Abstract
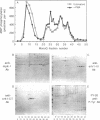
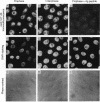
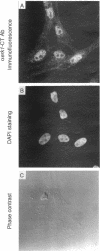
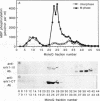
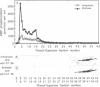
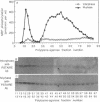
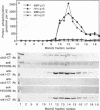
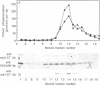
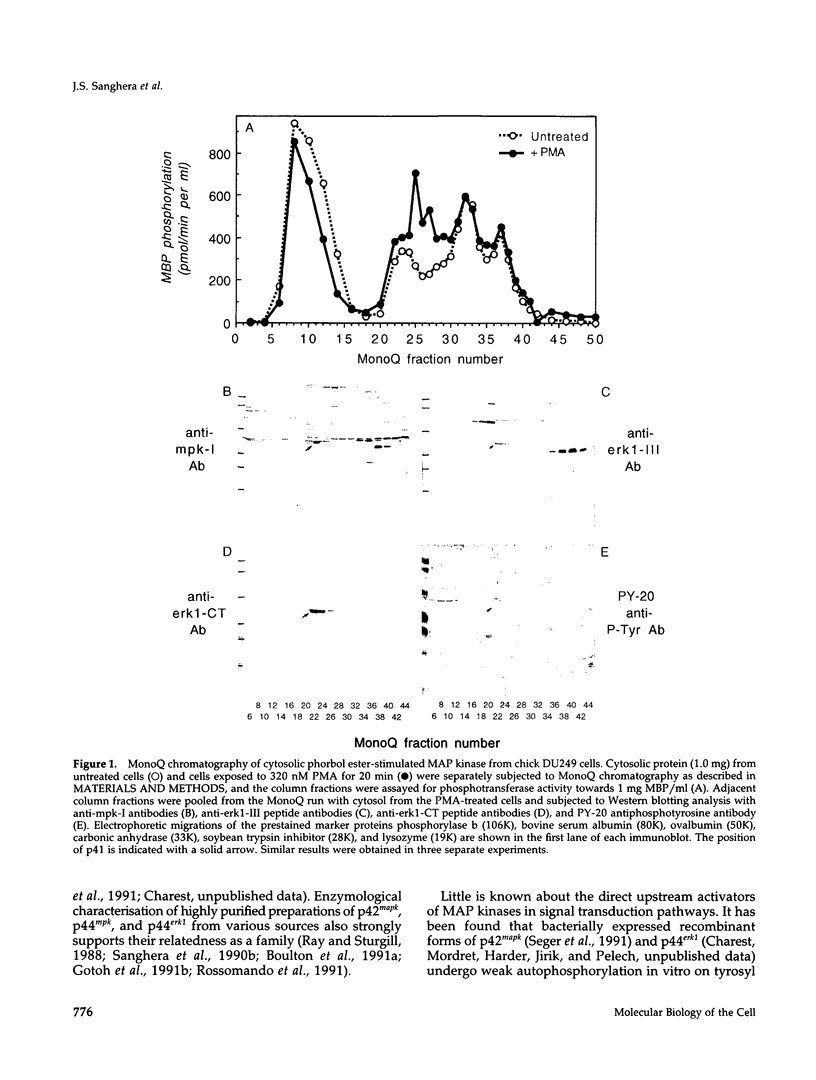
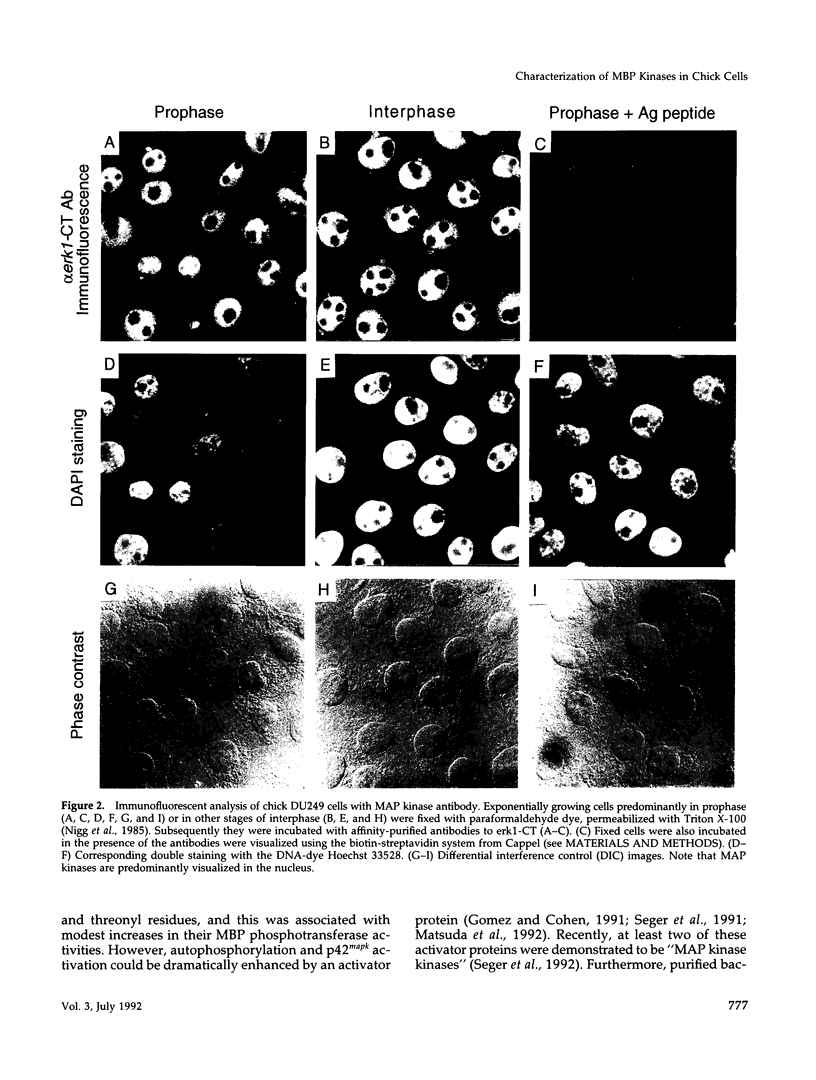
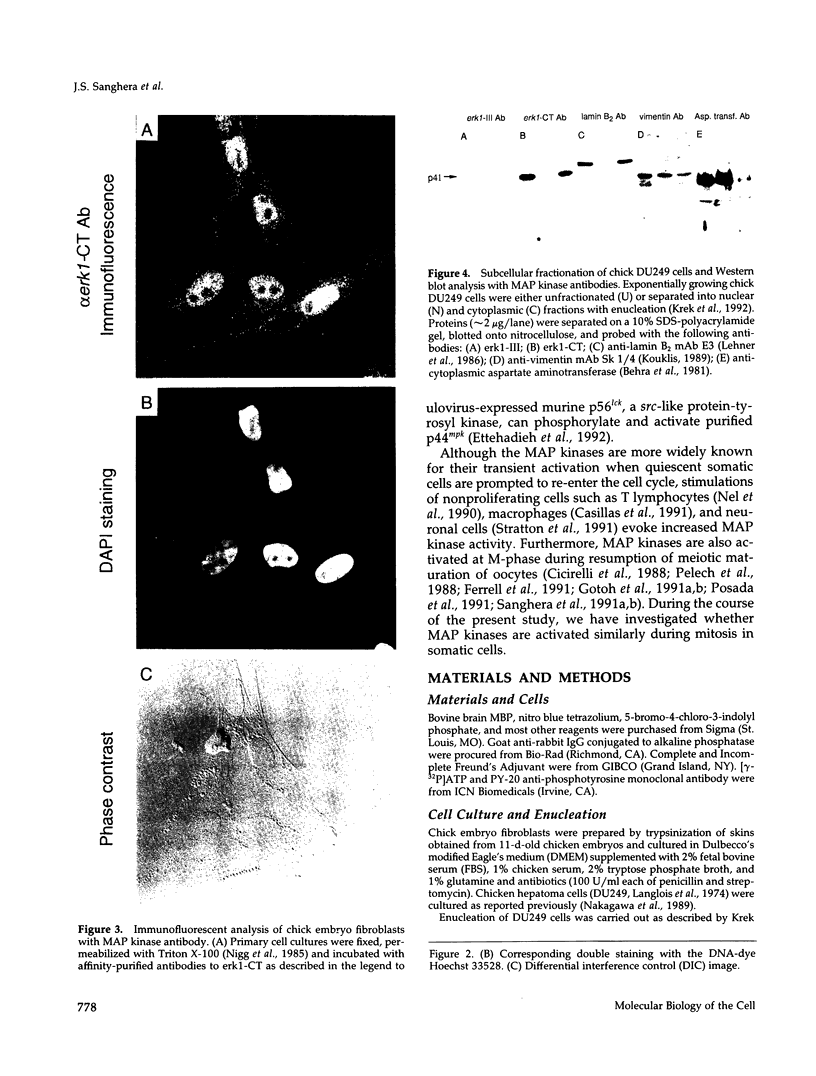
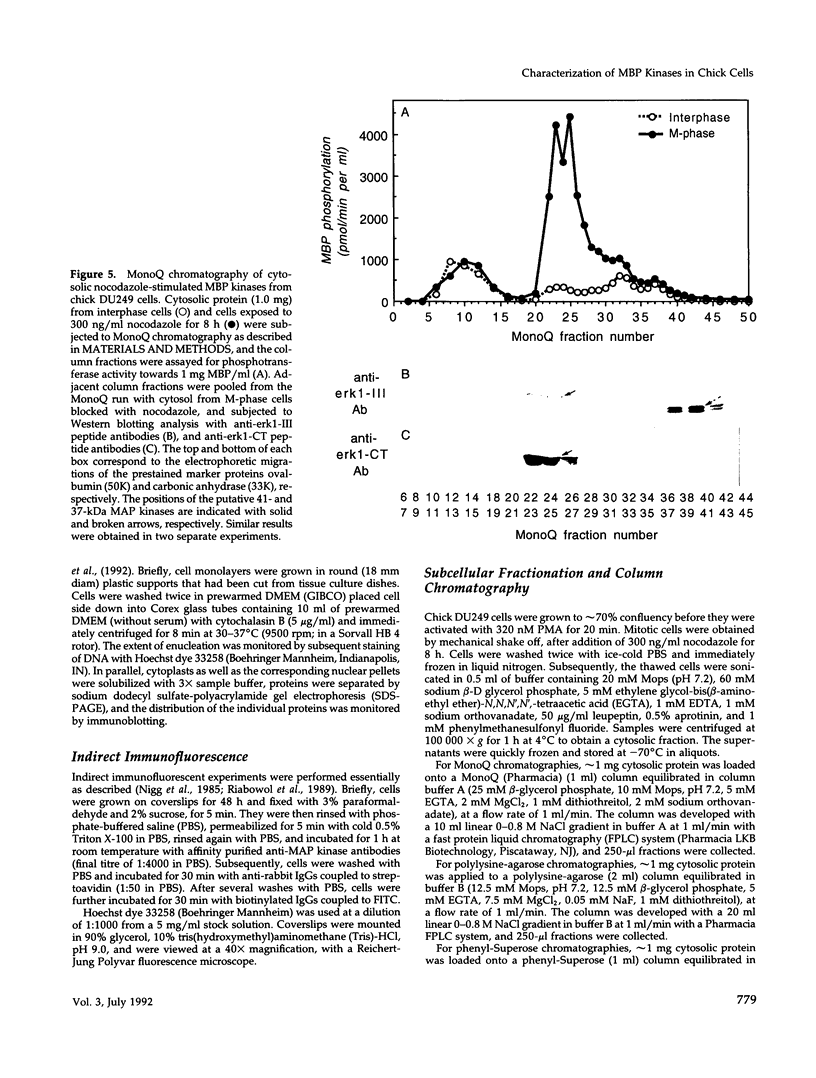
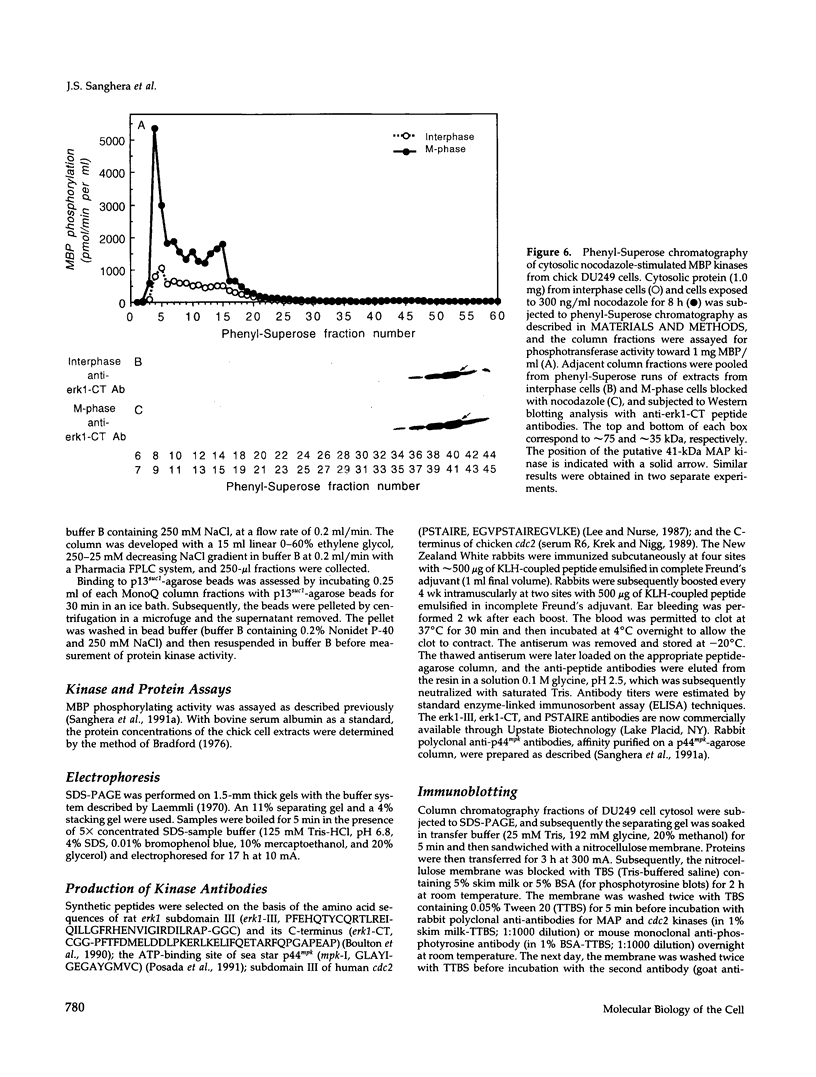
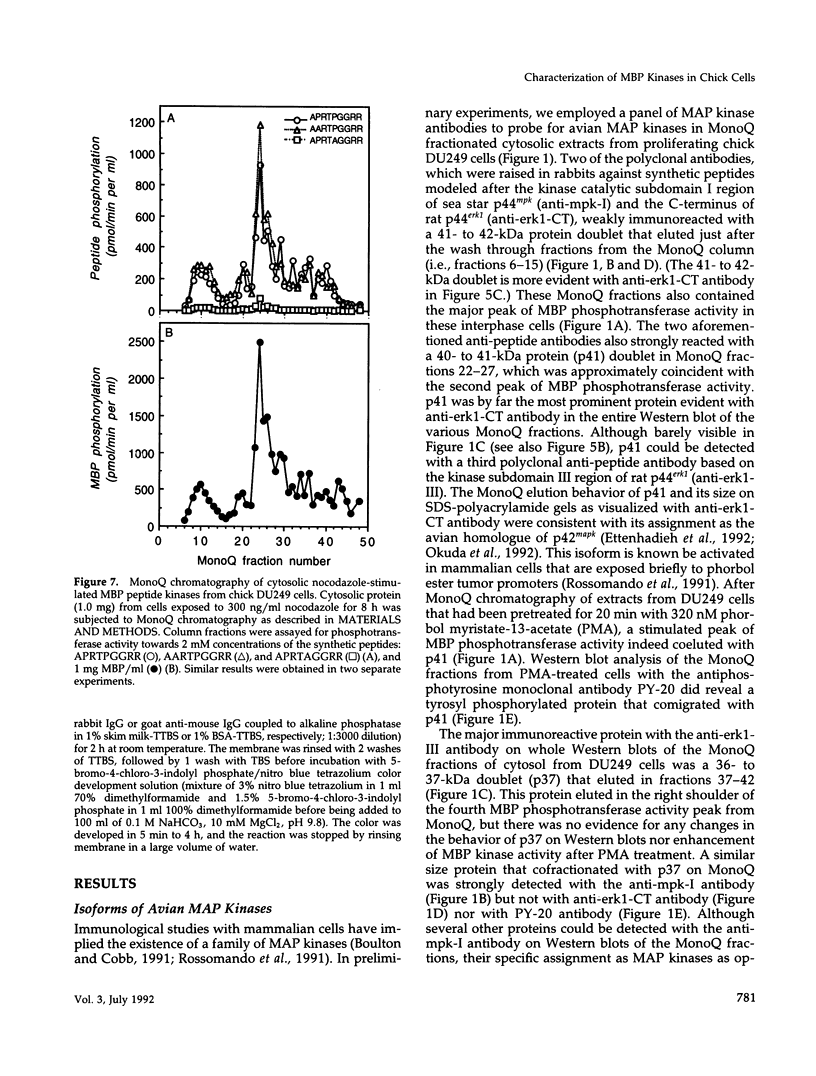
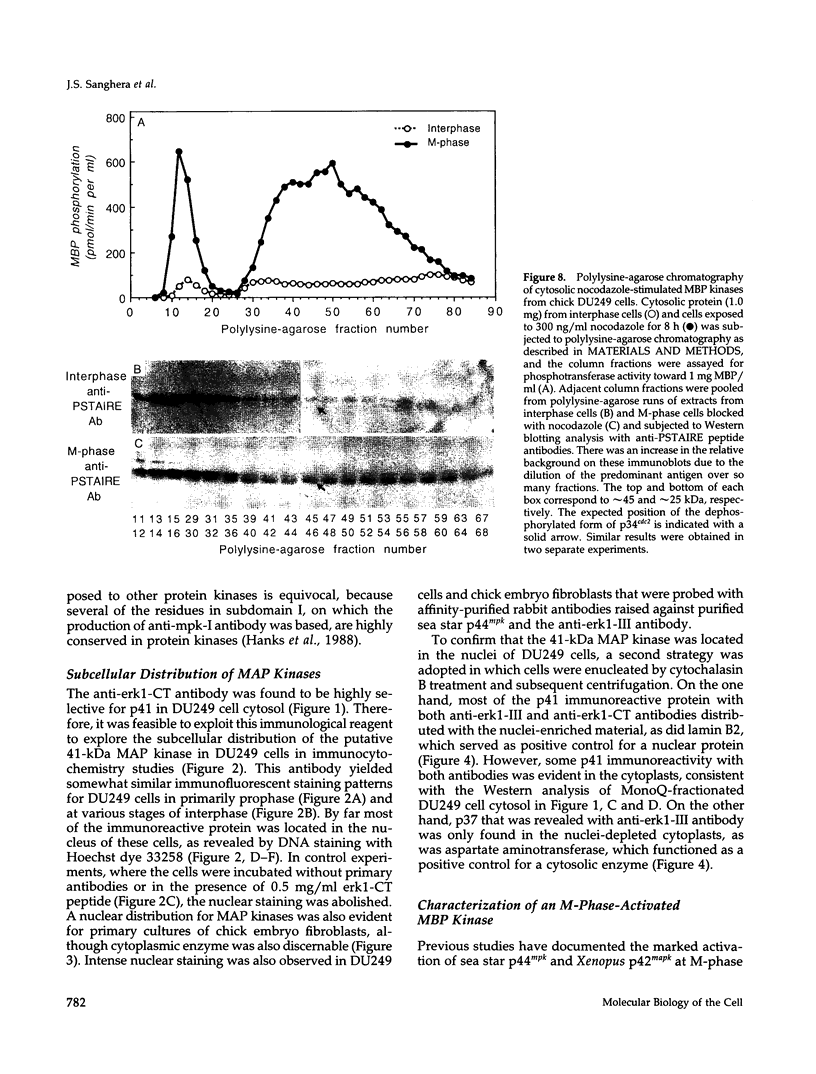
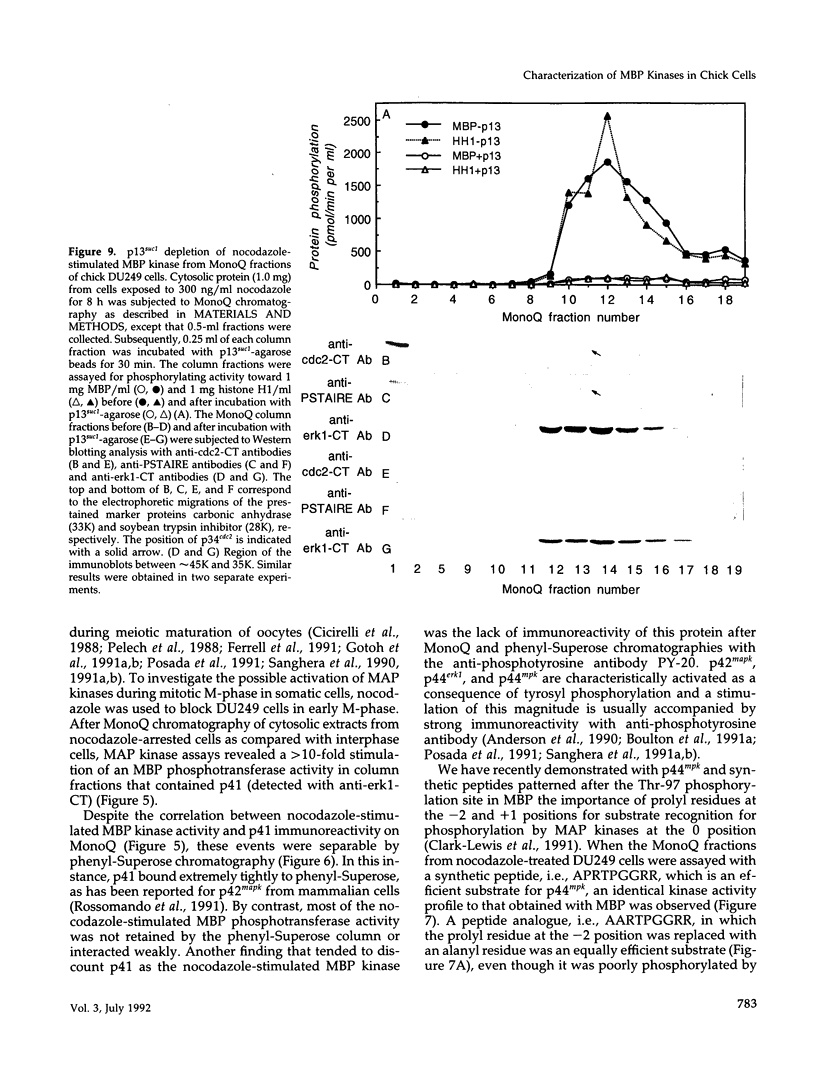
The subcellular distribution and regulation of MAP kinase isoforms in chicken hepatoma DU249 cells was investigated with antibodies directed against peptides patterned after sequences in the mitogen-activated protein (MAP) kinases, sea star p44mpk, and rat p44erk1. MonoQ chromatography of cytosol from these cells afforded the resolution of at least four peaks of myelin basic protein (MBP) phosphotransferase activity, but only one of these (peak II) was stimulated in extracts from phorbol ester-treated cells. A 40- to 41-kDa (p41) doublet on Western blots detected with three different MAP kinase antibodies was coincident with peak II, and it probably corresponded to the avian homolog of p42mapk/erk2. Immunofluorescent studies with DU249 cells and chicken embryo fibroblasts revealed that most of the cross-reactive