Abstract
Chiral odor pairs initially smelling the same become perceptually distinct following aversive Pavlovian conditioning, in parallel with spatial divergence of odor activity patterns in human olfactory (piriform) cortex, highlighting critical behavioral advantages when associative learning is accompanied by improved perceptual analysis of danger-predicting sensory signals.
Learning to associate sensory cues with threats is critical for minimizing aversive experience. The ecological benefit of associative learning relies upon accurate perception of predictive cues, but how aversive learning enhances perceptual acuity of sensory signals, particularly in humans, is unclear. We combined multivariate functional magnetic resonance imaging with olfactory psychophysics to show that initially indistinguishable odor enantiomers (mirror-image molecules) become discriminable following aversive conditioning, paralleling spatial divergence of ensemble activity patterns in primary olfactory (piriform) cortex. Our findings indicate that aversive learning induces piriform plasticity with corresponding gains in odor enantiomer discrimination, underscoring the capacity of fear conditioning to update perceptual representation of predictive cues, over and above its well-recognized role in the acquisition of conditioned responses. That completely indiscriminable sensations can be transformed into discriminable percepts further accentuates the potency of associative learning to enhance sensory cue perception and support adaptive behavior.
The ability to minimize contact with aversive experience is a hallmark of adaptive behavior. Via mechanisms of associative learning, organisms can use sensory information in the environment to predict impending danger and initiate fight-or-flight responses. The behavioral efficacy of associative learning thus hinges upon sensitive and accurate perceptual evaluation of sensory signals. In particular, the ability to discriminate between biologically meaningful cues (e.g., smell of a 175kg lion) and similar but irrelevant stimuli (e.g., smell of a 3kg housecat) maximizes an organism’s response sensitivity while minimizing hypervigilant and impulsive behaviors.
