (48) and, consistent with the differences in functional effects, the transcriptome regulated by NGF activation of TrkA is markedly different from the transcriptome regulated by BDNF activation of TrkB (49).
Using a regulated-TrkB expression model (43), the level of TrkB expression as well as the concentration of BDNF increase the survival of cells in a toxic environment (40). As in normal neurons, the survival of neuroblastoma tumor cells exposed to cytotoxic drugs is mediated in part by activation of the Trk-TK, via PI-3kinase (41) activation of Akt (50) and inactivation of GSK3β (51). These studies demonstrated that the effects of chemotherapeutic agents are attenuated even if a tumor cell with high levels of TrkB expression is in an environment with low levels of BDNF. More importantly, even if tumor cells expressed low levels of TrkB receptor, if cells were in a BDNF-rich environment, the effects of cytotoxic agents were decreased. This indicates that sites of relatively high levels of TrkB ligands (BDNF, NT-4) may serve as sanctuary sites for TrkB-expressing tumor cells. An example of this is the finding that bone marrow (BM) stroma produces BDNF capable of stimulating the survival and growth of TrkB-expressing multiple myeloma tumor cells. The BM-derived BDNF activation of TrkB via activation of the PI-3kinase/Akt pathway desensitizes myeloma tumor cells to the drugs clinically used to treat multiple myeloma (52).
TrkB has been shown to play a key role in metastasis of tumor cells. BDNF stimulates tumor cell disaggregation and increases the ability of TrkB-expressing neuroblastoma tumor cells to degrade and invade through extracellular matrix proteins (58). Elegant studies identified that TrkB expression by epithelial cells enabled them to resist anoikis (anchorage-dependent cell death) and metastasize (53). Subsequent studies have extended this to ovarian (54,55), pancreatic (56) and prostate tumor cells (57) as well hepatocellular (58), gastric (59) and head and neck squamous cell carcinomas (60). These findings indicate that TrkB-mediated resistance to anoikis may be a general property of epithelial-derived tumor cells. The importance of TrkB in survival has been extensively studied in neuroblastoma tumors (31,40-3,50,51), yet there are reports of a role for TrkB in the survival of cells derived from malignant B cells (52, 61), prostate (62), lung (63) breast cancer cells (64) and head and neck tumors (65). A provocative study indicates that the TrkB-mediated invasiveness in neuroblastoma cells is achieved via upregulation of Hepatocyte Growth Factor (Scatter Factor) and c-met. The siRNA-mediated silencing of c-met or antibodies to HGF diminish BDNF/TrkB-induced invasiveness (66). Whether this is the generalized mechanism by which TrkB mediates motility/invasiveness awaits evaluation in other model systems.
BDNF has been found to stimulate neovascularization via recruitment of TrkB-expressing endothelial progenitor cells (67), raising the possibility that tumor cells secreting TrkB ligands are able to induce angiogenesis. In neuroblastoma, angiogenesis may be more directly influenced by increases in VEGF that are mediated by the BDNF/TrkB-induced increases in HIF-1α (47,52). Moreover, hypoxia induces increases in NTRK2 (TrkB) gene expression that are mediated by HIF-1α binding to a hypoxia response element in the NTRK2 promoter region (48). Hypoxia-associated increases in neuroblastoma cell invasiveness can be blocked by inhibition of the Trk-TK(48), bringing full circle the degree of TrkB's involvement in the process of angiogenesis and metastasis. As hypoxia is a hallmark of many aggressive tumors, hypoxia-induced increases in TrkB may be the reason that so many different tumor types have elevated TrkB levels. Where studied, most reports have pointed to the importance the Trk-TK/PI-3 kinase/Akt pathway in mediating invasiveness, resistance to anoikis and production of VEGF.