protein with at least two different MAP kinase antibodies was distributed in the nucleus. Subcellular fractionation studies confirmed a predominantly nuclear localization for p41 MAP kinase. Nocodazole arrest of DU249 cells was exploited for the detection of an M-phase-activated MBP kinase that was resolved from p41 MAP kinase by phenyl-Superose chromatography. Western blotting analysis with antibodies for the cdc2-encoded protein kinase and p13suc1-agarose binding studies allowed positive identification of this MBP kinase as p34cdc2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez E., Northwood I. C., Gonzalez F. A., Latour D. A., Seth A., Abate C., Curran T., Davis R. J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991 Aug 15;266(23):15277–15285. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Behra R., Christen P., Sonderegger P. Independent quantitation of the mitochondrial and the cytosolic isoenzyme of aspartate aminotransferase in chicken tissues by radioimmunoassays. J Biol Chem. 1981 Apr 10;256(7):3381–3384. [PubMed] [Google Scholar]
- Blenis J. Growth-regulated signal transduction by the MAP kinases and RSKs. Cancer Cells. 1991 Nov;3(11):445–449. [PubMed] [Google Scholar]
- Boulton T. G., Cobb M. H. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991 May;2(5):357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T. G., Gregory J. S., Cobb M. H. Purification and properties of extracellular signal-regulated kinase 1, an insulin-stimulated microtubule-associated protein 2 kinase. Biochemistry. 