However, models of associative learning have traditionally focused on delineating the formation of associations between a sensory cue (the conditioned stimulus, or CS) and a biologically salient event (the unconditioned stimulus, or US) (1, 2), paying scant attention to perceptual changes in the CS itself. Several studies have considered how associative learning modifies cue-related tuning profiles in sensory cortex (3–8), though none has provided concomitant measures of sensory perception. As a consequence, direct links relating learning-induced changes in sensory cortex to perceptual gains in cue discrimination are unavailable, such that the functional significance of these neural effects upon behavior remains poorly characterized. Importantly, to the extent that conditioning can transform indiscriminable sensations into distinct percepts, such a mechanism would constitute a unique and potent means of optimizing adaptive behavior.
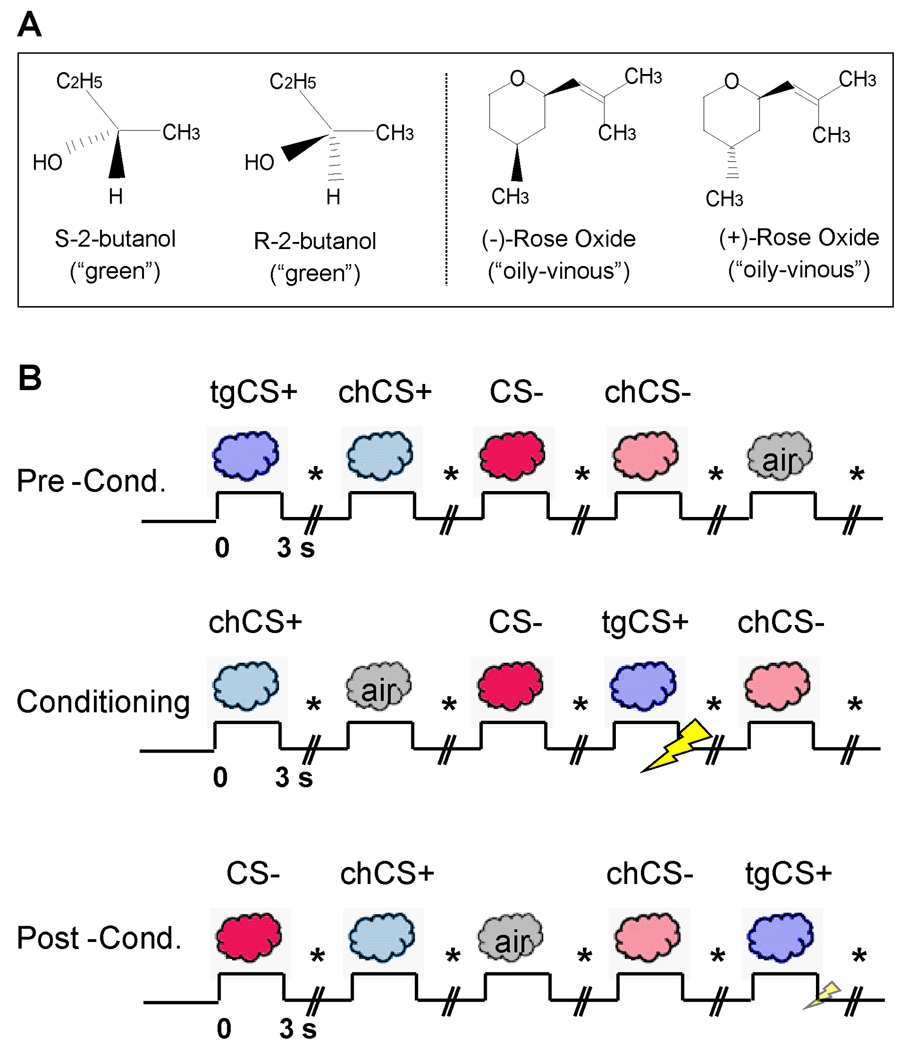
We combined functional magnetic resonance imaging (fMRI) with multivariate analytical techniques to explore the impact of aversive olfactory conditioning on perceptual and neural discrimination of predictive odor cues. Critically, the use of perceptually identical odor enantiomers (mirror-image molecules differing only in their chiral properties) (9, 10) enabled us to determine whether humans can acquire the ability to distinguish between odorous stimuli that initially smell the same. Subjects were presented with four enantiomers (two different pairs), one of which (the target CS+, or “tgCS+”) was repetitively paired with an electric shock (US) during a conditioning phase, whereas its chiral counterpart (“chCS+”) was not accompanied by shock (Fig. 1) (10). The second pair of odor enantiomers served as non-conditioned control stimuli (“CS−” and “chCS−”). The central prediction was that associative learning would enhance behavioral discrimination of related CS+ odorants, in parallel with reorganization of neural coding in human primary olfactory (piriform) cortex.
Fig 1.
Experimental paradigm. (A) Chemical structures of the enantiomer pairs. (B) Learning task. Odorants included: a target CS+ (tgCS+) destined for aversive conditioning, its chiral counterpart (chCS+), a non-conditioned control (CS−), and its chiral counterpart (chCS−). A baseline condition comprised odorless air. Stimuli were delivered during pre-conditioning, conditioning, and post-conditioning sessions. During conditioning, tgCS+ presentation co-terminated with electric shock (the US). During post-conditioning, the US was presented with tgCS+ on 4/19 trials to prevent extinction. On each trial participants indicated whether odor was present or absent (marked by *). SCR and respirations were continuously recorded.