After the discovery of the involvement of Trks in the biology of cancer, it was appreciated that inhibition of Trk activity might be beneficial in the clinical oncology setting (68,69). Most strategies have been aimed at targeting the Trk TK domain. Since there is a high degree of homology among Trk TK domains, there are currently no reported inhibitors that selectively block TrkA or TrkB kinase activity. The earliest Trk inhibitors to reach clinical trials were Cephalon's CEP-751 and CEP-701(lestaurtinib). These compounds were modeled on the indolocarbazole K252a from Nocardiopsis sp. and were relatively non-selective in inhibiting Trks, Flt3 and protein kinase C (PKC). CEP-751 and CEP-701 reduced tumor burden in xenograft models of prostate (68), pancreatic (70), neuroblastoma and medulloblastoma tumors (71), medullary thyroid carcinoma (72) and acute myeloid leukemia cells (73). A Phase I study of CEP-701 established the maximum tolerated dose and identified that toxicities such as nausea, gastrointestinal events and dyspepsia were relatively well-tolerated (74). CEP-701 Phase II studies are in progress. Given the important role Trk signaling plays in normal neurons, it is encouraging that neural toxicity has not been a dose-limiting toxicity. In pediatrics, the New Agents in Neuroblastoma Therapy (NANT) consortium has an open trial N2001-03: A Phase I study of CEP-701 in Patients with Refractory Neuroblastomaa.
Another series of Trk inhibitors came from the optimization of 4-aminopyrazolylpyrimidines from AstraZeneca, Inc. One such compound, AZ-23, inhibits TrkA/B in the nanomolar range and had reasonable pharmacodynamics and oral bioavailability in toxicology studies. Phase I testing in adults was initiated (75) but recently halted due to poor pharmacodynamic properties. Other congeners of 4-aminopyrazolylpyrimidines remain poised for clinical development.
Since mutations in Trk TK are rare, it is unclear how successful Trk kinase inhibitors as single agents will be. To date, small molecule inhibitors targeted to kinase domains have been most successful in cases where the targeted kinases contain activating mutations. In fact, CEP-701's activity as a single agent has been seen primarily in acute myeloid leukemias (AML) containing mutations in the Flt3 receptor (76). However, as the capability to perform high-density sequence analysis of tumors increases and the cost of “personalized” medicine decreases, it may be possible to specifically identify patients whose tumors contain Trk mutations and who therefore would be candidates for a Trk-targeted kinase inhibitor as part of their therapeutic regimen.
Another strategy suggested by preclinical modeling is to use TrkB kinase inhibitors in combination with standard chemotherapy (57-65). In a pre-clinical NB xenograft model, CEP-701 (or the active precursor CEP-751) significantly enhanced the activity of cyclophosphamide, as well as other cytotoxic drug combinations currently used in the treatment of high-risk NB (71 and personal communication, GM Brodeur). A clinical trial of CEP-701 with gemcitabine was undertaken to assess drug interactions in patients with advanced pancreatic cancer. The combination was well tolerated with no unexpected toxicities, but as the trial was limited and not designed to assess efficacy, further studies are planned (77).
Additional therapeutic options can be developed by moving downstream to the key intracellular mediators of activated TrkB's pro-survival and pro-metastatic signals, PI3K and Akt. Pharmacologic inhibitors of these kinases have been extensively pursued. Inhibitors of Akt enhance the efficacy of low-dose etoposide and cause regression of TrkB-expressing NB tumors in a xenograft model (Z. Li and C. Thiele, unpublished data). Such strategies may offer a broader spectrum of inhibitory activity than Trk kinase targeted agents and may also be useful when the seemingly inevitable resistance to targeted agents arises.