1991 Jan 8;30(1):278–286. doi: 10.1021/bi00215a038. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Yancopoulos G. D., Gregory J. S., Slaughter C., Moomaw C., Hsu J., Cobb M. H. An insulin-stimulated protein kinase similar to yeast kinases involved in cell cycle control. Science. 1990 Jul 6;249(4964):64–67. doi: 10.1126/science.2164259. [DOI] [PubMed] [Google Scholar]
- Casillas A., Hanekom C., Williams K., Katz R., Nel A. E. Stimulation of B-cells via the membrane immunoglobulin receptor or with phorbol myristate 13-acetate induces tyrosine phosphorylation and activation of a 42-kDa microtubule-associated protein-2 kinase. J Biol Chem. 1991 Oct 5;266(28):19088–19094. [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Chen R. H., Blenis J. Coordinate regulation of pp90rsk and a distinct protein-serine/threonine kinase activity that phosphorylates recombinant pp90rsk in vitro. Mol Cell Biol. 1991 Apr;11(4):1868–1874. doi: 10.1128/mcb.11.4.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Pelech S. L., Blenis J. Mitogen-activated Swiss mouse 3T3 RSK kinases I and II are related to pp44mpk from sea star oocytes and participate in the regulation of pp90rsk activity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4981–4985. doi: 10.1073/pnas.88.