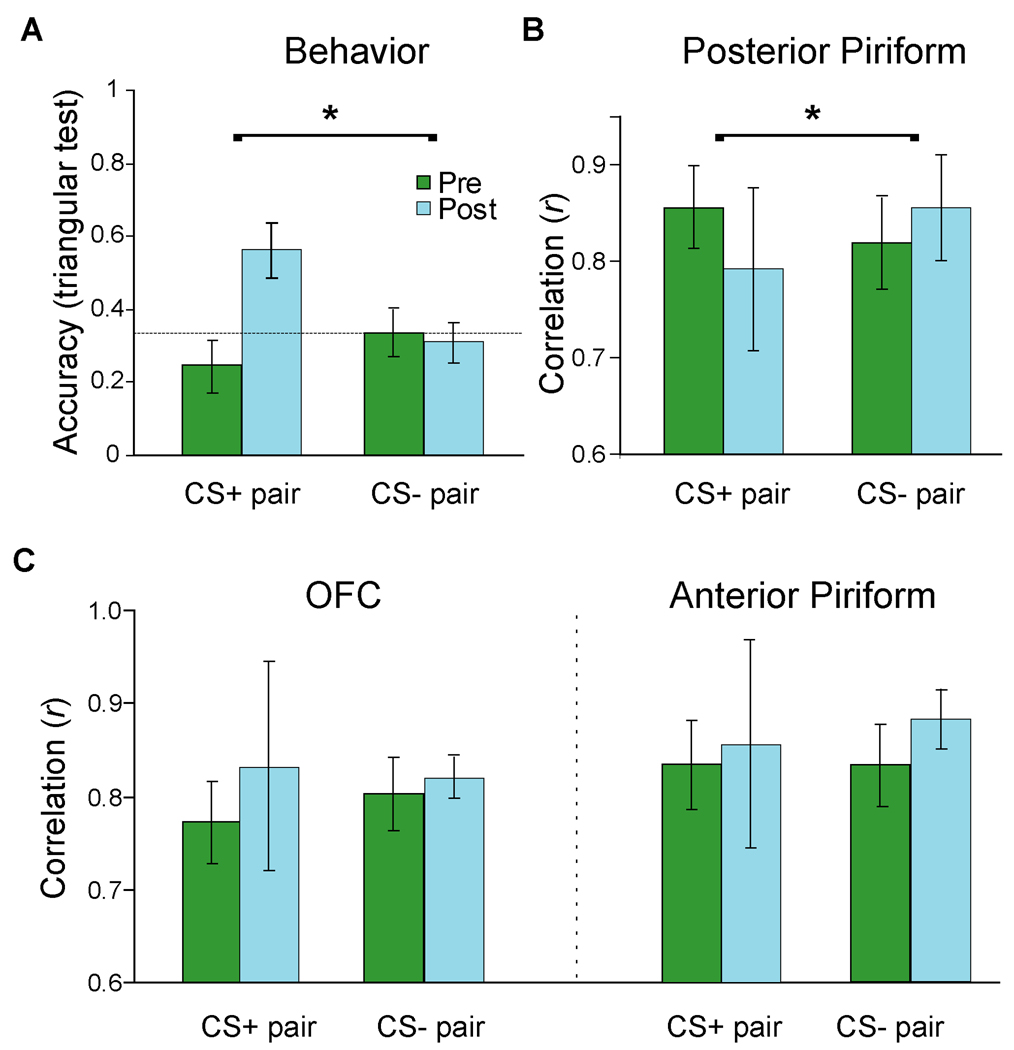
We first examined the behavioral effects of aversive conditioning on perceptual discrimination between the conditioned cue (tgCS+) and its related enantiomer (chCS+). We administered a triangular (triple-forced-choice) odor discrimination test (10, 11) to assess differences in perceived odor identity (i.e., the quality or character of a smell emanating from an odorous object). On each trial, subjects smelled sets of three bottles (two containing one odorant, the third containing its chiral opposite) and selected the odd stimulus. Before conditioning, discrimination accuracy was at chance (33%) for both CS+ and CS− enantiomer pairs, confirming that each pair was initially indistinguishable (Fig. 2A). After conditioning, behavioral accuracy for distinguishing between tgCS+ and chCS+ rose more than twofold, significantly exceeding both chance and pre-conditioning performance (p’s ≤ 0.01; Wilcoxon test, two-tailed), without any improvement in distinguishing between CS− and chCS−. Importantly, subjective ratings of odor intensity, valence, or familiarity (11) did not vary across conditions (p’s > 0.4), ruling out confounds of the triangular test due to these extraneous variables and accentuating the change in perceived odor identity. Associative learning thus can enhance perceptual discriminability between initially indistinguishable odors, and these effects are specific for the CS+.