Footnotes
References
- 1.Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319:743–8. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991a;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- 3.Tessarollo L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998;9:125–37. doi: 10.1016/s1359-6101(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 4.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene LA, Kaplan DR. Early events in neurotrophin signalling via Trk and p75 receptors. Curr Opinion Neurobio. 1995;5:579–87. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–91. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 7.Klesse FJ, Parada LJ. p21 Ras and Phosphatidylinositol-3 kinase are required for survival of wild-type and NF1 mutant sensory neurons. J Neuroscience. 1998;18:10420–8. doi: 10.1523/JNEUROSCI.18-24-10420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qui MS, Green SH. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992;9:705–17. doi: 10.1016/0896-6273(92)90033-a. [DOI] [PubMed] [Google Scholar]
- 9.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 10.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–22. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kharebava G, Makonchuk D, Kalita KB, Zheng JJ, Hetman M. Requirement of 3-phosphoinositide-dependent protein kinase-1 for BDNF-mediated neuronal survival. J Neurosci. 2008;28:11409–20. doi: 10.1523/JNEUROSCI.2135-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–23. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yano H, Ninan I, Zhang H, Milner TA, Arancio O, Chao MV. BDNF-mediated neurotransmission relies upon a myosin VI motor complex. Nature Neuro. 2006;9:1009–18. doi: 10.1038/nn1730. [DOI] [PubMed] [Google Scholar]
- 15.Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–9. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoilov P, Castren E, Stamm S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem Biophys Res Commun. 2002;290:1054–65. doi: 10.1006/bbrc.2001.6301. [DOI] [PubMed] [Google Scholar]
- 17.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 18.Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–7. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- 19.Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57:546–58. doi: 10.1016/j.neuron.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–33. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 22.Willnow TE, Petersen CM, Nykjaer A. WPS10P-domain receptors-regulators of neuronal viability and function. Nature Reviews Neuroscience. 2008;9:899–909. doi: 10.1038/nrn2516. [DOI] [PubMed] [Google Scholar]
- 23.Pflug BR, Dionne C, Kaplan DR, et al. Expression of a Trk high affinity nerve growth factor receptor in the human prostate. Endocrinology. 1995;136:262–8. doi: 10.1210/endo.136.1.7828539. [DOI] [PubMed] [Google Scholar]
- 24.Melamed I, Kelleher CA, Franklin RA, et al. Nerve growth factor signal transduction in human B lymphocytes is mediated by gp140trk. Eur J Immunol. 1996;26:1985–92. doi: 10.1002/eji.1830260903. [DOI] [PubMed] [Google Scholar]
- 25.Raap U, Deneka N, Bruder M, Kapp A, Wedi B. Differential up-regulation of neurotrophin receptors and functional activity of neurotrophins on peripheral blood eosinophils of patients with allergic rhinitis, atopic dermatitis and nonatopic subjects. Clin Exp Allergy. 2008;38:1493–8. doi: 10.1111/j.1365-2222.2008.03035.x. [DOI] [PubMed] [Google Scholar]
- 26.Kermani P, Rafii D, Jin DK, et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115:653–63. doi: 10.1172/JCI200522655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiltunen JO, Arumäe U, Moshnyakov M, Saarma M. Expression of mRNAs for neurotrophins and their receptors in developing rat heart. Circ Res. 1996;79:930–9. doi: 10.1161/01.res.79.5.930. [DOI] [PubMed] [Google Scholar]