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicirelli M. F., Pelech S. L., Krebs E. G. Activation of multiple protein kinases during the burst in protein phosphorylation that precedes the first meiotic cell division in Xenopus oocytes. J Biol Chem. 1988 Feb 5;263(4):2009–2019. [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Ettehadieh E., Sanghera J. S., Pelech S. L., Hess-Bienz D., Watts J., Shastri N., Aebersold R. Tyrosyl phosphorylation and activation of MAP kinases by p56lck. Science. 1992 Feb 14;255(5046):853–855. doi: 10.1126/science.1311128. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Wu M., Gerhart J. C., Martin G. S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991 Apr;11(4):1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Moriyama K., Matsuda S., Okumura E., Kishimoto T., Kawasaki H., Suzuki K., Yahara I., Sakai H., Nishida E. Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J. 1991 Sep;10(9):2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Gómez N., Cohen P. Dissection of the protein kinase cascade by which nerve growth factor activates MAP kinases. Nature. 1991 Sep 12;353(6340):170–173. doi: 10.1038/353170a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Her J. H., Wu J., Rall T. B., Sturgill T. W., Weber M. J. Sequence of pp42/MAP kinase, a serine/threonine kinase regulated by tyrosine phosphorylation. Nucleic Acids Res. 1991 Jul 11;19(13):3743–3743. doi: 10.1093/nar/19.13.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi M., Nishida E., Sakai H. Activation of a Ca2+-inhibitable protein kinase that phosphorylates microtubule-associated protein 2 in vitro by growth factors, phorbol esters, and serum in quiescent cultured human fibroblasts. J Biol Chem. 1988 Apr 15;263(11):5396–5401. [PubMed] [Google Scholar]