Fig. 2.
Parallel enhancement of perceptual and neural discrimination after aversive learning. (A) Odor discrimination accuracy was at chance (dashed line) for CS+ and CS− pairs before conditioning, but selectively improved for the CS+ pair after conditioning. Error bars, ± s.e.m. (B) Spatial patterns of fMRI activity in posterior piriform cortex between tgCS+ and chCS+ were highly correlated pre-conditioning, but became more distinct (less correlated) after conditioning, relative to the CS− pair. (C) Learning-induced effects on voxel-wise spatial activity in OFC (left) and anterior piriform cortex (right) indicated that post-conditioning patterns became more correlated (though not significantly) for both CS+ and CS− pairs.
We next clarified the neural mechanisms underlying learning-induced perceptual enhancement of the predictive cue (11). Because neural representations of odor identity are maintained in posterior piriform cortex (12–14), and given the highly distributed spatial organization of afferent projections into the piriform region (15–17), we used multivariate fMRI (18, 19) to test the hypothesis that spatially distributed patterns of neural activity in piriform cortex evoked by tgCS+ and chCS+ would be reorganized as a consequence of associative learning (Fig. S1).
By extracting the raw fMRI signal intensity from every activated piriform voxel (Fig. S2), we found that the spatial activity patterns in posterior piriform cortex strongly correlated for the CS+ pair (tgCS+:chCS+) and the control pair (CS−:chCS−) prior to odor-shock learning (Fig. 2B), corresponding to the high perceived similarity within each pair. However, following conditioning, these spatial correlations declined for the CS+ pair, particularly in comparison to the CS− pair (p < 0.05; Wilcoxon test, two-tailed) (Fig. 2B), in line with the learning-induced behavioral enhancement