- 28.Lin MI, Das I, Schwartz GM, et al. Trk C receptor signaling regulates cardiac myocyte proliferation during early heart development in vivo. Dev Biol. 2000;226:180–91. doi: 10.1006/dbio.2000.9850. [DOI] [PubMed] [Google Scholar]
- 29.Dissen GA, Hirshfield AM, Malamed S, Ejeda SR. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis. Endocrinology. 1995;136:4681. doi: 10.1210/endo.136.10.7664689. [DOI] [PubMed] [Google Scholar]
- 30.Pierotti MA, Greco A. Oncogenic rearrangements of the NTRK1/NGF receptor. Cancer Lett. 2006;232:90–8. doi: 10.1016/j.canlet.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993;328:847–54. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- 32.Yamashiro DJ, Nakagawara A, Ikegaki N, Liu XG, Brodeur GM. Expression of TrkC in favorable human neuroblastomas. Oncogene. 1996;12:37–41. [PubMed] [Google Scholar]
- 33.Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol. 1994;14:759–67. doi: 10.1128/mcb.14.1.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asgharzadeh S, Pique-Regi R, Sposto R, et al. Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN gene amplification. J Natl Cancer Inst. 2006;98:1193–203. doi: 10.1093/jnci/djj330. [DOI] [PubMed] [Google Scholar]
- 35.Segal RA, Goumnerova LC, Kwon YK, Stiles CD, Pomeroy SL. Expression of the neurotrophin receptor TrkC is linked to a favorable outcome in medulloblastoma. Proc Natl Acad Sci U S A. 1994;91:12867–71. doi: 10.1073/pnas.91.26.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGregor LM, McCune BK, Graff JR, et al. Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci U S A. 1999;96:4540–5. doi: 10.1073/pnas.96.8.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eggert A, Grotzer MA, Ikegaki N, et al. Expression of the neurotrophin receptor TrkB is associated with unfavorable outcome in Wilm's tumor. J Clin Oncol. 2001;19:689–96. doi: 10.1200/JCO.2001.19.3.689. [DOI] [PubMed] [Google Scholar]
- 38.Tacconelli A, Farina AR, Cappabianca L, et al. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell. 2004;6:347–60. doi: 10.1016/j.ccr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Knusel B, Rabin SJ, Hefti F, Kaplan DR. Regulated neurotrophin receptor responsiveness during neuronal migration and early differentiation. J Neurosci. 1994;14:1542–54. doi: 10.1523/JNEUROSCI.14-03-01542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scala S, Wosikowski K, Giannakakou P, et al. Brain-derived neurotrophic factor protects neuroblastoma cells from vinblastine toxicity. Cancer Res. 1996;56:3737–42. [PubMed] [Google Scholar]
- 41.Jaboin J, Kim CJ, Kaplan DR, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3′-kinase pathway. Cancer Res. 2002;62:6756–635. [PubMed] [Google Scholar]
- 42.Matsumoto K, Wada RK, Yamashiro JM, Kaplan DR, Thiele CJ. Expression of brain-derived neurotrophic factor and p145TrkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Res. 1995;55:1798–806. [PubMed] [Google Scholar]
- 43.Kim CJ, Matsuo T, Lee KH, Thiele CJ. Up-regulation of insulin-like growth factor-II expression is a feature of TrkA but not TrkB activation in SH-SY5Y neuroblastoma cells. Am J Pathol. 1999;155:1661–70. doi: 10.1016/S0002-9440(10)65481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249–55. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- 45.Martens LK, Kirschner KM, Warnecke C, Scholz H. Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activator of the TrkB neurotrophin receptor gene. J Biol Chem. 2007;282:14379–88. doi: 10.1074/jbc.M609857200. [DOI] [PubMed] [Google Scholar]