- Krek W., Maridor G., Nigg E. A. Casein kinase II is a predominantly nuclear enzyme. J Cell Biol. 1992 Jan;116(1):43–55. doi: 10.1083/jcb.116.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Structure and developmental expression of the chicken CDC2 kinase. EMBO J. 1989 Oct;8(10):3071–3078. doi: 10.1002/j.1460-2075.1989.tb08458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., Brautigan D. L., Ingebritsen T. S., Avruch J. pp54 microtubule-associated protein-2 kinase requires both tyrosine and serine/threonine phosphorylation for activity. J Biol Chem. 1991 Jun 5;266(16):10043–10046. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langlois A. J., Lapis K., Ishizaki R., Beard J. W., Bolognesi D. P. Isolation of a transplantable cell line induced by the MC29 avian leukosis virus. Cancer Res. 1974 Jun;34(6):1457–1464. [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Matsuda S., Kosako H., Takenaka K., Moriyama K., Sakai H., Akiyama T., Gotoh Y., Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992 Mar;11(3):973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa J., Kitten G. T., Nigg E. A. A somatic cell-derived system for studying both early and late mitotic events in vitro. J Cell Sci. 1989 Nov;94(Pt 3):449–462. doi: 10.1242/jcs.94.3.449. [DOI] [PubMed] [Google Scholar]