in odor discrimination between tgCS+ and chCS+. Additional analysis showed that within-pair correlations were significantly higher than across-pair correlations at pre-conditioning (p < 0.005, f = 0.66), suggesting our multivariate technique has satisfactory discriminant validity for distinguishing between odor classes (11). Finally, the absence of respiratory differences across conditions (11) suggests the imaging effects were not due to sniff-related confounds.
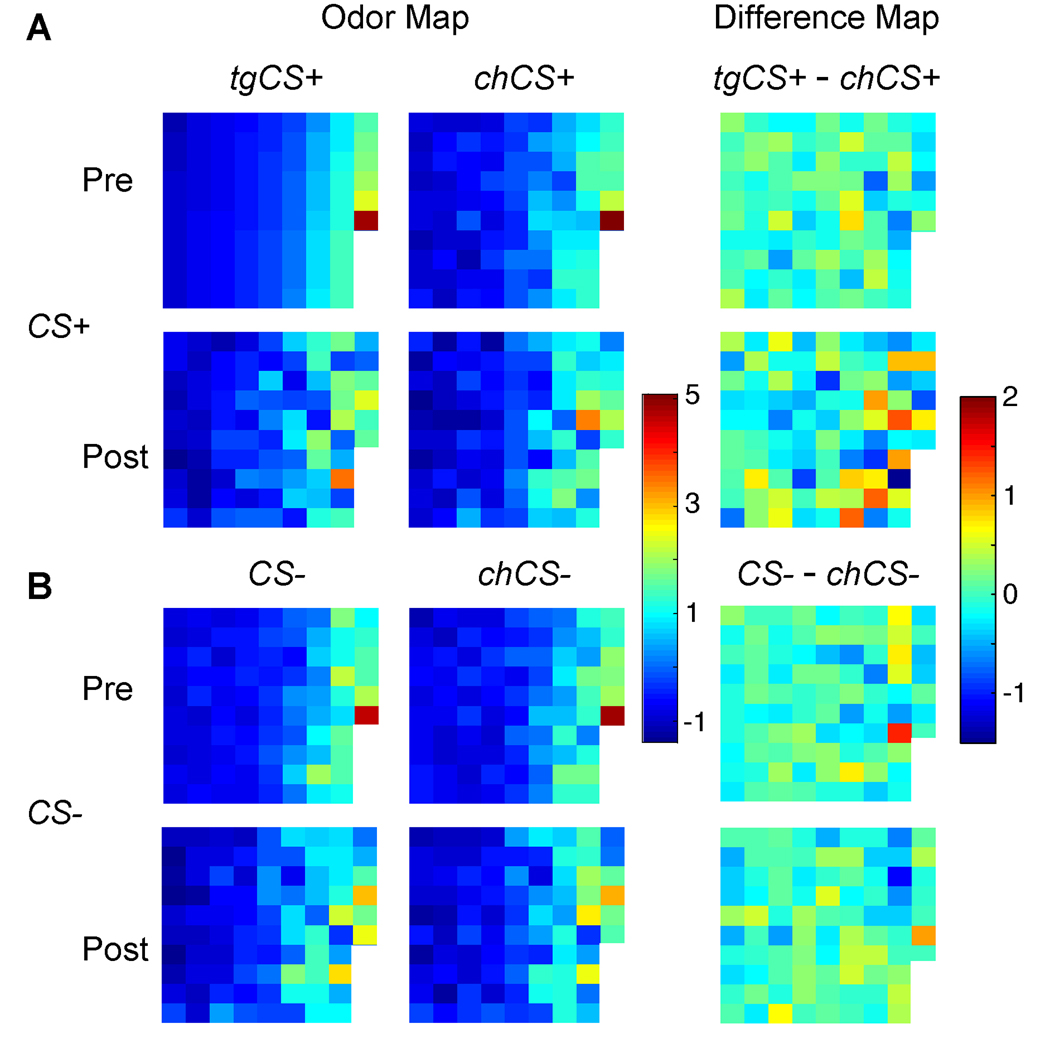
Condition-specific odor maps from one subject (Fig. 3) illustrate how spatial patterns of piriform activity were selectively reorganized from pre- to post-learning for the CS+ pair, whereas the patterns for the CS− pair remained highly coupled. Spatial ‘difference’ maps (Fig. 3, right column) further show that whereas there was minimal signal deviation (light-colored voxels) between CS− and chCS− at both pre- and post-conditioning, substantial pattern variation (dark-colored voxels) emerged between tgCS+ and chCS+ post-conditioning. Moreover, that the response in any given piriform voxel could change in either direction (activation or deactivation) exemplifies the sensitivity of multivariate pattern-based fMRI approaches to characterize neural information in human sensory cortex (20).
Fig. 3.
Spatial maps of posterior piriform activity from one subject. Condition-specific spatial patterns (left two columns) for the CS+ pair (A), but not the CS− pair (B), diverged following conditioning. Difference maps between odorant pairs (right column) highlight the selective differentiation within the CS+ pair at post-conditioning. Each square in the grid represents fMRI signal intensity from a different piriform voxel (n, 86 voxels), arranged in columns from top left to bottom right, in ascending order of signal intensity for tgCS+ in the pre-conditioning phase.