- 46.Woo CW, Lucarelli E, Thiele CJ. NGF activation of TrkA decreases N-myc expression via MAPK path leading to a decrease in neuroblastoma cell number. Oncogene. 2004;23:1522–30. doi: 10.1038/sj.onc.1207267. [DOI] [PubMed] [Google Scholar]
- 47.Eggert A, Grotzer MA, Ikegaki N, Liu XG, Evans AE, Brodeur GM. Expression of the neurotrophin receptor TrkA down-regulates expression and function of angiogenic stimulators in SH-SY5Y neuroblastoma cells. Cancer Res. 2002;62:1802–8. [PubMed] [Google Scholar]
- 48.Lucarelli E, Kaplan D, Thiele CJ. Activation of trk-A but not trk-B signal transduction pathway inhibits growth of neuroblastoma cells. Eur J Cancer. 1997;33:2068–70. doi: 10.1016/s0959-8049(97)00266-9. [DOI] [PubMed] [Google Scholar]
- 49.Schulte JH, Schramm A, Klein-Hitpass L, et al. Microarray analysis reveals differential gene expression patterns and regulation of single target genes contributing to the opposing phenotype of TrkA- and TrkB-expressing neuroblastomas. Oncogene. 2005;24:165–77. doi: 10.1038/sj.onc.1208000. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Jaboin J, Dennis PA, Thiele CJ. Genetic and pharmacologic identification of Akt as a mediator of brain-derived neurotrophic factor/TrkB rescue of neuroblastoma cells from chemotherapy-induced cell death. Cancer Res. 2005;65:2070–5. doi: 10.1158/0008-5472.CAN-04-3606. [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Tan F, Thiele CJ. Inactivation of glycogen synthase kinase-3beta contributes to brain-derived neutrophic factor/TrkB-induced resistance to chemotherapy in neuroblastoma cells. Mol Cancer Ther. 2007;6:3113–21. doi: 10.1158/1535-7163.MCT-07-0133. [DOI] [PubMed] [Google Scholar]
- 52.Pearce RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005;105:4429. doi: 10.1182/blood-2004-08-3096. [DOI] [PubMed] [Google Scholar]
- 53.Douma S, Van Laar T, Zevenhoven J, et al. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–9. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 54.Yu X, Liu L, Cai B, He Y, Wan X. Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Sci. 2008;99:543–52. doi: 10.1111/j.1349-7006.2007.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiu L, Zhou C, Sun Y, et al. Crosstalk between EGFR and TrkB enhances ovarian cancer cell migration and proliferation. Int J Oncol. 2006;29:1003–11. [PubMed] [Google Scholar]
- 56.Sclabas GM, Fujioka S, Schmidt C, et al. Overexpression of tropomysin-related kinase B in metastatic human pancreatic cancer cells. Clin Cancer Res. 2005;11:440–9. [PubMed] [Google Scholar]
- 57.Festuccia C, Muzi P, Gravina GL, et al. Tyrosine kinase inhibitor CEP-701 blocks the NTRK1/NGF receptor and limits the invasive capability of prostate cancer cells in vitro. Int J Oncol. 2007;18:503–11. [PubMed] [Google Scholar]
- 58.Zhang Z, Han L, Liu Y, Liang X, Sun W. Up-regulation of Tropomyosin related kinase B contributes to resistance to detachment-induced apoptosis in hepatoma multicellular aggregations. Mol Biol Rep. 2009;36:1211–6. doi: 10.1007/s11033-008-9299-z. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Fujiwara Y, Doki Y, et al. Overexpression of Tyrosine Kinase B Protein as a Predictor for Distant Metastases and Prognosis in Gastric Carcinoma. Oncology. 2008;75:17–26. doi: 10.1159/000151615. [DOI] [PubMed] [Google Scholar]
- 60.Kupferman ME, Zhou G, Feng L, et al. Critical Role of TrkB for invasion and EMT in HNSCC Proc Amer Assoc Cancer Res 5498. 2008. [Google Scholar]
- 61.D'Onofrio M, de Grazia U, Morrone S, et al. Expression of neurotrophin receptors in normal and malignant B lymphocytes. Eur Cytokine Netw. 2000;11:283–91. [PubMed] [Google Scholar]