- Nel A. E., Pollack S., Landreth G., Ledbetter J. A., Hultin L., Williams K., Katz R., Akerley B. CD-3-mediated activation of MAP-2 kinase can be modified by ligation of the CD4 receptor. Evidence for tyrosine phosphorylation during activation of this kinase. J Immunol. 1990 Aug 1;145(3):971–979. [PubMed] [Google Scholar]
- Nigg E. A., Schäfer G., Hilz H., Eppenberger H. M. Cyclic-AMP-dependent protein kinase type II is associated with the Golgi complex and with centrosomes. Cell. 1985 Jul;41(3):1039–1051. doi: 10.1016/s0092-8674(85)80084-2. [DOI] [PubMed] [Google Scholar]
- Northwood I. C., Gonzalez F. A., Wartmann M., Raden D. L., Davis R. J. Isolation and characterization of two growth factor-stimulated protein kinases that phosphorylate the epidermal growth factor receptor at threonine 669. J Biol Chem. 1991 Aug 15;266(23):15266–15276. [PubMed] [Google Scholar]
- Okuda K., Sanghera J. S., Pelech S. L., Kanakura Y., Hallek M., Griffin J. D., Druker B. J. Granulocyte-macrophage colony-stimulating factor, interleukin-3, and steel factor induce rapid tyrosine phosphorylation of p42 and p44 MAP kinase. Blood. 1992 Jun 1;79(11):2880–2887. [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. Mitogen-activated protein kinases: versatile transducers for cell signaling. Trends Biochem Sci. 1992 Jun;17(6):233–238. doi: 10.1016/s0968-0004(00)80005-5. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Tombes R. M., Meijer L., Krebs E. G. Activation of myelin basic protein kinases during echinoderm oocyte maturation and egg fertilization. Dev Biol. 1988 Nov;130(1):28–36. doi: 10.1016/0012-1606(88)90410-1. [DOI] [PubMed] [Google Scholar]