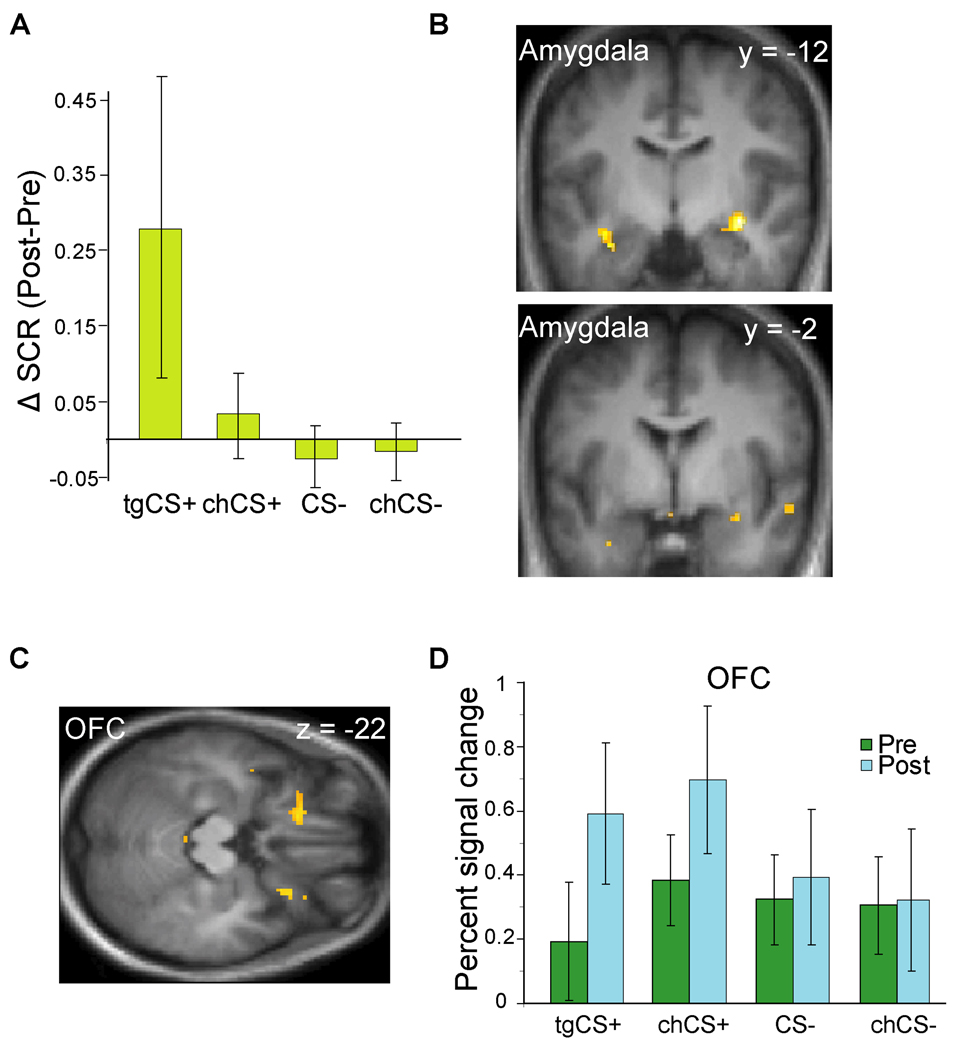
The post-learning changes described above were paralleled by robust evidence for aversive conditioning. First, online physiological measurements (11) of the odor-evoked skin conductance response (SCR) revealed significant enhancement to tgCS+ at post-versus pre-conditioning, when compared to the CS− odorants (Fig. 4A). These SCR changes however were not selective for the tgCS+, because tgCS+ and chCS+ changes did not significantly differ (p > 0.2). In fact there was a small but non-significant SCR increase to chCS+ relative to CS− odorants (p > 0.4). Second, learning-induced changes in amygdala and orbitofrontal cortex (OFC) (analyzed using conventional fMRI approaches) (11) paralleled the SCR effects. A condition-by-time interaction (11) demonstrated progressive decreases in amygdala activity evoked by tgCS+ (vs. CS− odors) as learning proceeded (Fig. 4B; Table S1), consistent wityh prior studies of aversive learning using visual CS+ stimuli (21, 22). Moreover, comparison of post- to pre-conditioning revealed increased mean responses to tgCS+ (vs. CS− odors) in the OFC bilaterally (Fig. 4C–D), another region implicated in associative learning (23, 24). Interestingly, fear conditioning partially generalized to the chCS+ odorant, which at reduced threshold (p < 0.005 uncorrected) showed similar profiles in amygdala and OFC (Table S2). These findings validate the efficacy of our paradigm to induce aversive olfactory learning, supporting the idea that the perceptual and neural changes in sensory discriminability were a consequence of associative learning. The results further demonstrate that fear conditioning to an odor cue recruits many of the same regions involved in conditioning to visual and auditory cues, emphasizing the multimodal versatility of these learning networks.