- 62.Weeraratna AT, Arnold JT, George DJ, DeMarzo A, Isaacs JT. Rational basis for Trk inhibition therapy for prostate cancer. Prostate. 2000;45:140–8. doi: 10.1002/1097-0045(20001001)45:2<140::aid-pros8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 63.Ricci A, Greco S, Mariotta S, et al. Neurotrophins and neurotrophin receptors in human lung cancer. Am J Respir Cell Mol Biol. 2001;25:439–46. doi: 10.1165/ajrcmb.25.4.4470. [DOI] [PubMed] [Google Scholar]
- 64.Cameron HL, Foster WG. Dieldrin promotes resistance to anoikis in breast cancer cells in vitro. Reprod Toxicol. 2008;25:256–62. doi: 10.1016/j.reprotox.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 65.Zhu L, Werner JA, Mandic R. Implications of tropomyosin-related kinase B (TrkB) in head and neck cancer. Anticancer Res. 2007;27:3121–6. [PubMed] [Google Scholar]
- 66.Hecht M, Schulte JH, Eggert A, Wilting J, Schweigerer L. The neurotrophin receptor TrkB cooperates with c-Met in enhancing neuroblastoma invasiveness. Carcinogenesis. 2005;26:2105–15. doi: 10.1093/carcin/bgi192. [DOI] [PubMed] [Google Scholar]
- 67.Kermani P, Rafii D, Jin DK, et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J Clin Invest. 2005;115:653–63. doi: 10.1172/JCI200522655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Camoratto AM, Jani JP, Angeles TS, et al. CEP-751 inhibits TRK receptor tyrosine kinase activity in vitro exhibits anti-tumor activity. Int J Cancer. 1997;72:673–9. doi: 10.1002/(sici)1097-0215(19970807)72:4<673::aid-ijc20>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 69.George DJ, Dionne CA, Jani J, et al. Sustained in vivo regression of Dunning H rat prostate cancers treated with combinations of androgen ablation and Trk tyrosine kinase inhibitors, CEP-751 (KT-6587) or CEP-701 (KT-5555) Cancer Res. 1999;59:2395–401. [PubMed] [Google Scholar]
- 70.Miknyoczki SJ, Dionne CA, Klein-Szanto AJ, Ruggeri BA. The novel Trk receptor tyrosine kinase inhibitor CEP-701 (KT-5555) exhibits antitumor efficacy against human pancreatic carcinoma (Panc1) xenograft growth and in vivo invasiveness. Ann N Y Acad Sci. 1999;880:252–62. doi: 10.1111/j.1749-6632.1999.tb09530.x. [DOI] [PubMed] [Google Scholar]
- 71.Evans AE, Kisselbach KD, Yamashiro DJ, Ikegaki N, Camoratto AM, Dionne CA, Brodeur GM. Antitumor activity of CEP-751 (KT-6587) on human neuroblastoma and medulloblastoma xenografts. Clin Cancer Res. 1999;5:3594–602. [PubMed] [Google Scholar]
- 72.Strock CJ, Park JI, Rosen M, Dionne C, Ruggeri B, Jones-Bolin S, Denmeade SR, Ball DW, Nelkin BD. CEP-701 and CEP-751 inhibit constitutively activated RET tyrosine kinase activity and block medullary thyroid carcinoma cell growth. Cancer Res. 2003;63:5559–63. [PubMed] [Google Scholar]
- 73.Levis M, Allebach J, Tse KF, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–91. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 74.Marshall JL, Kindler H, Deeken J, et al. Phase I trial of orally administered CEP-701, a novel neurotrophin receptor-linked tyrosine kinase inhibitor. Invest New Drugs. 2005;23:31–7. doi: 10.1023/B:DRUG.0000047103.64335.b0. [DOI] [PubMed] [Google Scholar]
- 75.Wang T, Lamb ML, Scott DA, Wang H, et al. Identification of 4-aminopyrazolylpyrimidines as potent inhibitors of Trk kinases. J Med Chem. 2008;51:4672–84. doi: 10.1021/jm800343j. [DOI] [PubMed] [Google Scholar]
- 76.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 77.Chan E, Mulkerin D, Rothenberg M, et al. A phase I trial of CEP-701 + gemcitabine in patients with advanced adenocarcinoma of the pancreas. Invest New Drugs. 2008;26:241–7. doi: 10.1007/s10637-008-9118-3. [DOI] [PubMed] [Google Scholar]