- Peter M., Sanghera J. S., Pelech S. L., Nigg E. A. Mitogen-activated protein kinases phosphorylate nuclear lamins and display sequence specificity overlapping that of mitotic protein kinase p34cdc2. Eur J Biochem. 1992 Apr 1;205(1):287–294. doi: 10.1111/j.1432-1033.1992.tb16779.x. [DOI] [PubMed] [Google Scholar]
- Posada J., Sanghera J., Pelech S., Aebersold R., Cooper J. A. Tyrosine phosphorylation and activation of homologous protein kinases during oocyte maturation and mitogenic activation of fibroblasts. Mol Cell Biol. 1991 May;11(5):2517–2528. doi: 10.1128/mcb.11.5.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Characterization of insulin-stimulated microtubule-associated protein kinase. Rapid isolation and stabilization of a novel serine/threonine kinase from 3T3-L1 cells. J Biol Chem. 1988 Sep 5;263(25):12721–12727. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Rossomando A. J., Sanghera J. S., Marsden L. A., Weber M. J., Pelech S. L., Sturgill T. W. Biochemical characterization of a family of serine/threonine protein kinases regulated by tyrosine and serine/threonine phosphorylations. J Biol Chem. 1991 Oct 25;266(30):20270–20275. [PubMed] [Google Scholar]