Fig. 4.
Effects of aversive olfactory conditioning. (A) SCR significantly increased for tgCS+ at post- vs. pre-conditioning, when compared to CS− stimuli (p = 0.05; Wilcoxon test, two-tailed). (B) During conditioning, tgCS+-evoked activity in amygdala exhibited significant time-dependent response decline, relative to the CS− pair. Activations superimposed on coronal T1-weighted scans (display threshold, p < 0.001). (C) From pre- to post-conditioning, bilateral OFC showed enhanced responses to tgCS+ relative to CS− (axial T1 section; threshold, p < 0.005). (D) Plots of percent signal change for peak activity in left OFC are shown for each condition.
The shock-dependent spatial modifications in posterior piriform cortex were seen in the absence of changes in the magnitude of mean activation. While the conventional (univariate) fMRI analysis revealed increased mean activity in OFC (cf. Fig. 4C), there was no evidence for similar mean changes in posterior piriform cortex, even at reduced threshold (p < 0.01 uncorrected). At the same time, fMRI multivariate (pattern) analysis of OFC (Fig. 2C, left) showed no evidence for enhanced spatial discrimination between the CS+ odorants, if anything showing further loss of coding specificity (more highly correlated patterns). In fact, the spatial correlation changes for the CS+ pair significantly differed between posterior piriform cortex and OFC (p = 0.01; Wilcoxon test), highlighting a regional specificity for the ensemble learning effect. This anatomical double dissociation suggests that fear conditioning recruits functionally distinct networks acting in concert to maximize adaptive behavior: an emotion system (e.g., amygdala and OFC) optimized to detect threat signals with high sensitivity, and a perceptual system (e.g., posterior piriform cortex) optimized to encode signal specificity.
We considered that aversive conditioning could have heightened attention (or arousal) to tgCS+, evoking response changes in olfactory cortex. However, anterior piriform cortex, the purported target of human olfactory attention (25), was not modulated in response to associative learning, either in univariate (at p < 0.01 uncorrected) or multivariate (Fig. 2C, right) analysis. By comparison, the idea that aversive learning updates odor quality representations in posterior piriform cortex would closely accord with its role in coding odor identity in both animal (13, 26) and human (12, 14) models of olfactory processing. It is therefore unlikely that attention or arousal directly modulates odor coding in posterior piriform, though it remains possible that these mechanisms could mediate olfactory perceptual plasticity indirectly.
Aversive conditioning therefore has a direct influence on how perceptual information about a CS+ is updated in sensory-specific cortex, providing a potent neural substrate to guide the behavioral discrimination of predictive cues. The spatial reorganization of sensory coding in piriform cortex may reflect changes in olfactory receptive-field tuning, leading to improved perception of odor cues, such that unique or “tagged” piriform representations might gain privileged access to critical nodes underlying aversion-minimizing behaviors.
Knowing what to avert presents behavioral challenges that an organism must solve to survive. Prior work of fear conditioning has focused on how the CS comes to produce behavioral (i.e., conditioned) responses, rather than how conditioning alters sensory processing of the CS itself, resulting in perceptual learning and enhanced discrimination. We hypothesize that the substantial effect of emotional experience on perceptual processing in sensory cortices should have a vital impact on adaptive behavior and should thus be considered an indispensable component for models of learning and decision-making (4, 27, 28). Clinically, our data raise the intriguing possibility that neurobiological derangements in the ability to distinguish between salient cues and perceptually related inconsequential stimuli may underlie the emergence of anxiety disorders characterized by exaggerated sensory sensitivity and hypervigilance. This may provide a unique mechanistic framework for the development of new therapeutic interventions.
Supplementary Material
References and Notes
- 1.Everitt BJ, Cardinal RN, Hall J, Parkinson JA, Robbins TW. The amygdala: a functional analysis. In: Aggleton J, editor. Differential Involvement of Amygdala Subsystems in Appetitive Conditioning and Drug Addiction. Oxford: Oxford University Press; 2000. pp. 353–390. [Google Scholar]
- 2.Balleine BW. Physiol Behav. 2005;86:717. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 3.Edeline JM. Prog Neurobiol. 1999;57:165. doi: 10.1016/s0301-0082(98)00042-2. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger NM. Learn Mem. 2007;14:1. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polley DB, Heiser MA, Blake DT, Schreiner CE, Merzenich MM. Proc Natl Acad Sci U S A. 2004;101:16351. doi: 10.1073/pnas.0407586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohl FW, Scheich H. Curr Opin Neurobiol. 2005;15:470. doi: 10.1016/j.conb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Morris JS, Friston KJ, Dolan RJ. Proc Biol Sci. 1998;265:649. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phelps EA, LeDoux JE. Neuron. 2005;48:175. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Linster C, et al. J Neurosci. 2001;21:9837. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laska M, Teubner P. Chem Senses. 1999;24:161. doi: 10.1093/chemse/24.2.161. [DOI] [PubMed] [Google Scholar]
- 11.Materials and methods are available as supporting material on Science Online.
- 12.Gottfried JA, Winston JS, Dolan RJ. Neuron. 2006;49:467. doi: 10.1016/j.neuron.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Kadohisa M, Wilson DA. Proc Natl Acad Sci U S A. 2006;103:15206. doi: 10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Luxenberg E, Parrish T, Gottfried JA. Neuron. 2006;52:1097. doi: 10.1016/j.neuron.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberly LB. The Synaptic Organization of the Brain. In: Shepherd GM, editor. Olfactory cortex. NY: Oxford University Press; 1998. [Google Scholar]
- 16.Illig KR. J Comp Neurol. 2005;488:224. doi: 10.1002/cne.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou Z, Li F, Buck LB. Proc Natl Acad Sci U S A. 2005;102:7724. doi: 10.1073/pnas.0503027102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Haxby JV, et al. Science. 2001;293:2425. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 19.Polyn SM, Natu VS, Cohen JD, Norman KA. Science. 2005;310:1963. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- 20.Kriegeskorte N, Bandettini P. Neuroimage. 2007;38:666. doi: 10.1016/j.neuroimage.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 21.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Neuron. 1998;20:937. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 22.Buchel C, Morris J, Dolan RJ, Friston KJ. Neuron. 1998;20:947. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 23.Schoenbaum G, Chiba AA, Gallagher M. J Neurosci. 1999;19:1876. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montague PR, King-Casas B, Cohen JD. Annu Rev Neurosci. 2006;29:417. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- 25.Zelano C, et al. Nat Neurosci. 2005;8:114. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]
- 26.Wilson DA, Stevenson RJ. Learning to Smell: Olfactory Perception from Neurobiology to Behavior. Baltimore: Johns Hopkins University Press; 2006. [Google Scholar]
- 27.Hall G. Q J Exp Psychol. 2003;56:43. doi: 10.1080/02724990244000151. [DOI] [PubMed] [Google Scholar]
- 28.McLaren IPL, Mackintosh NJ. Anim Learn Behav. 2000;28:211. [Google Scholar]
- 29.We thank T. Egner, M.M. Mesulam, J.S. Winston, and R.E. Zinbarg for helpful comments, E. Featherstone, E. Davchev, and V. Djoev for stimulator assembly, and M. Benton for assistance collecting data. This work was supported by NIH/NIDCD Grant #DC007653 (J.A.G.).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.