- Sanghera J. S., McNabb C. K., Tonks N., Pelech S. L. Tyrosyl phosphorylation and activation of the myelin basic protein kinase p44mpk during sea star oocyte maturation. Biochim Biophys Acta. 1991 Oct 26;1095(2):153–160. doi: 10.1016/0167-4889(91)90078-c. [DOI] [PubMed] [Google Scholar]
- Sanghera J. S., Paddon H. B., Bader S. A., Pelech S. L. Purification and characterization of a maturation-activated myelin basic protein kinase from sea star oocytes. J Biol Chem. 1990 Jan 5;265(1):52–57. [PubMed] [Google Scholar]
- Sanghera J. S., Paddon H. B., Pelech S. L. Role of protein phosphorylation in the maturation-induced activation of a myelin basic protein kinase from sea star oocytes. J Biol Chem. 1991 Apr 15;266(11):6700–6707. [PubMed] [Google Scholar]
- Seger R., Ahn N. G., Boulton T. G., Yancopoulos G. D., Panayotatos N., Radziejewska E., Ericsson L., Bratlien R. L., Cobb M. H., Krebs E. G. Microtubule-associated protein 2 kinases, ERK1 and ERK2, undergo autophosphorylation on both tyrosine and threonine residues: implications for their mechanism of activation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6142–6146. doi: 10.1073/pnas.88.14.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K. R., Worley P. F., Litz J. S., Parsons S. J., Huganir R. L., Baraban J. M. Electroconvulsive treatment induces a rapid and transient increase in tyrosine phosphorylation of a 40-kilodalton protein associated with microtubule-associated protein 2 kinase activity. J Neurochem. 1991 Jan;56(1):147–152. doi: 10.1111/j.1471-4159.1991.tb02574.x. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]